Abstract
Objectives
This study aimed to investigate the effects of Ramadan diurnal intermittent fasting (RDIF) on cognitive performance, sleep quality, daytime sleepiness, and insomnia in physically active and sedentary elderly individuals.
Methods
A total of 58 participants (62.93 ± 3.99 years) were assigned to one of the following two groups: a sedentary group (control group) who observed Ramadan (n = 32) and a physically active group (n = 26) who continued to train while observing Ramadan. Participants were assessed 2 weeks before Ramadan and during the fourth week of Ramadan. On each occasion, participants completed a digital assessment of their cognitive performance and responded to the Pittsburgh sleep quality index (PSQI), the insomnia severity index (ISI) and the Epworth sleepiness scale (ESS) questionnaires to assess sleep parameters.
Results
Compared to before Ramadan, performance in executive function (p = 0.035), attention (p = 0.005), inhibition (p = 0.02), associative memory (p = 0.041), and recognition memory (p = 0.025) increased significantly during Ramadan in the physically active group. For the sedentary group, associative learning performance decreased (p = 0.041), whilst performances in the remaining domains remained unchanged during Ramadan. Global PSQI, ISI, and ESS scores indicated both groups suffered from poor sleep quality and excessive daytime sleepiness, with significantly higher negative effects of RDIF observed in the sedentary group.
Conclusion
Older adults who continue to train at least three times per week during Ramadan may improve their cognitive performance, despite the impairment of sleep quality. Future studies in older adults during Ramadan including objective measures of sleep (e.g., polysomnography, actigraphy) and brain function (e.g., functional magnetic resonance imaging) are warranted.
Keywords: religious fasting, exercise, time-restricted eating, elderly, cognitive performance, sleep quality
Introduction
The incidence and severity of brain disease is steadily rising over the past decades and one of the leading causes of disability and death worldwide (1, 2).
Age-related cognitive decline is inextricably linked to the highly complicated biological process of brain aging (3), which is the primary risk factor for neurodegeneration (4). This brain health deterioration is characterized by neural loss, reduced neurogenesis, and limited neuronal plasticity (5), which are associated with subsequent declines in memory, executive function, attentional abilities, and processing speed (6, 7). Additionally, physiological alterations due to the aging process contribute to disturbances in the circadian rhythms (i.e., central and peripheral rhythms) (8), including a decrease in overall melatonin secretion (9–11), as well as metabolism-related rhythms (e.g., lipid metabolism, glucose control, xenobiotic detoxification), regulated by the liver and pancreatic clocks which can lead to the emergence of metabolic diseases (12).
Diet and the gut-brain axis have recently been recognized for their contribution to brain health and cognitive function (13, 14), thus the ability for dietary treatments to prevent and/or treat brain diseases is promising. As such, intermittent fasting which involves abstaining from meals or severely restricting caloric intake for durations ranging from 12 to 48 h, has demonstrated potential effects on brain health through the enhanced expression of brain-derived neurotrophic factor (BDNF), enhancing knowledge acquisition and retention, stimulating neurogenesis, and consequently, improving cognitive function (15–21).
Ramadan diurnal intermittent fasting (RDIF) is considered a unique model of intermittent fasting (i.e., religious and spiritual reasons) in which Muslims abstain from eating, drinking, smoking and sexual intercourses between dawn and sunset for a lunar month (i.e., 28–30 days) every year (22). In this context, Alsharidah et al. (23) demonstrated the beneficial effects of RDIF in adults on psychomotor function, attention, and processing speed. Likewise, Farooq et al. (24) showed a significant increase in performance for spatial planning, working memory tasks, and working memory capacity tests, at the end of Ramadan month. However, Ghayour Najafabadi et al. (25) showed RDIF has negative effects on short-term memory and cognitive flexibility function.
Several influencing factors can affect cognitive performance, such as disruption of sleep patterns, meal composition and schedule, and lifestyle changes (26–28).
Previous studies showed impairment of sleep quantity (i.e., nocturnal sleep duration) and quality and excessive daytime sleepiness (EDS) during Ramadan, possibly due to physiological and behavioral changes in diurnal and nocturnal activities. These activities include waking up for the predawn meal (Suhur) and dawn prayer, and practicing more activities at night such as social meetings, shopping, Quran reading groups, and prayers (e.g., Attarawih) (29–31).
Physical activity (PA) may attenuate some of the negative impacts that poor sleep has on cognition and improve cognitive function through physiological adaptations such as neurogenesis, angiogenesis, increases in cerebral blood flow, functional connectivity, and growth factors, while reducing inflammation and brain neuropathology accumulation (32–36). Furthermore, Küster et al. (37) concluded that a regular active lifestyle is associated with better global cognition in elderly individuals. However, during the month of Ramadan, Farooq et al. (38) showed a decrease in the objectively assessed habitual PA in adults. Similarly, Lessan et al. (39) reported that the total number of steps walked per day were significantly lower during Ramadan.
Some investigations have examined the effect of RDIF on cognitive function and sleep quality in adolescents (24, 40, 41) and athletes (42, 43), but not in physically active and sedentary older adults. Examining the effects of combining regular physical exercise and intermittent fasting in older adults may enable us to assess whether combining intermittent fasting and regular training is an effective therapeutic strategy for the prevention or even the improvement of cognitive function, thus potentially counteracting the adverse effects associated with the aging process.
Therefore, our primary objective is to investigate the effect of RDIF on cognitive function in active and sedentary older adults. Our secondary objective is to examine the influence of RDIF on sleep quality, daytime sleepiness, insomnia, and sleep-wake behaviors in active and sedentary older adults.
We hypothesized that from before to during RDIF, (i) active older adults would have better cognitive performance than their sedentary counterparts, and (ii) both groups would have a sleep quality impairment, higher sleepiness and insomnia more accentuated in the sedentary group.
Materials and methods
Participants
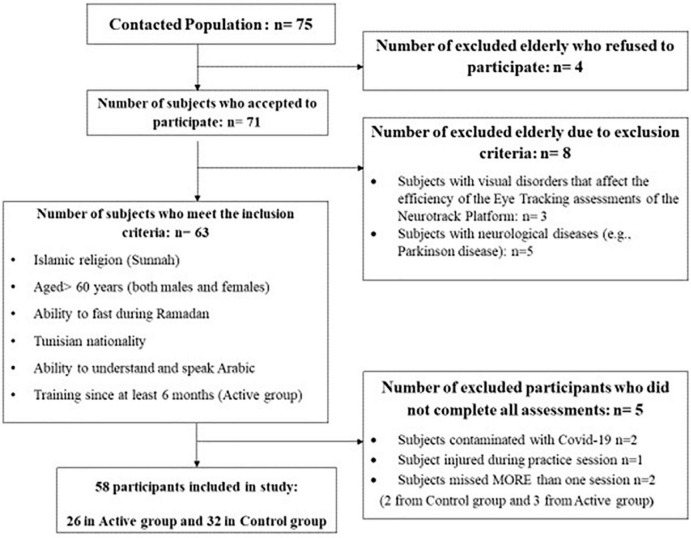
Seventy-one older adults (aged > 60 years) agreed to participate in this study. After the verification of eligibility criteria, 63 subjects signed an informed consent prior to the start of the evaluation process (Figure 1). Five participants were excluded because they did not complete all assessments. A total of 58 included participants (26 men and 32 women) aged between 60 and 79 years (62.9 ± 4.0 years) were assigned to one of the following two groups: Control group (n = 32; mean age: 62.71 ± 3.52 years; age range: 60–77) who did not follow any training program before Ramadan and physically active elderly group (active group) (n = 26; mean age: 63.19 ± 4.56 years; age range: 60–78) who (i) trained for at least 6 months prior to RDIF. The active group continued with the same training schedule while observing Ramadan (Table 1), and completed all tests at the final analysis. Participant characteristics are presented in Table 2. There were some participants with chronic and/or cardiovascular disease and, based on medical doctors’ authorization, their participation in the training program and/or the fasting during Ramadan does not pose any risk of danger to their health. The rate of compliance with the training program was 93.59%. The research ethics committee CPP SUD N°0322/2021 gave its approval to this project, and the present study was carried out according to the Declaration of Helsinki. The required sample size was calculated a priori for repeated measures analysis of variance (ANOVA) using the G* power software (version 3.1.9.2; Kiel University, Kiel, Germany) (44). Values for α were set at 0.05 and power at 0.90. Given the pioneer character of this study and since no previous study has evaluated the effects of RDIF on cognitive function in older adults, an effect size f of 0.4 was reported in the study of Tian et al. (42) for the verbal learning and memory assessed at16:00 h during Ramadan. A total sample size of 52 was required with at least 26 in each group to achieve the desired power for cognitive functions parameters. Concerning sleep components, based on the results of the meta-analysis of Trabelsi et al. (31), an effect size f of 0.41 was computed for the nocturnal sleep duration. The required sample size was 25 for each group for nocturnal sleep duration. To avoid potential risks of participant drop-out, a total of 58 participants were included.
