Abstract
Conjugation of various serotypes of pneumococcal polysaccharide (PnPS) to carrier protein enhances the magnitude of the polysaccharide-specific antibody response, presumably by eliciting T-cell help. However, variability in PnPS serotype-specific immunogenicity has been observed. CBA/J mice immunized with either 6B or 19F PnPS conjugated to the protein carrier Cross Reactive Material197 (CRM197) produce a strong anti-PnPS antibody response; however, when mice are immunized with 23F PnPS conjugated to CRM197, they fail to produce a significant anti-PnPS response. In order to determine whether this difference was related to alterations in antigen processing of the carrier protein and the subsequent T-cell responses, we studied proliferation of lymphocytes from CBA/J mice immunized with CRM197 alone or conjugated to 6B, 19F, or 23F PnPS. T-cell proliferative responses to synthetic peptides demonstrated that lymph node cells elicited by the poorly immunogenic conjugate 23F-CRM197 recognized many, but not all, of the epitopes recognized by lymph node cells elicited by 6B- and 19F-CRM197 as well as additional epitopes. Despite marked differences in PnPS-specific immunogenicity, all mice made high titers of CRM197 antibodies of the immunoglobulin G1 isotype. Cells from mice immunized with any of the conjugates yielded vigorous T-cell responses to whole antigen. We conclude that the serotype of PnPS can alter the peptide specificities of T-cell responses, but even a poorly immunogenic PnPS conjugate can elicit a significant T-cell response. Thus, conjugation of PnPS to a carrier protein that elicits carrier-specific T- and B-cell responses does not necessarily enhance PnPS immunogenicity.
Streptococcus pneumoniae remains a significant pathogen in children under the age of two, splenectomized individuals, and the elderly, despite the availability of a purified multivalent S. pneumoniae capsular polysaccharide (PnPS) vaccine (2, 5, 14, 22). Immunization with bacterial polysaccharide (PS) antigens typically induces a T-cell-independent type 2 antibody response characterized by high levels of immunoglobulin M (IgM), IgG antibodies primarily of the IgG3 subclass in mice and IgG2 in humans, an absent or blunted memory response, and no requirement for the direct involvement of T cells (16, 19, 20, 23, 24). Polysaccharides are thought to be unable to bind to class II major histocompatibility complex (MHC), and are thus poor inducers of T-cell responses (12, 13, 16, 23, 24). To overcome this limitation and to enhance immunogenicity, PSs have been conjugated to carrier proteins to make conjugate vaccines, an approach first reported in the 1920s and 1930s (3, 4, 9, 10). This strategy has been highly successful in the prevention of infection with Haemophilus influenzae type b (Hib) (1, 21).
While immunity to Hib requires antibodies to only one capsular PS serotype, there are at least 90 different S. pneumoniae capsular serotypes, more than 20 of which are considered clinically relevant (2). Therefore, pneumococcal conjugate vaccines will require multiple conjugates, each consisting of a different PnPS linked to a carrier protein. However, clinical trials with a heptavalent PnPS-protein conjugate vaccine, in which each PnPS was conjugated to the same carrier protein, showed that the monovalent components of the vaccine had widely varying abilities to elicit PnPS-specific antibodies (5, 7, 17). The reasons for such differences in immunogenicity are unclear, especially in instances where different PnPSs are attached by identical methods of conjugation to the same carrier protein.
In this report, we examine the immunogenicities of three PnPS-protein conjugate vaccines in a mouse model and investigate the mechanisms that might account for the significant differences observed in the magnitudes of PnPS-specific antibody responses despite linkage to the same carrier protein, Cross Reactive Material 197 (CRM197) (25). In particular, we address the hypothesis that conjugation of different PnPSs to a carrier protein such as CRM197 can change the T-cell response to the conjugate vaccine by altering the antigen processing of the carrier protein, thereby modifying T-cell help for B-cell production of PS-specific antibodies. S. pneumoniae capsular serotypes 6B, 19F, and 23F were chosen for study due to the high clinical incidence of disease caused by these three serotypes in humans and because of their inclusion as components in the new heptavalent pneumococcal conjugate vaccine undergoing clinical trials (7, 17). Our data show that conjugation of different PnPSs to the same carrier protein can alter the peptide specificity of T-cell responses. However, despite marked differences in the immunogenicity of the PnPS components of these pneumococcal conjugate vaccines in a mouse model, vigorous carrier protein-specific T-cell activation after immunization can be demonstrated with all three vaccines.
MATERIALS AND METHODS
Antigens.
