Abstract
This study demonstrates that intravenous infusion of the cell-penetrant thiol ester, L-cysteine ethyl ester (L-CYSee), to adult male Sprague-Dawley rats elicited (a) minor alterations in frequency of breathing, expiratory time, tidal volume, minute ventilation, or expiratory drive but pronounced changes in inspiratory time, end-inspiratory and expiratory pauses, peak inspiratory and expiratory flows, EF50, relaxation time, apneic pause, inspiratory drive and non-eupneic breathing index, (b) minimal changes in arterial blood-gas (ABG) chemistry (pH, pCO2, pO2, SO2) and Alveolar-arterial (A-a) gradient (index of alveolar gas exchange), and (c) minimal changes in antinociception (tail-flick latency). Subsequent injection of morphine (10 mg/kg, IV) elicited markedly smaller effects on the above parameters, ABG chemistry, and A-a gradient in rats receiving L-CYSee, whereas morphine antinociception was not impaired. Infusions of L-cysteine or L-serine ethyl ester (oxygen rather than sulfur moiety), did not affect morphine actions on ABG chemistry or A-a gradient. L-CYSee (250 μmol/kg, IV) injection elicited dramatic changes in ventilatory parameters given 15 min after injection of morphine in rats receiving L-CYSee. Our findings suggest that (a) L-CYSee acts in neurons that drive ventilation, (b) L-CYSee reversal of the adverse actions of morphine on ventilation, ABG chemistry and A-a gradient may be via modulation of intracellular signaling pathways activated by morphine rather than by direct antagonism of opioid receptors since morphine antinociception was not diminished by L-CYSee, and (c) the thiol moiety of L-CYSee is vital to efficacy, (d) intracellular conversion of L-CYSee to an S-nitrosylated form may be part of its mechanism of action.
Keywords: L-thiol ester infusion, Morphine, Ventilatory depression, Arterial blood-gas chemistry, Analgesia, Sprague-Dawley rats
1. Introduction
The clinical effectiveness of opioids as analgesics is often compromised by their ability to induce adverse effects on ventilatory parameters [1–6]. Although the administration of opioid receptor (OR) antagonists can in many cases reverse opioid-induced respiratory depression (OIRD), these antagonists also diminish the analgesic/antinociceptive actions of opioids, which may not be a problem in overdose situations, but which is contraindicated when analgesia is required during and after injuries and surgical procedures [2–4]. Over recent years, numerous classes of drugs that do not directly block ORs have been examined for their potential to overcome OIRD while preserving analgesia [2–8]. Most of these drugs did not reach the level of consideration for clinical trials in humans or did not meet expected therapeutic outcomes in such trials because of a lack of efficacy and/or unacceptable levels of toxicity or side effects [4,5,7,8]. The current opioid crisis has reinforced the urgent necessity to develop drugs that can effectively reverse OIRD by mechanisms independent of OR blockade for numerous reasons including that OR antagonists can elicit often life-threatening withdrawal symptoms in habitual opioid users [5,6].
Membrane-permeable thioesters such as L-cysteine ethyl ester (L-CYSee), D-cysteine ethyl ester (D-CYSee), L-cysteine methyl ester (L-CYSme), L-glutathione ethyl ester (L-GSHee), D-cystine dimethyl ester (D-CYSdime) and D-cystine diethyl ester (D-CYSdiee) readily gain access to central and peripheral tissues and have diverse pharmacological actions [9–14]. We have reported that these L-, D-thioesters prevent and/or reverse the adverse effects of opioids such as morphine and fentanyl on ventilation, arterial blood-gas (ABG) chemistry, and Alveolar-arterial (A-a) gradient (index of alveolar gas exchange in the lungs) in unanesthetized freely-moving rats whereas L- and D-thioesters minimally impact the sedative or analgesic effects of the opioids [10–14]. In contrast, the parent thiols, L- or D-cysteine, L-glutathione, and D-cystine, only minimally altered the effects of the opioids [9–14]. In initial studies that were designed to better understand structure-activity relationships of L, and D-thioesters, we demonstrated that N-acetyl-L-cysteine methyl ester [14] and L-methionine ethyl ester (unpublished observations) could not reverse any of the deleterious effects of morphine on breathing in unanesthetized rats. These findings suggest that modifications to the L-,D-cysteine backbone or sulfur moiety prevent L-,D-thioester efficacy. The mechanisms of action of these L-, D-thioesters could involve (i) direct interactions with yet to be determined functional proteins, (ii) binding to the putative L-,D-cysteine binding protein, myristoylated alanine-rich C-kinase substrate [15], (iii) interruption of OR-β-arrestin-coupled cell signaling processes to spare the analgesic G-protein-dependent actions of morphine [16,17], and/or (iv) conversion of reduced L-,D-thioesters to S-nitrosothiols via S-nitrosylation of the sulfur atom by nitric oxide synthase-dependent processes [15–21], which may act similarly S-nitroso-L-CYSee [22].
Our studies to date in freely-moving adult rats have only examined the effects of bolus intravenous injections of L-,D-thioesters against the ventilatory depressant effects of opioids [9–14]. We subsequently wanted to extend our knowledge about the pharmacological profile and efficacy of these L-,D-thioesters by determining whether continuous intravenous infusion of these agents would be efficacious against OIRD while sparing opioid analgesia. We began these studies by determining whether the effects of a bolus injection of morphine on ventilatory parameters, ABG chemistry, A-a gradient, and antinociception were altered in freely-moving adult male Sprague-Dawley rats receiving continuous intravenous infusion of L-CYSee. To extend our structure-activity knowledge about L-,D-thioesters, we also determined whether similar infusions of the cell penetrant L-thioester, L-serine ethyl ester (L-SERee) [9], which has an oxygen instead of a sulfur atom, could be as equally effective as L-CYSee in reversing morphine OIRD.
2. Methods
2.1. Permissions, rats, and surgical procedures
All studies were done in accordance with the NIH Guide for Care and Use of Laboratory Animals (NIH Publication No. 80–23) revised in 1996 and in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (http://www.nc3rs.org.uk/page.asp?id=1357). All protocols were approved by the Animal Care and Use Committees of Galleon Pharmaceuticals, the University of Virginia Case, and Case Western Reserve University. Adult male Sprague Dawley rats were purchased from Harlan Industries (Madison, WI, USA). After five days of recovery from transportation, the rats received femoral artery catheters and/or two jugular vein catheters under 2–3% isoflurane anesthesia [10–14, 23]. One of the jugular catheters was to allow for continuous infusion of L-CYSee or L-SERee and the other was to allow bolus injections of morphine (10 mg/kg, IV). The rats were given 4 days to recover from surgery. All femoral arterial catheters were flushed daily with a heparin solution (50 units in 0.1 M, pH 7.4 phosphate-buffered saline). On the day of the study, the arterial catheters were flushed with 0.3 ml of phosphate-buffered saline (0.1 M, pH 7.4) 2–3 h before the start of the protocols. The pH of all stock solutions of vehicle, L-CYSee, and L-SERee was adjusted to a pH of 7.3 with 0.125 M NaOH. All studies were done in a quiet laboratory room with a relative humidity of 49 ± 3% and room temperature of 21.3 ± 0.2 °C. The ventilatory studies, ABG chemistry studies, and antinociception studies were performed in separate groups of rats to not compromise each set of recordings. The plethysmography and antinociception recording sessions and arterial blood sampling studies (ABG assays) were done by one investigator whereas another filled the syringes with test agents, such that the investigator performing the experiment was blind to the protocol. In addition, the data files resulting from each experiment were collated and analyzed by yet another investigator in the group.
2.2. Protocols for whole-body plethysmography measurement of ventilatory parameters
Ventilatory parameters were recorded continuously in unrestrained freely-moving rats by a whole-body plethysmography system (PLY3223; Data Sciences International, St. Paul, MN) as described previously [9–14]. Directly recorded and derived parameters (see 53–56) are defined in Table 1. These parameters and their abbreviations are: frequency of breathing (Freq), tidal volume (TV), minute ventilation (MV), inspiratory time (Ti), expiratory time (Te), Ti/Te, end-inspiratory pause (EIP), end-expiratory pause (EEP), relaxation time (RT), peak inspiratory flow (PIF), peak expiratory flow (PEF), expiratory flow at 50% expired TV (EF50), point in expiration when PEF occurs expressed as a fraction of Te (Rpef), inspiratory drive (TV/Ti), expiratory drive (TV/Te), apneic pause [(Te/RT)−1], non-eupneic breathing index (NEBI) and NEBI corrected for Freq (NEBI/Freq). On the day of the study, each rat was placed in an individual plethysmography chamber and given 60–75 min to acclimatize so that baseline (pre) ventilatory parameter values could be defined. One group of rats (n = 5) received a continuous infusion of vehicle (20 μL/min, IV) and another received a continuous infusion of L-CYSee (14.3 μmol/kg/min, IV). Both infusions were maintained throughout the study. After 35 min of infusion, all rats received a bolus injection of morphine (10 mg/kg, IV). After, a further 15 min, the vehicle-infused rats received a bolus injection of vehicle whereas the L-CYSee-infused rats received a bolus injection of L-CYSee (250 μmol/kg, IV) and ventilatory parameters were recorded for a further 45 min. The body weights of the two groups were equivalent to one another (see Table 2) and as such, ventilatory parameters related to volumes such as TV, PIF, PEF, and EF50 are presented without correcting for body weight. FinePointe (DSI) software constantly corrected digitized ventilatory values originating from the respiratory waveforms for alterations in chamber humidity and temperature. Pressure changes associated with the respiratory waveforms were converted to volumes (e.g., TV, PIF, PEF, EF50) using the algorithms of Epstein and colleagues [24,25]. Factoring in chamber humidity and temperature, cycle analyzers filtered the acquired signals, and FinePointe algorithms generated an array of box flow data that identified waveform segments as acceptable breaths, and minimum and maximum values were determined. Flows at this point were box-flow signals and from this array, minimum and maximum box flow values were then determined and multiplied by a compensation factor from the algorithms [24,25] thus producing TV, PIF, PEF, and EF50 values used to determine non-eupneic breathing events expressed as NEBI, reported as the percentage of non-eupneic breathing events per each individual epoch [10–14, 23, 26]. Apneic pause was determined by the formula, (Te/RT) - 1 [10–14, 23].
Table 1.
Definition of ventilatory parameters.
| Parameter | Abbreviation | Units | Definition |
|---|---|---|---|
| Frequency of breathing | Freq | breaths/min | Frequency of breaths per min |
| Tidal volume | TV | ml | Volume of air per breath |
| *Minute ventilation | MV = Freq × TV | ml/min | Total volume of air breathed in per min |
| Inspiratory time | Ti | sec | Duration of inspiration |
| Expiratory time | Te | sec | Duration of expiration |
| End Inspiratory Pause | EIP | msec | Pause between the end of inspiration and start of expiration |
| End Expiratory Pause | EEP | msec | Pause between the end of expiration and start of inspiration |
| Relaxation time | RT | sec | Time to expire 64% of tidal volume: non-elastic phase |
| Peak inspiratory flow | PIF | ml/sec | Peak of inspiratory flow |
| Peak expiratory flow | PEF | ml/sec | Peak of expiratory flow |
| EF50 | EF50 | ml/sec | Air-flow at 50% of expired TV |
| Rpef | Rpef | none | Point in expiration when PEF occurs expressed as a fraction of Te |
| *Apneic Pause | AP = (Te/RT)−1 | none | Relative pause in expiration – index of increased airway resistance |
| *Inspiratory drive | TV/Ti | ml/sec | Drive to inspire air |
| *Expiratory drive | TV/Te | ml/sec | Drive to expire air |
| Non-eupneic breathing index | NEBI | % of breaths | Occurrence of non-eupneic breathing events such as apneas |
| *NEBI/Frequency of breathing | NEBI/Freq | %/(breaths/min) | NEBI adjusted for frequency of breathing |
Parameters denoted with an asterisk were computed from directly recorded parameters
Table 2.