FIGURE 1.
Flowchart outlining the selection criteria and decision-making process for the elderly who participated in the ongoing assessments.
TABLE 1.
Training program for active group before and during Ramadan.
| 3 sessions per week of 1 h interspersed with a recovery day | ||
| Session 1 | Session 2 | Session 3 |
| • 15 min warm-up | • 15 min warm-up in the pool | • 15 min warm-up |
| • 35 min team sports (i.e., volleyball or basketball) | • 35 min aquatic activities | • 35 min circuit training and ludic games. |
| • 10 min stretching | • 10 min stretching in the pool | • 10 min stretching |
TABLE 2.
Demographic baseline characteristics of the participants.
| Variables | Active group (n = 26) | Control group (n = 32) | |
| Mean (SD) | BMI | 26.6 (2.8) | 27.3 (2.7) |
| N (%) | Gender | ||
| Female | 13 (50%) | 18 (56.3%) | |
| Male | 13 (50%) | 14 (43.7%) | |
| Education level | |||
| University | 11 (42.3%) | 7 (21.9%) | |
| High school | 9 (34.6%) | 8 (25%) | |
| Mild school | 5 (19.2%) | 12 (37.5%) | |
| Primary school | 1 (3.8%) | 5 (15.6%) | |
| Health status | |||
| Healthy | 14 (53.8%) | 19 (59.4%) | |
| Chronic diseases | |||
| Type 2 diabetes | 5 (19.2%) | 6 (18.8%) | |
| Dyslipidemia | 1 (3.8%) | 1 (3.1%) | |
| Cardiovascular diseases | |||
| Coronary artery disease | 0 (0%) | 1 (3.1%) | |
| Hypertension | 3 (11.5%) | 2 (6.3%) | |
| Type 2 diabetes + hypertension | 1 (3.8%) | 2 (6.3%) | |
| Type 2 diabetes + dyslipidemia | 2 (7.7%) | 1 (3.1%) | |
BMI, Body mass index; SD, Standard deviation.
Experimental design
The study was carried out in Tunisia during the 2022 Ramadan month from 02 April to 02 May in the Spring, when daily fasting started from ∼04:35 a.m. and ended at ∼6:45 p.m. (i.e., fasting duration of approximately ∼14 h). Participants were assessed twice: 2 weeks prior to Ramadan (non-fasting condition), in which the mean temperature was 15 ± 2.1n°C and the mean humidity 76 ± 10.2%, and during the fourth week of Ramadan’s fast (fasting condition) with 18 ± 1.0°C of mean temperature and 72 ± 8.3% of mean humidity.
All tests were conducted at the same time of day between 4:00 and 6:00 p.m. for both groups before and during RDIF (before the break of fast). Participants had their last meal between 03:30 and 04:30 a.m. (i.e., Suhur), approximately 11–12 h before their assessment session.
The two sessions were conducted in a laboratory under the guidance of a qualified examiner who directed the evaluation process and provided subjects with instructions, cues, and information as needed. During Ramadan, participants (active group) continued to train in a fasted state (i.e., at the same time as before Ramadan (i.e., 5:30 p.m.) while the control group did not perform any type of physical training program. All subjects fasted during the entire month of Ramadan. No side effects (i.e., dizziness, hypoglycemia, headache) due to fasting were recorded in either group, suggesting the feasibility of the intervention.
Both groups performed the same evaluation procedure; each session included a digital cognitive assessment battery (Neurotrack Technologies, Redwood City, CA), an assessment of sleep, sleepiness and insomnia based on validated Arabic version questionnaires including the Pittsburgh sleep quality index (PSQI) (45, 46), the ESS (47, 48) and the insomnia severity index (ISI) (49, 50).
Cognitive assessments
Cognitive function was assessed using the Neurotrack digital cognitive battery. Neurotrack’s cognitive assessments are brief, reliable measures that are based on paradigms previously shown to be sensitive to fluctuations in cognitive function (51–53). Given the assessments are designed in American English, the battery was adapted to fit our sample population.
First, the assessments and instructions were converted into an explanatory video with an Arabic language voice. Second, the output responses on the keyboard buttons were colored and translated to Arabic (e.g., yes, no, left, right, equal, unequal). The translation-back translation was carried out by two native Arabic speakers who were also fluent in English. To ensure understanding and feasibility, the translated versions of the assessments were pilot-tested with 15 older adults who were not included in the final sample. The pilot feedback was incorporated into the translated assessments, which were then re-piloted until no additional changes were required based on subsequent feedback. This digital cognitive battery was performed in translated Arabic version as in the recent study conducted by Boujelbane et al. (54).
This digital cognitive battery consists of six tests, each with its own measurement domain:
-
–
Symbol Match, a digitized version of the traditional Digit Symbol Substitution test, is a task that assesses processing speed and executive functioning. During this test, scores are calculated based on speed and accuracy.
-
–
Item Price is a visual associative learning measure. Participants in a recognition trial are required to learn a succession of food/price pairs and then identify appropriately matched pairs. All of the items fall into the same semantic category (e.g., vegetables, and fruits). The accuracy of the answers determines the score.
-
–
Arrow Match, based on the traditional Choice Reaction Time paradigm, is a test of attention and processing speed. Five arrows appear in the center of the screen, and participants are asked to identify/determine the direction of the middle arrow. Speed and accuracy are used to calculate scores.
-
–
Path Points is an executive function and attention measure developed based on the Trail Making Test-Part B. Participants are instructed to alternately connect a succession of circles with numbers and letters (e.g., 1 connected to A, 2 connected to B, 3 connected to C, etc.). Speed determines the score.
-
–
Light Reaction is an inhibition measure based on the go/no-go paradigm. A positive (i.e., green light) or negative (i.e., red light) stimulus will be shown to participants. In the case of a positive stimulus appearance, the participant must press a button. However, in the case of the appearance of a negative stimulus appears, subjects are instructed not to press the button. Speed and accuracy are used to calculate scores.
-
–
Image Pairs is a test that assesses associative and recognition memory. The latter is assessed via eye-tracking movements (ETM) (55). Behavioral responses are used to assess the associative memory component of the assessment. Briefly, a series of object pairs are presented to subjects during the familiarization phase. Then, during the test phase, subjects are asked to identify the previously observed pairs. Participants are instructed to look at and focus just on the new (i.e., novel) image that has not previously been shown. The individual’s “novelty preference” is determined by the proportion of time spent viewing the new image relative to the overall gazing time. Accuracy is used to calculate scores.
The Epworth sleepiness scale
The subjective sleepiness was assessed using the Arabic validated version of the ESS questionnaire (56). On a four-point scale, participants indicated how prone they are to doze in eight different daily situations (circumstances). ESS scores were interpreted as follows—0–5: lower than normal daytime sleepiness; 6–10: higher than normal daytime sleepiness; 11–12: mild excessive daytime sleepiness (EDS); 13–15: moderate EDS and 16–24: severe EDS (57). In older men, the internal consistency is adequate (Cronbach’s α = 0.70) (58). For the construct validity, worse sleep on the PSQI is associated with greater sleepiness on the ESS (r = 0.13; p < 0.001) (58).
The Pittsburg sleep quality index
The PSQI questionnaire (45), extensively validated in various populations and cultures (59), was used to assess sleep quality over the preceding month. The Arabic-validated version of the PSQI was utilized (46). The questionnaire includes 19 questions, each representing one of the seven sleep quality components: subjective sleep quality, sleep duration, sleep onset latency (SOL), sleep disturbance, sleep efficiency, daytime dysfunction, and sleep medication intake (45). Global (total) PSQI scores > 5 indicate poor sleep quality, whilst global PSQI scores ≤ 5 indicate good sleep quality (45). The reliability of the test according to Cronbach’s α is 0.81 (60). In addition, the internal consistency of the PSQI was 0.81 and the scales correlation score ranged from 0.48 to 0.71 (60).
Insomnia severity index
The Arabic-validated version of the ISI questionnaire was utilized (50). The ISI is a self-reported 7-item questionnaire that assesses the nature, severity, and impact of insomnia. ISI scores range from 0 to 28. The total ISI scores of 8 or higher indicates insomnia. The total score is interpreted as follows: no insomnia (0–7); sub-threshold insomnia (8–14); moderate insomnia (15–21); and severe insomnia (22–28). The internal consistency reliability equals 0.91 (49, 61). Regarding validity, ISI shows a statistically significant positive correlation with Athens Insomnia scale-5 (r = 0.93) (49, 61).
The Arabic version of the physical activity scale for the elderly
The PASE is a brief, easy-to-score, reliable, and valid instrument used in epidemiologic investigations to assess PA in the elderly (62). Respondents are asked to report the number of days per week in which the activity was performed, as well as the number of hours per day spent on leisure activity items. PASE scores are calculated from weights and frequency values assigned to each of the 12 types of activity. The internal consistency of the physical activity scale for the elderly (PASE-A) components is good (Cronbach’s α 0.70–0.75), and the reliability of the components is excellent (ICC2,10.90–0.98) (63).
