Abstract
Older adults have been markedly impacted by the coronavirus disease 19 (COVID-19) pandemic, and many reports have cited concerns regarding potential psychiatric sequelae of coronavirus disease (COVID-19), but the actual effects of psychotropics on the COVID-19 are unclear. In this study, multivariate logistic regression was used to evaluate associations between the prescription of psychotropics and the risk of SARS-CoV-2 infection, and COVID-19-related death among the participants who were tested for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) before October 18, 2021, in UK Biobank. The psychotropics included 18 types of medications. Among 168,173 participants who underwent testing for SARS-CoV-2 RNA, 30,577 (18.2%) were positive, and 14,284 (8.5%) participants used psychotropics. Among 30,577 participants who were infected with SARS-CoV-2, 1,181 (3.9%) were COVID-19-related deaths, and 2,542 (8.3%) participants used psychotropics. In multivariate logistic regression, psychotropics use was significantly associated with the risk of SARS-CoV-2 infection (odds ratio [OR], 0.95; 95% confidence interval [CI], 0.88–0.98), and COVID-19-related death (OR, 0.78; 95% CI, 0.64–0.98). Interestingly, the use of diazepam was significantly associated with a 31% lower risk of SARS-CoV-2 infection (OR, 0.69; 95% CI, 0.53–0.88). The use of sertraline was significantly associated with a 89% lower risk of COVID-19-related death (OR, 0.11; 95% CI, 0.02–0.39). In conclusion, our findings suggested that the use of psychotropics was associated with a lower risk of SARS-CoV-2 infection and COVID-19-related deaths.
Keywords: Psychotropics, COVID-19, Pharmacoepidemiology
Abbreviations: COVID-19, coronavirus disease
1. Introduction
Coronavirus disease (COVID-19) is a highly contagious and life-threatening infection caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2, Mutesa et al., 2021). Potential explanations for the risk of severe illness from COVID-19 include, but are not limited to, older age, social vulnerability and economic status, psychiatric disorders, and comorbidities (Gupta et al., 2020; Toubasi et al., 2021). People with mental illnesses, such as schizophrenia, bipolar disorder, and major depressive disorder were more susceptible to SARS-CoV-2 (Fornaro et al., 2021; Fonseca et al., 2020). Moreover, aging has been proven to be one of the main risk factors for subsequent poor COVID-19 outcomes, and it is independent of other age-related comorbidities like diabetes, cardiovascular diseases, or obesity (Cao et al., 2022). The severity and mortality in patients with COVID-19 have been reported to increase with aging (Liu et al., 2020). Thus, identifying modifiable risk factors and finding existing drugs that are effective for treating COVID-19 among middle-aged and older adults would be of substantial public health benefit.
To date, several studies have shown that COVID-19 survivors appear to be at an increased risk of psychiatric sequelae and that psychiatric diagnosis might be an independent risk factor for COVID-19 (Taquet et al., 2021; Lo et al., 2021). A retrospective cohort study from New York found that a diagnosis of schizophrenia ranked behind age in the strength of an association with COVID-19-related mortality when compared with other risk factors (Nemani et al., 2021). Psychiatric disorders have been shown to be associated with an increased risk of altered immune responses and severe infections (Druss., 2020). Although psychiatric disorders contribute significantly to the global burden of the disease, over two-thirds of those affected do not receive any treatment. Only half of those with common mental disorders, such as depression or anxiety disorders, consulted their general practitioner (Armitage., 2021).
Psychotropics are the mainstay of treatment for patients with mental illnesses, many mental illnesses require lifelong medication to prevent recurrence (Reddy et al., 2020) While most of these agents have physiological effects on the brain, immune system, and gastrointestinal tract (Thorkelson et al., 2016). Previous studies have shown that atypical antipsychotic medications could reduce the mortality of COVID-19 because psychotropics exert the therapeutic effect of relieving mental symptoms by reducing the inflammatory response to modulate the body's immunity (Wohleb et al., 2016). However, there are increasing concerns that the adverse effects of psychotropic medications may worsen the course and outcome of underlying medical conditions (Ostuzzi et al., 2020). It is therefore necessary to explore the association between psychotropics and the risk of COVID-19 outcomes. To the best of our knowledge, this has rarely been investigated.
Thus, we aimed to investigate the association between the prescription of psychotropic medications and the risk of COVID-19 outcomes by taking advantage of the rich and continuously updated data on COVID-19 available in the UK Biobank.
2. Methods
2.1. Study design and participants
Data were obtained from the UK Biobank (https://www.ukbiobank.ac.uk/), using previously described methods (Sudlow et al., 2015). In brief, over 502,000 community-dwelling individuals were recruited for this study during 2006–2010, and these individuals underwent detailed medical assessments. Data were collected on a range of topics, including social and demographic factors, health, and behavioral risk factors, using standardized questionnaires administered by trained interviewers and self-completion by a computer. Before data collection, all participants provided written informed consent. The UK Biobank has full ethical approval from the NHS National Research Ethics Service (http://www.ukbiobank.ac.uk/ethics/). This work was conducted under the UK Biobank application number 45,676. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
We analyzed the association between psychotropic drug use and the risk of SARS-CoV-2 infection among the participants (n = 168,173) tested for SARS-CoV-2 RNA from March 16, 2020, to October 18, 2021, from England, Scotland, and Wales in the UK Biobank. The association between psychotropic drugs and COVID-19-related deaths was analyzed among participants who were tested positive for SARS-CoV-2 (n = 30,577).
2.2. Medication and history assessment
Use of psychotropic drugs in the UK Biobank was self-reported regular use (defined as most days of the week for the last 4 weeks) of psychotropic drugs and types, which was first recorded by a touchscreen questionnaire from participants, and then verified during a verbal interview with a trained nurse. Self-reported psychotropic drug use was first ascertained from participants using a touchscreen questionnaire at baseline and then verified by a verbal interview with a UK Biobank nurse. The information for regular psychotropic drug use was collected from the touchscreen questionnaire. In the section of ‘Do you regularly take any of the following?’ and then we could select more than one answer from a list of psychotropic drugs (including amitriptyline, citalopram, and so on). Regular use of psychotropic drugs was defined as 1=yes, 0=no, respectively. The list of psychotropic drugs is presented in the online supplemental Table S1. We subgrouped the psychotropic drug users into one and more than one type of psychotropic drug users and compared them to nonusers.
The history of the disease was defined as a self-reported physician-diagnosed primary care or hospital record at or before recruitment. We characterized the participants as having or not having a history of chronic obstructive pulmonary disease (COPD), chronic kidney disease, diabetes, cardiovascular diseases (CVD), asthma, depression, anxiety, substance misuse, psychotic disorders, dementia/delirium, epilepsy, neuropathic pain, multiple sclerosis (the International Classification of Diseases [ICD] codes and self-reported codes in the UK biobank were shown in Table S2).