Baseline (pre) values in the groups that received vehicle or L-CYSee.
| Parameter | Vehicle | L-CYSee |
|---|---|---|
| N | 5 | 6 |
| Age, days | 79.1 ± 0.3 | 78.7 ± 0.3 |
| Body weights, grams | 335 ± 2 | 333 ± 2 |
| Frequency, breaths/min | 94.8 ± 2.6 | 97.0 ± 5.2 |
| Tidal Volume (TV), ml | 2.40 ± 0.04 | 2.33 ± 0.09 |
| Minute Ventilation, ml/min | 228 ± 9 | 224 ± 5 |
| Inspiratory Time (Ti), sec | 0.24 ± 0.02 | 0.26 ± 0.01 |
| Expiratory Time (Te), sec | 0.41 ± 0.03 | 0.40 ± 0.03 |
| Inspiratory Time/Expiratory Time | 0.61 ± 0.07 | 0.66 ± 0.04 |
| Ti/(Ti + Te) | 37.4 ± 3.0 | 39.4 ± 1.5 |
| End Inspiratory Pause, msec | 8.1 ± 0.3 | 8.0 ± 0.3 |
| End Expiratory Pause, msec | 56.4 ± 4.8 | 58.8 ± 3.4 |
| Peak Inspiratory Flow, ml/sec | 14.6 ± 1.0 | 13.8 ± 0.6 |
| Peak Expiratory Flow, ml/sec | 10.9 ± 0.5 | 10.1 ± 0.6 |
| Rpef | 0.17 ± 0.02 | 0.17 ± 0.01 |
| EF50, ml/sec | 0.46 ± 0.03 | 0.46 ± 0.04 |
| Inspiratory Drive (TV/Ti), ml/sec | 10.3 ± 0.9 | 9.2 ± 0.3 |
| Inspiratory Drive (TV/Te), ml/sec | 6.1 ± 0.5 | 6.0 ± 0.3 |
| Apneic Pause, sec | 0.79 ± 0.06 | 0.86 ± 0.03 |
| Non-Eupneic Breathing Index (NEBI), % | 7.0 ± 0.6 | 6.5 ± 0.2 |
| NEBI/Freq, (%/(breaths/min)) × 100 | 7.4 ± 0.6 | 6.7 ± 0.4 |
L-CYSee, L-cysteine ethyl ester. There were 5 rats in the vehicle group and 6 rats in the L-CYSee group. The data are presented as mean ± SEM. There were no between group differences for any parameter (P > 0.05, for all comparisons).
2.3. Protocols for blood gas measurements and determination of Arterial-alveolar gradient
ABG chemistry parameters (pH, pCO2, pO2, sO2) and A-a gradients were recorded before (Pre) and 30 min after commencing continuous infusions of vehicle (20 μL/kg/min, IV; n = 9 rats, 84.4 ± 0.4 days of age; 341 ± 3 g), L-CYSee (7.15 μmol/kg, IV; 84.7 ± 0.3 days; 346 ± 3 g) or L-SERee (7.15 μmol/kg, IV; 84.3 ± 0.4 days; 341 ± 3 g) as described previously [9–14, 23]. All rats received a bolus injection of morphine (10 mg/kg, IV) and arterial blood samples (100 μL) were taken after 5, 15, 30, 45, and 60 min. The above samples of arterial blood were injected into a Radiometer blood-gas analyzer (ABL800 FLEX) to obtain ABG values. The A-a gradient defines the differences between alveolar O2 and arterial blood O2 concentrations (see 10–14, 23). A fall in PaO2, without change in A-a gradient, is due to hypoventilation, whereas a decrease in PaO2 with a concomitant elevation in A-a gradient indicates an ongoing mismatch in ventilation-perfusion in alveoli [10–14, 23]. A-a gradient = PAO2 − PaO2, where PAO2 is the partial pressure (p) of alveolar O2 and PaO2 is pO2 in the sampled arterial blood. PAO2 = [(FiO2 × (Patm − PH2O) - (PaCO2/respiratory quotient)], where FiO2 is the fraction of O2 in inspired air; Patm is atmospheric pressure; PH2O is the partial pressure of H2O in inspired air; PaCO2 is pCO2 in arterial blood; and respiratory quotient (RQ) is the ratio of CO2 eliminated/O2 consumed. We took FiO2 of room-air to be 21% = 0.21, Patm to be 760 mmHg, and PH2O to be 47 mmHg. We took the RQ value of our adult male rats to be 0.9 [10–14, 23, 27, 28].
2.4. Antinociception assessment by tail-flick latency assay
We recorded TFL values before (−15 and 0 min, Pre values), and at 15 and 35 min during the continuous infusion of vehicle (20 μL/kg/min, IV; 81.9 ± 0.3 days; 325 ± 3 g), L-CYSee (14.3 μL/kg/min, IV; 82.2 ± 0.4 days; 327 ± 3 g) or L-SERee (14.3 μmol/kg/min, IV; 81.8 ± 0.2 days; 323 ± 3 g). Both groups of rats received an injection of morphine (10 mg/kg, IV) after the TFL recording at 35 min of infusion, and TFL values were recorded again after 15, 30, and 60 min. TFL values were determined using a Tail-Flick Analgesia Meter (IITC Life Science Inc., USA) as detailed previously [10–14, 23]. The procedure involved a minor degree of manual restraint while positioning the tail to apply a thermal beam sufficient to induce a latency of tail withdrawal of approximately 2.5 s. TFL data are shown as actual TFL (sec) and as maximum possible effect (%MPE) determined by the formula, %MPE = [(post-injection TFL − baseline TFL)/(12 − baseline TFL)] × 100 [10–14, 23].
2.5. Data analyses
Directly recorded and arithmetically-derived ventilatory parameters (1 min bins) were taken for statistical analyses. Pre-drug 1 min bins excluded occasional marked deviations from resting values due to abrupt rat movements such as scratching. All ventilatory, ABG chemistry, A-a gradient, and TFL are presented as mean ± SEM and were evaluated using one-way and two-way ANOVA followed by Bonferroni corrections for multiple comparisons between means using the error mean square terms from each ANOVA analysis [29–32] as detailed previously [9–14]. A P < 0.05 value denoted an initial level of statistical significance that was modified according to the number of comparisons between means as described by Wallenstein et al. [31]. The modified t-statistic is t = (mean group 1 − mean group 2)/[s × (1/n1 + 1/n2)1/2] where s2 = the mean square within groups term from the ANOVA (the square root of this value is used in the modified t-statistic formula) and n1 and n2 are the number of rats per group being compared. Based on elementary inequality called Bonferroni’s inequality, a conservative critical value for modified t-statistics from tables of t-distribution using a significance level of P/m, where m is the number of comparisons between groups to be performed [32]. The degrees of freedom are those of the mean square for within-group variation from ANOVA tables. The critical Bonferroni value cannot be found in conventional tables of the t-distribution but can be approximated from tables of the normal curve by t * = z + (z + z3)/4 n, with n being the degrees of freedom and z being the critical normal curve value for P/m [29–32]. Wallenstein et al. [31] first demonstrated that the Bonferroni procedure is preferable for general use since it has the widest range of applications and because it provides critical values that are lower than those of other procedures when the investigator can limit the number of comparisons (and will be slightly larger than other procedures if many comparisons are made. As mentioned, a value of P < 0.05 was taken as the initial level of statistical significance [29–31] and the statistical analyses were performed with the aid of GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Ventilatory responses elicited by morphine in rats receiving infusion of L-CYSee
Baseline parameters in the two groups of rats prior to them receiving infusions of vehicle or L-CYSee are summarized in Table 1. As can be seen, there were no between-group differences for any parameter (P > 0.05, for all comparisons). Freq, TV, and MV values during the various stages of the experiment are summarized in the left-hand panels of Fig. 1. The infusion of L-CYSee (14.3 μmol/kg/min, IV) elicited relatively minor increases in Freq (Panel A) and minor decreases in TV (Panel C) such that there were only trivial changes in MV (Panel E). A bolus injection of morphine (10 mg/kg, IV) into rats receiving vehicle infusion elicited a decrease in Freq and more sustained decreases in TV and MV. In rats receiving an infusion of L-CYSee, the injection of morphine elicited a pronounced increase in Freq, a smaller but distinct reduction in TV than in vehicle-infused rats such that morphine elicited trivial decreases in MV. The injection of L-CYSee (250 μmol/kg, IV) given 15 min after the injection of morphine elicited an increase in Freq and TV resulting in a sustained increase in MV. The right-hand panels summarize the sum of the responses during each phase of the protocol. Infusion (columns designated as INF) of L-CYSee elicited an increase in Freq (Panel B), a decrease in TV (Panel D) and no change in MV (Panel F). The total decreases in Freq elicited by morphine in vehicle-infused rats (first 15 min, columns designated as MOR) were converted to pronounced increases in the L-CYSee-infused rats. The total decreases in TV elicited by morphine were much smaller in the L-CYSee-infused rats and as such the total changes in MV were not significant. The injection of L-CYSee (250 μmol/kg, IV) maintained the increase in Freq, restored TV to baseline values resulting in a pronounced total increase in MV.
Fig. 1.
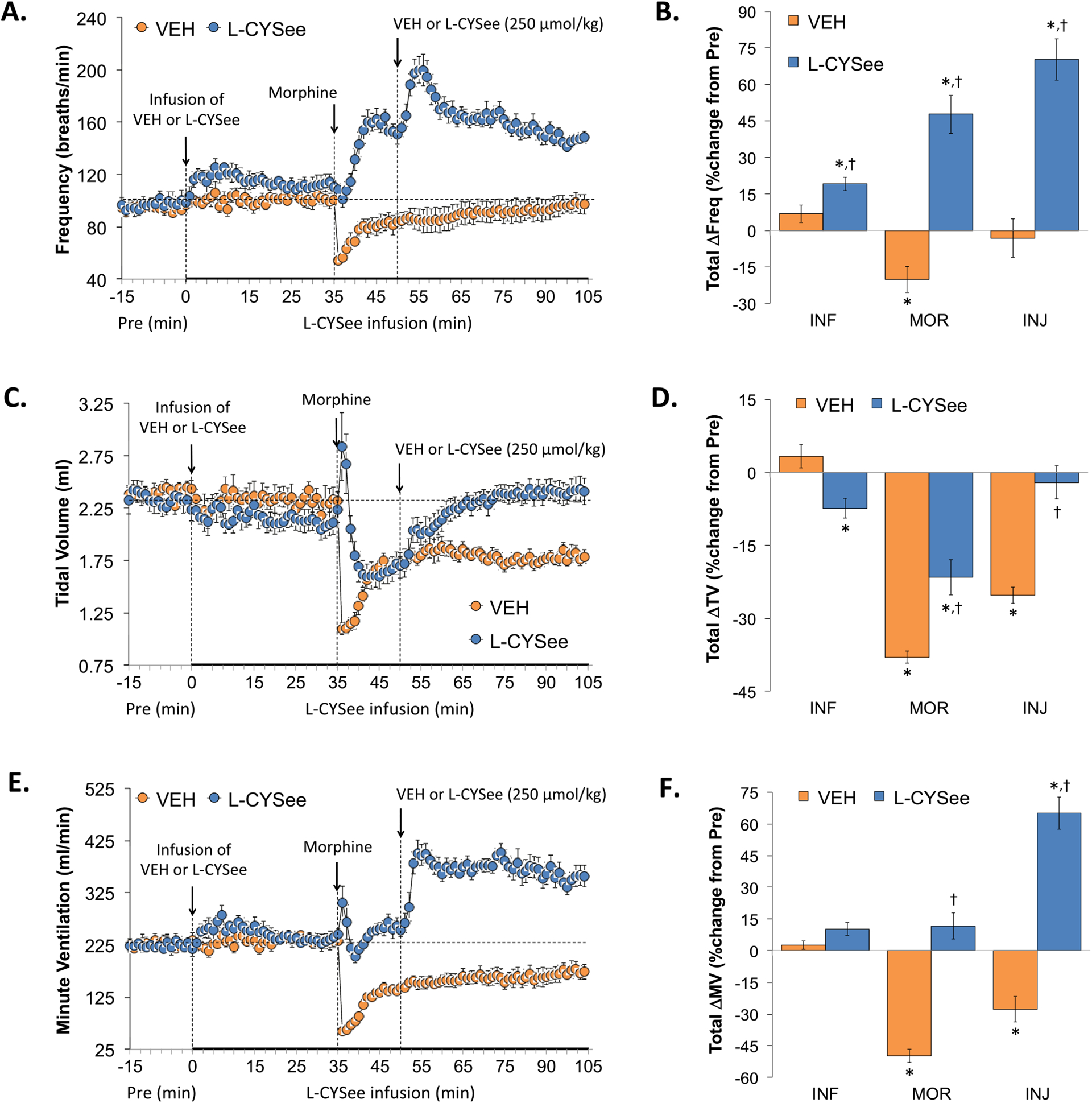
Changes in frequency of breathing (Freq), tidal volume (TV), and minute ventilation (MV). Panels A, C, and E: Actual Freq, TV, and MV values during the various stages of the experiment, respectively. Panels B, D, and F: Sum of the Freq, TV, and MV responses recorded during infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV) upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group. *P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
Ti, Te, and Ti/Te (respiratory quotient) values during the various stages of the experiment are summarized in the left-hand panels of Fig. 2. The infusion of L-CYSee elicited a substantial decrease in Ti (Panel A) and minor changes in Te (Panel C) resulting in a decrease in Ti/Te (Panel E). Injection of morphine into vehicle-infused rats elicited a sustained increase in Ti, a transient increase followed by a sustained increase in Te, and a sustained increase in Ti/Te. In rats receiving L-CYSee, the injection of morphine elicited an increase in Ti, a transient increase followed by a sustained decrease in Te resulting in a gradual and sustained increase in Ti/Te paralleling that in vehicle-infused rats. Subsequent bolus injection of L-CYSee did not exert substantial changes in these parameters. With respect to the total changes in each parameter, the infusion (columns INF) of L-CYSee elicited a decrease in Ti (Panel B), no change in Te (Panel D), and therefore a decrease in Ti/Te (Panel F). Total increases in Ti elicited by morphine in vehicle-infused rats (first 15 min, MOR columns) were converted to increases in L-CYSee-infused rats. Total increases in Te elicited by morphine were converted to decreases in L-CYSee-infused rats and as such, the total changes in Ti/Te were not different from those in vehicle-infused rats. The injection of L-CYSee (250 μmol/kg, IV) maintained the changes in Ti, Te and Ti elicited by morphine in L-CYSee-infused rats.