Experimental lots of unconjugated CRM197 and 6B-CRM197, 19F-CRM197, and 23F-CRM197 conjugate vaccines were the generous gift of Wyeth-Lederle Vaccines (West Henrietta, N.Y.). PnPSs were individually conjugated to CRM197 by reductive amination. The PS/protein ratios of the experimental vaccine lots were as follows: 6B-CRM197, 0.69; 19F-CRM197, 0.66; and 23F-CRM197, 0.52 (8). These experimental lots did not contain any adjuvant, as is the case with the commercially available Hib-CRM197 conjugate vaccine HibTITER. Unconjugated 6B, 19F, and 23F PnPSs were obtained from American Type Culture Collection (Rockville, Md.). These PnPS preparations are similar to those used in the conjugation procedure. Pneumococcal cell wall polysaccharide (C-PS) was obtained from the University of Rochester (Rochester, N.Y.). A series of 16-mer CRM197 peptides with an overlap of 12 amino acids was produced by multipin synthesis (Chiron Technologies, Raleigh, N.C.). All conjugates and unconjugated PnPS, CRM197, and peptides were determined to contain less than 0.1 U of endotoxin per ml of sample by using a Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.).
Immunization of mice.
Six-week-old female CBA/J mice (Jackson Laboratories, Bar Harbor, Maine) were immunized intraperitoneally (i.p.) on days 0 and 14 with 11 μg (protein content) of CRM197, 10 μg (PS content) of 6B-CRM197, 19F-CRM197, and 23F-CRM197, or 10 μg of 6B, 19F, or 23F unconjugated PnPS in phosphate-buffered saline (PBS). The dose of unconjugated CRM197 was approximately equivalent to the amount of CRM197 injected into the conjugate-immunized mice. Ten micrograms of PnPS was chosen as the amount to be injected since dose response experiments demonstrated this dose to yield optimal antibody titers. Mice immunized with sterile PBS served as negative controls. CBA/J mice were chosen based on the availability of reagents for studying murine antigen processing and T cells, specifically the H-2k system (11), and as a result of the observed differences in the immunogenicities of the different serotypes of pneumococcal conjugate vaccines. These differences in PnPS immunogenicity are similar to those observed in humans (17). Mice were bled from the tail vein weekly for 5 weeks, and the sera were screened for anti-PnPS antibodies via enzyme-linked immunosorbent assay (ELISA) as described below.
ELISA for antibodies against polysaccharides and carrier protein.
Ninety-six-well PolySorp plates (Nunc, Roskilde, Denmark) were coated with 100 μl of 6B, 19F, or 23F PnPS (obtained from the American Type Culture Collection) at 10 μg/ml of PBS. These plates were previously found to bind all of these PnPS serotypes (26). After plates were blocked with 200 μl of PBS containing 1% bovine serum albumin and 1% NaN3, a 100-μl sample was added to each well. Sera, standards, and controls were diluted in PBS containing 1% bovine serum albumin and 1% NaN3. Fifty micrograms of C-PS/ml of sera was added to absorb anti-C-PS antibodies (15). Serum samples diluted 1:100 were used to assess relative differences in antibody production over time. To detect CRM197-specific antibodies, 96-well high-binding plates (Corning Glass Works, Corning, N.Y.) were coated with 100 μl of a solution containing 1 μg of CRM197 per ml of a coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3, pH 9.6). Serum samples were prepared as for the PnPS ELISA, except that C-PS was not added. The relative titers of IgM and IgG1 PS- or CRM197-specific antibodies were determined for serum samples obtained 2 weeks after the secondary immunization. Serial dilutions of these sera were used in the ELISA. Antisera derived after hyperimmunization of BALB/c mice with 6B-CRM197, 19F-CRM197, or 23F-CRM197 in monophosphoryl lipid-A (RibiImmunoChem Research, Hamilton, Mont.) served as positive controls. Detection of total PnPS- or CRM197-specific serum antibodies was performed by using goat anti-mouse kappa antibodies conjugated to alkaline phosphatase (AP) (Southern Biotech, Birmingham, Ala.). Anti-kappa antibodies were chosen since the vast majority of murine PS-specific antibodies contain kappa light chains. Serum antibodies of specific isotypes were detected by using goat anti-mouse-IgG1–AP and -IgM–AP antibodies. The plates were washed and developed with p-nitrophenyl phosphate (Sigma, St. Louis, Mo.) as the substrate, and absorbances were read as optical densities at 410 nm.
Lymph node proliferation assay.
Female CBA/J mice were immunized in the hind footpads with 100 μl of antigen (CRM197, 6B-CRM197, 19F-CRM197, 23F-CRM197, or hen egg lysozyme [HEL]) in complete Freund’s adjuvant (CFA, Sigma) at a final concentration of 800 μg/ml (protein content). HEL-immunized mice served as a negative control. Nine days later, the popliteal lymph nodes were removed, and the cells were suspended in standard media (Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, penicillin, streptomycin, l-glutamine, and sodium pyruvate [Hyclone, Logan, Utah]) and plated at a concentration of 4 × 105 cells/well in 96-well tissue culture plates with antigen at 0, 1, 3, and 10 μg/ml. Peptide-specific lymph node cell proliferation was assessed with each CRM197 peptide at a concentration of 5 μM. A solution containing 10 μg of CRM197 per ml served as a positive control in these experiments. The plates were incubated at 37°C in 5% CO2 for 4 days and then [3H]thymidine (1 μCi/well) was added. The following day the cells were harvested and [3H]thymidine incorporation was determined by a scintillation counter (Packard, Walkersville, Md.).
