Abstract
Background and aims
Sarcopenia has been demonstrated to be closely associated with nonalcoholic fatty liver disease (NAFLD). However, whether there are causal relationships between sarcopenia and NAFLD remains undetermined. Here, we aim to address the question using a two-sample bidirectional Mendelian randomization (MR) analysis approach.
Methods
We performed a two-sample bidirectional MR study using summary-level data from genome-wide association studies (GWAS) of the whole body lean mass (n = 38,292), appendicular (arms and legs) lean mass (n = 28,330), and NAFLD (1,483 biopsy-proven NAFLD cases and 17,781 controls). We first conducted MR analysis with five single nucleotide polymorphisms (SNPs) as genetic instruments for whole body lean mass and three SNPs as instruments for appendicular lean mass to estimate the causal effect of genetically predicted sarcopenia on the risk of NAFLD using the inverse-variance weighted (IVW) method. Then we performed reverse MR analysis with four SNPs as instruments to examine the causality of genetically predicted NAFLD with whole body lean mass and appendicular lean mass. Further sensitivity analysis was conducted to testify the reliability of the MR results.
Results
Genetic predisposition to decreased whole body lean mass was not associated with NAFLD [IVW-random effects, odds ratio (OR) = 1.054, 95%CI: 0.750–1.482, P = 0.761]. Similar results were observed using genetic instruments of appendicular lean mass (IVW-random effects, OR = 0.888, 95%CI: 0.386–2.042, P = 0.780). Reverse MR analysis revealed that genetically predicted NAFLD using four genetic instruments was not associated with whole body lean mass (IVW, β = −0.068, 95%CI: −0.179 to 0.043, P = 0.229) and appendicular lean mass (IVW, β = −0.020, 95%CI: −0.092 to 0.051, P = 0.574). MR analyses using other methods and sensitivity analysis showed consistent results.
Conclusion
These results suggested no causal relationships between sarcopenia and NAFLD, indicating that sarcopenia may not be directly involved in the pathogenesis of NAFLD and vice versa.
Keywords: sarcopenia, nonalcoholic fatty liver disease—NAFLD, Mendelian randomization (MR), lean body mass, causality
Introduction
Nonalcoholic fatty liver disease (NAFLD) has emerged as the most common chronic liver disease worldwide with a prevalence of about 25% in adult population (1). The spectrum of NAFLD comprises of nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH) and NASH-related end-stage liver diseases such as cirrhosis and hepatocellular carcinoma (HCC). It is considered to be a multisystem disease and hepatic component of metabolic syndrome (2). The pathogenesis of NAFLD has been demonstrated to be multifactorial, in which insulin resistance and genetic predisposition are regarded as two critical driven factors. One of the hallmarks of NAFLD is the interaction between the environment and susceptible polygenic host background which endows varied disease phenotype and influences disease progression (3). Over the past decades, accumulating evidences have established links between NAFLD and extrahepatic diseases such as cardiovascular disease (CVD), and type 2 diabetes mellitus (T2DM) (4, 5). Both epidemiological and mechanical investigations reveal mutual effect of NAFLD on other metabolic disorders suggesting NAFLD could serve as both cause and consequence of extrahepatic metabolic diseases (6, 7).
Sarcopenia is known as a reduction in skeletal muscles, which has been described in not only aging but also a variety of pathological conditions outside the elderly population (8). In recent years, the role of sarcopenia in NAFLD has attracted substantial attentions. A series of researches have shown that sarcopenia is not only closely related to the presence of NAFLD but also an independent risk factor of the advancement and deterioration of the disease (9–11). Similar with NAFLD, sarcopenia is also a result of the interaction between genetic and environmental factors (12). The main characteristic of sarcopenia is loss of lean body mass, notably skeletal muscle, which is commonly measured by dual energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA). Although an inverse correlation between lean body mass and risk of NAFLD has been observed, the causal relationships between sarcopenia and NAFLD are hardly investigated.
Two-sample MR is an approach utilizing genetic variants as instrumental variables (IVs) for exposure to investigate the causal inference between the exposure and outcome. MR analysis has advantages to overcome the issue of confounding and reverse causality and thus widely applied to assess the causal relationships (13). In the present study, we performed a two-sample bidirectional MR investigation with genome-wide association studies (GWAS) summary data of NAFLD, whole body lean mass and appendicular lean mass to explore the causal relationships.