Fig. 2.
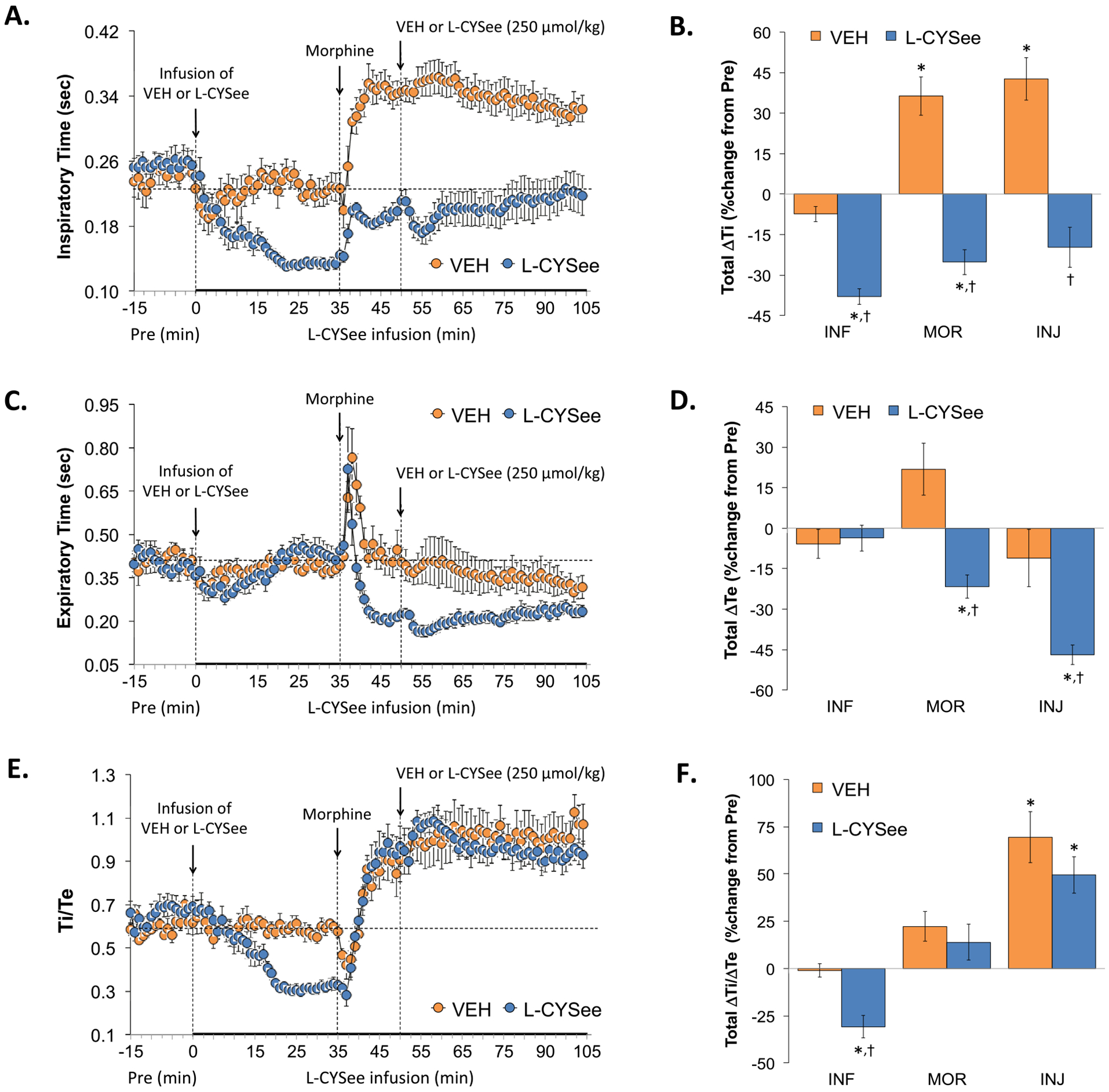
Changes in inspiratory time (Ti), expiratory time (Te), and Ti/Te. Panels A, C, and E: Actual Ti, Te, and Ti/Te values during the various stages of the experiment, respectively. Panels B, D, and F: Sum of the Ti, Te, and Ti/Te responses recorded during the infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV), upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
EIP and EEP values are summarized in the left-hand panels of Fig. 3. Infusion of L-CYSee elicited a substantial decrease in EIP (Panel A) but a substantial and sustained increase in EEP (Panel C). Injection of morphine into vehicle-infused rats elicited a sustained increase in EIP and a transient increase in EEP. In rats receiving L-CYSee, morphine elicited an increase in EIP that was similar to vehicle-infused rats. Morphine also elicited pronounced and sustained decrease in EEP. The subsequent bolus injection of L-CYSee elicited minor changes in these parameters. With respect to the total changes in EIP and EEP, the infusion (columns INF) of L-CYSee elicited a decrease in EIP (Panel B) but a large increase in EEP (Panel D). The total increases in EIP elicited by morphine in vehicle-infused rats (first 15 min, MOR columns) were absent in L-CYSee-infused rats. The total increases in EEP elicited by morphine were not greatly affected by L-CYSee-infusion. The injection of L-CYSee (250 μmol/kg, IV) did not further influence EIP or EEP.
Fig. 3.
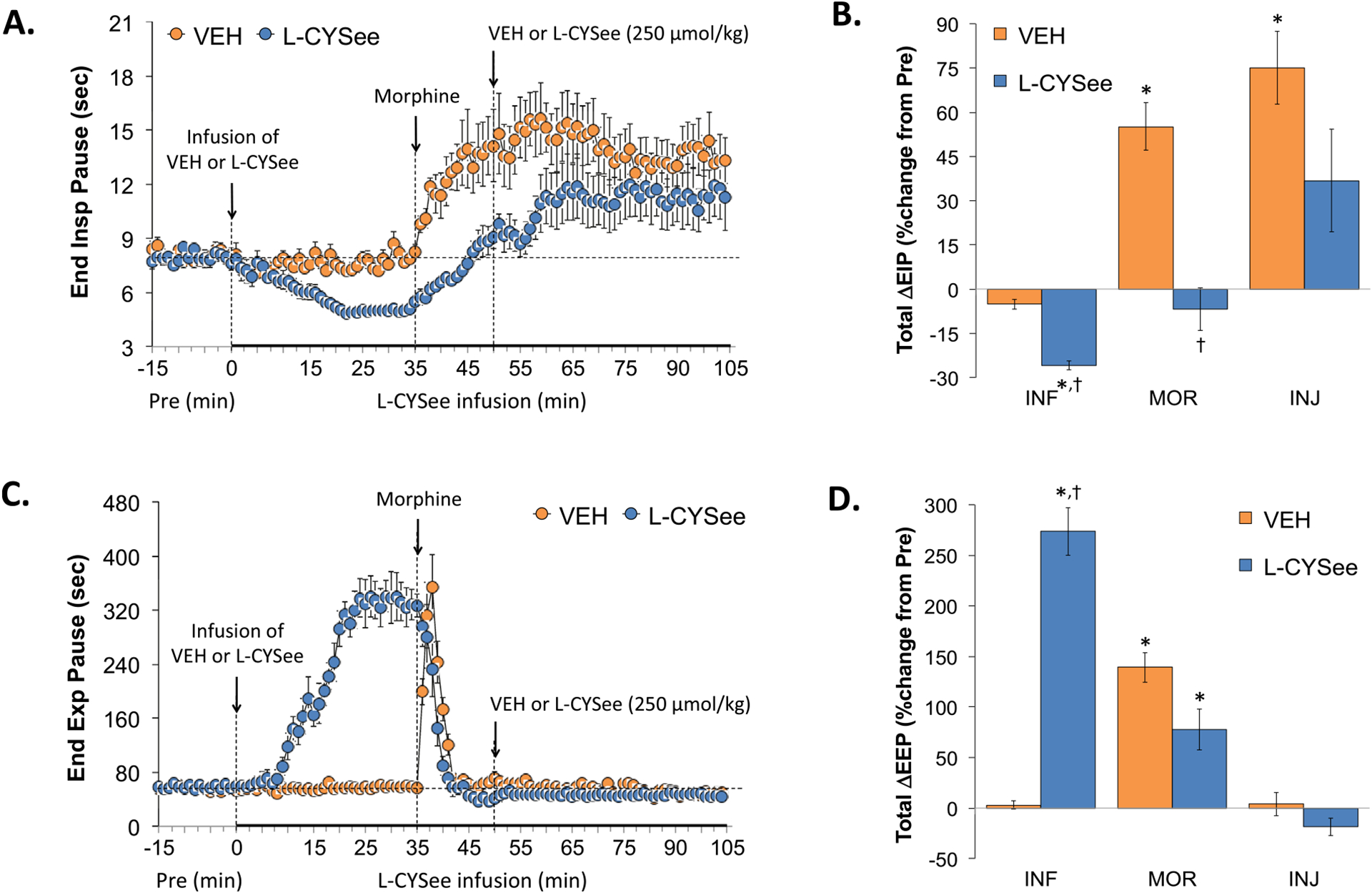
Changes in end inspiratory pause (EIP) and end expiratory pause (EEP). Panels A and C: Actual EIP and EEP values during the various stages of the experiment, respectively. Panels B and D: Sum of the EIP and EEP responses recorded during the infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV), upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
PIF, PEF, and Rpef values are summarized in the left-hand panels of Fig. 4. The infusion of L-CYSee elicited substantial increases in PIF (Panel A) and PEF (Panel C) and substantial and sustained decreases in Rpef (Panel E). Injection of morphine into vehicle-infused rats caused sustained decreases in PIF, a transient decrease in PEF, and restored Rpef values to pre-morphine values. In L-CYSee-infused rats, the injection of morphine elicited a pronounced decrease in PIF and PEF to pre-morphine levels whereas it caused a return of Rpef to pre-morphine values. The subsequent injection of L-CYSee elicited relatively minor but sustained increases in PIF, PEF, and Rpef. With respect to the total changes in each parameter, the infusion of L-CYSee (columns INF) elicited pronounced increases in PIF (Panel B) and PEF (Panel D) and a decrease in Rpef (Panel F). The total increases in PIF and PEF elicited by morphine in vehicle-infused rats (first 15 min, MOR columns) were converted to increases in PIF and PEF in L-CYSee-infused rats. The total increases in Te elicited by morphine were similar in the two groups. The injection of L-CYSee maintained the effects of L-CYSee infusion on PIF and PEF and augmented the effects of the infusion on Rpef.
Fig. 4.