Statistical methods.
An analysis of variance was used to determine statistical differences between experimental groups in the lymph node proliferation assays. Differences within groups were analyzed by the use of the Tukey multiple comparison method. The threshold for statistical significance was taken as p ≤ 0.05.
RESULTS
Immunization with 6B-CRM197 and 19F-CRM197, but not 23F-CRM197, results in high titers of PnPS-specific antibodies.
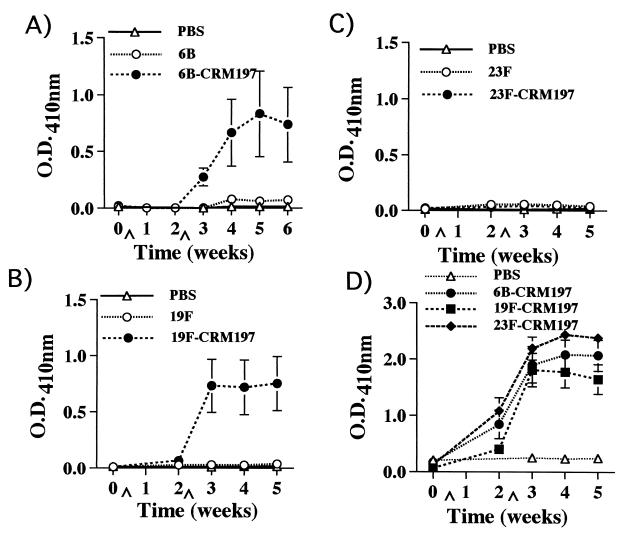
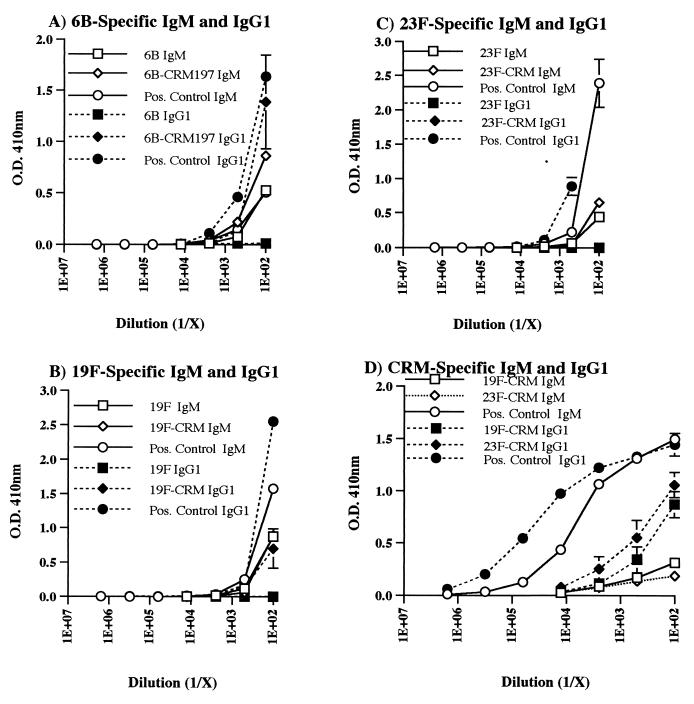
CBA/J mice were immunized i.p. with 6B, 6B-CRM197, 19F, 19F-CRM197, 23F, or 23F-CRM197. Sera were obtained weekly for 5 weeks and then tested by ELISA for the presence of PnPS- and CRM197-specific antibodies. High-titer 6B- and 19F-specific antibodies were detected in mice immunized with 6B-CRM197 or 19F-CRM197, in contrast to the low titers of 6B- and 19F-specific antibodies detected in mice immunized with unconjugated PnPS (Fig. 1A and B). In addition, the total 6B- and 19F-specific serum antibody levels elicited by the conjugates, but not by the unconjugated PnPS, rose significantly after secondary immunization, as expected. The PnPS-specific antibodies elicited by immunization with the conjugates were primarily of the IgM and IgG1 isotypes, in contrast to the predominantly IgM antibodies elicited by the unconjugated PnPS (Fig. 2A and B). PnPS-specific IgG3 was not detected in any of the sera tested.
FIG. 1.
Total serum Ig kappa chain reactivities of (A) 6B-specific, (B) 19F-specific, (C) 23F-specific, and (D) CRM197-specific antibodies over time in CBA/J mice as detected by PnPS solid-phase ELISA. Data shown are from a representative experiment that was repeated three times with similar results. Sera were diluted 1:100, and the mean absorbances ± standard errors of the means (SEMs) of six mice per group are shown. ∧ indicates time point of immunization. O.D., optical density.
FIG. 2.
Levels of (A) 6B-specific, (B) 19F-specific, (C) 23F-specific, and (D) CRM197-specific IgM and IgG1 in CBA/J mice were detected by PnPS or CRM197 solid-phase ELISA using isotype-specific conjugates. Reactivities of PnPS-specific IgG1 and IgM are shown as a function of serial serum dilution 14 days after secondary immunization. Points represent means ± SEMs of six mice per group, as in Fig. 1.