Methods
Study design
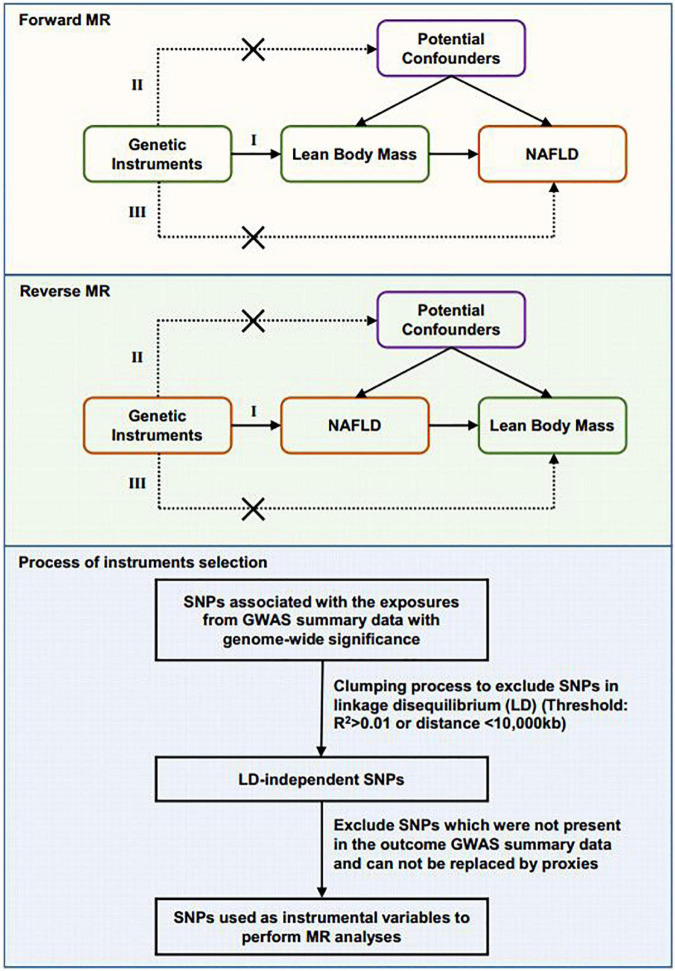
We used a two-sample MR design in which the genetic instruments of exposure and outcome are extracted from independent GWAS data sources. The MR analyses were bidirectional. First, we assessed the causal effect of the whole body lean mass and appendicular lean mass variables on NAFLD. Then we investigated the reverse causal effect of genetically predicted NAFLD on whole body lean mass and appendicular lean mass. The MR analyses conform to three assumptions: (1) genetic variants are associated with the exposure; (2) genetic instruments are not associated with the outcome via confounders; (3) genetic instruments do not affect the outcome directly, only possibly via the exposure (Figure 1). All cited GWAS had been approved by a relevant review board. Ethical approval was not required for this MR study based on summary-level data.
FIGURE 1.
Schematic diagram showing the design of the bidirectional MR analysis and the process of instrument selection. Assumption I: The selected genetic variants are associated with exposure. Assumption II: The genetic instruments are not associated with potential confounders. Assumption III: The genetic instruments are associated with outcome only through the pathway from exposure. The flow chart of genetic instruments selection is shown.
Data sources for lean body mass
Data regarding the lean body mass were obtained from the up-to-date largest meta-analysis of GWAS, which identified five single nucleotide polymorphisms (SNPs) associated with whole body lean mass in 38,292 individuals of European ancestry and three SNPs associated with appendicular lean mass in 28,330 individuals of European ancestry either genome wide (P < 5 × 10–8) or suggestively genome wide (P < 2.3 × 10–6) (14). The identified SNPs were further meta-analyzed and verified in replication cohorts and joint analyses with a total sample size of over 100,000 from 53 studies (14). The lean body mass was measured using DXA or BIA and adjusted for potential confounders such as fat mass, height, sex, age and other study-specific covariates. Then we queried these SNPs in the PhenoScanner database to identify their association with GWAS traits that are potential confounding phenotypes or introduce horizontal pleiotropy. Linkage disequilibrium (defined as R2 > 0.01 or clump distance < 10,000 kb) between SNPs was assessed based on the 1,000 Genomes European reference panel. Qualified SNPs are selected as instruments after these steps.
Data source for nonalcoholic fatty liver disease
Summary data of NAFLD were extracted from a large GWAS by Anstee et al. which included 1,483 biopsy-proven NAFLD cases and 17,781 genetically matched controls of European ancestry (15). We estimated the potential bias caused by participants overlap in the data sources of lean body mass and NAFLD which may introduce inflate type I error rate by an online tool.1 The results showed low bias risk from sample overlap. Candidate SNPs which met a genome-wide significance threshold (P < 5 × 10–8) were screened. Linkage disequilibrium was assessed as described above. For SNPs in linkage disequilibrium, those with the strongest association with the exposure were retained. SNPs that were unavailable in the outcome dataset were replaced by suitable proxies (R2 ≥ 0.8) where available. Palindromic SNPs were also replaced by suitable proxies (R2 ≥ 0.8). To avoid potential bias from population stratification, we selected proxies using European population reference.