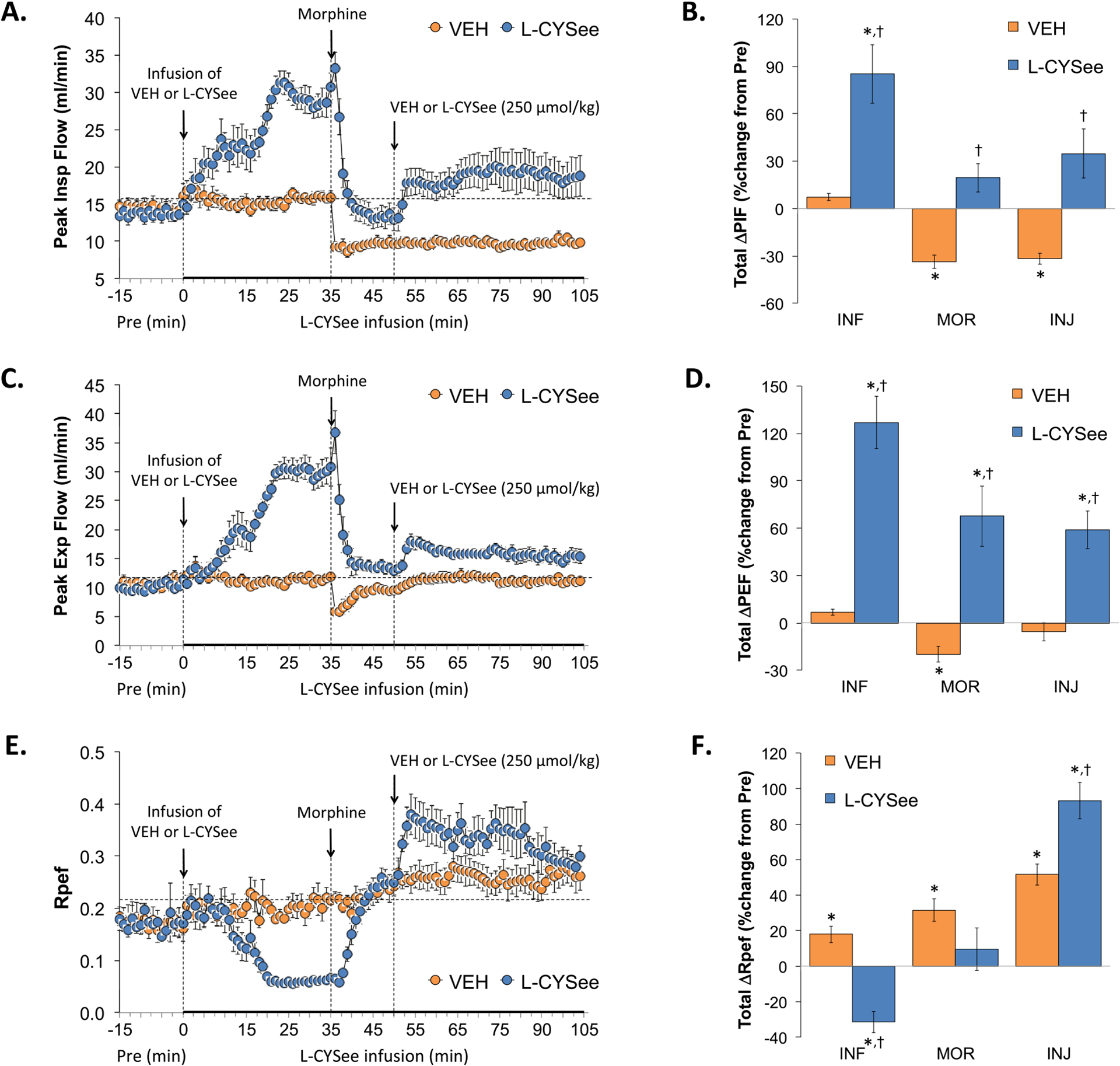
Changes in peak inspiratory flow (PIF), peak expiratory flow (PEF), and rate of achieving PEF (Rpef). Panels A, C, and E: Actual PIF, PEF, and Rpef values during the various stages of the experiment, respectively. Panels B, D, and F: Sum of the PIF, PEF, and Rpef responses recorded during the infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV), upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
EF50, Relaxation Time, and Apneic Pause values are summarized in the left-hand panels of Fig. 5. The infusion of L-CYSee elicited a substantial increase in EF50 (Panel A), a substantial decrease in Relaxation Time (Panel C), and a substantial increase in Apneic Pause (Panel E). The injection of morphine into vehicle-infused rats elicited a transient decrease in EF50, a sustained decrease in Relaxation Time, and transient increases in Apneic Pause. In L-CYSee-infused rats, the injection of morphine elicited a pronounced decrease in EF50 to values still above pre-morphine levels whereas it had minimal initial effects on Relaxation Time. The injection of morphine elicited a pronounced and sustained decrease in Apneic Pause values back to pre-L-CYSee-infusion values. Subsequent bolus injection of L-CYSee elicited a minor but sustained increase in EF50, and minor changes in Relaxation Time and Apneic Pause. Regarding the total changes in each parameter, the infusion of L-CYSee (columns INF) elicited pronounced increases in PIF (Panel B), decreases in Relaxation Time (Panel D), and increases in Apneic Pause (Panel F). The total increases in EF50 and decreases in Relaxation Time elicited by L-CYSee infusion were sustained following the injections of morphine and L-CYSee. The total increase in Apneic Pause elicited by the infusion of L-CYSee was diminished by the injection of morphine and absent upon bolus injection of L-CYSee.
Fig. 5.
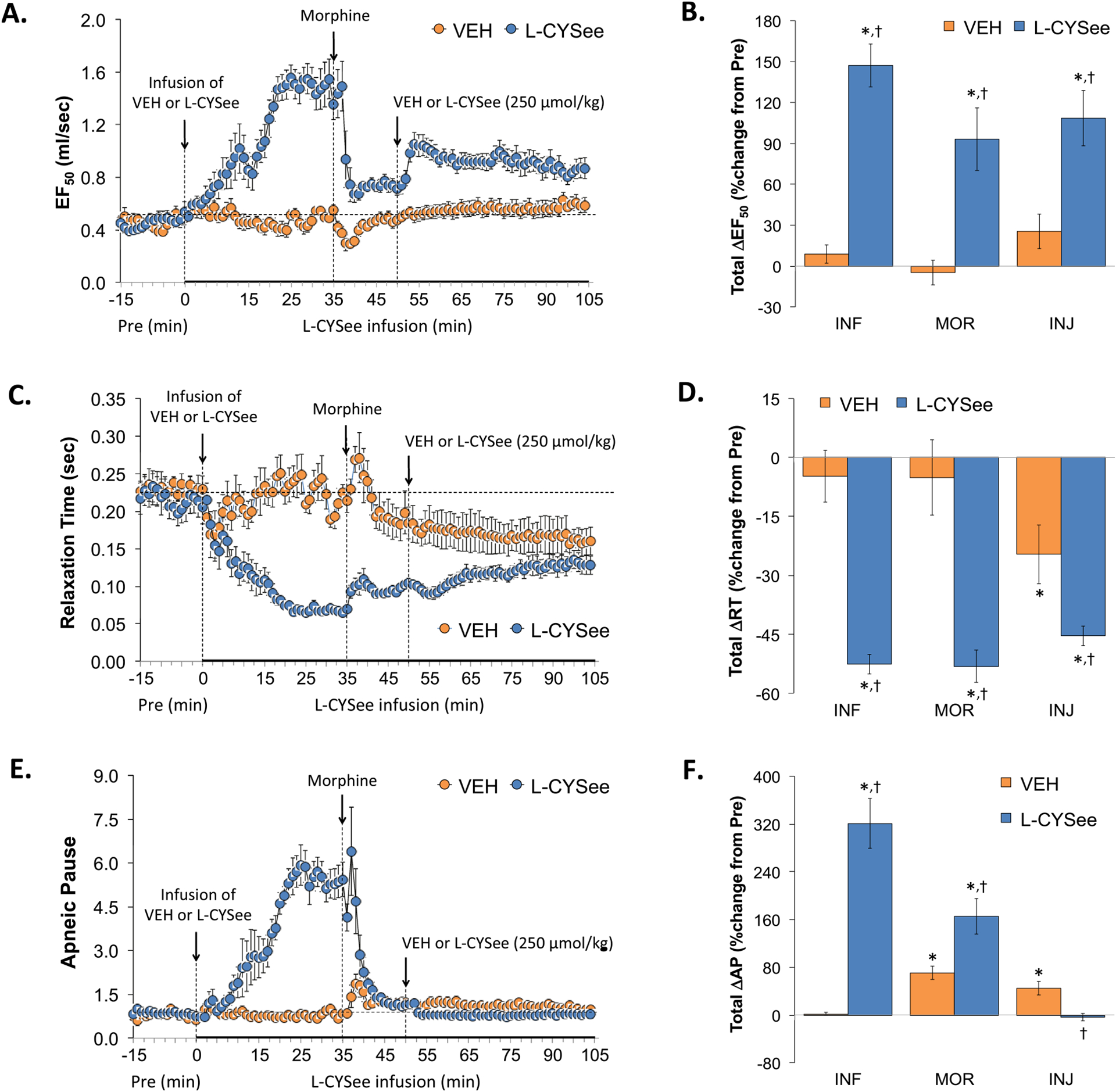
Changes in expiratory flow at 50% expired tidal volume (EF50), relaxation time (RT), and apneic pause (ApP). Panels A, C, and E: Actual EF50, RT, and ApP values during the various stages of the experiment, respectively. Panels B, D, and F: Sum of EF50, RT, and ApP responses recorded during the infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV), upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
Inspiratory Drive (TV/Ti) and Expiratory Drive (TV/Te) values are summarized in the left-hand panels of Fig. 6. L-CYSee infusion elicited substantial increases in Inspiratory Drive (Panel A) but minor changes in Expiratory Drive (Panel C). The injection of morphine into vehicle-infused rats caused sustained decreases in Inspiratory Drive and transient decreases in Expiratory Drive. In L-CYSee-infused rats, morphine elicited a rapid decrease in Inspiratory Drive toward pre-morphine levels whereas it caused an initial decrease followed by a sustained increase in Expiratory Drive. Subsequent injection of L-CYSee elicited a relatively minor but sustained increase in Inspiratory Drive and robust and sustained increases in Expiratory Drive. With respect to the total changes in each parameter, the of L-CYSee infusion (columns INF) elicited pronounced increases in Inspiratory Drive (Panel B) but minimal changes in Expiratory Drive (Panel D). The total increases in Inspiratory and Expiratory Drive elicited by morphine in vehicle-infused rats (first 15 min, MOR columns) were absent in L-CYSee-infused rats. The injection of L-CYSee maintained the effects of L-CYSee infusion on Inspiratory Drive but elicited a sustained increase in Expiratory Drive.
Fig. 6.
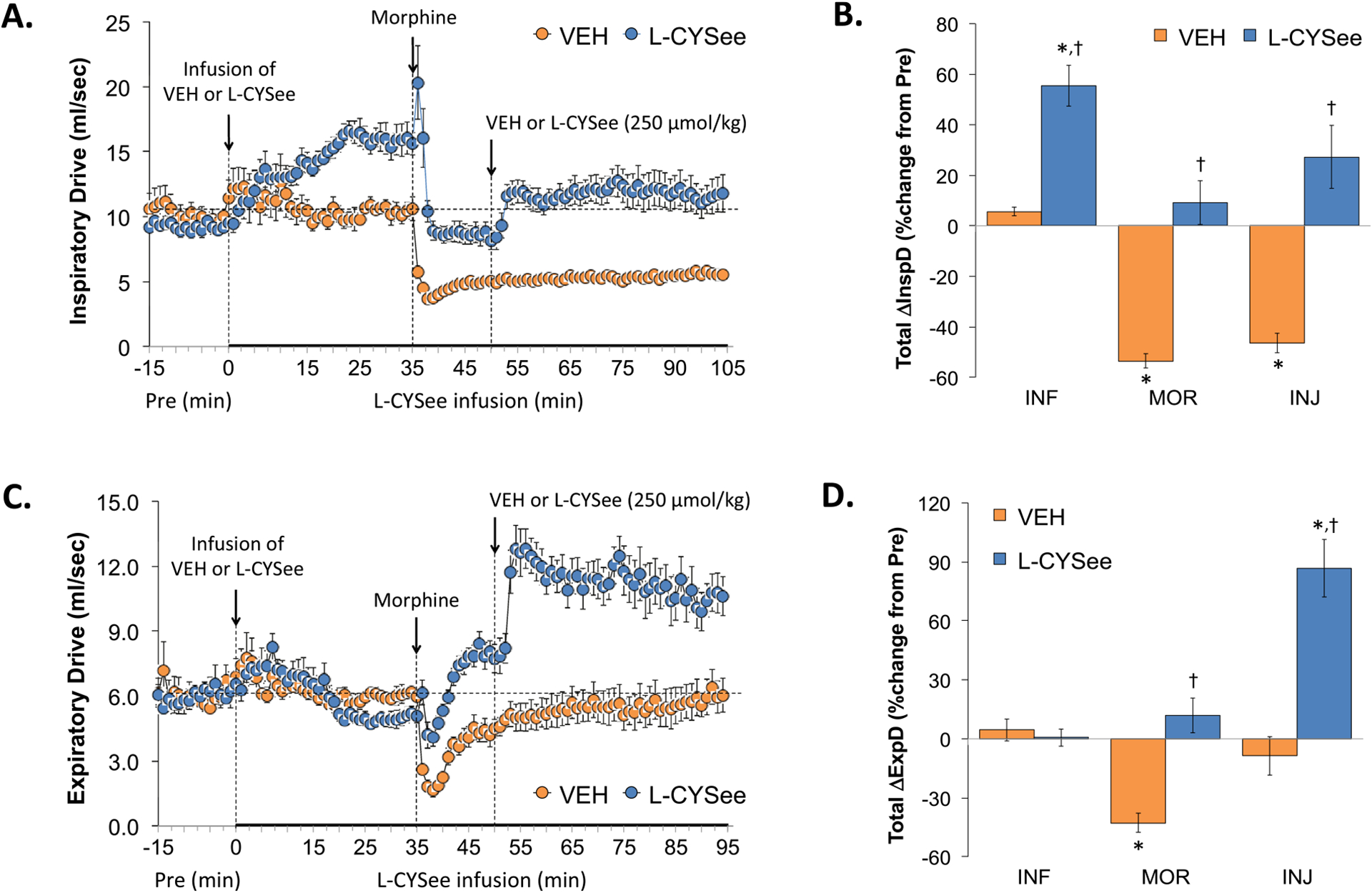
Changes in inspiratory drive (InspD) and expiratory drive (ExpD). Panels A and C: Actual InspD and ExpD values during the various stages of the experiment, respectively. Panels B and D: Sum of InspD and ExpD responses recorded during infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV), upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
NEBI and NEBI/Freq values during the various stages of the experiment are summarized in the left-hand panels of Fig. 7. The infusion of L-CYSee elicited substantial decreases in NEBI (Panel A) and NEBI/Freq (Panel C). The injection of morphine into vehicle-infused rats elicited a rapid and pronounced increase in NEBI and NEBI/Freq that rapidly decreased toward zero levels. In L-CYSee-infused rats, the injection of morphine did not affect the markedly diminished NEBI and NEBI/Freq values. The subsequent injection of L-CYSee elicited minimal responses in either group. Regarding the total changes in NEBI and NEBI/Freq, the infusion of L-CYSee (columns INF) elicited pronounced falls in NEBI (Panel B) and NEBI/Freq (Panel D) that were minimally affected by the subsequent bolus injections of morphine or L-CYSee.