Statistical analysis
All statistical analyses were performed using STATISTICA software (StatSoft, version 12, Paris, France). Data are presented as means and standard deviations. The variation (Δ) recorded from before Ramadan session to during Ramadan session is calculated as follows: Δ = During Ramadan – Before Ramadan. The percentage of variation (Δ%) recorded from before Ramadan session to during Ramadan session is calculated by the following formula: Δ% = (Δ/Before Ramadan) × 100. The normality of the distributions was tested by the Shapiro-Wilks test.
Pearson’s chi-squared test was used to compare the demographic baseline characteristics of the participants including gender, education level, and health status of the two groups. Because the age and the body mass index (BMI) were not normally distributed, the non-parametric Mann-Whitney U-test was used.
From all investigated parameters, processing speed, associative learning, executive function, attention, and ESS scores are normally distributed. Therefore, a two-way mixed analysis of variance (ANOVA) was applied 2 groups (Sedentary vs. Active) × 2 Ramadan (Before Ramadan vs. During Ramadan) for these variables. When the ANOVA showed a significant interaction, pairwise comparison is carried out by a Bonferroni post-hoc test, otherwise by an independent or paired Student’s t-test.
For other parameters where normality is not verified, non-parametric tests are used as follows: Wilcoxon test was made for the comparison from before to during Ramadan for each group and the Mann-Whitney U-test is carried out for the comparison between the means of the two groups, before Ramadan, during Ramadan and the Δ variation. All statistics are considered significant for a probability threshold of 5% (p < 0.05).
To determine the meaningfulness of significant findings for the ANOVA Group × Ramadan interaction, effect sizes (ES) were calculated as partial eta-squared ηp2 (64, 65). Small, moderate, and large ES are represented by ηp2 values of 0.01, 0.06, and 0.14, respectively (65). Furthermore, the magnitude of the change in score was determined using Cohen’s d (66), and it was interpreted using the following criteria: 0.2 (small), 0.5 (moderate), and 0.8 (large). The matched rank biserial correlation was used to measure ES for non-parametric tests (67). ESs were classified as small (0.10–0.30), medium (0.30–0.50), and large (0.50) (67).
Results
Baseline characteristics of participants
There were no significant differences between active and sedentary participants with regard to gender, education level, and health status. Likewise, the Mann-Whitney U-test reported no significant difference in age and BMI between groups.
Cognitive function
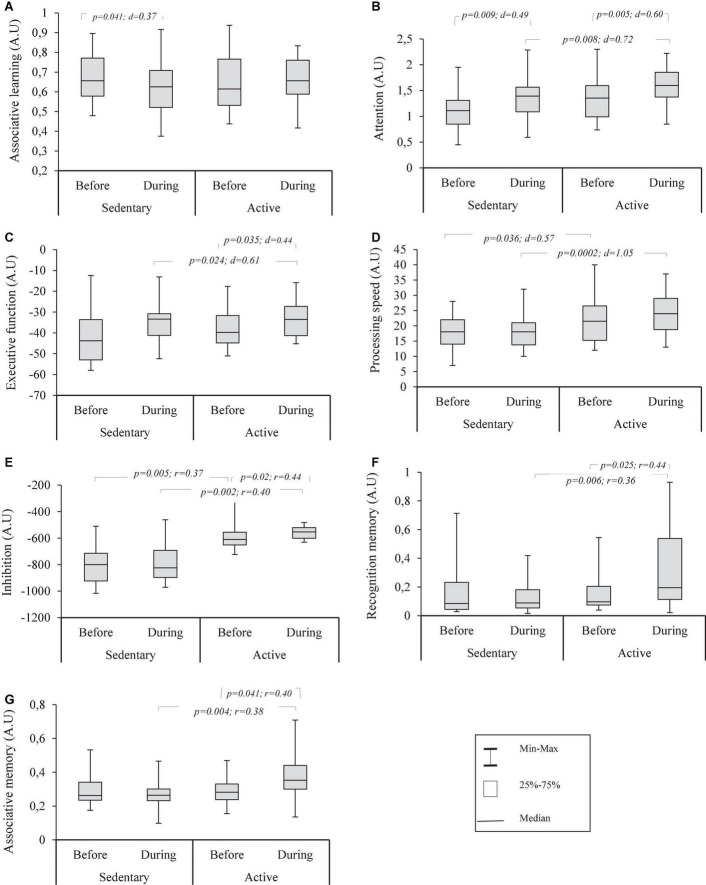
Cognitive function before and during Ramadan are shown in Figure 2. The two-way ANOVA (Ramadan × group) for associative learning (i.e., Figure 2A) showed no significant effect for Ramadan × group interaction [F(1,56) = 2.12; p = 0.151; ηp2 = 0.04]. Paired samples t-test revealed that associative learning decreased significantly in the control group with no change in the active group (p = 0.738; d = 0.066). Independent samples t-test showed no significant differences in this variable between the two groups at any time period.
FIGURE 2.
Box plots distribution of recognition memory (A), associative memory (B), inhibition (C), processing speed (D), associative learning (E), executive function (F) and attention (G) assessed using Neurotrack’s digital cognitive battery in sedentary and active groups before and during Ramadan fasting.
For attention (i.e., Figure 2B), there was no significant Ramadan × group interaction [F(1,56) = 0.31; p = 0.581; ηp2 = 0.005]. From before to during RDIF, a significant increase in both groups was shown with greater scores for the active group during RDIF. Before RDIF, no significant difference between the two groups was recorded (p = 0.056; d = 0.516).
Executive function (i.e., Figure 2C) revealed no significant effect for Ramadan × group interaction [F(1,56) = 2.77; p = 0.102; ηp2 = 0.047]. However, a significant increase from before to during RDIF was found in the active group, whereas this variable remained unchanged in the control group during the same time period (p = 0.584; d = 0.098). Moreover, no significant difference between the groups existed before RDIF (p = 0.933; d = 0.022), while better scores were observed in the active group during RDIF.
Likewise, there was no significant effect for Ramadan × group interaction [F(1,56) = 1.98; p = 0.165; ηp2 = 0.034] on processing speed (i.e., Figure 2D). No change from before to during RDIF either for active (p = 0.101; d = 0.334) and control groups (p = 0.701; d = 0.068). Furthermore, a significant difference between the two groups before and during RDIF was observed.
With regard to inhibitory function (i.e., Figure 2E), the Mann–Whitney U-test revealed no significant effect for Ramadan × group interaction (z = 0.899; p = 0.369; r = 0.118). The Wilcoxon test showed inhibitory function increased significantly in the active group while there was no change from before to during RDIF for the control group (p = 0.614; r = 0.089). Compared to the sedentary group, higher scores were recorded for the active group before and during RDIF.
For recognition memory (i.e., Figure 2F), a significant effect for Ramadan × group interaction (z = 2.291; p = 0.022; r = 0.301) was shown. The Bonferroni post-hoc test revealed a significant increase from before to during RDIF for the active group; this parameter remained unchanged for the control group (p = 0.466; r = 0.129) throughout the study. No difference between groups was found before RDIF (p = 0.477; r = 0.093), whereas higher scores were recorded for the active group during RDIF compared to the control group.
For associative memory (i.e., Figure 2G), there was a significant effect for Ramadan × group interaction (z = 2.556; p = 0.011; r = 0.336). The Bonferroni post hoc test revealed a significant increase throughout the investigation for the active group, while no change was observed for the sedentary group (p = 0.130; r = 0.268). Furthermore, no difference between groups was shown before RDIF (p = 0.969; r = 0.005), whilst the active group had a greater score during RDIF than the control group.
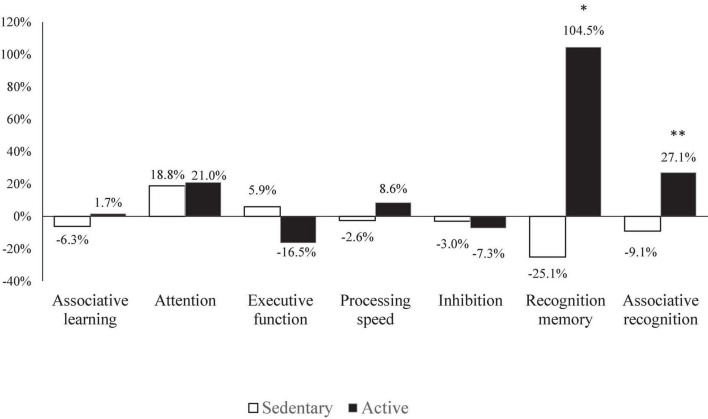
Figure 3 shows the percent change (Δ%) of cognitive function from before to during RDIF in both groups. There was a significant effect for Ramadan × group interaction between sedentary and active groups in the memory parameters (i.e., recognition memory, associative recognition), with a higher positive Δ% observed in the active group.