2.3. Outcomes of COVID-19
The primary outcome was the risk of SARS-CoV-2 infection, and the secondary outcome was the risk of COVID-19-related death. SARS-CoV-2 infection was defined as a positive reverse transcription polymerase chain reaction (RT-PCR) test result on the Public Health England microbiology database. The ICD-10 codes denoting COVID-19-related death were U07.1 (n = 1173, virus identified in laboratory testing) and U07.2 (n = 8, clinical or epidemiological diagnosis of COVID-19 where laboratory testing was inconclusive or not available).
2.4. Statistical analysis
To reduce the confounding effects of potential risk factors on outcomes, we used multivariate logistic regression models that adjusted for the following variables: demographic characters (age, sex, race, or ethnicity), lifestyle factors (smoking history, alcohol consumption history), socioeconomic factors (Townsend deprivation index [TDI], educational attainment, annual household income), body mass index (BMI), history of COPD, chronic kidney disease, diabetes, CVD, asthma, depression, anxiety, substance misuse, psychotic disorders, dementia/delirium, epilepsy, neuropathic pain, and multiple sclerosis. TDI is widely used as a measure of area-level socioeconomic deprivation, with higher scores representing greater deprivation. If the covariate information was missing, we classified it into a separate category.
We assessed the association between psychotropics and the risk of SARS-CoV-2 infection by conducting multivariate logistic regression analyses among the participants with COVID-19 test results. For the association between psychotropic drug use and the risk of COVID-19-related death, a multivariate logistic regression analysis was performed among the participants who were tested positive for SARS-CoV-2. Multivariable logistic regression analysis was performed to calculate odds ratios (ORs), and 95% confidence intervals (CIs), which assess the effects of one or more types of psychotropics on SARS-CoV-2 infection and COVID-19-related deaths. In addition, psychotropic drugs were retained in the models to adjust for polypharmacy due to the participants might be exposed to multiple medications. A Bonferroni-type correction was used to adjust for multiple comparisons within medication classes.
We performed stratified analyses to evaluate potential modification effects according to age, sex, race or ethnicity, BMI, smoking status, alcohol drinking status, TDI, education, household income, number of somatic comorbidities, and mental disorders.
We performed a sensitivity analysis among overall participants in the UK biobank (n = 471,607), excluding loss to follow-up (n = 1,392), and death before March 16, 2020 (n = 29,508). We used multiple imputations to handle missing values as sensitivity analysis. In addition, we conducted an analysis by using inverse probability of treatment weighting (IPTW), and including the propensity score as an additional covariate. A two-tailed P-value < 0.05 was considered statistically significant. All statistical analyses were performed with R statistical software, version 4.0.3.
3. Results
3.1. Characteristics of the participants
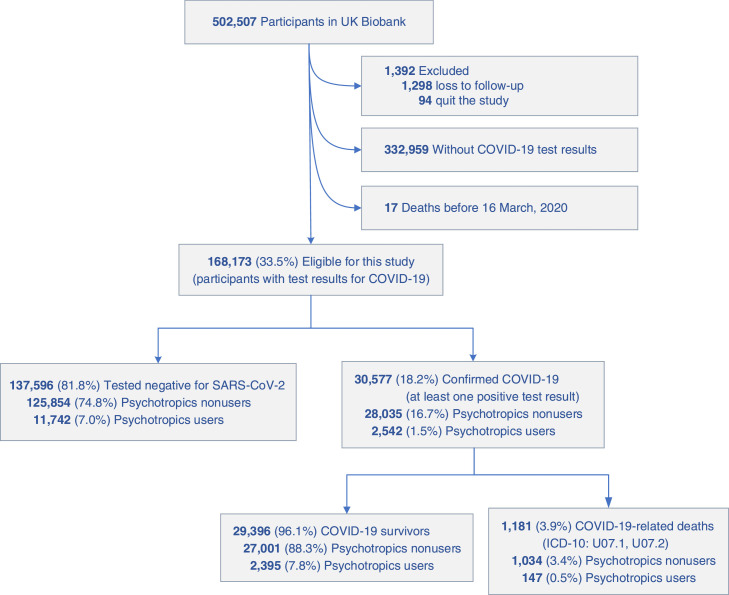
The flowchart of the population screening process was shown in Fig. 1 , and the characteristics of participants by two COVID-19 outcomes are summarized in Table 1 . The total sample included 168,173 participants with a SARS-CoV-2 RNA test in UK Biobank. Of the 168,173 participants, 77,084 (45.8%) were men, the mean age (SD) was 69.9 years (8.22), and the age ranged from 50 to 88 years old (Fig. 1). We identified 14,284 (8.5%) individuals were psychotropic drug users, and 153,889 (91.5%) individuals were nonusers. According to the SARS-CoV-2 test records, 30,577 and 1181 cases of SARS-CoV-2 infection and COVID-19-related deaths were determined, respectively. The mean age (SD) of the participants confirmed COVID-19 was 67.28 years (8.52), which was younger than the total participants. However, the mean age (SD) of the participants who died of COVID-19 was 76.22 years (6.05), and 827 (70.0%) participants were ≥75 years old. The participants with the lowest annual household income (< 18,000 £), higher BMI, previous/current smoking, never/previous alcohol drink, and comorbidities had a higher prevalence of COVID-19 outcomes than did the others (Table 1).
Fig. 1.
Flowchart of the participants selected in the study.
Table 1.
Demographic and clinical characteristics of the study cohort from UK Biobank.