Fig. 7.
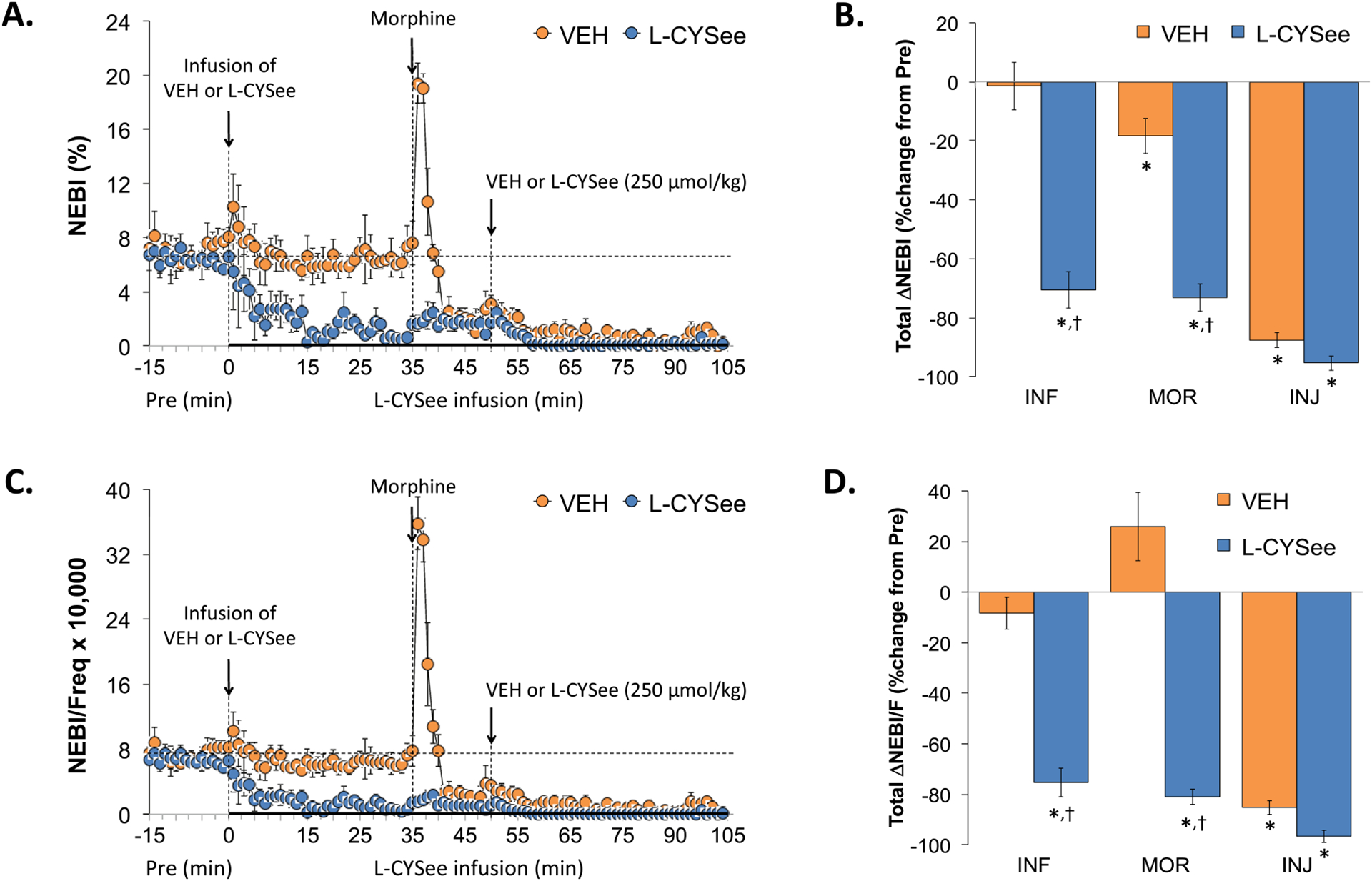
Changes in non-eupneic breathing index (NEBI) and NEBI/frequency of breathing (NEBI/Freq). Panels A and C: NEBI and NEBI/Freq values during various stages of the experiment, respectively. Panels B and D: Sum of NEBI and NEBI/Freq responses recorded during infusion of vehicle (VEH; 20 μL/min, IV) or L-CYSee (INF; 14.3 μmol/kg/min, IV), upon injection of morphine (MOR, 10 mg/kg, IV) and following injection of VEH (100 μL/100 g body weight, IV) or L-CYSee (250 μmol/kg, IV), respectively. Data are presented as mean ± SEM. There were 5 rats in the vehicle-infusion group and 6 rats in the L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
ABG chemistry parameters (pH, pCO2, pO2, and sO2) during the experiments with L-CYSee and L-SERee are summarized in Fig. 8. The infusion of L-CYSee (7.15 μmol/kg, IV) or L-SERee (7.15 μmol/kg, IV) did not (as recorded after 30 min of infusion) affect pH (Panel A), pCO2 (Panel B), pO2 (Panel C) and sO2 (Panel D). The injection of morphine (10 mg/kg, IV) elicited large and sustained reductions in pH, pO2, and sO2 that were accompanied by equally pronounced increases in pCO2 in vehicle-infused rats. The ABG chemistry values following injection of morphine in rats receiving L-SERee were very similar to those in vehicle-infused rats. In contrast, the changes in pH, pCO2, pO2, and sO2 were dramatically diminished in rats receiving the infusion of L-CYSee. A-a gradient values during the L-CYSee and L-SERee studies are summarized in Fig. 9. The infusion of L-CYSee (7.15 μmol/kg, IV) or L-SERee (7.15 μmol/kg, IV) did not (as recorded after 30 min of infusion) affect A-a gradient. The injection of morphine (10 mg/kg, IV) elicited large and sustained increases in A-a gradient that were of similar magnitude in vehicle-infused and L-SER-infused rats. In contrast, the changes in A-a gradient were markedly reduced in rats receiving L-CYSee infusion.
Fig. 8.
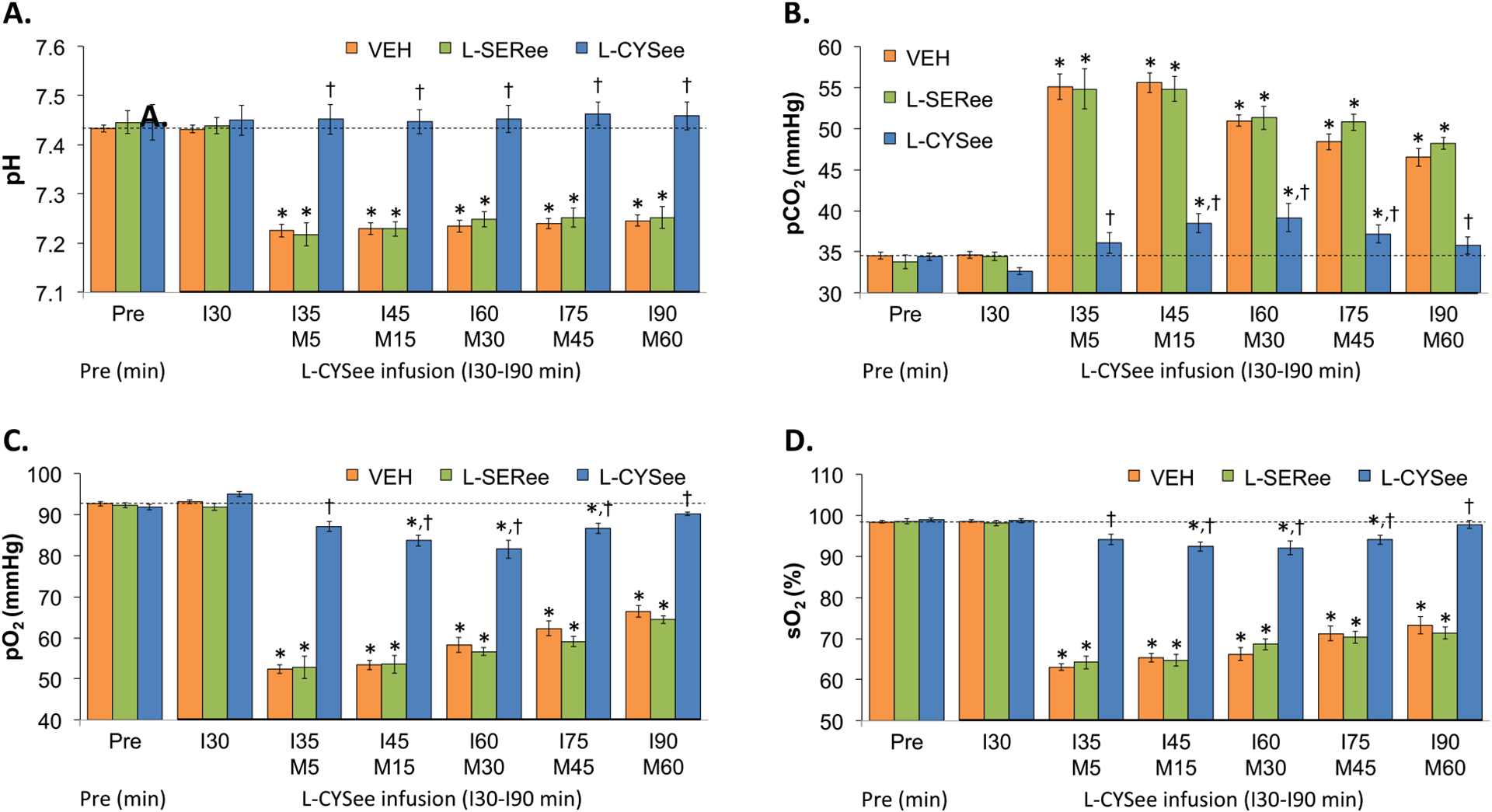
Actual values for pH (Panel A), pCO2 (Panel B), pO2 (Panel C), and sO2 (Panel D) prior to (Pre) following a bolus injection of morphine (M5–M60; 10 mg/kg, IV) in freely-moving rats receiving a continuous infusion (I30-I90) of vehicle (VEH; 10 μL/min), L-serine ethyl ester (L-SERee, 7.15 μmol/kg/min, IV) or L-cysteine ethyl ester (L-CYSee, 7.15 μmol/kg/min, IV). The data are presented as mean ± SEM. There were 8 rats in the vehicle-infusion group and 6 rats in the L-SERee-infusion and L-CYSee-infusion group.*P < 0.05, significant change from Pre values. †P < 0.05, L-CYSee versus VEH.
Fig. 9.