FIGURE 3.
Percent change (Δ%) of cognitive functions for sedentary and active groups. *Significant difference: p < 0.05; **significant difference: p < 0.001.
Effects of Ramadan on sleep quality quantitative sleep measures, daytime sleepiness, insomnia, and physical activity level
The means scores of quantitative measures of PSQI questionnaires, daytime sleepiness, insomnia, and PA level before and during Ramadan are presented in Table 3.
TABLE 3.
Means and standards deviations (SD) of questionnaire findings before and during Ramadan for sedentary and active groups.
| Parameters | Groups | Means ± SD | Ramadan × group interaction | |||||
|
|
|
|||||||
| Before-R | During-R | Delta | Δ % | F/Z | P-value | ηp2/r | ||
| ESS score (a.u.) | Sedentary | 6.41 ± 2.96 | 7.69 ± 3.12a | 1.28 ± 2.22 | 20.00% | F = 0.030 | 0.859 | ηp2 = 0.001 |
| Active | 4.46 ± 1.79b | 5.65 ± 2.28ab | 1.19 ± 1.36 | 26.70% | ||||
| ISI score (a.u.) | Sedentary | 5.91 ± 2.9 | 6.84 ± 3.61a | 0.94 ± 2 | 15.90% | z = 0.133 | 0.894 | r = 0.017 |
| Active | 3.58 ± 2.8b | 4.46 ± 2.89ab | 0.88 ± 1.88 | 24.70% | ||||
| Global PSQI score (a.u.) | Sedentary | 6.38 ± 2.15 | 9.69 ± 1.96a | 3.31 ± 1.69 | 52.00% | z = 2.494 | 0.013 | r = 0.327 |
| Active | 5.81 ± 2.17 | 8.04 ± 2.34ab | 2.23 ± 2.14 | 38.40% | ||||
| Subjective sleep quality (a.u.) | Sedentary | 0.88 ± 0.79 | 1.66 ± 0.94a | 0.78 ± 1.01 | 89.30% | z = 1.243 | 0.214 | r = 0.163 |
| Active | 0.54 ± 0.71 | 1.12 ± 1.11ab | 0.58 ± 0.9 | 107.10% | ||||
| Sleep disturbances (a.u.) | Sedentary | 0.53 ± 0.51 | 1.22 ± 0.55a | 0.69 ± 0.59 | 129.40% | z = 0.571 | 0.568 | r = 0.075 |
| Active | 0.31 ± 0.47 | 0.85 ± 0.46ab | 0.54 ± 0.71 | 175.00% | ||||
| Daytime dysfunction (a.u.) | Sedentary | 0.47 ± 0.67 | 1.34 ± 0.65a | 0.88 ± 0.71 | 186.70% | z = 1.415 | 0.157 | r = 0.186 |
| Active | 0.12 ± 0.33 | 0.77 ± 0.51ab | 0.65 ± 0.49 | 566.70% | ||||
| Sleep latency (min) | Sedentary | 18 ± 11.6 | 25.8 ± 13.6a | 7.78 ± 8.64 | 43.20% | z = 1.767 | 0.077 | r = 0.232 |
| Active | 19 ± 12.6 | 23.1 ± 11a | 4.12 ± 7.48 | 21.70% | ||||
| Sleep efficiency (%) | Sedentary | 89.4 ± 8.2 | 84.2 ± 9.3a | –5.28 ± 7.22 | –5.90% | z = 1.681 | 0.093 | r = 0.221 |
| Active | 85.7 ± 10 | 84.3 ± 7.5 | –1.43 ± 9.38 | –1.70% | ||||
| Nocturnal sleep duration (hours) | Sedentary | 7.22 ± 1.11 | 6.17 ± 0.96a | –1.05 ± 1.09 | –14.50% | z = 2.853 | 0.004 | r = 0.375 |
| Active | 7.02 ± 1.07 | 6.73 ± 1.03b | –0.29 ± 0.75 | –4.10% | ||||
| Bedtime (h:mm) | Sedentary | 23:30 ± 1:07 | 00:39 ± 1:22a | 1:09 ± 1:04 | 4.9% | F = 5.39 | 0.024 | ηp2 = 0.088 |
| Active | 23:06 ± 1:05 | 23:40 ± 0:53ab | 0:34 ± 0:48 | 2.4% | ||||
| Wake-up (h:mm) | Sedentary | 6:43 ± 1:07 | 6:49 ± 1:20 | 0:06 ± 1:00 | 1.5% | F = 0.79 | 0.377 | ηp2 = 0.014 |
| Active | 6:07 ± 1:04b | 6:25 ± 0:57a | 0:18 ± 0:33 | 4.8% | ||||
| Use of sleep medication (a.u.) | Sedentary | 0.09 ± 0.3 | 0.06 ± 0.25 | –0.03 ± 0.31 | –33.30% | z = 0.031 | 0.975 | r = 0.004 |
| Active | 0.08 ± 0.39 | 0.04 ± 0.2 | –0.04 ± 0.2 | –50.00% | ||||
| PASE score (a.u.) | Sedentary | 101.23 ± 20.6 | 84.383 ± 13.11a | –16.84 ± 12.52 | –20% | z = 2.447 | 0.014 | r = 0.321 |
| Active | 153.81 ± 11.14b | 119.55 ± 11.25ab | –34.26 ± 16.22 | –28.70% | ||||
a: significantly different from before Ramadan at p < 0.05; b: significantly different from sedentary group at p < 0.05. Before-R, before Ramadan; During-R, during Ramadan; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth sleepiness scale; ISI, insomnia severity index; PASE, Physical activity scale for elderly; Δ, difference between during and before Ramadan; SD, standard deviation; a.u., arbitrary unit.
Before RDIF, there were significant differences between the two groups in ESS (p = 0.003; d = 0.578), ISI (p = 0.013; r = 0.440) and PASE (p < 0.001; r = 0.853) scores, in which control group had worst scores compared to active group.
From before to during RDIF, ESS score, ISI score, Global PSQI score, subjective sleep quality, sleep disturbances, daytime dysfunction increased in active group (p < 0.001; d = 0.879, p = 0.031; r = 0.422, p < 0.001; r = 0.781, p = 0.007; r = 0.531, p = 0.005; r = 0.552, p < 0.001; r = 0.710, respectively) and control group (p = 0.003; d = 0.578, p = 0.013; r = 0.440, p < 0.001; r = 0.852, p = 0.001; r = 0.580, p < 0.001; r = 0.683, p < 0.001; r = 0.717, respectively), with significantly higher negative effects observed during RDIF in control group (p = 0.007; d = 0.733, p = 0.016; r = 0.315, p = 0.006; r = 0.361, p = 0.043; r = 0.266, p = 0.038; r = 0.273, p = 0.004; r = 0.380, respectively).
Furthermore, sleep latency increased in active (p = 0.025; r = 0.439) and sedentary (p < 0.001; r = 0.672) groups, with no significant differences between the two groups at any time periods. Moreover, there was a significant increase in habitual sleep efficiency only for the control group (p < 0.001; r = 0.718), while for the active group this parameter remained unchanged (p = 0.411; r = 0.161).
Sleep duration decreased significantly from before to during RDIF in the control group (p < 0.001; r = 0.770), while no significant change observed in the active group (p = 0.057; r = 0.373). The sedentary population had lower sleep duration during RDIF (p = 0.036; r = 0.275).
Bedtime was delayed by 0.56 h during vs. before Ramadan in active group (p = 0.002, d = 0.7). Likewise, the inactive group showed a delay in bedtime by 1.15 h from before to during Ramadan (p < 0.001, d = 1.1). A significant difference was found between the two groups during Ramadan (p = 0.003, d = 0.8). However, no significant difference between active and sedentary groups was shown before Ramadan.
Active group awakened 0.3 h later during vs. before Ramadan (p = 0.01, d = 0.5), whereas no significant difference was found in the sedentary group (p = 0.580, d = 0.1). No significant difference was found between the two groups during Ramadan.
No significant change and/or difference was recorded in both groups for the use of sleep medication (p > 0.05).
Finally, PASE total score decreased significantly in active (p < 0.001; r = 0.874) and sedentary (p < 0.001; r = 0.803) groups from pre to during RDIF, with preservation of greater values during RDIF in active group (p < 0.001; r = 0.827).
Discussion
The present study is the first to evaluate the effects of RDIF on cognitive function, sleep quality, daytime sleepiness, insomnia, and sleep-wake behaviors for active and inactive elderly populations.
Before RDIF, significant differences in questionnaires baseline scores including ESS, ISI, and PASE as well as for some cognitive function domains (i.e., inhibition and processing speed) were reported. This may be explained by the physical training effects in the active group. Previous reports suggest a beneficial role of chronic training schedule on inhibitory function (68, 69), processing speed (70), insomnia, daytime sleepiness, and sleep quality (71, 72).