In comparison, mice immunized with either 23F or 23F-CRM197 had very low serum titers of 23F-specific antibodies (Fig. 1C and 2C). 23F-specific antibodies were, however, detected in sera from BALB/c mice immunized with 23F-CRM197, demonstrating that the appropriate 23F PnPS B-cell epitopes were not destroyed by the conjugation process (data not shown). Finally, CBA/J mice immunized with the conjugates in CFA according to the protocol for the lymph node proliferation assays had antibody titers similar to those of mice immunized i.p. without adjuvant. This immunization protocol also elicited relatively low titers of 23F-specific antibodies (data not shown).
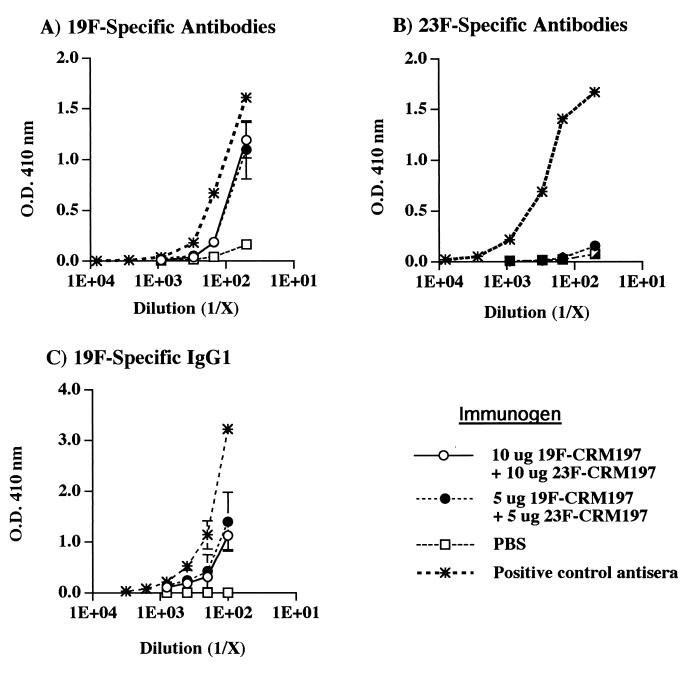
When mice were immunized simultaneously with both 19F-CRM197 and 23F-CRM197, the levels of 23F-specific antibody were very low (Fig. 3B). These same mice, however, produced significant levels of 19F-specific antibodies, including antibodies of the IgG1 isotype, production of which generally indicates the presence of T-cell help (Fig. 3A and C). Thus, despite the evidence of carrier-specific T-cell help, 23F-specific antibody levels remained very low.
FIG. 3.
Polysaccharide-specific antibody levels in mice immunized simultaneously with either 10 or 5 μg each of 19F-CRM197 and 23F-CRM197. Total serum Ig kappa chain reactivities of (A) 19F-specific and (B) 23F-specific antibodies in CBA/J mice as detected by PnPS solid-phase ELISA. 19F-specific IgG1 detected in the same sera is also shown (C). Reactivities of PnPS-specific antibodies are shown as a function of serum dilution. The mean absorbances ± SEMs of groups of three mice are shown. O.D., optical density.
In contrast to PnPS-specific antibody responses, carrier-specific (CRM197) antibody responses were equivalent in sera from mice immunized with 6B-CRM197, 19F-CRM197, and 23F-CRM197 (Fig. 1D). The predominant isotype of the CRM197-specific antibodies, following two immunizations, was IgG1 (Fig. 2D). These results indicate that an immunogenic form of CRM197 was administered to all of the mice, that CRM197 was processed and presented by antigen-presenting cells, and that T-cell help was elicited after immunization with any of the three conjugate vaccines used.
Conjugation of different PnPSs to CRM197 yields similar magnitudes of lymph node cell proliferation upon restimulation in vitro.