| Characteristics n (%) |
Total (n = 168,173) |
SARS-CoV-2 infection |
Covid-19-related death |
||
|---|---|---|---|---|---|
| No (n = 137,596) |
Yes (n = 30,577) |
No (n = 29,396) |
Yes (n = 1,181) |
||
| Age, years, mean (SD) | 69.92 (8.22) | 70.51 (8.04) | 67.28 (8.52) | 66.92 (8.41) | 76.22 (6.05) |
| Age category | |||||
| <65 | 51,203 (30.4) | 37,623 (27.3) | 13,580 (44.4) | 13,506 (45.9) | 74 (6.3) |
| 65–74 | 56,607 (33.7) | 47,513 (34.5) | 9,094 (29.7) | 8,814 (30.0) | 280 (23.7) |
| ≥75 | 60,363 (35.9) | 52,460 (38.1) | 7,903 (25.8) | 7,076 (24.1) | 827 (70.0) |
| Sex (male) | 77,084 (45.8) | 62,621 (45.5) | 14,463 (47.3) | 13,711 (46.6) | 752 (63.7) |
| Race (white) | 158,325 (94.1) | 130,323 (94.7) | 28,002 (91.6) | 26,927 (91.6) | 1,075 (91.0) |
| TDI, mean (SD) | 3.02 (1.42) | 2.98 (1.42) | 3.18 (1.42) | 3.17 (1.42) | 3.48 (1.41) |
| Education | |||||
| College degree | 19,651 (11.7) | 16,904 (12.3) | 2,747 (9.0) | 2,652 (9.0) | 95 (8.0) |
| A-level | 4,953 (2.9) | 4,185 (3.0) | 768 (2.5) | 743 (2.5) | 25 (2.1) |
| O-level | 30,293 (18.0) | 25,241 (18.3) | 5,052 (16.5) | 4,905 (16.7) | 147 (12.4) |
| CSE or equivalent | 11,173 (6.6) | 8,273 (6.0) | 2,900 (9.5) | 2,863 (9.7) | 37 (3.1) |
| NVQ or equivalent | 23,927 (14.2) | 18,654 (13.6) | 5,273 (17.2) | 5,120 (17.4) | 153 (13.0) |
| Other professional | 74,920 (44.5) | 61,732 (44.9) | 13,188 (43.1) | 12,504 (42.5) | 684 (57.9) |
| Annual household income, £ | |||||
| < 18,000 | 30,672 (18.2) | 24,951 (18.1) | 5,721 (18.7) | 5,275 (17.9) | 446 (37.8) |
| 18,000–30,999 | 35,538 (21.1) | 29,121 (21.2) | 6,417 (21.0) | 6,172 (21.0) | 245 (20.7) |
| 31,000–51,999 | 37,430 (22.3) | 30,090 (21.9) | 7,340 (24.0) | 7,197 (24.5) | 143 (12.1) |
| 52,000–100,000 | 30,351 (18.0) | 24,901 (18.1) | 5,450 (17.8) | 5,375 (18.3) | 75 (6.4) |
| > 100,000 | 9,657 (5.7) | 8,282 (6.0) | 1,375 (4.5) | 1,360 (4.6) | 15 (1.3) |
| BMI, kg/m² | |||||
| < 18.5 | 673 (0.4) | 574 (0.4) | 99 (0.3) | 94 (0.3) | 5 (0.4) |
| 18.5–24.9 | 50,172 (29.8) | 42,007 (30.5) | 8,165 (26.7) | 7,992 (27.2) | 173 (14.6) |
| 25.0–29.9 | 70,565 (42.0) | 57,697 (41.9) | 12,868 (42.1) | 12,413 (42.2) | 455 (38.5) |
| ≥ 30.0 | 43,306 (25.8) | 34,473 (25.1) | 8,833 (28.9) | 8,339 (28.4) | 494 (41.8) |
| Smoking status | |||||
| Never | 89,487 (53.2) | 73,034 (53.1) | 16,453 (53.8) | 16,015 (54.5) | 438 (37.1) |
| Previous | 60,568 (36.0) | 49,818 (36.2) | 10,750 (35.2) | 10,202 (34.7) | 548 (46.4) |
| Current | 17,096 (10.2) | 13,898 (10.1) | 3,198 (10.5) | 3,017 (10.3) | 181 (15.3) |
| Alcohol status | |||||
| Never | 7,492 (4.5) | 5,862 (4.3) | 1,630 (5.3) | 1,543 (5.2) | 87 (7.4) |
| Previous | 5,932 (3.5) | 4,814 (3.5) | 1,118 (3.7) | 1,022 (3.5) | 96 (8.1) |
| Current | 154,515 (91.9) | 126,726 (92.1) | 27,789 (90.9) | 26,798 (91.2) | 991 (83.9) |
| Comorbidity | |||||
| COPD | 8,991 (5.3) | 7,444 (5.4) | 1,547 (5.1) | 1,271 (4.3) | 276 (23.4) |
| CKD | 8,615 (5.1) | 7,158 (5.2) | 1,457 (4.8) | 1,182 (4.0) | 275 (23.3) |
| Diabetes | 16,701 (9.9) | 13,571 (9.9) | 3,130 (10.2) | 2,742 (9.3) | 388 (32.9) |
| CVD | 35,774 (21.3) | 30,113 (21.9) | 5,661 (18.5) | 4,951 (16.8) | 710 (60.1) |
| Asthma | 17,666 (10.5) | 14,371 (10.4) | 3,295 (10.8) | 3,098 (10.5) | 197 (16.7) |
| Depression | 11,321 (6.7) | 9,197 (6.7) | 2,124 (6.9) | 1,948 (6.6) | 176 (14.9) |
| Anxiety | 8,519 (5.1) | 7,051 (5.1) | 1,468 (4.8) | 1,366 (4.6) | 102 (8.6) |
| Psychotic disorder | 715 (0.4) | 573 (0.4) | 142 (0.5) | 121 (0.4) | 21 (1.8) |
| Substance misuse | 11,500 (6.8) | 9,538 (6.9) | 1,962 (6.4) | 1,777 (6.0) | 185 (15.7) |
| Multiple sclerosis | 811 (0.5) | 700 (0.5) | 111 (0.4) | 96 (0.3) | 15 (1.3) |
| Neuropathic pain | 17,217 (10.2) | 14,464 (10.5) | 2,753 (9.0) | 2,547 (8.7) | 206 (17.4) |
| Dementia/delirium | 6,606 (3.9) | 5,002 (3.6) | 1,604 (5.2) | 1,221 (4.2) | 383 (32.4) |
| Epilepsy | 2,736 (1.6) | 2,232 (1.6) | 504 (1.6) | 448 (1.5) | 56 (4.7) |
| Psychotropics users | 14,284 (8.5) | 11,742 (8.5) | 2,542 (8.3) | 2,395 (8.1) | 147 (12.4) |
Abbreviations: TDI: Townsend deprivation index. CSE: Certificate of Secondary Education. NVQ: National Vocation Qualifications. BMI: Body mass index. COPD: Chronic obstructive pulmonary disease. CKD: Chronic kidney disease. CVD: Cardiovascular disease.
3.2. Association between each type of psychotropics and the risk of COVID-19
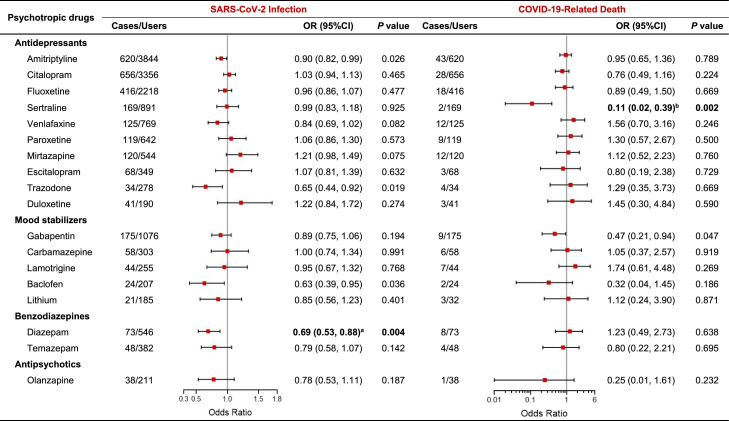
In this study, we filtered all participants in the UK Biobank using psychotropic drugs, and the 18 types of psychotropic medications along with the number of users were listed in Supplemental Table S1. The associations between each psychotropic drug and the risk of COVID-19 outcomes were shown in Fig. 2 . Additionally, the association between one or more types of psychotropic drug use and the risk of COVID-19 outcomes were shown in Table S4.