Furthermore, from before to during RDIF, our results showed an improvement in the majority of cognitive function variables for the active group, including attention, executive function, inhibition, recognition memory, and associative memory; there were no changes for other cognitive domains including processing speed and associative learning. However, the control group showed a significant decline in associative learning from before to during RDIF, unlike other cognitive domains which remained unchanged overtime period. These findings partially verified our first hypothesis. Furthermore, we observed an overall deterioration of sleep quality and an increase in daytime sleepiness for both groups. The reported scores of PSQI and ESS confirmed both groups suffered from poor sleep quality, which confirmed our second hypothesis.
The average overall PSQI score (PSQI score ≥ 5) recorded before and during Ramadan is indicative of poor sleep quality (43, 73), which could be associated with volume loss in several hippocampal subfields triggered by the aging process (74). The greater increase in the global PSQI score during RDIF (PSQI score > 8) (75) for the control group suggests a worsened sleep quality and thus potential depressive syndrome (76). Based on the results of a recent systematic review (77), it is likely that maintaining a moderate intensity PA during Ramadan could have reduced the negative effect of Ramadan on sleep quality. Furthermore, several studies have shown exercise may modulate the circadian rhythm by serving as a time cue, modifying the phase of a molecular clock in peripheral tissues (78–80). More precisely, endurance and resistance exercise may stimulate the expression of core molecular clock genes, through the influence on exercise-sensitive genes such as AMPK, HIF-1α, and PGC1α. Increased activation of AMPK modifies the stability of Per and Cry, modulating the expression of clock genes (78–81). Moreover, adopting PA can potentially resynchronize peripheral muscle and vascular clock misalignment, which could improve vascular health and, consequently, skeletal muscle function (82).
Regarding PSQI components, nocturnal sleep duration, which is a critical element in assessing the restorative effects of sleep (83), decreased more in the control group (<7 h/night) by ∼1 h during RDIF, as older adults require about the same amount of sleep as all adults (i.e., 7–9 h/night) (84). The decrease in nocturnal sleep duration was previously reported during Ramadan (30, 85) and could be explained by the occurrence of several lifestyle changes in nocturnal and diurnal activities (i.e., zeitgeber factors), such as rising early for the Suhur meal and engaging in more social activities after the break of fast (e.g., Quran reading groups, social gatherings, shopping, prayers) (30, 86, 87). Furthermore, the greater than normal food consumption at night as well as eating close to bedtime could have decreased nocturnal sleep duration by negatively affecting circadian rhythm and hormone excretion (88, 89).
Sleep disturbances increased significantly in both groups during RDIF. Nocturnal urination is known as a potential cause of sleep disturbances in the elderly (90), and increased water intake after the break of fast during RDIF could have exacerbated the effect of nocturnal urination on sleep disturbances. Additionally, rising early for the Suhur and prayers could explain, at least in part, the previous finding.
SOL, another component of PSQI, increased in both groups. Additionally, average SOL values reported during Ramadan in the two groups were higher than the cut-off of 20 min indicative of usual SOL (91), suggesting our participants have difficulty initiating sleep during Ramadan. The increased exposure to evening light during Ramadan (e.g., family commitment, watching TV programs, shopping) could have prolonged subsequent SOL (i.e., taking longer to fall asleep during Ramadan) in both groups. As a result of sleep impairment, daytime dysfunction increased during Ramadan in the two groups; this may increase the loss of daily structure during Ramadan (92).
ESS scores increased during Ramadan in the two groups. However, it is worth mentioning that the average ESS score recorded in the sedentary group is indicative of higher than normal daytime sleepiness, whilst daytime sleepiness in the active group is lower than normal. The increase in daytime sleepiness could be explained by sleep insufficiency during Ramadan, as previously reported by Faris et al. (30). In this context, a comparable increase in daytime sleepiness levels during Ramadan was previously reported by the systematic review and meta-analysis of Faris et al. (30). Chasens et al. (93) reported daytime sleepiness in older adults is negatively associated with some measures of PA. It appears practicing PA during Ramadan may explain lower ESS scores observed in the active compared to the sedentary group. Nevertheless, the mechanism(s) explaining the relationship between daytime sleepiness and PA activity level is not fully understood.
Despite the increase in ISI total scores in both groups from before Ramadan to during Ramadan, it remained clinically non-significant (i.e., total ISI score < 7), and this finding could be related to the inexistence of very high mean age in both groups (i.e., mean age < 65 years). Some previous studies suggest the rise of insomnia severity problems is associated with getting old and that about 12–25% of individuals aged more than 65 years old suffer from insomnia problems (94, 95).
The deterioration of sleep-wake patterns may lead to a subsequent decrease in cognitive function during RDIF in both groups. The digital cognitive assessment battery revealed enhancements in several cognitive domains, in the active elderly group compared to the control group. These improvements may be attributed to the effects of regular PA, combined with intermittent fasting.
Associative learning is defined as, “learning about the connection between two disparate stimuli” (96). There are not yet any studies investigating the effects of RDIF on associative learning, but we suggest the performance decrease in the control group could be attributed to the aging process, which is related to dysfunction in dopamine receptors and transmitter substances (97, 98). This may lead to the disruption in dopamine receptor stimulation levels in the prefrontal cortex, which may worsen the balance of cognitive stability and flexibility (99), consequently disturbing associative learning modulation (100). Future investigations based on clinical data regarding the dopamine pathways and their interactions with associative learning for elderly individuals are warranted. Moreover, the dysfunction of nitric oxide (NO) activity in the brain due to the aging process may also explain the learning impairment (101–103). However, regular PA reshapes the system balance, leading to the regulation of dopamine levels in the brain and the availability of more dopamine receptors (104), as well as increasing the production of NO release from endothelial cells, favoring vascular function and decreasing NO from inducible NO synthase in the brain (103, 105, 106), potentially reducing the harmful effect of aging. This is evident for active elderly who maintain the associative learning level during RDIF.
Attention refers to the collection of adapted brain functions that enable effective and adaptive behavioral selection (107). According to the BOLD-fMRI research, compensatory mechanisms exist to help attention and focus and inhibit extraneous mental processes induced by sleep deprivation, which may mitigate some of their adverse effects and add flexibility in tasks that deserve attention and vigilance (108–110). Despite the sleep deterioration observed in the sedentary group during Ramadan, attention scores increased. This may be explained by the compensatory mechanisms enabling attention improvement during Ramadan. It should be acknowledged attention scores increased in the active group, but with a higher effect size (i.e., d = 0.60) compared to that of the sedentary group (i.e., d = 0.49) and this highlights the role of continuous PA in potentially enhancing attention in the elderly (111, 112).
Furthermore, our results are in accordance with the study of Tian et al. (42), which revealed a beneficial effect of RDIF on visual attention for Muslim athletes. However, other studies revealed a significant decline in attentional performance induced by RDIF in sedentary individuals (88, 113, 114).
For executive function, which is defined as a set of mental aptitudes that incorporate working memory, adaptable reasoning, and self-control (115), physically active older adults performed better during RDIF compared to sedentary participants who did not record a change from before to during RDIF. This stagnation in the control group is in concert with the findings of Harder-Lauridsen et al. (116), who revealed no effects of intermittent fasting on executive function. Furthermore, the significant increase of executive function values for the active group reinforces existing literature suggesting regular physical exercise may buffer the impacts of aging on cognitive decrements, especially with regard to executive function (117–119). Moreover, the recent meta-analysis of Xiong et al. (120) confirmed the beneficial effects of regular physical activities for improving cognitive flexibility, working memory, and inhibitory control of executive function in cognitively healthy older adults.
Inhibitory control or inhibition refers to the ability to suppress or control impulsive (or instinctive) responses and produce replies by employing attention and logic (121). In this context, inferior inhibitory control shown in the control group, compared to physically active elderly before and during RDIF, may explain the decrease of sleep efficiency for sedentary participants (122). Ghayour Najafabadi et al. (25) showed no adverse effects of RDIF on inhibitory performance in female athletes; thereby we suggest that regular exercise may preserve inhibitory control, exhibited by faster response speeds during inhibition tasks. This is related to greater productivity of neural circuits (123), suggesting the synthesis of BDNF as a vital component underlying the impact of PA on brain structure and capacities evidently on inhibitory function (124).
Processing speed, which reflects the amount of time needed to react to and/or process information to perform an intellectual task (125), remained unchanged from before to during RDIF for both groups. Our results are contradictory to those of Tian et al. (42). This latter showed a significant effect of RDIF on psychomotor function in athletes who had poorer scores in the afternoon (i.e., at 16:00 h) than morning (i.e., at 09:00 h) (42), which could explain the reason we found no significant changes in the active group since our testing was performed in the afternoon. Additionally, higher processing speed values recorded before and during RDIF in the active group may explain the positive effects of PA training on processing speed (126). Moreover, the decrease, even though non-significant, shown in the control group between before to during RDIF, may be attributed to decreased integrity of the cerebral white matter associated to the aging process (127). Reducing this decline is important as it may cause several problems in daily life. In this context, decreases in processing speed are the strongest indicator of driving cessation in older adults (128).