Statistical analysis
To evaluate the weak instrument bias, we first calculated the F statistics using the formula F = , where N is the sample size, K is the number of IVs, and R2 is the proportion of the variability of the exposure explained by IVs. And the statistical power was estimated using an online tool (16). We used the inverse variance-weighted (IVW) method under random or fixed effects as the primary statistical method for the bidirectional MR analysis. Cochran’s Q-test was calculated to assess the heterogeneity across the individual effect estimates derived from each genetic variant. If significant heterogeneity (P < 0.05) was observed, a random-effect IVW model was applied. Other methods we used for MR included Weighted median regression, MR-Egger regression, MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) analysis, and Weighted mode to examine the consistency of results and potential pleiotropy. Weighted median method was used to provide a causal estimate assuming more than half of the weight in the analysis comes from valid IVs (17, 18). MR-Egger analysis is applied to detect horizontal pleiotropy by its intercept (P < 0.05 for intercept indicates pleiotropy) and generate estimates after correcting for pleiotropic effects (19). MR-PRESSO includes detection of horizontal pleiotropy (MR-PRESSO global test), correction for horizontal pleiotropy via outlier removal (MR-PRESSO outlier test), and testing of significant differences in the causal estimates before and after correction for outliers (MR-PRESSO distortion test). The MR-PRESSO outlier test requires that at least 50% of the variants are valid instruments, has balanced pleiotropy, and relies on the Instrument Strength Independent of Direct Effect (InSIDE) condition that instrument-exposure and pleiotropic effects are uncorrelated. Weighted mode method estimates the causal effect of the subset with the largest number of SNPs by clustering the SNPs into subsets resting on the resemblance of causal effect (20). All analyses were performed using the TwoSampleMR and MR-PRESSO packages in R software (v4.1.3, R Foundation for Statistical Computing, Vienna, Austria) (21, 22).
Results
Effect of genetically predicted lean body mass on nonalcoholic fatty liver disease
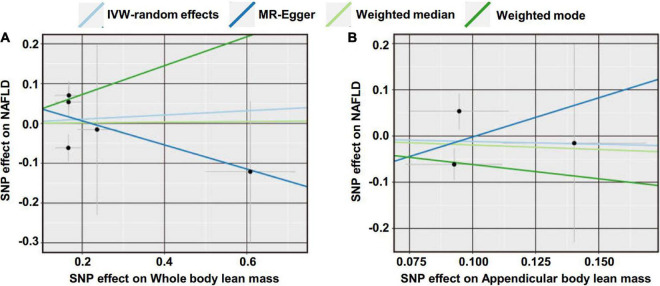
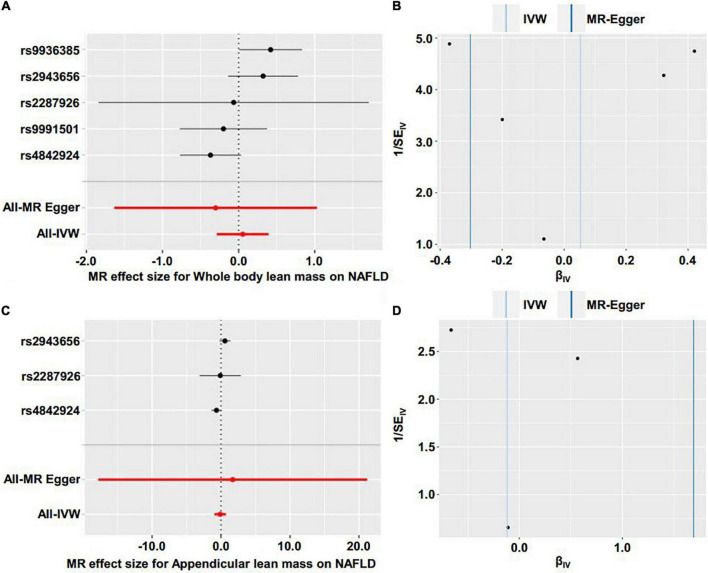
We first investigated the causality of genetically predicted whole body lean mass and appendicular lean mass on NAFLD. For whole body lean mass, five SNPs were selected as IVs. These included rs2943656 in/near IRS1, rs9991501 in/near HSD17B11, rs2287926 in/near VCAN, rs4842924 in/near ADAMTSL3, and rs9936385 in/near FTO which are all available in NAFLD GWAS (Table 1). Forward MR analysis results are shown in Table 2 and Figure 2, which showed no causal effect of whole body lean mass on NAFLD in IVW-random effects method (OR = 1.054, 95%CI: 0.750–1.482, P = 0.761). MR analyses in other methods showed consistent results (Table 2 and Figures 2A, 3A,B). Significant heterogeneity was detected (MR-Egger Q = 8.534, P = 0.036; IVW Q = 9.379, P = 0.052) which suggested the use of random effect model of IVW. No horizontal pleiotropy was observed by intercept estimated from MR-Egger regression (Table 2) and MR-PRESSO global test (P = 0.099).
TABLE 1.