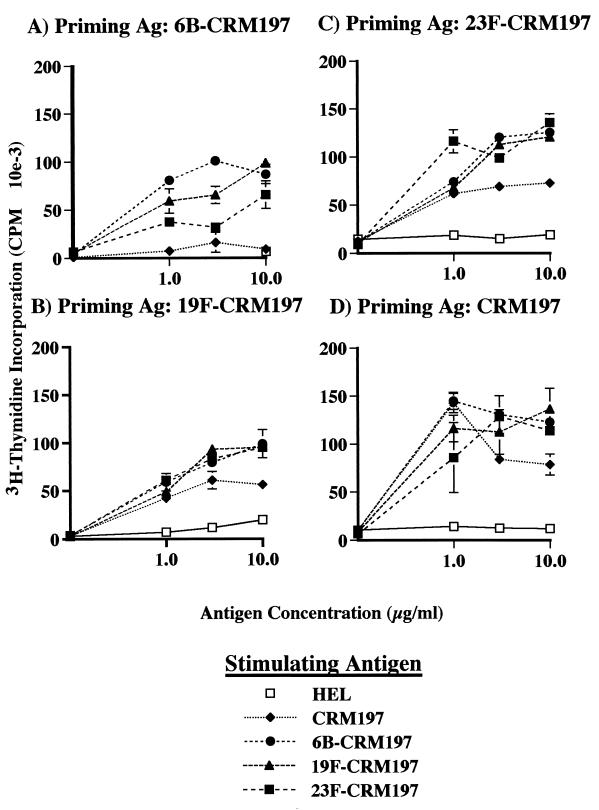
To more directly determine whether the serotype-specific difference in the ability to elicit PnPS-specific antibodies could be due to differential T-cell activation for the various conjugates, lymph node cells were obtained from mice immunized with 6B-CRM197, 19F-CRM197, 23F-CRM197, or unconjugated CRM197 in CFA. These cells were incubated in vitro with CRM197, 6B-CRM197, 19F-CRM197, or 23F-CRM197, and cellular proliferation, an indicator of antigen recognition by T cells, was measured by [3H]thymidine incorporation (18). Cells from mice primed with 6B-CRM197 proliferated more in response to the conjugates than in response to CRM197 alone (p ≤ 0.0011). Although CRM197 was less effective at eliciting T-cell proliferation after immunization with 19F-CRM197 or 23F-CRM197, these differences did not reach statistical significance (Fig. 4A, B, C, and D). In addition, the responses of lymph node cells elicited by CRM197, 6B-CRM197, 19F-CRM197, or 23F-CRM197 to any of the conjugates did not significantly differ between immunization groups (analysis of variance, p > 0.45). Control lymph node cells elicited by HEL only responded to in vitro stimulation with HEL and not to any of the conjugates (data not shown), indicating the specificity of the lymph node cells and the inability of the conjugates to act as nonspecific mitogens. Similarly, the PSs alone were unable to stimulate the lymph node cells (data not shown). Finally, in vitro stimulation with a mixture of CRM197 and each of the conjugates did not decrease proliferation, showing that nonspecific inhibition of lymphocyte proliferation by the unconjugated CRM197 preparation did not occur (data not shown). The equivalent proliferation of T cells and the three PnPS-CRM197 conjugates used as recall antigens suggests that similar numbers of peptide-MHC II complexes were generated by antigen processing of carrier protein in the conjugates.
FIG. 4.
Proliferation of lymph node cells from mice immunized with (A) 6B-CRM197, (B) 19F-CRM197, (C) 23F-CRM197, or (D) CRM197 as determined by [3H]thymidine incorporation. Lymph node cells were stimulated in vitro with whole antigen (CRM197, 6B-CRM197, 19F-CRM197, or 23F-CRM197). Data shown are derived from three mice per group and are representative of four experiments that yielded similar results. Values represent means ± standard deviations (S.D.s) from triplicate wells. Baseline proliferation in these experiments for lymph node cells stimulated in vitro with media alone was approximately 2,000 cpm.
Lymph node cells elicited by 23F-CRM197 recognize different sets of CRM197 peptide epitopes.
In order to determine if conjugation of PnPS to CRM197 altered the peptides recognized by carrier-specific T cells, lymph node cells from mice immunized with CRM197, 6B-CRM197, 19F-CRM197, or 23F-CRM197 in CFA were challenged in vitro with a series of synthetic 16-mer peptides, overlapping by 12 amino acids and covering the entire CRM197 amino acid sequence. This peptide library was expected to contain all possible linear T-cell epitopes of the A and B fragments of CRM197.
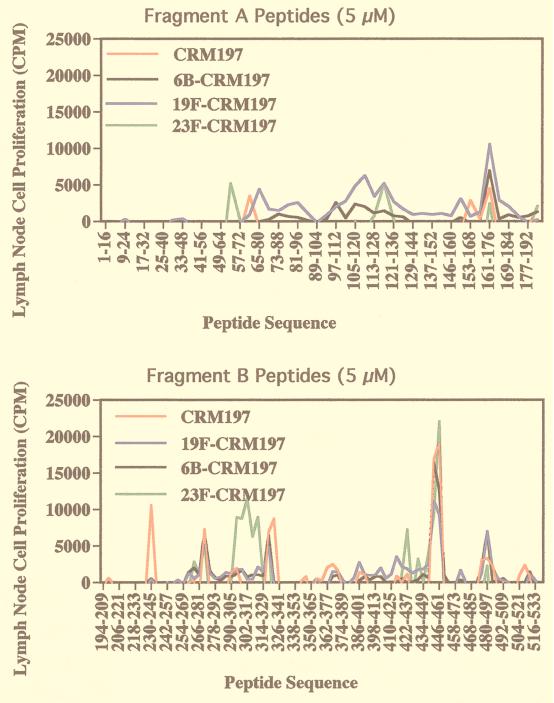
Lymph node cells elicited by CRM197 recognized a number of peptide sequences, including amino acids 360 to 380 of fragment B, a previously described CRM197 T-cell epitope in H-2s mice (6). However, lymph node cells elicited by 23F-CRM197 demonstrated several major changes in peptide reactivity in comparison with lymph node cells elicited by CRM197, 6B-CRM197, and 19F-CRM197 (Fig. 5). First, there was an expansion of the sequence of amino acids 438 to 465 recognized by lymph node cells elicited by 19F-CRM197, to 414 to 465 for lymph node cells elicited by 23F-CRM197. Second, lymph node cells elicited by 23F-CRM197 shifted recognition from positions 318 to 331, as seen in the case of lymph node cells elicited by CRM197 or 19F-CRM197, to amino acids 290 to 333 and with a different peak response. Lymph node cells elicited by 23F-CRM197 also recognized the sequence of amino acids 53 to 