To the best of our knowledge, the present study is the first to assess memory function based on eye tracking in older adults during RDIF. Novelty preference score is an operation of recognition memory based on ETM which is the capacity to identify already experienced objects (129), in addition to associative memory which is defined as the capacity to learn and retain the connection between unrelated items. Our results revealed a better increase during RDIF for active subjects compared to sedentary older adults. Similar to this unchanged value in the control group, Doniger et al. (130) found no evidence of a substantial impact of fasting (i.e., religious orthodox Jewish day of fast) on verbal recognition memory performance based on computerized cognitive assessment for normal individuals. However, a recent study conducted by Dias et al. (131) affirmed the protentional effects of intermittent fasting including several neurological modifications on memory consolidation.
Furthermore, the continuous physical training program based on several tactical schemes and combinations of team sport and repetitive movements on circuit training, as in our research, could have positively consolidated and strengthened memory functions in elder adults (132–134).
Limitations
Despite the novelty of our study, some limitations should be acknowledged and addressed in future investigations. First, we did not evaluate sleep quality and daytime sleepiness using objective measurements (e.g., actigraphy, polysomnography, the Multiple Sleep Latency Test). Although there is disagreement about what exactly constitutes excellent sleep, sleep quality was previously described as a subjective perception (135). Interestingly, the used questionnaires include a standardized scoring strategy which seems to offer more prominent reliability and reproducibility in further investigations. The second issue is that the Neurotrack battery’s cultural adaptation did not cover all of the assessments’ components (e.g., the items on display when taking the exam). It is worth noting that for adapting cognitive assessments to different cultural contexts, there are currently no established standards guidelines, or criteria (136). Nevertheless, an exact transcribing methodology was performed to consider any potential cultural context-related performance bias. In this regard, methodological variations and the absence of sensitive computerized neuropsychological tools (i.e., the lack of objective assessment) in some studies may be to blame for the contradictory results. Third, the evident decrease in PASE scores in both groups could not be due to the decline of PA level, specifically for the active group, since coaches prioritize maintenance during RDIF to the same duration and intensity in training sessions as before Ramadan month. This energy expenditure decline may be attributed to the decrease in daily life behaviors (e.g., gardening). In this regard, raising these daily activities to normal rates is recommended. Fourth, we did not investigate hydration status, nutritional intake, and glycemia before and during Ramadan as well as in pre-dawn meal and when breaking fast, despite the existence of several studies in the elderly population confirming the strong relationship between hypohydration, nutrition and poor cognitive performance (137–142), as well as between glycemic control and cognitive functions (143, 144). More rigorous studies investigating these parameters before and during overall RDIF are needed. Other factors such as environmental variables (e.g., noise and number of the household in the same house), as well as the emotional and psychological status (e.g., satisfaction, depression), may have influenced the results. Therefore, these confounding factors should be assessed/controlled during future investigations. Furthermore, the results of our study pertain only to older adults. Muslim and could not be generalized to non-Muslim older adults given that intermittent fasting performed by our participants is of a religious type. More empirical studies with largest number of participants in non-Muslim countries should be investigated. Moreover, experimental investigations based on clinical data (e.g., biochemical and cardiometabolic parameters) are warranted to better understand the mechanisms, and objectively discuss the variation in the outcomes of interests in order to reach more definitive conclusions.
Conclusion
Ramadan observance worsened sleep quality and increased daytime sleepiness in both groups; however, this was more prominent in the control group. Likewise, more pronounced adverse impacts on cognitive functions were recorded for inactive subjects compared to active older adults. Therefore, continuous PA practice during the month of Ramadan may counteract the potential negative effect of RDIF on sleep and cognitive performance.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee CPP SUD N°0322/2021 gave its approval to this project, and the present study was carried out according to the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
Authors JG and KB were employees in Neurotrack Technologies. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Castillo X, Castro-Obregón S, Gutiérrez-Becker B, Gutiérrez-Ospina G, Karalis N, Khalil AA, et al. Re-thinking the etiological framework of neurodegeneration. Front Neurosci. (2019) 13:728. 10.3389/fnins.2019.00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:459–80. 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murman DL. The impact of age on cognition. Semin Hear. (2015) 36:111–21. 10.1055/s-0035-1555115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. (2020) 21:93–102. 10.1038/s41583-019-0255-9 [DOI] [PubMed] [Google Scholar]
- 5.Foster TC. Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs. (2006) 20:153–66. 10.2165/00023210-200620020-00006 [DOI] [PubMed] [Google Scholar]
- 6.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The course of cognitive impairment in preclinical alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. (2000) 57:839–44. 10.1001/archneur.57.6.839 [DOI] [PubMed] [Google Scholar]
- 7.Gazzaley A, Sheridan MA, Cooney JW, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. (2007) 21:532–9. 10.1037/0894-4105.21.5.532 [DOI] [PubMed] [Google Scholar]
- 8.Kessel L, Lundeman JH, Herbst K, Andersen TV, Larsen M. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. (2010) 36:308–12. 10.1016/j.jcrs.2009.08.035 [DOI] [PubMed] [Google Scholar]
- 9.Arendt J. Melatonin and human rhythms. Chronobiol Int. (2006) 23:21–37. 10.1080/07420520500464361 [DOI] [PubMed] [Google Scholar]
- 10.Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. (2017) 127:437–46. 10.1172/JCI90328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale JA, Bonato M, La Torre A, Banfi G. The role of the molecular clock in promoting skeletal muscle growth and protecting against sarcopenia. Int J Mol Sci. (2019) 20:4318. 10.3390/ijms20174318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle. (2015) 5:17. 10.1186/s13395-015-0039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudden J, Arias Vasquez A, Bloemendaal M. The effects of intermittent fasting on brain and cognitive function. Nutrients. (2021) 13:3166. 10.3390/nu13093166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longo VD, Anderson RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. (2022) 185:1455–70. 10.1016/j.cell.2022.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi MC, Morales FR, Chase MH. Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the latero-dorsal tegmental nucleus of the cat. Brain Res. (2001) 901:259–64. 10.1016/s0006-8993(01)02317-4 [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. (2005) 25:237–60. 10.1146/annurev.nutr.25.050304.092526 [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. (2017) 39:46–58. 10.1016/j.arr.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. (2019) 381:2541–51. 10.1056/NEJMra1905136 [DOI] [PubMed] [Google Scholar]
- 19.Francis N. Intermittent fasting and brain health: efficacy and potential mechanisms of action. OBM Geriatr. (2020) 4:1–15. 10.21926/obm.geriatr.2002121 [DOI] [Google Scholar]
- 20.Mattson MP. The intermittent fasting revolution: The science of optimizing health and enhancing performance. Cambridge: MIT Press; (2022). [Google Scholar]
- 21.Seidler K, Barrow M. Intermittent fasting and cognitive performance – targeting BDNF as potential strategy to optimise brain health. Front Neuroendocrinol. (2022) 65:100971. 10.1016/j.yfrne.2021.100971 [DOI] [PubMed] [Google Scholar]
- 22.Lessan N, Ali T. Energy metabolism and intermittent fasting: the Ramadan perspective. Nutrients. (2019) 11:1192. 10.3390/nu11051192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsharidah AM, Murtaza G, Alsharidah MM, Bashir S. Fasting in Ramadan affects cognitive and physiological function in normal subjects. (Pilot Study). Neurosci Med. (2016) 7:60–5. 10.4236/nm.2016.72007 [DOI] [Google Scholar]