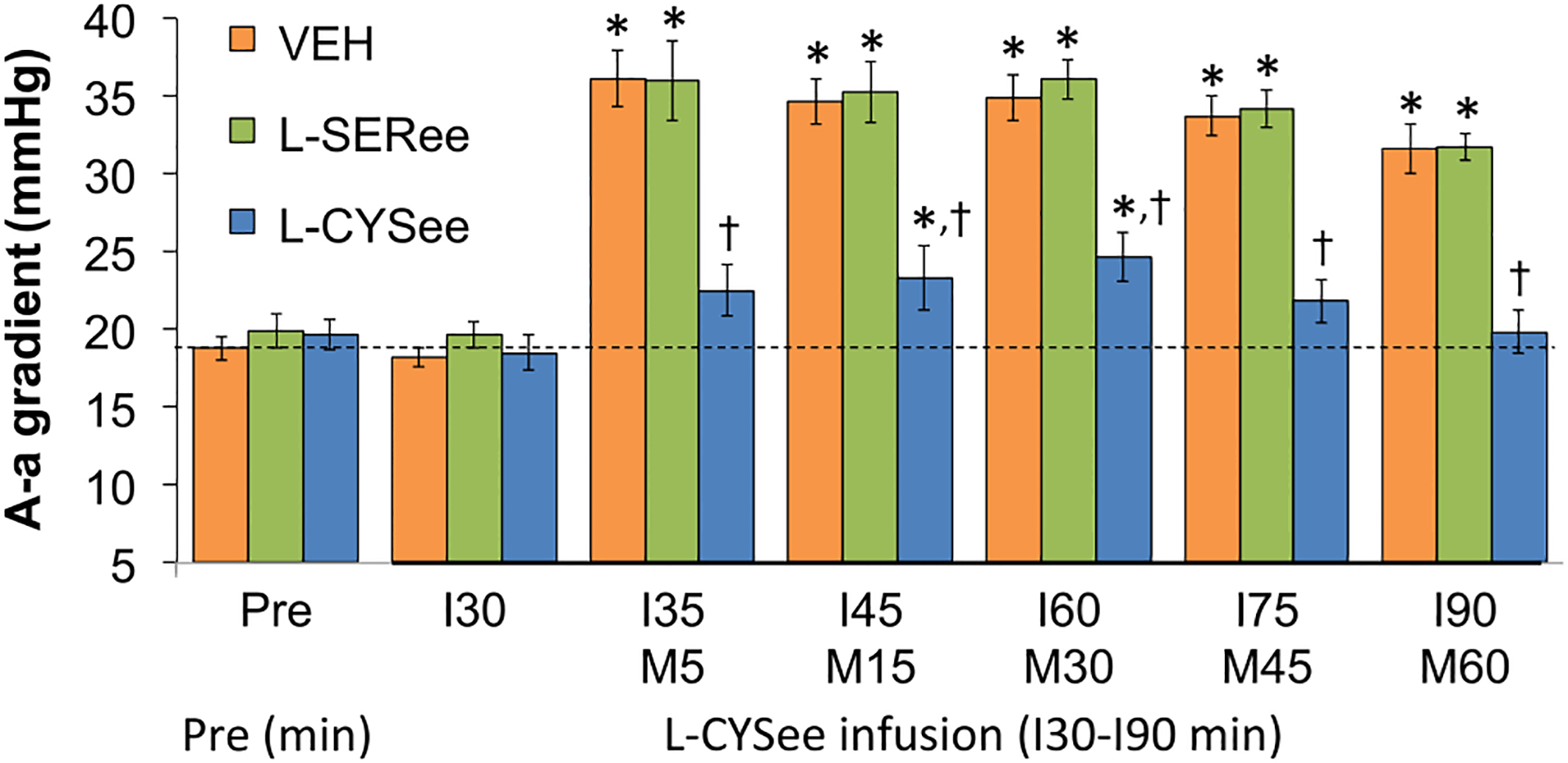
Actual values for Alveolar-arterial (A-a) gradient prior to (Pre) following an injection of morphine (M5–M60; 10 mg/kg, IV) in freely-moving rats receiving a continuous infusion (I30-I90) of vehicle (VEH; 10 μL/min), L-serine ethyl ester (L-SERee, 7.15 μmol/kg/min, IV) or L-cysteine ethyl ester (L-CYSee, 7.15 μmol/kg/min, IV). The data are presented as mean ± SEM. There were 8 rats in the vehicle-infusion group and 6 rats in the L-SERee-infusion and L-CYSee-infusion group.* P < 0.05, significant change from Pre values. † P < 0.05, L-CYSee versus VEH.
TFL values during studies with L-CYSee and L-SERee are summarized in Fig. 10. The injection of morphine (10 mg/kg, IV) elicited sustained increases in TFL that were of similar magnitude in rats receiving infusions of vehicle, L-SERee (14.3 μmol/kg/min, IV), and L-CYSee (14.3 μmol/kg/min, IV). An important note is that the injection of morphine (10 mg/kg, IV) to vehicle-infused rats caused immediate sedation with the rats most often lying still on their sides or belly. It took about 65–75 min for the rats to regain their feet and move around the chamber. The sedative effects of morphine were similar in rats receiving an infusion of L-CYSee (14.3 μmol/kg/min, IV) and the bolus injection of L-CYSee (250 μmol/kg, IV).
Fig. 10.
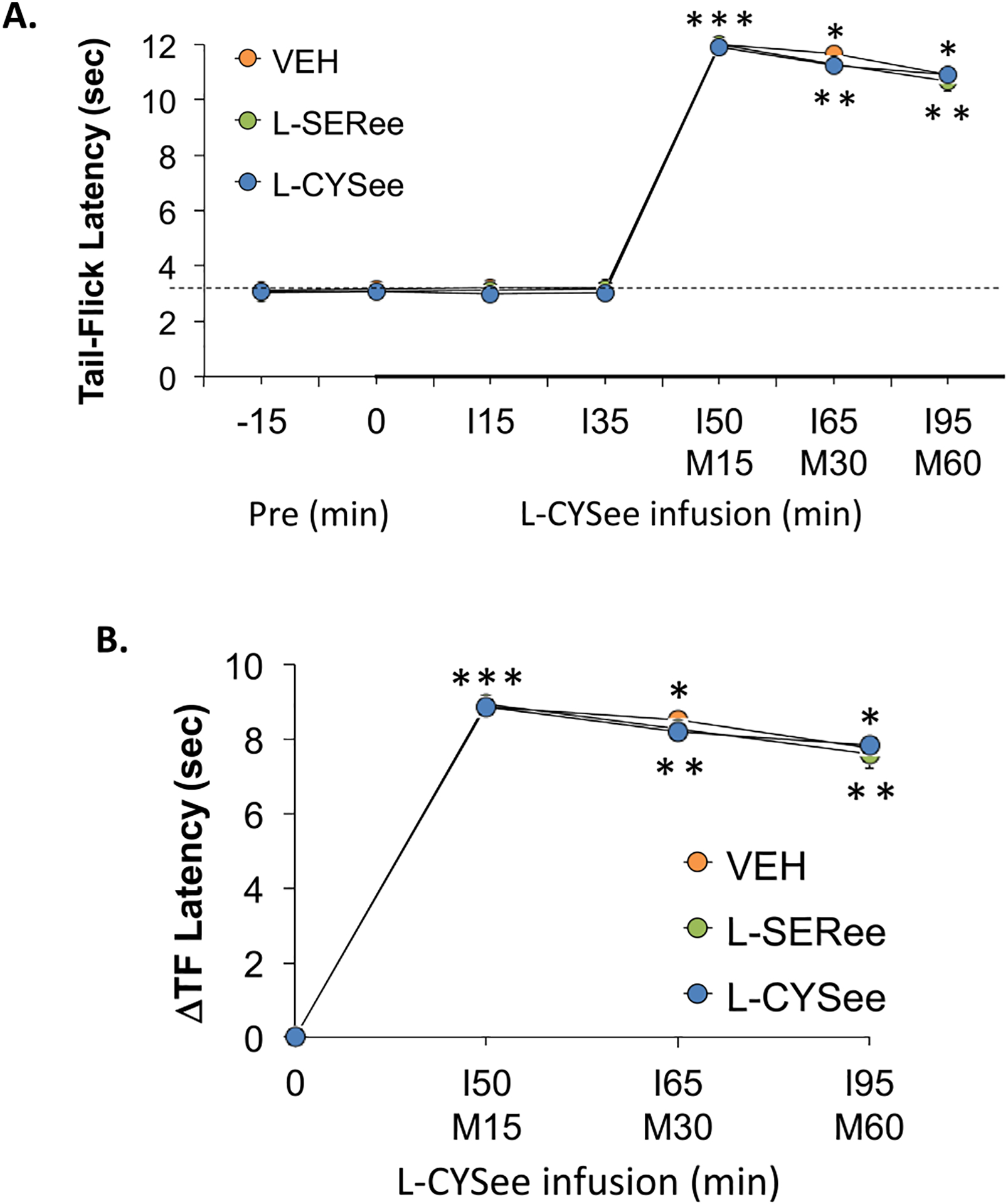
Panel A: Actual tail-flick (TF) latency values prior to (Pre) following an injection of morphine (M15-M60; 10 mg/kg, IV) in freely-moving rats receiving a continuous infusion (I15-I95) of vehicle (VEH; 20 μL/min), L-serine ethyl ester (L-SERee, 14.3 μmol/kg/min, IV) or L-cysteine ethyl ester (L-CYSee, 14.3 μmol/kg/min, IV). Panel B: Arithmetic changes in TF latency. The data are presented as mean ± SEM. There were 6 rats in each group.* P < 0.05, a significant change from Pre values. † P < 0.05, L-CYSee versus VEH.
4. Discussion
This study demonstrates that the deleterious effects of morphine on ventilatory parameters, ABG chemistry, and A-a gradient (alveolar gas exchange) were markedly diminished in rats receiving a continuous intravenous infusion of the highly membrane-permeable, L-thioester, L-CYSee [9, 33–47]. With respect to the plethysmography studies, the infusion rate of L-CYSee (14.3 μmol/kg/min, IV) was chosen based on preliminary studies, which determined that it would elicit minimal effects on Freq, TV, and therefore MV. More specifically, this infusion rate was chosen to establish whether the L-thioester could interfere with the ventilatory depressant effects of morphine by processes that are independent of simple stimulation of breathing. Indeed, the present study confirmed that infusion of L-CYSee at 14.3 μmol/kg/min elicited relatively minor increases in Freq, TV, and MV and that the bolus injection of morphine elicited substantially smaller reductions in these parameters in these rats. As such, on face value, it would appear that L-CYSee does interfere with the processes by which morphine decreases Freq and TV and therefore MV.
The finding that L-CYSee infusion did not interfere with the antinociceptive or sedative effects of morphine argues that L-CYSee does not directly block ORs and that the ready access of L-CYSee to peripheral and central sites [36], especially those controlling Freq and TV, modulates the signaling processes that mediate the effects of morphine on breathing. However, the relatively minor effects of L-CYSee infusion on Freq, TV, and MV (the most widely studied of all ventilatory parameters) contrasted with the substantial effects of L-CYSee infusion on other, less well-reported parameters. Specifically, L-CYSee infusion elicited a fall in Ti without having a major effect on Te demonstrating that the L-thioester selectively modulates (presumably) central pathways controlling inspiration [48–50]. The observation that L-CYSee elicited robust increases in Inspiratory Drive but no change in Expiratory Drive reinforces the concept that the L-CYSee selectively affects processes controlling inspiratory timing. The ability of L-CYSee to differentially affect ventilatory timing is also evident in that it decreased EIP (reduced the interval between the end of inspiration and the start of expiration) whereas it increased EEP (increased the interval between the end of expiration and the start of inspiration). Moreover, whereas L-CYSee increased Apneic Pause this was due primarily to a pronounced decrease in decreased Relaxation Time rather than Te. In addition, L-CYSee elicited remarkable increases in PIF, PEF, and EF50, while as would be expected a robust decrease in Rpef, clearly suggesting that L-CYSee enhances both inspiratory and expiratory ventilatory mechanics. Whether these effects involve enhancement of neurotransmitter release at neuromuscular junctions and/or enhanced responsiveness of skeletal muscle in the diaphragm and internal and external intercostal muscles remain to be determined.
A remarkable finding was that the L-CYSee infusion elicited a marked drop in ventilatory instability as measured by pronounced falls in NEBI and NEBI/Freq. The low level of baseline NEBI in these (mostly quietly resting) rats is likely due to the occurrence of apneas and type 1 and 2 sighs [10–12, 26] and the ability of L-CYSee to stabilize breathing may involve effects on brainstem and suprapontine pathways that control the ventilatory network [51–53]. With respect to the effects of morphine on ventilatory parameters other than Freq, TV, or MV, it was apparent that L-CYSee infusion markedly blunted the ability of morphine to decrease Ti whereas L-CYSee enhanced morphine-induced decrease in Te. Morphine reversed the pronounced increase in EEP observed during L-CYSee infusion whereas the ability of morphine to increase EIP was not affected by L-CYSee. Possibly due to the great increases in baseline PIF, PEF, EF50, and Expiratory Drive values observed during the L-CYSee infusion, morphine did not decrease these parameters below baseline levels as in vehicle-infused rats. Morphine had minor effects on the pronounced decrease in Relaxation Time in the L-CYSee-infused rats and restored the substantial increase in Apneic Pause seen during L-CYSee infusion to pre-infusion levels. L-CYSee prevented the initial increase in NEBI elicited by morphine whereas the subsequent and profound decrease in NEBI elicited by morphine occurred in both vehicle- and L-CYSee-infused rats. Finally, the ability of the bolus injection of L-CYSee in rats receiving the L-CYSee infusion to further enhance Freq, TV, MV, PIF, PEF, EF50, Inspiratory Drive, and Expiratory Drive suggests that the L-thioester has enormous potential in overcoming morphine-induced respiratory depression.