Fig. 2.
Association between psychotropics and the risk of COVID-19 outcomes. The analysis used multivariate logistic regression models, and adjusted for age, sex, race, smoking history, alcohol consumption history, Townsend deprivation index (TDI), educational attainment, annual household income, body mass index (BMI), history of chronic obstructive pulmonary disease (COPD), chronic kidney disease, diabetes, cardiovascular disease (CVD), asthma, depression, anxiety, substance misuse, psychotic disorders, dementia/delirium, epilepsy, neuropathic pain, multiple sclerosis, and psychotropic medications. aP < 0.03, where α’ = 0.05/2 = 0.025 to correct for multiple comparisons. bP < 0.005, where α’ = 0.05/10 = 0.005 to correct for multiple comparisons.
As shown in Fig. 2, the odds ratios (ORs) of SARS-CoV-2 infection and COVID-19-related death associated with psychotropic drug therapy were 0.95 (95% confidence interval [CI], 0.88–0.98) and 0.78 (95% CI, 0.64–0.98) among the participants, respectively. After Bonferroni correction, benzodiazepines (OR, 0.72; 95% CI, 0.58–0.87) were associated with a decreased risk of SARS-CoV-2 infection, in which diazepam (OR, 0.69; 95% CI, 0.53–0.88) were associated with a 31% lower risk of SARS-CoV-2 infection. We found that the use of sertraline was related to an 89% lower risk of COVID-19-related death (OR, 0.11; 95% CI, 0.02–0.39). Similar results were observed among the individuals only using sertraline (OR, 0.13; 95% CI, 0.02–0.45; Table S4).
3.3. Age, psychotropics, and risk of COVID-19
We conducted a stratification analysis to determine whether the characteristics of participants modified the association between the use of psychotropics and the risk of SARS-CoV-2 infection and COVID-19-related deaths. We found that the decreased risk of COVID-19 observed in participants using psychotropic medications did not vary by sex, race, TDI, educational attainment, annual household income, BMI, smoke status, alcohol intake status, or mental disorders (P interaction > 0.05, Table 2 ). A significant interaction was observed between psychotropic medications and the number of somatic comorbidities on the risk of COVID-19-related death (P interaction < 0.001, Table 2), in which the protective effect of psychotropic drugs was more evident among participants with somatic comorbidities compared to the participants without comorbidities. Additionally, we found that the OR for SARS-CoV-2 infection (OR, 0.87; 95% CI, 0.80–0.94; P interaction < 0.001) was lower among individuals younger than 65 years, and the OR for COVID-19-related death (OR, 0.76; 95% CI, 0.61–0.95; P interaction = 0.01) were lower among the elderly population.
Table 3.
Risk of COVID-19 among individuals treated with psychotropic medications by different characteristics compared to nonusers.
| SARS-CoV-2 Infection (N = 3,591) |
COVID-19-Related Death (N = 1,881) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Users (n/N = 2,542/ 14,284) |
Nonusers (n/N = 28,035/ 153,889) |
OR (95%CI) | P interaction | Users (n/N = 147/ 2542) |
Nonusers (n/N = 1,034/ 28,035) |
OR (95%CI) | P interaction | |
| Age, years | <0.001 | 0.010 | ||||||
| < 65 | 979/4,151 (23.6) | 12,601/47,052 (26.8) | 0.87 (0.80, 0.94) | 12/979 (1.2) | 62/12,601 (0.5) | 0.96 (0.43, 2.01) | ||
| ≥ 65 | 1,563/10,133 (15.4) | 15,434/106,837 (14.4) | 1.04 (0.98, 1.10) | 135/1,563 (8.6) | 972/15,434 (6.3) | 0.76 (0.61, 0.95) | ||
| Sex | 0.387 | 0.485 | ||||||
| Men | 1,716/4,557 (37.7) | 13,637/72,527 (18.8) | 1.01 (0.95, 1.07) | 75/826 (9.1) | 677/13,637 (5.0) | 0.83 (0.63, 1.07) | ||
| Women | 826/9,727 (8.5) | 14,398/81,362 (17.7) | 0.92 (0.86, 0.99) | 72/1,716 (4.2) | 357/14,398 (2.5) | 0.75 (0.54, 0.99) | ||
| Race or ethnicity | 0.354 | 0.887 | ||||||
| White | 2,396/13,669 (17.5) | 25,606/144,656 (17.7) | 1.00 (0.96, 1.06) | 136/2,396 (5.7) | 939/25,606 (3.7) | 0.79 (0.62, 0.99) | ||
| Others | 131/531 (24.7) | 2,291/8,478 (27.0) | 0.79 (0.49, 1.25) | 10/131 (7.6) | 84/2,291 (3.7) | 0.89 (0.37, 2.00) | ||
| Townsend deprivation index | 0.922 | 0.230 | ||||||
| < Median | 982/6,246 (15.7) | 12,763/77,727 (16.4) | 0.99 (0.88, 1.11) | 62/1118 (5.5%) | 423/14,148 (3.0) | 1.04 (0.73, 1.46) | ||
| ≥ Median | 1,554/8,010 (19.4) | 15,236/75,964 (20.1) | 0.99 (0.93, 1.06) | 85/1418 (6.0) | 610/13,851 (4.4) | 0.64 (0.48, 0.85) | ||
| Educational attainment | 0.194 | 0.894 | ||||||
| College degree | 164/1,280 (12.8) | 2,583/18,371 (14.1) | 0.83 (0.70, 0.99) | 10/164 (6.1) | 85/2,583 (3.3) | 0.59 (0.24, 1.34) | ||
| A-level | 70/384 (18.2) | 698/4,569 (15.3) | 1.33 (0.99, 1.78) | 2/70 (2.9) | 23/698 (3.3) | 0.57 (0.08, 2.56) | ||
| O-level | 402/2,389 (16.8) | 4,650/27,904 (16.7) | 1.05 (0.93, 1.18) | 21/402 (5.2) | 126/4,650 (2.7) | 0.89 (0.47, 1.60) | ||
| CSE or equivalent | 250/1,053 (23.7) | 2,650/10,120 (26.2) | 1.04 (0.88, 1.22) | 4/250 (1.6) | 33/2,650 (1.2) | 0.43 (0.09, 1.55) | ||
| NVQ or equivalent | 434/1,948 (22.3) | 4,839/21,979 (22.0) | 1.07 (0.95, 1.21) | 18/434 (4.1) | 135/4,839 (2.8) | 0.69 (0.34, 1.32) | ||
| Other professional | 1,174/6,941 (16.9) | 12,014/67,979 (17.7) | 0.97 (0.90, 1.04) | 89/1,174 (7.6) | 595/12,014 (5.0) | 0.72 (0.52, 0.98) | ||