- 24.Farooq A, Herrera CP, Almudahka F, Mansour RA. prospective study of the physiological and neurobehavioral effects of Ramadan fasting in preteen and teenage boys. J Acad Nutr Diet. (2015) 115:889–97. 10.1016/j.jand.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 25.Ghayour Najafabadi M, Rahbar Nikoukar L, Memari A, Ekhtiari H, Beygi S. Does Ramadan fasting adversely affect cognitive function in young females? Scientifica. (2015) 2015:432428. 10.1155/2015/432428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leigh Gibson E, Green MW. Nutritional influences on cognitive function: mechanisms of susceptibility. Nutr Res Rev. (2002) 15:169–206. 10.1079/NRR200131 [DOI] [PubMed] [Google Scholar]
- 27.Lieberman HR, Bathalon GP, Falco CM, Kramer FM, Morgan CA, Niro P. Severe decrements in cognition function and mood induced by sleep loss, heat, dehydration, and undernutrition during simulated combat. Biol Psychiatry. (2005) 57:422–9. 10.1016/j.biopsych.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Bahammam AS, Alaseem AM, Alzakri AA, Sharif MM. The effects of Ramadan fasting on sleep patterns and daytime sleepiness: an objective assessment. J Res Med Sci Off J Isfahan Univ Med Sci. (2013) 18:127–31. [PMC free article] [PubMed] [Google Scholar]
- 29.Boukhris O, Trabelsi K, Shephard RJ, Hsouna H, Abdessalem R, Chtourou L, et al. Sleep patterns, alertness, dietary intake, muscle soreness, fatigue, and mental stress recorded before, during and after Ramadan observance. Sports Basel Switz. (2019) 7:118. 10.3390/sports7050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faris MAIE, Jahrami HA, Alhayki FA, Alkhawaja NA, Ali AM, Aljeeb SH, et al. Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep Breath Schlaf Atm. (2020) 24:771–82. 10.1007/s11325-019-01986-1 [DOI] [PubMed] [Google Scholar]
- 31.Trabelsi K, Ammar A, Glenn JM, Boukhris O, Khacharem A, Bouaziz B, et al. Does observance of Ramadan affect sleep in athletes and physically active individuals? A systematic review and meta-analysis. J Sleep Res. (2022) 31:e13503. 10.1111/jsr.13503 [DOI] [PubMed] [Google Scholar]
- 32.Stillman CM, Cohen J, Lehman ME, Erickson KI. Mediators of physical activity on neurocognitive function: a review at multiple levels of analysis. Front Hum Neurosci. (2016) 10:626. 10.3389/fnhum.2016.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage. (2016) 131:142–54. 10.1016/j.neuroimage.2015.10.084 [DOI] [PubMed] [Google Scholar]
- 34.Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A. How does exercise reduce the rate of age-associated cognitive decline? A Review of Potential Mechanisms. J Alzheimers Dis JAD. (2017) 55:1–18. 10.3233/JAD-160665 [DOI] [PubMed] [Google Scholar]
- 35.Brown BM, Peiffer J, Rainey-Smith SR. Exploring the relationship between physical activity, beta-amyloid and tau: a narrative review. Ageing Res Rev. (2019) 50:9–18. 10.1016/j.arr.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 36.Sewell KR, Erickson KI, Rainey-Smith SR, Peiffer JJ, Sohrabi HR, Brown BM. Relationships between physical activity, sleep and cognitive function: a narrative review. Neurosci Biobehav Rev. (2021) 130:369–78. 10.1016/j.neubiorev.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 37.Küster OC, Fissler P, Laptinskaya D, Thurm F, Scharpf A, Woll A, et al. Cognitive change is more positively associated with an active lifestyle than with training interventions in older adults at risk of dementia: a controlled interventional clinical trial. BMC Psychiatry. (2016) 16:315. 10.1186/s12888-016-1018-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farooq A, Basterfield L, Adamson AJ, Pearce MS, Hughes AR, Janssen X, et al. Failure to launch: predictors of unfavourable physical activity and sedentary behaviour trajectories from childhood to adolescence: the gateshead millennium study. Int J Environ Res Public Health. (2021) 18:13283. 10.3390/ijerph182413283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lessan N, Saadane I, Alkaf B, Hambly C, Buckley AJ, Finer N, et al. The effects of Ramadan fasting on activity and energy expenditure. Am J Clin Nutr. (2018) 107:54–61. 10.1093/ajcn/nqx016 [DOI] [PubMed] [Google Scholar]
- 40.Roky R, Chapotot F, Hakkou F, Benchekroun MT, Buguet A. Sleep during Ramadan intermittent fasting. J Sleep Res. (2001) 10:319–27. 10.1046/j.1365-2869.2001.00269.x [DOI] [PubMed] [Google Scholar]
- 41.Pourabbasi A, Ebrahimnegad Shirvani MS, Shams AH. Does Islamic fasting affect cognitive functions in adolescents? A systematic review. J Pak Med Assoc. (2019) 69:1164–9. [PubMed] [Google Scholar]
- 42.Tian HH, Aziz AR, Png W, Wahid MF, Yeo D, Constance Png AL. Effects of fasting during Ramadan month on cognitive function in muslim athletes. Asian J Sports Med. (2011) 2:145–53. 10.5812/asjsm.34753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipert A, Kozłowski R, Rasmus P, Marczak M, Timler M, Timler D, et al. Sleep quality and performance in professional athletes fasting during the month of Ramadan. Int J Environ Res Public Health. (2021) 18:6890. 10.3390/ijerph18136890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 46.Suleiman KH, Yates BC, Berger AM, Pozehl B, Meza J. Translating the pittsburgh sleep quality index into arabic. West J Nurs Res. (2010) 32:250–68. 10.1177/0193945909348230 [DOI] [PubMed] [Google Scholar]
- 47.Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. (1991) 14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 48.Riachy M, Juvelikian G, Sleilaty G, Bazarbachi T, Khayat G, Mouradides C. Validation of the arabic version of the epworth sleepiness scale: multicentre study. Rev Mal Respir. (2012) 29:697–704. 10.1016/j.rmr.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 49.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 50.Suleiman KH, Yates BC. Translating the insomnia severity index into Arabic. J Nurs Scholarsh. (2011) 43:49–53. 10.1111/j.1547-5069.2010.01374.x [DOI] [PubMed] [Google Scholar]
- 51.Gills JL, Glenn JM, Madero EN, Bott NT, Gray M. Validation of a digitally delivered visual paired comparison task: reliability and convergent validity with established cognitive tests. GeroScience. (2019) 41:441–54. 10.1007/s11357-019-00092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gills JL, Bott NT, Madero EN, Glenn JM, Gray M. A short digital eye-tracking assessment predicts cognitive status among adults. GeroScience. (2021) 43:297–308. 10.1007/s11357-020-00254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers JR, Glenn JM, Madero EN, Anderson J, Mak-McCully R, Gray M, et al. Asynchronous remote assessment for cognitive impairment: reliability verification of the neurotrack cognitive battery. JMIR Form Res. (2022) 6:e34237. 10.2196/34237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boujelbane MA, Trabelsi K, Boukhris O, Kacem FH, Ammar A, Charfi I, et al. The use of digital technology to assess cognitive function in Tunisian adults. J Alzheimers Dis JAD. (2022) 88:1545–52. 10.3233/JAD-220398 [DOI] [PubMed] [Google Scholar]
- 55.Zola SM, Manzanares CM, Clopton P, Lah JJ, Levey AI. A behavioral task predicts conversion to mild cognitive impairment and alzheimer’s disease. Am J Alzheimers Dis Other Demen. (2013) 28:179–84. 10.1177/1533317512470484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed AE, Fatani A, Al-Harbi A, Al-Shimemeri A, Ali YZ, Baharoon S, et al. Validation of the arabic version of the epworth sleepiness scale. J Epidemiol Glob Health. (2014) 4:297–302. 10.1016/j.jegh.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johns M. The Official Website of the Epworth Sleepiness Scale (ESS) and the Epworth Sleepiness Scale For Children And Adolescents (Ess-Chad). (2019). Available online at: https://epworthsleepinessscale.com/about-the-ess (accessed July 30, 2022). [Google Scholar]
- 58.Spira AP, Beaudreau SA, Stone KL, Kezirian EJ, Lui LY, Redline S, et al. Reliability and validity of the pittsburgh sleep quality index and the epworth sleepiness scale in older men. J Gerontol A Biol Sci Med Sci. (2012) 67:433–9. 10.1093/gerona/glr172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the persian version of the pittsburgh sleep quality index. (PSQI-P). Sleep Breath Schlaf Atm. (2012) 16:79–82. 10.1007/s11325-010-0478-5 [DOI] [PubMed] [Google Scholar]
- 60.Chehri A, Nourozi M, Eskandari S, Khazaie H, Hemati N, Jalali A. Validation of the persian version of the pittsburgh sleep quality index in elderly population. Sleep Sci. (2020) 13:119–24. 10.5935/1984-0063.20190134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sierra JC, Guillén-Serrano V, Santos-Iglesias P. Insomnia severity index: some indicators about its reliability and validity on an older adults sample. Rev Neurol. (2008) 47:566–70. [PubMed] [Google Scholar]
- 62.Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the physical activity scale for the elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. (1997) 50:541–6. 10.1016/s0895-4356(97)00010-3 [DOI] [PubMed] [Google Scholar]
- 63.Alqarni AM, Vennu V, Alshammari SA, Bindawas SM. Cross-cultural adaptation and validation of the arabic version of the physical activity scale for the elderly among community-dwelling older adults in saudi arabia. Clin Interv Aging. (2018) 13:419–27. 10.2147/CIA.S157007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keppel G. Design and Analysis: A Researcher’s Handbook. Englewood Cliffs, NJ: Prentice Hall; (1991). [Google Scholar]