Genetic instruments for lean body mass and their associations with NAFLD.
| SNP | Chr | Pos | Gene | EA | OA | Whole body lean mass |
NAFLD |
||||
| Beta | SE | P | OR | SE | P | ||||||
| rs2943656 | 2 | 226,830,162 | IRS1 | A | G | –0.167 | 0.032 | 2.536 × 10–7 | 0.948 | 0.039 | 0.169 |
| rs9991501 | 4 | 88,477,507 | HSD17B11 | T | C | –0.609 | 0.110 | 2.897 × 10–8 | 1.129 | 0.178 | 0.495 |
| rs2287926 | 5 | 82,851,164 | VCAN | A | G | 0.236 | 0.048 | 8.592 × 10–7 | 0.985 | 0.214 | 0.943 |
| rs4842924 | 15 | 82,378,611 | ADAMTSL3 | T | C | –0.166 | 0.031 | 1.375 × 10–7 | 0.940 | 0.034 | 0.071 |
| rs9936385 | 16 | 52,376,670 | FTO | T | C | –0.168 | 0.034 | 1.115 × 10–6 | 1.073 | 0.035 | 0.046 |
|
| |||||||||||
| SNP | Chr | Pos | Gene | EA | OA |
Appendicular lean mass
|
NAFLD
|
||||
| Beta | SE | P | OR | SE | P | ||||||
|
| |||||||||||
| rs2943656 | 2 | 226,830,162 | IRS1 | A | G | –0.095 | 0.020 | 1.141 × 10–6 | 0.948 | 0.039 | 0.169 |
| rs2287926 | 5 | 82,851,164 | VCAN | A | G | 0.140 | 0.029 | 8.120 × 10–7 | 0.985 | 0.214 | 0.943 |
| rs4842924 | 15 | 82,378,611 | ADAMTSL3 | T | C | –0.093 | 0.019 | 1.232 × 10–6 | 0.940 | 0.034 | 0.071 |
SNP, single nucleotide polymorphism; Chr, chromosome; Pos, position based on hg19; EA, effect allele; OA, other allele; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
TABLE 2.
MR estimates from different methods assessing the causal effect of lean body mass on NAFLD.
| Exposure | Outcome | Method | OR | 95%CI | P |
| Whole body lean mass (5 SNPs, F statistic = 26.682, MR power = 0.05) |
NAFLD | IVW-random effects | 1.054 | 0.750–1.482 | 0.761 |
| Weighted median | 1.008 | 0.741–1.371 | 0.961 | ||
| MR-Egger | 0.739 | 0.195–2.799 | 0.686 | ||
| Cochran’s Q = 8.533 (P = 0.036); MR-Egger intercept = 0.067 (P = 0.624) | |||||
| MR-PRESSO | 1.054 | 0.750–1.482 | 0.776 | ||
| Weighted mode | 1.437 | 0.813–2.541 | 0.280 | ||
| Appendicular lean mass (3 SNPs, F statistic = 23.830, MR power = 0.06) |
NAFLD | IVW-random effects | 0.888 | 0.386–2.042 | 0.780 |
| Weighted median | 0.823 | 0.389–1.739 | 0.610 | ||
| MR-Egger | 5.432 | 1.866 × 10–8–1.581 × 109 | 0.893 | ||
| Cochran’s Q = 4.801 (P = 0.028); MR-Egger intercept = −0.171 (P = 0.885) | |||||
| Weighted mode | 0.540 | 0.217–1.346 | 0.317 | ||
OR, odds ratio; CI, confidence interval; SNP, single nucleotide polymorphism; NAFLD, nonalcoholic fatty liver disease; IVW, inverse variance weighted; PRESSO, pleiotropy residual sum and outlier.
FIGURE 2.
Scatter plot of the causal effect of lean body mass on NAFLD using different MR methods. (A) Causal effect of whole body lean mass on NAFLD. (B) Causal effect of appendicular lean mass on NAFLD.
FIGURE 3.
Forest plot and funnel plot of the causal effect of lean body mass on NAFLD. (A) Forest plot of the causal effect of whole body lean mass on NAFLD. (B) Funnel plot of the causal effect of whole body lean mass on NAFLD. (C) Forest plot of the causal effect of appendicular lean mass on NAFLD. (D) Funnel plot of the causal effect of appendicular lean mass on NAFLD.
For appendicular lean mass, rs2943656, rs2287926, and rs4842924 were selected as IVs to conduct the forward MR analysis. Similarly, no evidence supporting the causal effect of appendicular lean mass on NAFLD was obtained in IVW-random effects method (OR = 0.888, 95%CI: 0.386–2.042, P = 0.780) and in other MR methods (Table 2 and Figures 2B, 3C,D). The heterogeneity test showed significant heterogeneity in MR-Egger Cochran’s Q-test (Q = 4.801, P = 0.028) and borderline significant heterogeneity of estimates derived from the three SNPs in IVW Cochran’s Q-test (Q = 4.961, P = 0.084). Horizontal pleiotropy test showed no significant results by MR-Egger intercept (Table 2). MR-PRESSO global test was unable to be conducted due to the lack of enough IVs.