| Annual household income, £ | 0.070 | 0.804 | ||||||
| < 18,000 | 788/4,335 (18.2) | 4,933/26,337 (18.7) | 0.99 (0.90, 1.08) | 64/788 (8.1) | 382/4,933 (7.7) | 0.71 (0.51, 0.97) | ||
| 18,000–30,999 | 563/3,244 (17.4) | 5,854/32,294 (18.1) | 1.01 (0.91, 1.12) | 33/563 (5.9) | 212/5,854 (3.6) | 1.19 (0.73, 1.87) | ||
| 31,000–51,999 | 439/2,499 (17.6) | 6,901/34,931 (19.8) | 0.89 (0.82, 0.99) | 15/439 (3.4) | 128/6,901 (1.9) | 1.06 (0.51, 2.03) | ||
| 52,000–100,000 | 274/1,492 (18.4) | 5,176/28,859 (17.9) | 1.04 (0.90, 1.20) | 4/274 (1.5) | 71/5,176 (1.4) | 0.52 (0.12, 1.68) | ||
| > 100,000 | 62/368 (16.8) | 1,313/9,289 (14.1) | 1.18 (0.87, 1.58) | 2/62 (3.2) | 13/1,313 (1.0) | 0.35 (0.01, 8.77) | ||
| (Table 2 continues on next page) | ||||||||
| Body mass index, kg/m² | 0.611 | 0.401 | ||||||
| <18.5 | 14/80 (17.5) | 85/593 (14.3) | 1.10 (0.54, 2.11) | 0/14 | 5/85 (5.9) | NA | ||
| 18.5–24.9 | 517/3,337 (0.5) | 7,648/46,835 (16.3) | 0.87 (0.77, 0.98) | 18/517 (3.5) | 155/7,648 (2.0) | 1.09 (0.59, 1.92) | ||
| 25.0–29.9 | 935/5,313 (17.6) | 11,933/65,252 (18.3) | 0.97 (0.87, 1.07) | 49/935 (5.2) | 406/11,933 (3.4) | 0.94 (0.64, 1.34) | ||
| ≥ 30·0 | 993/5,072 (19.6) | 7,840/38,234 (20.5) | 1.01 (0.93, 1.09) | 70/993 (7.0) | 424/7,840 (5.4) | 0.64 (0.46, 0.88) | ||
| Smoking status | 0.726 | 0.163 | ||||||
| Never | 1,203/6,732 (17.9) | 15,250/82,755 (18.4) | 0.99 (0.92, 1.06) | 59/1,203 (4.9) | 379/15,250 (2.5) | 1.04 (0.73, 1.45) | ||
| Previous | 962/5,334 (18.0) | 9,788/55,234 (17.7) | 1.02 (0.94, 1.10) | 62/962 (6.4) | 486/9,788 (5.0) | 0.67 (0.48, 0.93) | ||
| Current | 361/2,134 (16.9) | 2,837/14,962 (19.0) | 0.93 (0.81, 1.06) | 25/361 (6.9) | 156/2,837 (5.5) | 0.62 (0.36, 1.05) | ||
| Alcohol drinking status | 0.146 | 0.081 | ||||||
| Never | 159/848 (18.8) | 1,471/6,644 (22.1) | 0.89 (0.73, 1.08) | 17/159 (10.7) | 70/1,471 (0.3) | 1.14 (0.56, 2.22) | ||
| Previous | 226/1,220 (18.5) | 892/4,712 (18.9) | 0.99 (0.82, 1.18) | 22/226 (9.7) | 74/892 (8.3) | 0.60 (0.31, 1.11) | ||
| Current | 2,149/12,172 (17.7) | 25,640/142,343 (18.0) | 1.00 (0.95, 1.06) | 107/2,149 (5.0) | 884/25,640 (3.4) | 0.75 (0.58, 0.95) | ||
| Mental disorders a | 0.389 | 0.077 | ||||||
| No | 1,051/5,963 (17.6) | 22,137/119,924 (18.5) | 0.90 (0.84, 0.96) | 23/1,051 (2.2) | 464/22,137 (2.1) | 0.82 (0.50, 1.26) | ||
| Yes | 1,491/8,321 (17.9) | 5,898/33,965 (17.4) | 0.98 (0.91, 1.04) | 124/1,491 (8.3) | 570/5,898 (9.7) | 0.85 (0.69, 1.04) | ||
| Number of somatic comorbidities | 0.131 | < 0.001 | ||||||
| 0 | 739/4,016 (18.4) | 16,706/86,937 (19.2) | 0.93 (0.85, 1.00) | 9/739 (1.2) | 131/16,706 (0.8) | 1.52 (0.70, 2.91) | ||
| 1 | 567/3,230 (17.6) | 6118/36,409 (16.8) | 0.98 (0.89, 1.08) | 12/567 (2.1) | 231/6,118 (3.8) | 0.60 (0.31, 1.04) | ||
| 2 | 432/2,696 (16.0) | 2,702/16,718 (16.2) | 0.94 (0.84, 1.05) | 21/432 (4.9) | 227/2,702 (8.4) | 0.63 (0.38, 0.98) | ||
| ≥ 3 | 804/4,342 (18.5) | 2,509/13,825 (18.1) | 0.99 (0.91, 1.07) | 105/804 (13.1) | 445/2,509 (17.7) | 0.85 (0.67, 1.09) | ||
Data are presented as n/N (%). ORs (Odds ratio, 95% CI) were derived from logistic regression models. NA means that OR = 0 (none of the participants in the group had the event). CSE: Certificate of Secondary Education. NVQ: National Vocation Qualifications. COPD, chronic obstructive pulmonary disease. CKD, chronic kidney disease. CVD, cardiovascular disease. a Mental disorders consist of the indications for the use of psychiatric drugs, including depression, anxiety, psychotic disorder, substance misuse, multiple sclerosis, neuropathic pain, dementia/delirium, and epilepsy.
Table 2.
Incidence and Adjusted Odds Ratios (ORs) of COVID-19 outcomes by psychotropic drugs.
| SARS-CoV-2 infection |
COVID-19-Related Death |
||||||
|---|---|---|---|---|---|---|---|
| No. of participants | Cases | OR (95%CI)† | P value | Cases | OR (95%CI)† | P value | |
| Psychotropics | |||||||
| Yes | 14,284 | 2,542 | 0.95 (0.88, 0.98)a | 0.043 | 147 | 0.78 (0.64, 0.98)a | 0.038 |
| No | 153,889 | 28,035 | 1 [reference] | 1,034 | 1 [reference] | ||
| Antidepressants | |||||||
| Yes | 12,363 | 2,253 | 0.98 (0.93, 1.03) | 0.348 | 121 | 0.83 (0.65, 1.05) | 0.119 |
| No | 155,810 | 28,324 | 1 [reference] | 1,060 | 1 [reference] | ||
| Mood stabilizers | |||||||
| Yes | 1,953 | 320 | 0.86 (0.76, 0.98) | 0.022 | 26 | 0.81 (0.50, 1.27) | 0.378 |
| No | 166,220 | 30,257 | 1 [reference] | 1,155 | 1 [reference] | ||
| Benzodiazepines | |||||||
| Yes | 901 | 118 | 0.72 (0.58, 0.87)b | 0.001 | 11 | 1.08 (0.52, 2.08) | 0.817 |
| No | 167,272 | 30,459 | 1 [reference] | 1,170 | 1 [reference] | ||
| Antipsychotics | |||||||
| Yes | 211 | 38 | 0.79 (0.54, 1.12) | 0.201 | 1 | 0.29 (0.01, 1.64) | 0.251 |
| No | 167,962 | 30,539 | 1 [reference] | 1,180 | 1 [reference] | ||
Adjusted for age, sex, race and ethnicity, lifestyle factors (smoking history, alcohol consumption history), socioeconomic factors (TDI, educational attainment, annual household income), BMI, history of COPD, chronic kidney disease, diabetes, CVD, asthma, depression, anxiety, substance misuse, psychotic disorders, dementia/delirium, epilepsy, neuropathic pain, multiple sclerosis, and psychotropic medications in the model.