An important set of findings was that the ability of morphine (10 mg/kg, IV) to adversely affect ABG chemistry and A-a gradient was markedly diminished in rats that were receiving a lower infusion concentration of L-CYSee (7.15 μmol/kg/min) than used in the plethysmography studies (i.e., 50% of the 14.3 μmol/kg/min infusion concentration used in the plethysmography studies). This again demonstrates the obvious ability of L-CYSee to prevent the adverse effects of morphine on breathing and alveolar gas exchange and raises the possibility that even lower concentrations of L-CYSee may have efficacy. A key finding was that the infusion of L-SERee was without effect and as such, it appears that the sulfur atom of L-CYSee is essential to its activity. There are numerous explanations for these findings including that (i) L-CYSee can bind to functional proteins important for ventilatory control processes and especially those involved in the ability of morphine to depress breathing (see [54–57]) whereas L-SERee cannot, (ii) the degradation of L-CYSee to L-cysteine which then enters numerous metabolic pathways that L-serine cannot [58–66], and (iii) the formation of S-nitroso-L-CYSee [22] and/or S-nitroso-L-cysteine, an endogenous S-nitrosothiol [67–69] with many substantial roles in intracellular signaling cascades (see [70–72]) including those controlling cardiorespiratory function (see [73–77]) and those involved in attenuating OIRD [78,79]. With respect to potential mechanisms by which the infusion of L-CYSee inhibits the adverse effects of morphine on breathing, we have a tentative proposal that (i) morphine-activated μ-ORs recruit histidine triad nucleotide-binding protein 1 (HINT1) while simultaneously activating the neuronal form of NOS (nNOS). HINT1 recruits nNOS to the μ-OR-HINT1 complex [80,81], (ii) an essential process is that nNOS produces small molecule S-nitrosothiols and S-nitrosylated proteins which protect against/reverse morphine-induced respiratory depression, (iii) morphine-activated μ-ORs may recruit HINT1 and initiate the process by which L-CYSee is nitrosylated to S-nitroso-L-CYSee, (iv) such production of S-nitroso-L-CYSee has numerous effects including promoting formation of a μ-OR-NMDA (N-methyl-D-aspartate) receptor super complex, which is a possible mechanism for potentiation of morphine-induced respiratory depression in the absence of S-nitrosothiols [82,83].
A more simplified pathway is presented in the graphical abstract (note that the alpha subunit of G protein is not displayed for the sake of clarity) for why L-CYSee does not affect morphine antinociception/analgesia (upper panel) whereas it does affect the signaling events driving morphine-induced depression of breathing. The upper panel shows that the signaling pathways responsible for morphine-induced antinociception/analgesia involves the ability of morphine to block pain signaling via inhibiting Ca2+-entry into neurons processing nociceptive signals. This involves morphine binding to and activating the μ-OR (Step 1), the βγ subunits releasing from the receptor complex and binding to and inhibited Ca2+-channels, thereby blocking signal transduction responsible for pain (Step 2) and then β-arrestin (β-arr) binding to the μ-OR triggering endocytosis, and the μ-OR recycling to the cell surface (Step 3). None of these events are modulated by L-CYSee which freely-enters the cell but which cannot bind with efficacy to any of these signaling proteins. The bottom panel depicts how L-CYSee prevents the signaling events driving morphine depression of breathing. The presence of nitric oxide synthase (NOS) in these cells allows for the conversion of L-CYSee to S-nitroso-L-CYSee (SNO-L-CYSee), which binds to the βγ subunits of the μ-OR upon morphine binding (Step 1). The entry of Ca2+ is an essential component of the signaling processes that drive breathing. The binding of SNO-L-CYSee prevents the βγ subunits from inhibiting Ca2+ entry thereby maintaining normal signal transduction processes and preventing morphine suppression of breathing (Step 2). β-arr still binds the complex and triggers endocytosis, but the presence of SNO-L-CYSee prevents recycling to the membrane, reducing available μ-OR at the cell surface (Step 3).
Finally, although it has been established that L-CYSee readily enters peripheral and central tissues following systemic administration to rats [36] and increases levels of L-cysteine and L-cysteine in peripheral tissues in these animals [33], it remains to be established how L-CYSee affects brain regional levels of L-cysteine, L-cystine and L-glutathione or the functional status of S-glutathionylated proteins, in naïve or morphine-treated rats. Such information will be vital to understand the biochemical effects of L-CYSee and its potential impact on redox homeostasis.
Acknowledgments
The authors wish to thank the expert technical veterinarians and staff at the animal care facilities at Galleon Pharmaceuticals, Inc., the University of Virginia, and Case Western Reserve University for their caring assistance. The authors also thank David Kalergis (CEO, Atelerix Life Sciences) for his perspectives related to clinical importance of our findings. These studies were funded partially by an NIH/NIDA grant to SJL (U01DA051373, Optimization of Novel Thiolesters as a Therapeutic Strategy for Combating Opioid Overdoses and Abuse) and funding from Galleon Pharmaceuticals, Inc. The leadership of Galleon Pharmaceuticals was not directly involved in any of the aspects of this study as a commercial entity. Only the principal scientists of Galleon Pharmaceuticals were involved in study design, collection, analysis, interpretation of data, writing of this article and the decision to submit it for publication. The remaining authors declare that the research described in this manuscript was performed in the absence of commercial or financial relationships that could be construed as a potential conflict of interest. All authors declare no other competing interests.
Funding
This work was supported by NIH/NIDA grant U01DA051373.
Footnotes
CRediT authorship contribution statement
Tristan H.J. Lewis: Conceptualization of study, reduction to practice. Investigation - performance of the experiments, Writing – review & editing. Walter J. May: Conceptualization of study, reduction to practice. Investigation - performance of the experiments, Writing – review & editing. Alex P. Young: Conceptualization of study, reduction to practice. Investigation - performance of the experiments, Writing – review & editing. James N. Bates: Conceptualization of study, Analyses of data, Writing – original draft and editing. Santhosh M. Baby: Conceptualization: reduction to practice. Investigation - performance of the experiments, Writing – review & editing. Paulina M. Getsy: Analyses of data, Writing – original draft and editing, Writing – review & editing. Rita M. Ryan: Analyses of data, Writing – original draft and editing, Writing – review & editing. Yee-Hsee Hsieh: Conceptualization of the study, Writing – review & editing. Stephen J. Lewis: Conceptualization of the study, Investigation - performance of the experiments, Analyses of data, Writing – original draft and editing. Writing – review & editing.
Conflict of Interest Statement
The authors declare that they have no competing financial interests or personal relationships that would have influenced the studies that are described in this manuscript.
Data Availability
The datasets generated by this investigation are available freely when requested by contacting the author for correspondence.
References
- [1].van Dorp E, et al. , Naloxone treatment in opioid addiction: the risks and benefits, Expert Opin. Drug Saf 6 (2007) 125–132, 10.1517/14740338.6.2.125. [DOI] [PubMed] [Google Scholar]
- [2].Boom M, et al. , Non-analgesic effects of opioids: opioid-induced respiratory depression, Curr. Pharm. Des 18 (2012) 5994–6004, 10.2174/138161212803582469. [DOI] [PubMed] [Google Scholar]
- [3].Dahan A, et al. , Incidence, reversal, and prevention of opioid-induced respiratory depression, Anesthesiology 112 (2010) 226–338, 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- [4].Dahan A, et al. , Averting opioid-induced respiratory depression without affecting analgesia, Anesthesiology 128 (2018) 1027–1037, 10.1097/ALN.0000000000002184. [DOI] [PubMed] [Google Scholar]
- [5].Algera MH, et al. , Opioid-induced respiratory depression in humans: a review of pharmacokinetic-pharmacodynamic modelling of reversal, Br. J. Anaesth 122 (2019) e168–e179, 10.1016/j.bja.2018.12.023. [DOI] [PubMed] [Google Scholar]
- [6].Arendt F, The opioid-overdose crisis and fentanyl: the role of online information seeking via internet search engines, Health Commun. 36 (2021) 1148–1154, 10.1080/10410236.2020.1748820. [DOI] [PubMed] [Google Scholar]
- [7].van der Schier R, et al. , Opioid-induced respiratory depression: reversal by non-opioid drugs, F1000Prime Rep. 6 (2014) 79, 10.12703/P6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Imam MZ, et al. Countering opioid-induced respiratory depression by non-opioids that are respiratory stimulants. 2020;F1000Res. 9:F1000 Faculty Rev-91. doi: 10.12688/f1000research.21738.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mendoza J, et al. , L-Cysteine ethyl ester reverses the deleterious effects of morphine on, arterial blood-gas chemistry in tracheotomized rats, Respir. Physiol. Neurobiol 189 (2013) 136–143, 10.1016/j.resp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Getsy PM, et al. L-cysteine methyl ester reverses the deleterious effects of morphine on ventilatory parameters and arterial blood-gas chemistry in unanesthetized rats. Front Pharmacol, 2022; submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Getsy PM, et al. D-Cysteine ethylester and D-cystine dimethylester reverse the deleterious effects of morphine on arterial blood-gas chemistry and Alveolar-arterial gradient in anesthetized rats. Resp Physiol Neurobiol, 2022; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Getsy PM, et al. D-Cysteine ethyl ester reverses the deleterious effects of morphine on breathing in freely-moving rats while not negating the antinociceptive effects of the opioid. Front Pharmacol, 2022; submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jenkins MW, et al. , Glutathione ethyl ester reverses the deleterious effects of fentanyl on ventilation and arterial blood-gas chemistry while prolonging fentanyl-induced analgesia, Sci. Rep 11 (2021) 6985, 10.1038/s41598-021-86458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gaston B, et al. , D-Cystine di(m)ethyl ester reverses the deleterious effects of morphine on ventilation and arterial blood gas chemistry while promoting antinociception, Sci. Rep 11 (2021) 10038, 10.1038/s41598-021-89455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Semenza ER, Harraz MM, Abramson E, Malla AP, Vasavda C, Gadalla MM, Kornberg MD, Snyder SH, Roychaudhuri R, D-cysteine is an endogenous regulator of neural progenitor cell dynamics in the mammalian brain, e2110610118, Proc. Natl. Acad. Sci. USA 118 (2021), 10.1073/pnas.2110610118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM, Bias factor and therapeutic window correlate to predict safer opioid analgesics, e13, Cell 171 (2017) 1165–1175, 10.1016/j.cell.2017.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grim TW, Acevedo-Canabal A, Bohn LM, Toward directing opioid receptor signaling to refine opioid therapeutics, Biol. Psychiatry 87 (2020) 15–21, 10.1016/j.biopsych.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Perissinotti LL, Turjanski AG, Estrin DA, Doctorovich F, Transnitrosation of nitrosothiols: characterization of an elusive intermediate, J. Am. Chem. Soc 127 (2005) 486–487, 10.1021/ja044056v. [DOI] [PubMed] [Google Scholar]
- [19].Hess DT, Stamler JS, Regulation by S-nitrosylation of protein post-translational modification, J. Biol. Chem 287 (2012) 4411–4418, 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stomberski CT, Hess DT, Stamler JS, Protein S-nitrosylation: determinants of specificity and enzymatic regulation of S-nitrosothiol-based signaling, Antioxid. Redox Signal 30 (2019) 1331–1351, 10.1089/ars.2017.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Seckler JM, Grossfield A, May WJ, Getsy PM, Lewis SJ, Nitrosyl factors play a vital role in the ventilatory depressant effects of fentanyl in unanesthetized rats, Biomed. Pharmacother 146 (2022), 112571, 10.1016/j.biopha.2021.112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clancy R, Cederbaum AI, Stoyanovsky DA, Preparation and properties of S-nitroso-L-cysteine ethyl ester, an intracellular nitrosating agent, J. Med. Chem 44 (2001) 2035–2038, 10.1021/jm000463f. [DOI] [PubMed] [Google Scholar]
- [23].Henderson F, et al. , Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats, Respir. Physiol. Neurobiol 191 (2014) 95–105, 10.1016/j.resp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Epstein MA, Epstein RA, A theoretical analysis of the barometric method for measurement of tidal volume, Respir. Physiol 32 (1978) 105–120, 10.1016/0034-5687(78)90103-2. [DOI] [PubMed] [Google Scholar]