Effect of genetically predicted nonalcoholic fatty liver disease on lean body mass
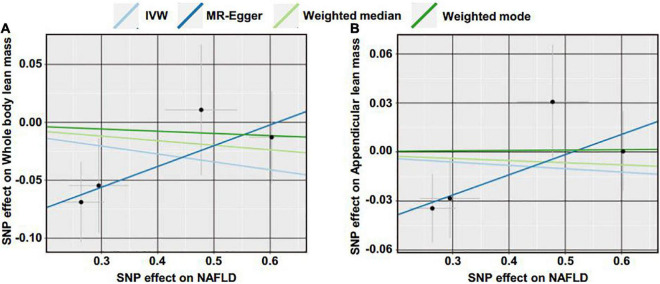
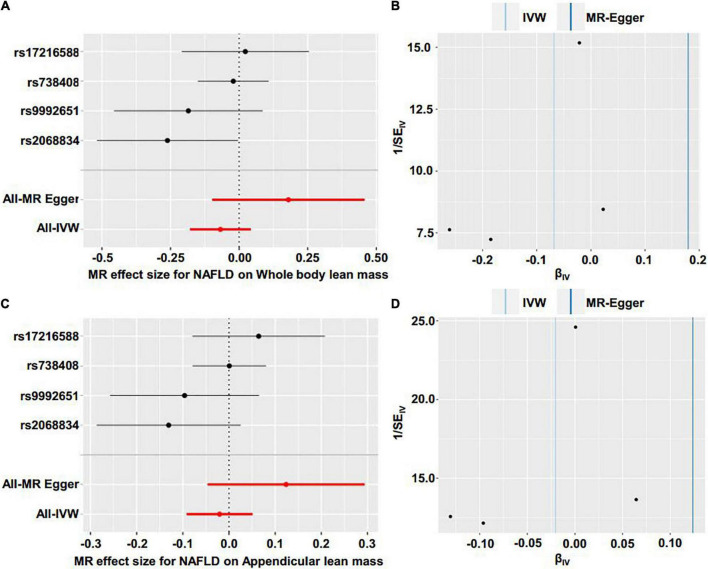
Reverse MR analyses were then conducted to assess the causal effect of NAFLD on lean body mass. Four SNPs including rs2068834 in/near ZNF512, rs9992651 in/near HSD17B13, rs17216588 in/near CLIP2, and rs738408 in/near PNPLA3 were utilized as IVs of NAFLD, in which rs738408 was used as a proxy for rs738409 in the MR analyses as the latter is a palindromic SNP (Table 3). MR analysis by IVW method showed that there was no causal effect of NAFLD on whole body lean mass (β = −0.068, 95%CI: −0.179 to 0.043, P = 0.229). Consistent results were obtained using other MR methods (Table 4 and Figures 4A, 5A,B). Heterogeneity analysis showed no significant results (MR-Egger Q = 0.499, P = 0.779; IVW Q = 3.975, P = 0.264). Moreover, there was no horizontal pleiotropy tested by intercept estimated from MR-Egger regression (Table 4) and MR-PRESSO global test (P = 0.376).
TABLE 3.
Genetic instruments for NAFLD and their associations with lean body mass.
| SNP | Chr | Pos | Gene | EA | OA | NAFLD |
Whole body lean mass |
||||
| OR | SE | P | Beta | SE | P | ||||||
| rs2068834 | 2 | 27839539 | ZNF512 | T | C | 1.302 | 0.041 | 8.486 × 10–11 | 0.069 | 0.035 | 0.046 |
| rs9992651 | 4 | 88232510 | HSD17B13 | A | G | 0.744 | 0.053 | 2.769 × 10–8 | 0.055 | 0.041 | 0.182 |
| rs17216588 | 19 | 19664077 | CILP2 | T | C | 1.612 | 0.064 | 7.245 × 10–14 | 0.011 | 0.057 | 0.849 |
| rs738408 | 22 | 44324730 | PNPLA3 | T | C | 1.827 | 0.041 | 1.591 × 10–49 | −0.013 | 0.040 | 0.748 |
|
| |||||||||||
| SNP | Chr | Pos | Gene | EA | OA |
NAFLD
|
Appendicular lean mass
|
||||
| OR | SE | P | Beta | SE | P | ||||||
|
| |||||||||||
| rs2068834 | 2 | 27839539 | ZNF512 | C | T | 1.302 | 0.041 | 8.486 × 10–11 | 0.035 | 0.021 | 0.099 |
| rs9992651 | 4 | 88232510 | HSD17B13 | A | G | 0.744 | 0.053 | 2.769 × 10–8 | 0.028 | 0.024 | 0.242 |
| rs17216588 | 19 | 19664077 | CILP2 | T | C | 1.612 | 0.064 | 7.245 × 10–14 | 0.031 | 0.035 | 0.380 |
| rs738408 | 22 | 44324730 | PNPLA3 | T | C | 1.827 | 0.041 | 1.591 × 10–49 | 0.000 | 0.025 | 0.987 |
SNP, single nucleotide polymorphism; Chr, chromosome; Pos, position based on hg19; EA, effect allele; OA, other allele; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
TABLE 4.