P < 0.05.
P < 0.01, where α’ = 0.05/4 = 0.013 to correct for multiple comparisons.
Based on the above results, we analyzed the association between the use of psychotropic drugs and the risk of COVID-19 outcomes among middle-aged participants and old participants to determine whether the effects of different types of psychotropics on the outcome of COVID-19 vary with age. As shown in Table S6, for the middle-aged participants (age < 65 years old), using benzodiazepines (OR, 0.59; 95% CI, 0.40–0.85), especially diazepam (OR, 0.56; 95% CI, 0.35–0.86) were associated with a lower risk of SARS-CoV-2 infection. While for the old participants (age ≥ 65 years old), using mirtazapine was associated with a 53% higher risk of SARS-CoV-2 infection. It is worth noting that sertraline was associated with an extremely lower risk of COVID-19-related death among the middle-aged and older participants (Table S6).
3.4. Sensitivity analyses
As shown in Table S3, IPTW, propensity score analysis, and analyzed with data after multiple imputed all yielded similar results that the use of psychotropic drugs was significantly associated with lower risks of SARS-CoV-2 infection and COVID-19-related death (P < 0.05).
4. Discussionde
In this large-scale community-based cohort in the UK, we observed that treatment with psychotropic drugs was associated with a 5% decrease in SARS-CoV-2 infection and a 22% decrease in COVID-19-related death risk. Additionally, the risk of SARS-CoV-2 infection was substantially lower in patients who were treated with diazepam, and the risk of COVID-19-related death was substantially lower in patients who were treated with sertraline compared to nonusers.
Psychotropic drugs mentioned in our study were associated with a significantly lower risk of SARS-CoV-2 infection among the whole participants and associated with a significantly lower risk of COVID-19-related death among the participants infected with SARS-CoV-2, respectively. Compared to nonusers, psychotropic drug users are more likely to trust their healthcare professionals and are potentially more likely to follow medical guidance. Additionally, studies showed that taking psychotropic drugs could decrease the level of activation of the hypothalamic-pituitary-adrenal axis in individuals with psychiatric disorders, leading to the modulation of circulating glucocorticoids and improvement in cell-mediated and humoral immunity (Thorkelson et al., 2016; Han et al., 2020). As a result, psychotropic drugs might reduce the risk of SARS-CoV-2 infection and COVID-19-related death in individuals who are susceptible to SARS-CoV-2 by modulating immune system function, especially in older adults with weakened immune systems. As we saw in the stratified analysis of this study, patients with a high number of comorbidities and older persons who used psychotropic drugs had a lower COVID-19-related mortality compared to the nonusers.
Using psychotropics may help the population with a weakened immune system. The anti-inflammatory effects of selective serotonin reuptake inhibitors (SSRIs), could be especially important in the elderly, given their compromised immune response (Costa et al., 2020). Some clinical and in vitro experimental studies have evaluated the effect of SSRIs on COVID-19. For example, Lenze et al. (Lenze et al., 2020) conducted a double-blind, randomized, fully remote clinical trial of fluvoxamine vs. placebo among outpatients with symptomatic COVID-19. They found that patients treated with fluvoxamine, a type of SSRI, given during mild COVID-19 illness, had a lower likelihood of clinical deterioration compared to placebo. However, the study was limited by the small sample size of 152 patients and the short follow-up duration. Another in vitro experiment reported that amitriptyline, fluoxetine, sertraline, and escitalopram pharmacologically inhibited acid sphingomyelinase and genetic downregulated enzymes to prevent cultured cells or fresh isolated human nasal epithelial cells from being infected with SARS-CoV-2 (Carpinteiro et al., 2020).
The ability of SSRIs to reduce the rate of clinical deterioration in patients with COVID-19 may be due to their agonistic effects on the sigma-1 receptor (S1R), and stimulation of the S1R receptor is reported to reduce the damaging effects of the inflammatory response (Rosen et al., 2019). A study found that sertraline could block SARS-CoV-2 S protein-mediated cell fusion by high-throughput screening of approximately 1700 US FDA-approved compounds that can effectively inhibit the replication of SARS-CoV-2 (Xiao et al., 2020). Benzodiazepines are widely used in the treatment of anxiety and sleep problems (Olfson et al., 2015). A case study reported that benzodiazepines improved COVID-19 symptoms and COVID-19-associated psychosis in a 36-year-old woman (Smith et al., 2020). Another multicentre observational study performed at Greater Paris University hospitals indicated that most benzodiazepines were associated with increased mortality among patients with COVID-19, except for diazepam, which may be associated with reduced mortality compared with other benzodiazepines (Hoertel et al., 2022).
Despite studies finding protective effects of psychiatric drugs on COVID-19, there are concerns about interactions between psychotropic medications and COVID-19 medication (Gannon et al., 2020). The University of Liverpool provided an overview of possible interactions with COVID-19 medications, including psychotropics (http://www.covid19-druginteractions.org/). Most antipsychotic drugs and COVID-19 medications utilize cytochrome P450 (CYP) enzymes for their metabolism. Given the tolerability and minimal P450 interactions, antidepressants, and olanzapine are considered safe in combination with COVID-19 medication (Simon et al., 2020; Zhang et al., 2020).
Patients with mental disorders were thought to be at an increased risk of infection for the following reasons: non-compliance to protective measures, delayed access to health services due to social discrimination, confined conditions in psychiatric units favoring the dissemination of infections, and a high prevalence of high-risk comorbidities (Villoutreix et al., 2020). Indeed, French researchers found an intriguing relationship appeared between SARS-CoV-2 infection and psychiatric disorders. Alarmed by these high-risk situations, psychiatric departments in France created specialized COVID-19 units dedicated to psychiatric patients. However, these units remained nearly empty during the lockdown period since only a small proportion of psychiatric patients were found to have COVID-19, suggesting that these patients under treatment surveillance may be at reduced risk of SARS-CoV-2 infection. They then demonstrated that among the 18 most commonly prescribed psychotropic drugs, 10 have documented in vitro antiviral activity, while four of the eight remaining compounds were found to be structurally very similar to compounds with known in vitro antiviral activity by using various clustering approaches (Guy et al., 2020).