- [25].Epstein RA, et al. , Practical implementation of the barometric method for measurement of tidal volume, J. Appl. Physiol 49 (1980) 1107–1115, 10.1152/jappl.1980.49.6.1107. [DOI] [PubMed] [Google Scholar]
- [26].Getsy PM, et al. , Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice, Respir. Physiol. Neurobiol 204 (2014) 147–159, 10.1016/j.resp.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stengel A, et al. , Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats, Endocrinology 151 (2010) 4224–4235, 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chapman CD, et al. , Paraventricular nucleus anandamide signaling alters eating and substrate oxidation, Neuroreport 23 (2012) 425–429, 10.1097/WNR.0b013e32835271d1. [DOI] [PubMed] [Google Scholar]
- [29].Ludbrook J, Multiple comparison procedures updated. Clin. Exp. Pharm. Physiol 25 (1998) 1032–1037, 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- [30].McHugh ML, Multiple comparison analysis testing in ANOVA, Biochem. Med. (Zagreb) 21 (2011) 203–209, 10.11613/bm.2011.029. [DOI] [PubMed] [Google Scholar]
- [31].Wallenstein S, et al. , Some statistical methods useful in circulation research, Circ. Res 47 (1980) 1–9, 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- [32].Winer BJ, Statistical Principles of Experimental Design, McGraw-Hill Book Co,, 1971, pp. 752–809. [Google Scholar]
- [33].Hobbs MJ, Butterworth M, Cohen GM, Upshall DG, Structure-activity relationships of cysteine esters and their effects on thiol levels in rat lung in vitro, Biochem. Pharmacol 45 (1993) 1605–1612, 10.1016/0006-2952(93)90301-c. [DOI] [PubMed] [Google Scholar]
- [34].Hu TM, Ho SC, Similarity and dissimilarity of thiols as anti-nitrosative agents in the nitric oxide-superoxide system, Biochem. Biophys. Res. Commun 404 (2011) 785–789, 10.1016/j.bbrc.2010.12.059. [DOI] [PubMed] [Google Scholar]
- [35].Ding H, Demple B, Thiol-mediated disassembly and reassembly of [2Fe-2S] clusters in the redox-regulated transcription factor SoxR, Biochemistry 37 (1998) 17280–17286, 10.1021/bi980532g. [DOI] [PubMed] [Google Scholar]
- [36].Servin AL, Goulinet S, Renault H, Pharmacokinetics of cysteine ethyl ester in rat, Xenobiotica 18 (1988) 839–847, 10.3109/00498258809041722. [DOI] [PubMed] [Google Scholar]
- [37].McNiff EF, Cheng LK, Woodfield HC, Fung HL, Effects of L-cysteine, L-cysteine derivatives and ascorbic acid on lead excretion in rats, Res. Commun. Chem. Pathol. Pharmacol 20 (1978) 131–137. [PubMed] [Google Scholar]
- [38].Haas DJ, Intermolecular complexes. 3. l-Cysteine ethyl ester hydrochloride-urea (1:1), Acta Crystallogr 19 (1965) 860–861, . [DOI] [PubMed] [Google Scholar]
- [39].Goto K, Hisadome M, Kawazoe Y, Tsumagari T, Effect of cysteine ethylester hydrochloride (Cystanin) on host defense mechanism, Nihon Yakurigaku Zasshi 82 (1983) 27–35. [PubMed] [Google Scholar]
- [40].Hisadome M, Nakamura Y, Okumoto T, Ikegami K, Effect of cysteine ethylester hydrochloride (Cystanin) on host defense mechanisms (II): Restorative effects on the suppression of antibody production, Nihon Yakurigaku Zasshi 88 (1986) 349–354, 10.1254/fpj.88.349. [DOI] [PubMed] [Google Scholar]
- [41].Hisadome M, Nakamura Y, Okumoto T, Ikegami K, Effect of cysteine ethylester hydrochloride (Cystanin) on host defense mechanisms (III): Potentiating effects on phagocytosis and nitroblue tetrazolium (NBT) reduction by leukocytes of mice and guinea pigs, Nihon Yakurigaku Zasshi 88 (1986) 369–374, 10.1254/fpj.88.369. [DOI] [PubMed] [Google Scholar]
- [42].Hisadome M, Kimura Y, Ikegami K, Terasawa M, Effect of cysteine ethylester hydrochloride (Cystanin) on host defense mechanism (IV): Potentiating effects on the function of peritoneal or spleen macrophages, Jpn. J. Pharmacol 47 (1988) 379–385, 10.1254/jjp.47.379. [DOI] [PubMed] [Google Scholar]
- [43].Schöneich C, Dillinger U, von Bruchhausen F, Asmus KD, Oxidation of polyunsaturated fatty acids and lipids through thiyl and sulfonyl radicals: reaction kinetics, and influence of oxygen and structure of thiyl radicals, Arch. Biochem. Biophys 292 (1992) 456–467, 10.1016/0003-9861(92)90016-p. [DOI] [PubMed] [Google Scholar]
- [44].Susa N, Ueno S, Furukawa Y, Protective effects of thiol compounds on chromate-induced cytotoxicity in HeLa cells, J. Vet. Med. Sci 54 (1992) 281–288, 10.1292/jvms.54.281. [DOI] [PubMed] [Google Scholar]
- [45].Susa N, Ueno S, Furukawa Y, Protective effects of thiol compounds on chromate-induced toxicity in vitro and in vivo, Environ. Health Perspect 102 (Suppl 3) (1994) 247–250, 10.1289/ehp.94102s3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pagani R, Leoncini R, Terzuoli L, Pizzichini M, Marinello E, The regulation of alanine and aspartate aminotransferase by different aminothiols and by vitamin B-6 derivatives, Biochim. Biophys. Acta 1204 (1994) 250–256, 10.1016/0167-4838(94)90015-9. [DOI] [PubMed] [Google Scholar]
- [47].Arias JM, Díaz SB, Ben Altabef A, Dupuy FG, Interaction of cysteine and its derivatives with monolayers of dipalmitoylphosphatidylcholine, Colloids Surf. B. Biointerfaces 184 (2019), 110548, 10.1016/j.colsurfb.2019.110548. [DOI] [PubMed] [Google Scholar]
- [48].Mortola JP, How to breathe? Respiratory mechanics and breathing pattern, Respir. Physiol. Neurobiol 261 (2019) 48–54, 10.1016/j.resp.2018.12.005. [DOI] [PubMed] [Google Scholar]
- [49].Butler JE, Drive to the human respiratory muscles, Respir. Physiol. Neurobiol 159 (2) (2007) 115–126, 10.1016/j.resp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [50].Butler JE, Gandevia SC, The output from human inspiratory motoneurone pools, J. Physiol 586 (5) (2008) 1257–1264, 10.1113/jphysiol.2007.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Horn EM, Waldrop TG, Suprapontine control of respiration, Respir. Physiol 114 (3) (1998) 201–211, 10.1016/s0034-5687(98)00087-5. [DOI] [PubMed] [Google Scholar]
- [52].Moreira TS, Takakura AC, Damasceno RS, Falquetto B, Totola LT, Sobrinho CR, Ragioto DT, Zolezi FP, Central chemoreceptors and neural mechanisms of cardiorespiratory control, Braz. J. Med Biol. Res 44 (9) (2011) 883–889, . [DOI] [PubMed] [Google Scholar]
- [53].Poon CS, Lin SL, Knudson OB, Optimization character of inspiratory neural drive, J. Appl. Physiol. (1985) 72 (5) (1992) 2005–2017, 10.1152/jappl.1992.72.5.2005. [DOI] [PubMed] [Google Scholar]
- [54].Al-Hasani R, Bruchas MR, Molecular mechanisms of opioid receptor-dependent signaling and behavior, Anesthesiology 115 (6) (2011) 1363–1381, 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ, Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance, Pharm. Rev 65 (2013) 223–254, 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Law PY, Wong YH, Loh HH, Molecular mechanisms and regulation of opioid receptor signaling, Annu Rev. Pharm. Toxicol 40 (2000) 389–430, 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- [57].Pineyro G, Nagi K, Signaling diversity of mu- and delta- opioid receptor ligands: Re-evaluating the benefits of β-arrestin/G protein signaling bias, Cell Signal 80 (2021), 109906, 10.1016/j.cellsig.2020.109906. [DOI] [PubMed] [Google Scholar]
- [58].Gupta V, Carroll KS, Sulfenic acid chemistry, detection and cellular lifetime, Biochim Biophys. Acta 1840 (2014) 847–875, 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Roos G, Messens J, Protein sulfenic acid formation: from cellular damage to redox regulation, Free Radic. Biol. Med 51 (2011) 314–326, 10.1016/j.freeradbiomed.2011.04.031. [DOI] [PubMed] [Google Scholar]
- [60].Joseph CA, Maroney MJ, Cysteine dioxygenase: structure and mechanism, Chem. Commun. (Camb.) 32 (2007) 3338–3349, 10.1039/b702158e. [DOI] [PubMed] [Google Scholar]
- [61].Stipanuk MH, Ueki I, Dominy JE Jr, Simmons CR, Hirschberger LL, Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels, Amino Acids 37 (2009) 55–63, 10.1007/s00726-008-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Murphy B, Bhattacharya R, Mukherjee P, Hydrogen sulfide signaling in mitochondria and disease, FASEB J. 33 (2019) 13098–13125, 10.1096/fj.201901304R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kumar M, Sandhir R, Hydrogen sulfide in physiological and pathological mechanisms in brain, CNS Neurol. Disord. Drug Targets 17 (2018) 654–670, 10.2174/1871527317666180605072018. [DOI] [PubMed] [Google Scholar]
- [64].Gotor C, García I, Aroca Á, Laureano-Marín AM, Arenas-Alfonseca L, Jurado-Flores A, Moreno I, Romero LC, Signaling by hydrogen sulfide and cyanide through post-translational modification, J. Exp. Bot 70 (2019) 4251–4265, 10.1093/jxb/erz225. [DOI] [PubMed] [Google Scholar]
- [65].Olas B, Hydrogen sulfide in signaling pathways, Clin. Chim. Acta 439 (2015) 212–218, 10.1016/j.cca.2014.10.037. [DOI] [PubMed] [Google Scholar]
- [66].Kimura H, Hydrogen sulfide and polysulfide signaling, Antioxid. Redox Signal 27 (2017) 619–621, 10.1089/ars.2017.7076. [DOI] [PubMed] [Google Scholar]
- [67].Myers PR, Minor RL Jr, Guerra R Jr, Bates JN, Harrison DG, Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide, Nature 345 (1990) 161–163, 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- [68].Seckler JM, Shen J, Lewis THJ, Abdulameer MA, Zaman K, Palmer LA, Bates JN, Jenkins MW, Lewis SJ, NADPH diaphorase detects S-nitrosylated proteins in aldehyde-treated biological tissues, Sci. Rep 10 (2020) 21088, 10.1038/s41598-020-78107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Seckler JM, Meyer NM, Burton ST, Bates JN, Gaston B, Lewis SJ, Detection of trace concentrations of S-nitrosothiols by means of a capacitive sensor, grime0187149, PLoS One 12 (2017), 10.1371/journal.pone.0187149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS, A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds, Nature 364 (1993) 626–632, 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- [71].Foster MW, Hess DT, Stamler JS, Protein S-nitrosylation in health and disease: a current perspective, Trends Mol. Med 15 (2009) 391–404, 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hess DT, Stamler JS, Regulation by S-nitrosylation of protein post-translational modification, J. Biol. Chem 287 (2012) 4411–4418, 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lewis SJ, Owen JR, Bates JN, S-nitrosocysteine elicits hemodynamic responses similar to those of the Bezold-Jarisch reflex via activation of stereoselective recognition sites, Eur. J. Pharmacol 531 (2006) 254–258, 10.1016/j.ejphar.2005.11.027. [DOI] [PubMed] [Google Scholar]
- [74].Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B, S-nitrosothiols signal the ventilatory response to hypoxia, Nature 413 (2001) 171–174, 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- [75].Gaston B, Smith L, Bosch J, Seckler J, Kunze D, Kiselar J, Marozkina N, Hodges CA, Wintrobe P, McGee K, Morozkina TS, Burton ST, Lewis T, Strassmaier T, Getsy P, Bates JN, Lewis SJ, Voltage-gated potassium channel proteins and stereoselective S-nitroso-l-cysteine signaling, JCI Insight 5 (2020), e134174, 10.1172/jci.insight.134174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gaston B, Singel D, Doctor A, Stamler JS, S-nitrosothiol signaling in respiratory biology, Am. J. Respir. Crit. Care Med 173 (2006) 1186–1193, 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gaston B, May WJ, Sullivan S, Yemen S, Marozkina NV, Palmer LA, Bates JN, Lewis SJ, Essential role of hemoglobin beta-93-cysteine in post-hypoxia facilitation of breathing in conscious mice, J. Appl. Physiol 116 (2014) 1290–1299, 10.1152/japplphysiol.01050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Getsy PM Young AP, Gaston B, Bates JN, Baby SM, Seckler JM, Grossfield A, Jenkins MW, Hsieh Y-H, and Lewis SJ (2022). S-Nitroso-L-Cysteine stereoselectively blunts the negative effects of morphine on breathing and arterial blood gas chemistry while promoting analgesia. Biomedicine & Pharmacotherapy, under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Getsy PM, Baby SM, Gruber RB, Gaston B, Lewis THJ, Grossfield A, Seckler JM, Hsieh Y-H, Bates JN, Lewis SJ, S-nitroso-L-cysteine stereoselectively blunts the negative effects of fentanyl on breathing while augmenting fentanyl antinociception in freely-moving rats, Front. Pharmacol (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rodríguez-Muñoz M, de la Torre-Madrid E, Sánchez-Blázquez P, Garzón J, NO-released zinc supports the simultaneous binding of Raf-1 and PKCγ cysteine-rich domains to HINT1 protein at the mu-opioid receptor, Antioxid. Redox Signal 14 (2011) 2413–2425, 10.1089/ars.2010.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J, Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling, PLoS One 5 (2010), e11278, 10.1371/journal.pone.0011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rodríguez-Muñoz M, Garzón J, Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling, Mol. Neurobiol 48 (2013) 769–782, 10.1007/s12035-013-8465-z. [DOI] [PubMed] [Google Scholar]
- [83].Shah RM, Peterson C, Strom A, Dillenburg M, Finzel B, Kitto KF, Fairbanks C, Wilcox G, Wagner CR, Inhibition of HINT1 modulates spinal nociception and NMDA evoked behavior in mice, ACS Chem. Neurosci 10 (2019) 4385–4393, 10.1021/acschemneuro.9b00432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated by this investigation are available freely when requested by contacting the author for correspondence.