MR estimates from different methods assessing the causal effect of NAFLD on lean body mass.
| Exposure | Outcome | Method | Beta | 95%CI | P |
| NAFLD (4 SNPs, F statistic = 74.299, MR power = 0.43) |
Whole body lean mass | IVW | –0.068 | −0.179 to 0.043 | 0.229 |
| Weighted median | –0.040 | −0.150 to 0.071 | 0.484 | ||
| MR-Egger | 0.180 | -0.098 to 0.457 | 0.333 | ||
| Cochran’s Q = 0.499 (P = 0.779); MR-Egger intercept = −0.110 (P = 0.203) | |||||
| MR-PRESSO | –0.068 | −0.179 to 0.043 | 0.315 | ||
| Weighted mode | –0.019 | −0.142 to 0.104 | 0.780 | ||
| NAFLD (4 SNPs, F statistic = 74.299, MR power = 0.08) |
Appendicular lean mass | IVW | –0.020 | −0.092 to 0.051 | 0.574 |
| Weighted median | –0.013 | −0.082 to 0.056 | 0.709 | ||
| MR-Egger | 0.124 | -0.046 to 0.294 | 0.290 | ||
| Cochran’s Q = 1.230 (P = 0.541); MR-Egger intercept = −0.063 (P = 0.218) | |||||
| MR-PRESSO | –0.020 | −0.092 to 0.051 | 0.613 | ||
| Weighted mode | 0.002 | −0.076 to 0.081 | 0.955 | ||
OR, odds ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; SNP, single nucleotide polymorphism; IVW, inverse variance weighted; PRESSO, pleiotropy residual sum and outlier.
FIGURE 4.
Scatter plot of the causal effect of NAFLD on lean body mass using different MR methods. (A) Causal effect of NAFLD on whole body lean mass. (B) Causal effect of NAFLD on appendicular lean mass.
FIGURE 5.
Forest plot and funnel plot of the causal effect of NAFLD on lean body mass. (A) Forest plot of the causal effect of NAFLD on whole body lean mass. (B) Funnel plot of the causal effect of NAFLD on whole body lean mas. (C) Forest plot of the causal effect of NAFLD on appendicular lean mass. (D) Funnel plot of the causal effect of NAFLD on appendicular lean mass.
Next, we examined the causal effect of genetically predicted NAFLD on appendicular lean mass. No evidence was shown supporting the causality by IVW method (β = −0.020, 95%CI: −0.092 to 0.051, P = 0.574) and by other methods (Table 4 and Figures 4B, 5C,D). There were no significant heterogeneity (MR-Egger Q = 1.230, P = 0.541; IVW Q = 4.374, P = 0.224) and horizontal pleiotropy (MR-Egger intercept = −0.063, P = 0.218; MR-PRESSO global test P = 0.324) in this section.
Discussion
Although a close link between sarcopenia and NAFLD has been established, the causal relationships between the two pathological conditions are not examined. Here, we first investigated the causality by a two-sample bidirectional MR approach and discovered no causal relationships between lean body mass and NAFLD.
Recent studies have identified the association of sarcopenia with NAFLD and NAFLD-related advanced fibrosis, independent of insulin resistance and obesity (9–11, 23). Furthermore, sarcopenia has been shown be a risk factor of poor outcomes in NAFLD and other chronic liver diseases (24–26). However, whether sarcopenia directly contributes to NAFLD or vice versa is not yet clarified. Indeed, mechanistic studies may provide more insights into the direct link between the two pathological conditions. Our analyses provided evidences from genetic perspectives using the MR approach which diminishing reverse causality and minimizing residual confounding. The results of our study seemed to be inconsistent with the clinical observations mentioned above, in which no causal link between sarcopenia and NAFLD was established. A variety of reasons may underlie the inconsistency. Firstly, the association between sarcopenia and NAFLD observed by the cross-sectional studies may be mediated by potential confounders. Although some confounding factors such as obesity, T2DM and other components of metabolic syndrome may have been adjusted in some investigations, potential confounders may still exist. In fact, substantial factors have been shown to be involved in both sarcopenia and NAFLD and are considered to be shared pathological foundations. For instance, insulin resistance has been demonstrated to be correlated with both sarcopenia and NAFLD (27, 28). Insulin resistance promotes glycogenesis, accelerates protein degradation, reduces protein synthesis and induces myostatin which lead to the decline of skeletal muscle. In turn, the loss of skeletal muscle exacerbates insulin resistance as skeletal muscle accounts for about 80% of glucose disposal (29, 30). Similarly, insulin resistance is able to initiate and facilitate the progression of NAFLD by multiple pathways including promotion of hepatic de novo lipogenesis, activation of adipose tissue lipolysis and so on (31). And NAFLD also contributes to insulin resistance by combined mechanisms such as increasing lipid metabolites including ceramides and diacylglycerols which inhibit insulin signaling (31). Due to the fact that insulin resistance interferes with both sarcopenia and NAFLD, it can mediate the establishment of association between the two conditions without causal relationships. Furthermore, genetically determined NAFLD may differ from metabolically determined NAFLD in the pathogenic basis. It has been found that variants in PNPLA3 is strongly associated with NAFLD in the absence of insulin resistance or dyslipidemia (32, 33). Therefore, the association between genetically determined NAFLD and sarcopenia need further explorations.