Due to the limitation of medication data in the UK biobank, we can not cover all antipsychotic drugs in this study. However, a retrospective cohort study conducted in New York sheds light on the association between antipsychotic drugs and the risk of COVID-19. This study claimed that antipsychotic drugs (including first-generation and second-generation antipsychotics) or mood stabilizers (including valproic acid, lithium, lamotrigine, and topiramate) were associated with the risk of COVID-19 outcomes. And the use of second-generation antipsychotic drugs, especially paliperidone, was associated with a decreased risk of COVID-19 infection, whereas the use of valproic acid was associated with an increased risk (Nemani et al., 2022).
Additionally, the association between psychotropics and subsequent infections has been shown in previous experimental research. For instance, some psychotropics can bind to S1Rs and protect patients from psychiatric SARS-CoV-2 infection via modulation of the endo-lysosomal pathway, membrane fusion, and yet-to-be-characterized interactions with specific receptors (Artese et al., 2020). A recent experimental study showed that fluoxetine, a widely used antidepressant, could inhibit acute SARS-CoV-2 infection in a dose-dependent manner (Schloer et al., 2020), but we did not find any association between fluoxetine and the risk of COVID-19. Notably, the use of clozapine is widely reported to be associated with a higher risk of pneumonia and fatal outcomes than other second-generation antipsychotics, although we did not find any medication record of clozapine in our study.
The major merit of our study is the detailed and validated data in a well-characterized cohort including types of psychotropic medications and potential confounding risk factors for middle-aged and old participants. This study highlights the protective effects of psychotropics on the risk of COVID-19, especially among the elderly population. The present work has some limitations that should be considered. First, the UK Biobank only recruited 5.5% of the invited population in the United Kingdom. Second, the participants were predominantly white (94.3%) and therefore did not represent the global population. Third, UK Biobank did not collect specific information on dosage, brand, frequency, or duration of the psychotropic drugs. Finally, we did not consider any medication interactions between COVID-19 treatment and psychotropic medications in our study.
In conclusion, we found that the use of psychotropics was associated with a lower risk of confirmed COVID-19 and COVID-19-related deaths. Moreover, the use of gabapentin was associated with a lower risk of COVID-19-related morbidity and death, and the use of sertraline was associated with an extremely lower risk of COVID-19-related death. The large variety of psychotropic medications offers a toolbox of potential antivirals for host-directed therapy, and more clinical trials are needed to confirm our findings.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Ethnic approval
UK Biobank has ethics approval from the North West Multi-centre Research Ethics Committee (11/NW/0382). Appropriate informed consent was obtained from participants and ethical approval was covered by the UK Biobank. This research has been conducted using the UK Biobank Resource under the project number of 45676.
Data availability
Data from the UK Biobank can not be shared publicly. However, data are available from the UK Biobank Institutional Data Access / Ethics Committee (contact via http://www.ukbiobank.ac.uk/ or contact by email at access@ukbiobank.ac.uk) for researchers who meet the criteria for access to confidential data.
Contributors
YW directed the study. YW and YM designed the study and analyzed data. YM developed the first manuscript draft. YW, SL, YZ, XZ and HY edited the manuscript. All authors have critically revised the manuscript, and all authors have contributed to the final version.
Role of funding source
This study was supported by The National Natural Science Foundation of China (grant number: 71910107004), received by Yaogang Wang.
Acknowledgments
The present analyses were conducted using the UK Biobank Resource under Application 45676. We would like to thank all UK Biobank participants and staff, and all healthcare workers involved in the diagnosis and treatment of COVID-19 patients. We acknowledge the support to Y.G.W. funded by the National Natural Science Foundation of China (71910107004). The funding sources had no involvement in the study design, collection, analysis, or interpretation of data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2022.11.009.
Appendix. Supplementary materials
References
- Armitage R. Antidepressants, primary care, and adult mental health services in England during COVID-19. Lancet Psychiatry. 2021;8(2):e3. doi: 10.1016/S2215-0366(20)30530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artese A., Svicher V., Costa G., Salpini R., Di Maio V.C., Alkhatib M., Ambrosio F.A., Santoro M.M., Assaraf Y.G., Alcaro S., Ceccherini-Silberstein F. Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses. Drug. Resist. Updat. 2020;53 doi: 10.1016/j.drup.2020.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Li W., Wang T., Ran D., Davalos V., Planas-Serra L., Pujol A., Esteller M., Wang X., Yu H. Accelerated biological aging in COVID-19 patients. Nat. Commun. 2022;13(1):2135. doi: 10.1038/s41467-022-29801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S., Sehl C., Soddemann M., Wilker B., Kamler M., Bertsch T., Lang K.S., Patel S., Wilson G.C., Walter S., Hengel H., Pöhlmann S., Lang P.A., Kornhuber J., Becker K.A., Ahmad S.A., Fassbender K., Gulbins E. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell. Rep. Med. 2020;1(8) doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa L.H.A., Santos B.M., Branco L.G.S. Can selective serotonin reuptake inhibitors have a neuroprotective effect during COVID-19? Eur. J. Pharmacol. 2020;889 doi: 10.1016/j.ejphar.2020.173629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druss B.G. Addressing the COVID-19 Pandemic in Populations With Serious Mental Illness. JAMA Psychiatry. 2020;77(9):891–892. doi: 10.1001/jamapsychiatry.2020.0894. 2020. [DOI] [PubMed] [Google Scholar]
- Fonseca L., Diniz E., Mendonça G., Malinowski F., Mari J., Gadelha A. Schizophrenia and COVID-19: risks and recommendations. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999) 2020;42(3):236–238. doi: 10.1590/1516-4446-2020-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M., De Prisco M., Billeci M., Ermini E., Young A.H., Lafer B., Soares J.C., Vieta E., Quevedo J., de Bartolomeis A., Sim K., Yatham L.N., Bauer M., Stein D.J., Solmi M., Berk M., Carvalho A.F. Implications of the COVID-19 pandemic for people with bipolar disorders: a scoping review. J. Affect. Disord. 2021;295:740–751. doi: 10.1016/j.jad.2021.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J.M., Conlogue J., Sherwood R., Nichols J., Ballough J.R., Fredrick N.M., Chengappa K. Long acting injectable antipsychotic medications: ensuring care continuity during the COVID-19 pandemic restrictions. Schizophr Res. 2020;222:532–533. doi: 10.1016/j.schres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., Reiser J., Bansal A., Srivastava A., Zhou Y., Sutherland A., Green A., Shehata A.M., Goyal N., Vijayan A., Velez J. STOP-COVID Investigators. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA. Intern. Med. 2020;180(11):1436–1447. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R.K., DiPaola R.S., Romanelli F., Dutch R.E. Rapid repurposing of drugs for COVID-19. Science. 2020;368(6493):829–830. doi: 10.1126/science.abb9332. [DOI] [PubMed] [Google Scholar]