62, which was not recognized by lymph node cells elicited by the other conjugates. Lymph node cells elicited by 23F-CRM197 did not react to 54 other peptides that were recognized by lymph node cells elicited by 6B-CRM197 or 19F-CRM197 (Table 1). However, 38 of the 41 peptides that were recognized by lymph node cells elicited by 23F-CRM197 were also recognized by lymph node cells elicited by 6B-CRM197 and 19F-CRM197. In summary, lymph node cells from mice immunized with 23F-CRM197 were unable to recognize some peptides derived from the CRM197 amino acid sequence that were recognized by cells from mice immunized with the other conjugates. In addition, cells from mice immunized with 23F-CRM197 recognized additional peptides that were not recognized by lymph node cells from mice immunized with CRM197, 6B-CRM197, or 19F-CRM197. Thus, conjugation of CRM197 to 23F PnPS, as opposed to 6B or 19F PnPS, was associated with a different pattern of T-cell reactivity for CRM197-derived peptide epitopes, although there was substantial overlap in peptide reactivities.
FIG. 5.
Proliferation of lymph node cells from mice immunized with CRM197, 6B-CRM197, 19F-CRM197, or 23F-CRM197 to 16-mer peptides derived from the CRM197 fragment A (A) and fragment B (B) amino acid sequences as measured by [3H]thymidine incorporation. For clarity, only every third peptide in the series is marked on the x axis. Data shown are from a experiment that was repeated three times, yielding similar results each time. Values represent means from triplicate wells minus baseline proliferation plus two times the S.D.s. Only significant peaks are shown.
TABLE 1.
Comparative peptide reactivities of lymph node cellsa
| Antigen | Totalb | CRM197
|
6B-CRM197
|
19F-CRM197
|
23F-CRM197
|
||||
|---|---|---|---|---|---|---|---|---|---|
| +c | −d | + | − | + | − | + | − | ||
| CRM197 | 50 | 50 | 0 | 40 | 41 | 36 | 56 | 23 | 18 |
| 6B-CRM197 | 81 | 40 | 10 | 81 | 0 | 73 | 19 | 38 | 3 |
| 19F-CRM197 | 92 | 36 | 14 | 73 | 8 | 92 | 0 | 38 | 3 |
| 23F-CRM197 | 41 | 23 | 27 | 38 | 43 | 38 | 54 | 41 | 0 |
Lymph node cell proliferation in response to CRM197 peptides from mice immunized with carrier or PnPS conjugate vaccine. Proliferation to a peptide was defined as a cpm value greater than background plus two times the S.D.
Total number of peptides recognized by lymph node cells elicited by antigens listed in the first column.
+, Number of peptides recognized in common by lymph node cells elicited by antigens in left and top columns.
−, Number of peptides recognized by lymph node cells elicited by immunization with antigen at the top but not recognized by lymph node cells elicited by antigen at the left.
DISCUSSION
PS-protein conjugate vaccines have the potential to dramatically decrease the incidence of infection with PS-encapsulated bacteria such as pneumococcus. The Hib vaccines, the prototypes of successful conjugate vaccines, have resulted in the virtual eradication of disease due to Hib in the United States and much of the developed world. However, the Hib conjugate vaccines require multiple doses to achieve protection, and are thus costly, precluding widespread usage in the developing world. More detailed information on the properties of conjugate vaccines that contribute to greater immunogenicity may lead to improved designs for future conjugate vaccines.
Current hypotheses as to the mechanism by which the carrier protein enhances PS-specific immunogenicity envision internalization of the PS-carrier complex by the PS-specific B cells and proteolysis of the carrier protein, providing peptides able to bind noncovalently to class II MHC. The carrier confers on the PS-specific B cell the ability to activate helper T cells through the presentation of carrier-derived, class II MHC-bound peptides. The carrier-dependent boost to PS-specific immunogenicity is thus attributed primarily to carrier-dependent T-cell help. It was thus assumed that linking different PnPSs to the same immunogenic carrier protein would comparably enhance PS-specific antibody titers.