Moreover, most of the coexistence of sarcopenia and NAFLD were studied in patients with advanced stage of NAFLD, which cannot reflect sarcopenia’s contribution to the susceptibility of NAFLD. Intriguingly, a recent prospective investigation revealed that myosteatosis but not sarcopenia predisposes early stage NAFLD patients to early NASH and fibrosis progression, suggesting myosteatosis may play a more important role in the development and progression of early stage NAFLD (34). Therefore, the role of sarcopenia and myosteatosis in the pathogenesis of NAFLD should be further determined by more evidences from clinical and mechanistic studies. Our results also showed that there was no causal effect of lean body mass on NAFLD, which implied that sarcopenia may not contribute to the predisposition of NAFLD. More importantly, most of the clinical investigations on the association of sarcopenia with NAFLD were conducted in Asian countries while our MR analyses used data sources from GWAS studies in European ancestry, which may cause discrepancy of the results between our MR analyses and clinical studies in Asian populations. Therefore, large-scale GWAS studies on sarcopenia and NAFLD in Asian populations are needed to further examine the causal relationships between sarcopenia and NAFLD in Asian ancestry. Moreover, the SNPs we chose to genetically predict NAFLD were limited by the GWAS summary data we utilized for the MR analysis. More GWAS studies with large sample size would help to identify SNPs to better genetically predict NAFLD and facilitate examining our results with other proxy SNPs. Notably, a recent investigation have made further analyses based on the former GWAS data and discovered that the SNPs of lean body mass may have different and even contradictory effects on the metabolic phenotypes (35). Therefore, further GWAS analyses on the genetic characteristics of lean body mass are needed to identify the intrinsic link with metabolic phenotype and provide more suitable SNP candidates to conduct MR investigations on the causal effect of lean body mass on metabolic disorders including NAFLD.
The major finding of the present MR study is that there are no causal relationships between genetically predicted lean body mass and NAFLD. These results indicate that sarcopenia and NAFLD may not share common genetic background although certain metabolic disorders such as insulin resistance and adiposity are supposed to be involved in both pathological conditions. Therefore, the link between sarcopenia and NAFLD is likely to be established by modifiable factors that can be subjected to clinical interventions. There are some limitations that should be considered when interpreting the results of the present MR study. Firstly, the statistical power of the MR analyses were relatively low due to the limited sample size which implies that further MR studies based on larger scale GWAS of sarcopenia and NAFLD are needed to verify the causality. Secondly, epidemiological studies have shown that the risks of both sarcopenia and NAFLD are affected by gender and age (36–39). However, we could not assess the causality between genetically predicted sarcopenia and NAFLD in different genders and age groups due to the lack of gender and age-stratified data. Thirdly, as we have mentioned above, the effects of SNPs on the traits may be inconsistent or even contradictory which makes it hard to select suitable instruments that serve as true proxies for exposures. Therefore, function investigations of the SNPs may be needed to help interpret the genetic characteristics of sarcopenia and NAFLD and facilitate more precise selection of instruments.
In summary, the present two-sample bidirectional MR study suggested that there are no causal relationships between sarcopenia and NAFLD. The results provide novel insights into the pathogenesis of sarcopenia and NAFLD from genetic perspectives and further studies based on larger scale GWAS are needed to verify the conclusions.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Z-HZ, Y-CF, and KW contributed to the study conception, design, and manuscript drafting. Z-HZ, JZ, and XH contributed to the acquisition and analysis of data. All authors approved the final manuscript.
Acknowledgments
We thank all participants and investigators involved in the Anstee et al. GWAS, and the GEnetic Factors for OSteoporosis (GEFOS) Consortium for sharing data.