- Han Y., Sun C.Y., Meng S.Q., Tabarak S., Yuan K., Cao L., Yan W., Xu L.Z., Deng J.H., Zhu W.L., Li J.L., Lu L., Xue Y.X., Shi J. Systemic immunization with altered myelin basic protein peptide produces sustained antidepressant-like effects. Mol. Psychiatry. 2020;25(6):1260–1274. doi: 10.1038/s41380-019-0470-9. [DOI] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Gulbins E., Kornhuber J., Vernet R., Beeker N., Neuraz A., Blanco C., Olfson M., Airagnes G., Lemogne C., Alvarado J.M., Arnaout M., Cougoule C., Meneton P., Limosin F., AP-HP/Université de Paris/INSERM COVID-19 Research Collaboration/AP-HP COVID CDR Initiative/‘Entrepôt de Données de Santé’ AP-HP Consortium. Association between benzodiazepine receptor agonist use and mortality in patients hospitalised for COVID-19: a multicentre observational study. Epidemiol. Psychiatr. Sci. 2022;31:e18. doi: 10.1017/S2045796021000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., Miller J.P., Yang L., Yingling M., Avidan M.S., Reiersen A.M. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: a Randomized Clinical Trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Mao B., Liang S., Yang J.W., Lu H.W., Chai Y.H., Wang L., Zhang L., Li Q.H., Zhao L., He Y., Gu X.L., Ji X.B., Li L., Jie Z.J., Li Q., Li X.Y., Lu H.Z., Zhang W.H., Song Y.L., Shanghai Clinical Treatment Experts Group for COVID-19 Association between age and clinical characteristics and outcomes of COVID-19. The Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y.F., Yang F.C., Huang J.S., Lin Y.S., Liang C.S. Disentangling the complex bidirectional associations between COVID-19 and psychiatric disorder. The Lancet. Psychiatry. 2021;8(3):179. doi: 10.1016/S2215-0366(20)30565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutesa L., Ndishimye P., Butera Y., Souopgui J., Uwineza A., Rutayisire R., Ndoricimpaye E.L., Musoni E., Rujeni N., Nyatanyi T., Ntagwabira E., Semakula M., Musanabaganwa C., Nyamwasa D., Ndashimye M., Ujeneza E., Mwikarago I.E., Muvunyi C.M., Mazarati J.B., Nsanzimana S., Turok N., Ndifon W. A pooled testing strategy for identifying SARS-CoV-2 at low prevalence. Nature. 2021;589(7841):276–280. doi: 10.1038/s41586-020-2885-5. [DOI] [PubMed] [Google Scholar]
- Nemani K., Li C., Olfson M., Blessing E.M., Razavian N., Chen J., Petkova E., Goff D.C. Association of Psychiatric Disorders With Mortality Among Patients With COVID-19. JAMA Psychiatry. 2021;78(4):380–386. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani K., Williams S.Z., Olfson M., Leckman-Westin E., Finnerty M., Kammer J., Smith T.E., Silverman D.J., Lindenmayer J.P., Capichioni G., Clelland J., Goff D.C. Association Between the Use of Psychotropic Medications and the Risk of COVID-19 Infection Among Long-term Inpatients With Serious Mental Illness in a New York State-wide Psychiatric Hospital System. JAMA. Netw. Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., King M., Schoenbaum M. Benzodiazepine use in the United States. JAMA. psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- Ostuzzi G., Papola D., Gastaldon C., Schoretsanitis G., Bertolini F., Amaddeo F., Cuomo A., Emsley R., Fagiolini A., Imperadore G., Kishimoto T., Michencigh G., Nosé M., Purgato M., Serdar D., Stubbs B., Taylor D., Thornicroft G., Ward P.B., Hiemke C., Correll C.U., Barbui C. Safety of Psychotropic Medications in People With COVID-19: evidence Review and Practical Recommendations. Focus (Am Psychiatr Publ) 2020;18(4):466–481. doi: 10.1176/appi.focus.18308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M.S., Bobba N.S., Reddy S. Reevaluating the concept of lifelong mood stabilizers in a bipolar I disorder patient: A 25 year case study. Asian. J. Psychiatr. 2020;54 doi: 10.1016/j.ajp.2020.102422. [DOI] [PubMed] [Google Scholar]
- Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., Gaultier A. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 2019;11(478):eaau5266. doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloer S., Brunotte L., Goretzko J., Mecate-Zambrano A., Korthals N., Gerke V., Ludwig S., Rescher U. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg. Microbes. Infect. 2020;9(1):2245–2255. doi: 10.1080/22221751.2020.1829082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.M., Komisar J.R., Mourad A., Kincaid B.R. COVID-19-associated brief psychotic disorder. BMJ. Case. Rep. 2020;13(8) doi: 10.1136/bcr-2020-236940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N.M., Saxe G.N., Marmar C.R. Mental Health Disorders Related to COVID-19-Related Deaths. JAMA. 2020;324(15):1493–1494. doi: 10.1001/jama.2020.19632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M., Liu B., Matthews P., Ong G., Pell J., Silman A., Young A., Sprosen T., Peakman T., Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS. Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. The Lancet. Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorkelson G., Bielefeldt K., Szigethy E. Empirically Supported Use of Psychiatric Medications in Adolescents and Adults with IBD. Inflamm. Bowel. Dis. 2016;22(6):1509–1522. doi: 10.1097/MIB.0000000000000734. [DOI] [PubMed] [Google Scholar]
- Toubasi A.A., AbuAnzeh R.B., Tawileh H., Aldebei R.H., Alryalat S. A meta-analysis: the mortality and severity of COVID-19 among patients with mental disorders. Psychiatry. Res. 2021;299 doi: 10.1016/j.psychres.2021.113856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villoutreix B.O., Beaune P.H., Tamouza R., Krishnamoorthy R., Leboyer M. Prevention of COVID-19 by drug repurposing: rationale from drugs prescribed for mental disorders. Drug. Discov. Today. 2020;25(8):1287–1290. doi: 10.1016/j.drudis.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Franklin T., Iwata M., Duman R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016;17(8):497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- Xiao X., Wang C., Chang D., Wang Y., Dong X., Jiao T., Zhao Z., Ren L., Dela Cruz C.S., Sharma L., Lei X., Wang J. Identification of Potent and Safe Antiviral Therapeutic Candidates Against SARS-CoV-2. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.586572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Zhou X., Liu H., Hashimoto K. Treatment concerns for psychiatric symptoms in patients with COVID-19 with or without psychiatric disorders. Br. J. Psychiatry. 2020;217(1):351. doi: 10.1192/bjp.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the UK Biobank can not be shared publicly. However, data are available from the UK Biobank Institutional Data Access / Ethics Committee (contact via http://www.ukbiobank.ac.uk/ or contact by email at access@ukbiobank.ac.uk) for researchers who meet the criteria for access to confidential data.