Given the success of the Hib vaccine, it would seem straightforward to create a series of PnPS-protein conjugate vaccines that consist of capsular PS from common pathogenic serotypes conjugated to the same carrier proteins successfully used in Hib conjugates. In fact, recent clinical trials with a heptavalent vaccine, using CRM197 as the carrier protein, showed clinical efficacy in preventing invasive pneumococcal disease from vaccine serotypes in children (7). However, it is intriguing that in clinical studies significant variations in immunogenicity of the serotype-specific PnPS components of these vaccines utilizing the same carrier protein have been observed (17). In the present study, we also observed pronounced differences in immunogenicity, in that the 23F-CRM197 conjugate vaccine elicited substantially less PnPS-specific antibody than the 6B-CRM197 and 19F-CRM197 vaccines in CBA/J mice. Thus, while these mice were perfectly capable of making antibodies to two of the PnPSs, one PnPS serotype was a poor immunogen even when conjugated to the same immunogenic carrier protein. We chose to explore the possibility that variation in T-cell responses to the carrier protein caused by conjugation to a PS of different structure yielded differences in antigen processing that might account for the difference in PS-specific conjugate immunogenicity. Therefore, we immunized mice with the three conjugate vaccines or carrier alone and determined the reactivity of lymph node cells following restimulation in vitro with each of the conjugates or with carrier protein. Lymph node cells from mice immunized with any of the conjugate vaccines, including 23F-CRM197, proliferated similarly upon restimulation with any of the conjugates. A vigorous T-cell recall response was obtained even when the PnPS-specific antibody response was poor. Thus, these results do not provide evidence for a defect in the ability to activate carrier protein-specific T cells uniquely associated with the 23F-CRM197 conjugate as an explanation for the observed deficiency in 23F PnPS-specific immunogenicity.
Lymph node cells from mice immunized with 6B-CRM197 proliferated significantly more upon restimulation with conjugates than when stimulated with carrier alone, suggesting that antigen processing of the carrier protein linked to 6B PnPS yielded more peptide-MHC II complexes than were generated from the unconjugated carrier protein. Lymph node cells from mice immunized with 19F-CRM197 or 23F-CRM197 demonstrated a similar trend, but statistical significance was not achieved. Further studies will be required to determine if conjugation of the CRM197 carrier protein to PnPS directly alters the efficiency of antigen processing by PS-specific B cells.
We next carried out a higher-resolution analysis by determining lymph node cell proliferative responses to 16-mer peptides (overlapping by 12 amino acids) spanning the entire primary structures of the A and B fragments of CRM197. Lymph node cells from mice immunized with CRM197, 6B-CRM197, or 19F-CRM197 exhibited similar patterns of peptide reactivity. Lymph node cells from mice immunized with 23F-CRM197 reacted with many of the same peptides that elicited responses from the CRM197-, 6B-CRM197-, or 19F-CRM197-immune cells but also reacted with additional groups of peptides. In addition, lymph node cells elicited with 23F-CRM197 did not react to many of the peptide epitopes recognized by lymph node cells elicited by the other conjugates. Conjugation of PnPS to CRM197 is not site specific, and the 23F PnPS may be bound to CRM197 so as to change the patterns of proteolysis during processing, yielding presentation of a different set of epitopes than the other two pneumococcal conjugate vaccines containing structurally distinct PnPS. Since the 23F PnPS conjugate exhibits the lowest PS-specific immunogenicity, it is possible that 23F-CRM197 elicited T cells that were not optimal for stimulation of 23F PnPS-specific B cells. However, the ability of 23F-CRM197 to elicit a strong CRM197-specific antibody response as well as strong lymph node cell recall proliferative responses to the whole vaccines suggests that the 23F-CRM197 vaccine activated helper T cells comparably to 19F-CRM197 and 6B-CRM197.
Measurement of the antibody responses to the carrier protein following immunization with PnPS-CRM197 conjugates indicated that all three PnPS conjugate vaccines elicited approximately equivalent amounts of total carrier-specific antibody and carrier-specific IgG1 antibody. These results imply that the failure of the 23F conjugate to induce PnPS-specific antibody or PnPS-specific IgG in this model was not due to failures of antigen administration or antigenic integrity of the carrier protein unique to that conjugate. Furthermore, since the response to the carrier protein is T cell dependent, as supported by the significant production of carrier-specific IgG1, these results suggest that the carrier in the 23F conjugate was competent to induce helper T cells. Thus, simple measurement of antibody or T-cell responses to carrier proteins of conjugate vaccines may not predict the immunogenicity of the PS.
In conclusion, our results suggest that variations in carrier-induced T-cell help may contribute to differences in the PS-specific immunogenicity of conjugate vaccines. However, the similarities in lymph node proliferation and CRM197 immunogenicity between all the conjugates studied suggest that other mechanisms may also explain the poor immunogenicity of the 23F PnPS conjugate vaccine. Differences in PnPS-specific B-cell precursor frequency or PnPS-CRM197-induced patterns of cytokine production could contribute to the observed variation in the PnPS-specific immunogenicity of PnPS-CRM197 conjugate vaccines.
ACKNOWLEDGMENTS
N.S.G. and J.R.S. shared senior authorship of this work.
This work was supported by National Institutes of Health grants AI 32596 and AI 27862 (to J.R.S.), AI 41657 (to N.S.G.), and AI 35726 (to C.V.H.) and by a grant from Wyeth-Lederle vaccines.