Footnotes
Funding
This work was supported by National Key Research and Development Program of China (2021YFC2301800), the National Natural Science Foundation of China (82272313, 82270631, 81970522, 82000542, and 82000968), Shandong Natural Science Foundation Program (ZR201911030252), ECCM program of Clinical Research Center of Shandong University (2021SDUCRCB006), Shandong University Multidisciplinary Research and Innovation Team of Young Scholars (2020QNQT11), the Young Taishan Scholars (tsqn202103169), China Postdoctoral Science Foundation (2020M672074), and the Open Research Project of Shanghai Key Laboratory of Sleep Disordered Breathing (SHKSDB-KF-20-02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.971913/full#supplementary-material
References
- 1.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. (2017) 67:862–73. 10.1016/j.jhep.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat Rev Gastroenterol Hepatol. (2022) 19:60–78. [DOI] [PubMed] [Google Scholar]
- 3.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. (2018) 68:268–79. 10.1016/j.jhep.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:948–63. 10.1016/j.jacc.2018.11.050 [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus – mechanisms and treatments. Nat Rev Gastroenterol Hepatol. (2021) 18:599–612. 10.1038/s41575-021-00448-y [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. (2017) 66:1138–53. 10.1136/gutjnl-2017-313884 [DOI] [PubMed] [Google Scholar]
- 7.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. (2018) 68:335–52. 10.1016/j.jhep.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 8.Puchades Renau L, Herreras Lopez J, Cebria IIMA, Cezon Serrano N, Di Maira T, Berenguer M. Frailty and sarcopenia in acute-on-chronic liver failure. Hepatol Commun. (2021) 5:1333–47. 10.1002/hep4.1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean sarcopenic obesity study. Hepatology. (2014) 59:1772–8. 10.1002/hep.26716 [DOI] [PubMed] [Google Scholar]
- 10.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. (2017) 66:123–31. 10.1016/j.jhep.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 11.Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011). Hepatology. (2016) 63:776–86. 10.1002/hep.28376 [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro SM, Kehayias JJ. Sarcopenia and the analysis of body composition. Adv Nutr. (2014) 5:260–7. 10.3945/an.113.005256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey Smith G, Hemani G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. 10.1093/hmg/ddu328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zillikens MC, Demissie S, Hsu YH, Yerges-Armstrong LM, Chou WC, Stolk L, et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat Commun. (2017) 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort(). J Hepatol. (2020) 73:505–15. 10.1016/j.jhep.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 16.Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. 10.1093/ije/dyt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. 10.1097/EDE.0000000000000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol. (2015) 63:486–93. 10.1016/j.jhep.2015.02.051 [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Wijarnpreecha K, Sandhu KK, Cholankeril G, Ahmed A. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int. (2021) 41:1832–40. 10.1111/liv.14852 [DOI] [PubMed] [Google Scholar]
- 25.Moon JH, Koo BK, Kim W. Non-alcoholic fatty liver disease and sarcopenia additively increase mortality: A Korean nationwide survey. J Cachexia Sarcopenia Muscle. (2021) 12:964–72. 10.1002/jcsm.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. (2022) 76:588–99. 10.1016/j.jhep.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J Endocrinol. (2016) 229:R67–81. 10.1530/JOE-15-0533 [DOI] [PubMed] [Google Scholar]
- 28.Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. (2019) 40:1367–93. 10.1210/er.2019-00034 [DOI] [PubMed] [Google Scholar]
- 29.Son JW, Lee SS, Kim SR, Yoo SJ, Cha BY, Son HY, et al. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia. (2017) 60:865–72. 10.1007/s00125-016-4196-9 [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. (2021) 13:3519. 10.3390/nu13103519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. (2019) 70:711–24. 10.1002/hep.30429 [DOI] [PubMed] [Google Scholar]
- 32.Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. (2009) 58:2616–23. 10.2337/db09-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. (2008) 40:1461–5. 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh YC, Joo SK, Koo BK, Lin HC, Lee DH, Chang MS, et al. Myosteatosis, but not sarcopenia, predisposes NAFLD subjects to early steatohepatitis and fibrosis progression. Clin Gastroenterol Hepatol. (2022) S1542-3565:00074–X. 10.1016/j.cgh.2022.01.020 [DOI] [PubMed] [Google Scholar]
- 35.Karasik D, Zillikens MC, Hsu YH, Aghdassi A, Akesson K, Amin N, et al. Disentangling the genetics of lean mass. Am J Clin Nutr. (2019) 109:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw SC, Dennison EM, Cooper C. Epidemiology of sarcopenia: Determinants throughout the lifecourse. Calcif Tissue Int. (2017) 101:229–47. 10.1007/s00223-017-0277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choe HJ, Cho BL, Park YS, Roh E, Kim HJ, Lee SG, et al. Gender differences in risk factors for the 2 year development of sarcopenia in community-dwelling older adults. J Cachexia Sarcopenia Muscle. (2022) 13:1908–18. 10.1002/jcsm.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burra P, Bizzaro D, Gonta A, Shalaby S, Gambato M, Morelli MC, et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. (2021) 41:1713–33. 10.1111/liv.14943 [DOI] [PubMed] [Google Scholar]
- 39.Sheedfar F, Di Biase S, Koonen D, Vinciguerra M. Liver diseases and aging: Friends or foes? Aging Cell. (2013) 12:950–4. 10.1111/acel.12128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.