REFERENCES
- 1.Anderson P, Pichichero M E, Insel R A. Immunogens consisting of oligosaccharides from the capsule of Haemophilus influenzae type b coupled to diphtheria toxoid or the toxin protein CRM197. J Clin Investig. 1985;76:52–59. doi: 10.1172/JCI111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austrian R. Prevention of pneumococcal infection by immunization with capsular polysaccharides of Streptococcus pneumoniae: current status of polyvalent vaccines. J Infect Dis. 1977;136:S38–S42. doi: 10.1093/infdis/136.supplement.s38. [DOI] [PubMed] [Google Scholar]
- 3.Avery O T, Goebel W F. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery O T, Goebel W F. Chemo-immunological studies on conjugated carbohydrate-proteins. II. Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett D J, Lee C G, Ammann A J, Ayoub E M. IgG and IgM pneumococcal polysaccharide antibody responses in infants. Pediatr Res. 1984;18:1067–1071. doi: 10.1203/00006450-198411000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bixler G S J, Eby R, Dermody K M, Woods R M, Seid R C, Pillai S. Synthetic peptide representing a T-cell epitope of CRM197 substitutes as carrier molecule in a Haemophilus influenzae type b (HIB) conjugate vaccine. In: Atassi M Z, editor. Immunobiology of Proteins and Peptides. V. Vaccines. New York, N.Y: Plenum Publishing Corp.; 1989. pp. 175–180. [DOI] [PubMed] [Google Scholar]
- 7.Black S, Shinefield H, Ray P, Lewis E, Fireman B, Group K P V S, Austrian R, Siber G, Hackell J, Kohberger R, Chang I. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, D.C. 1998. Efficacy of heptavalent conjugate pneumococcal vaccine (Wyeth-Lederle) in 37,000 infants and children: results of the northern California Kaiser Permanente efficacy trial, abstr. LB-9; p. 23. [Google Scholar]
- 8.Eby, R. (Wyeth-Lederle Vaccines). Personal communication.
- 9.Goebel W F, Avery O T. Chemo-immunological studies on conjugated carbohydrate-proteins. I. The synthesis of p-aminophenol β-glucoside, p-aminophenol β-galactoside, and their coupling with serum globulin. J Exp Med. 1929;50:521–531. doi: 10.1084/jem.50.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goebel W F, Avery O T. Chemo-immunological studies on conjugated carbohydrate-proteins. IV. The synthesis of the p-aminobenzyl ether of the soluble specific substance of type III pneumococcus and its coupling with protein. J Exp Med. 1931;54:431–436. doi: 10.1084/jem.54.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin J, Chu R, Harding C. Early endosomes and a late endocytic compartment generate different peptide-class II MHC complexes via distinct processing mechanisms. J Immunol. 1997;158:1523–1532. [PubMed] [Google Scholar]
- 12.Harding C V, Roof R W, Allen P M, Unanue E R. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci USA. 1991;88:2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishioka G Y, Lamont A G, Thomson D, Bulbow N, Gaeta F C A, Sette A, Grey H. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992;148:2446–2451. [PubMed] [Google Scholar]
- 14.Klein J, Mortimer E J. Use of pneumococcal vaccine in children. Pediatrics. 1978;61:321–322. [PubMed] [Google Scholar]
- 15.Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1987;6:519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 17.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 18.Rosenwasser L J, Rosenthal A S. Adherent cell function in murine T lymphocyte antigen recognition. I. A macrophage-dependent T cell proliferation assay in the mouse. J Immunol. 1978;120:1991–1995. [PubMed] [Google Scholar]
- 19.Rubinstein L, Stein K. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. II. Specificity. J Immunol. 1988;141:4357–4362. [PubMed] [Google Scholar]
- 20.Rubinstein L, Stein K. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. I. Ontogeny. J Immunol. 1988;141:4352–4356. [PubMed] [Google Scholar]
- 21.Schneerson R, Barrera O, Sutton A, Robbins J. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber J R, Jacobs M R. Antibiotic-resistant pneumococci. In: Schreiber J R, Goldman D, editors. Pediatric clinics of North America. Vol. 42. Philadelphia, Pa: W. B. Saunders; 1995. pp. 519–537. [DOI] [PubMed] [Google Scholar]
- 23.Snapper C, Kehry M, Castle B, Mond J. Multivalent, but not divalent, antigen receptor cross-linkers synergize with CD40 ligand for induction of Ig synthesis and class switching in normal murine B cells: a redefinition of the TI-2 vs. T cell-dependent antigen dichotomy. J Immunol. 1995;154:1177–1187. [PubMed] [Google Scholar]
- 24.Stein K E. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165:S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 25.Uchida T, Pappenheimer J, Harper A A. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972;175:901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- 26.Zielen S, Broker M, Strnad N, Schwenen L, Schon P, Gottwald G, Hofmann D. Simple determination of polysaccharide specific antibodies by means of chemically modified ELISA plates. J Immunol Methods. 1996;193:1–7. doi: 10.1016/0022-1759(96)00033-6. [DOI] [PubMed] [Google Scholar]