- 65.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. (2013) 4:863. 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Routledge Academic; (1988). [Google Scholar]
- 67.King BM, Rosopa PJ, Minium EW. Statistical Reasoning in the Behavioral Sciences. 6th ed. Hoboken: John Wiley; (2011). [Google Scholar]
- 68.Boucard GK, Albinet CT, Bugaiska A, Bouquet CA, Clarys D, Audiffren M. Impact of physical activity on executive functions in aging: a selective effect on inhibition among old adults. J Sport Exerc Psychol. (2012) 34:808–27. 10.1123/jsep.34.6.808 [DOI] [PubMed] [Google Scholar]
- 69.Tsai CL, Pan CY, Chen FC, Tseng YT. Open- and closed-skill exercise interventions produce different neurocognitive effects on executive functions in the elderly: a 6-month randomized, controlled trial. Front Aging Neurosci. (2017) 9:294. 10.3389/fnagi.2017.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nouchi R, Taki Y, Takeuchi H, Sekiguchi A, Hashizume H, Nozawa T, et al. Four weeks of combination exercise training improved executive functions, episodic memory, and processing speed in healthy elderly people: evidence from a randomized controlled trial. Age. (2014) 36:787–99. 10.1007/s11357-013-9588-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fank F, Pereira FDS, Dos Santos L, de Mello MT, Mazo GZ. Effects of exercise on sleep in older adults: an overview of systematic reviews and meta-analyses. J Aging Phys Act. (2022) 1–17. [Epub Online ahead of print]. 10.1123/japa.2021-0444 [DOI] [PubMed] [Google Scholar]
- 72.Silva VPO, Silva MPO, Silva VLDS, Mantovani DBC, Mittelmann JV, Oliveira JVV, et al. Effect of physical exercise on sleep quality in elderly adults: a systematic review with a meta-analysis of controlled and randomized studies. J. Ageing Longev. (2022) 2:85–97. 10.3390/jal2020008 [DOI] [Google Scholar]
- 73.Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the pittsburgh sleep quality index (PSQI). Sleep. (1991) 14:331–8. [PubMed] [Google Scholar]
- 74.Liu C, Lee SH, Hernandez-Cardenache R, Loewenstein D, Kather J, Alperin N. Poor sleep is associated with small hippocampal subfields in cognitively normal elderly individuals. J Sleep Res. (2021) 30:e13362. 10.1111/jsr.13362 [DOI] [PubMed] [Google Scholar]
- 75.Zhou Y, Yang Y, Shi T, Song Y, Zhou Y, Zhang Z, et al. Prevalence and demographic correlates of poor sleep quality among frontline health professionals in liaoning province, china during the covid-19 outbreak. Front Psychiatry. (2020) 11:520. 10.3389/fpsyt.2020.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y, Zhu M. Increased global PSQI score is associated with depressive symptoms in an adult population from the united states. Nat Sci Sleep. (2020) 12:487–95. 10.2147/NSS.S256625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang F, Boros S. The effect of physical activity on sleep quality: a systematic review. Eur J Physiother. (2021) 23:11–8. 10.1080/21679169.2019.1623314 [DOI] [Google Scholar]
- 78.Dickinson JM, D’Lugos AC, Naymik MA, Siniard AL, Wolfe AJ, Curtis DR, et al. Transcriptome response of human skeletal muscle to divergent exercise stimuli. J Appl Physiol. (2018) 124:1529–40. 10.1152/japplphysiol.00014.2018 [DOI] [PubMed] [Google Scholar]
- 79.Lassiter DG, Sjögren RJO, Gabriel BM, Krook A, Zierath JR. AMPK activation negatively regulates GDAP1, which influences metabolic processes and circadian gene expression in skeletal muscle. Mol Metab. (2018) 16:12–23. 10.1016/j.molmet.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolff CA, Esser KA. Exercise timing and circadian rhythms. Curr Opin Physiol. (2019) 10:64–9. 10.1016/j.cophys.2019.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. (2017) 25:86–92. 10.1016/j.cmet.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva BSA, Uzeloto JS, Lira FS, Pereira T, Coelho-E-Silva MJ, Caseiro A. Exercise as a peripheral circadian clock resynchronizer in vascular and skeletal muscle aging. Int J Environ Res Public Health. (2021) 18:12949. 10.3390/ijerph182412949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samuels C. Sleep, recovery, and performance: the new frontier in high-performance athletics. Neurol Clin. (2008) 26:169–80. 10.1016/j.ncl.2007.11.012 [DOI] [PubMed] [Google Scholar]
- 84.Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the american academy of sleep medicine and sleep research society. Sleep. (2015) 38:843–4. 10.5665/sleep.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Rawi N, Madkour M, Jahrami H, Salahat D, Alhasan F, BaHammam A, et al. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: a prospective observational study. PLoS One. (2020) 15:e0237922. 10.1371/journal.pone.0237922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bahammam A. Does Ramadan fasting affect sleep? Int J Clin Pract. (2006) 60:1631–7. 10.1111/j.1742-1241.2005.00811.x [DOI] [PubMed] [Google Scholar]
- 87.Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath Schlaf Atm. (2017) 21:577–86. 10.1007/s11325-017-1473-x [DOI] [PubMed] [Google Scholar]
- 88.Roky R, Iraki L, HajKhlifa R, Lakhdar Ghazal N, Hakkou F. Daytime alertness, mood, psychomotor performances, and oral temperature during Ramadan intermittent fasting. Ann Nutr Metab. (2000) 44:101–7. 10.1159/000012830 [DOI] [PubMed] [Google Scholar]
- 89.Almeneessier AS, BaHammam AS. How does diurnal intermittent fasting impact sleep, daytime sleepiness, and markers of the biological clock? Current insights. Nat Sci Sleep. (2018) 10:439–52. 10.2147/NSS.S165637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Med. (2009) 10:540–8. 10.1016/j.sleep.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the apnea positive pressure long-term efficacy study (APPLES). Sleep. (2012) 35:1593–602. 10.5665/sleep.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. (2017) 18:61–71. 10.1002/jgf2.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chasens ER, Sereika SM, Houze MP, Strollo PJ. Subjective and objective appraisal of activity in adults with obstructive sleep apnea. J Aging Res. (2011) 2011:751819. 10.4061/2011/751819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry. (1985) 42:225–32. 10.1001/archpsyc.1985.01790260019002 [DOI] [PubMed] [Google Scholar]
- 95.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. (1989) 262:1479–84. 10.1001/jama.262.11.1479 [DOI] [PubMed] [Google Scholar]
- 96.Kirkpatrick K, Balsam PD. Associative learning and timing. Curr Opin Behav Sci. (2016) 8:181–5. 10.1016/j.cobeha.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bäckman L, Nyberg L, Lindenberger U, Li S-C, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. (2006) 30:791–807. 10.1016/j.neubiorev.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 98.Dohrn I-M, Papenberg G, Winkler E, Welmer A-K. Impact of dopamine-related genetic variants on physical activity in old age- a cohort study. Int J Behav Nutr Phys Act. (2020) 17:68. 10.1186/s12966-020-00971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. (2011) 69:e113–25. 10.1016/j.biopsych.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Puig MV, Antzoulatos EG, Miller EK. Prefrontal dopamine in associative learning and memory. Neuroscience. (2014) 282:217–29. 10.1016/j.neuroscience.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paul V, Ekambaram P. Involvement of nitric oxide in learning & memory processes. Indian J Med Res. (2011) 133:471–8. [PMC free article] [PubMed] [Google Scholar]
- 102.Torregrossa AC, Aranke M, Bryan NS. Nitric oxide and geriatrics: implications in diagnostics and treatment of the elderly. J Geriatr Cardiol. (2011) 8:230–42. 10.3724/SP.J.1263.2011.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, Bernatoniene J. An overview of NO signaling pathways in aging. Molecules. (2021) 26:4533. 10.3390/molecules26154533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci. (2013) 3:39–53. 10.3390/brainsci3010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr. (2017) 60:180–6. 10.3164/jcbn.16-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Facioli TP, Buranello MC, Regueiro EMG, Basso-Vanelli RP, Durand MT. Effect of physical training on nitric oxide levels in patients with arterial hypertension: an integrative review. Int J Cardiovasc Sci. (2021) 35:253–64. 10.36660/ijcs.20200244 [DOI] [Google Scholar]
- 107.Krauzlis RJ, Wang L, Yu G, Katz LN. What is attention? Wiley Interdiscip Rev Cogn Sci. (2021) e1570. [Epub ahead of print]. 10.1002/wcs.1570 [DOI] [PubMed] [Google Scholar]
- 108.Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res. (2001) 10:85–92. 10.1046/j.1365-2869.2001.00245.x [DOI] [PubMed] [Google Scholar]
- 109.Drummond SPA, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. (2005) 140:211–23. 10.1016/j.pscychresns.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 110.Beebe DW, Difrancesco MW, Tlustos SJ, McNally KA, Holland SK. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct BBF. (2009) 5:9. 10.1186/1744-9081-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. (2004) 101:3316–21. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Angevaren M, Aufdemkampe G, Verhaar HJJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. (2008) 2:CD005381. 10.1002/14651858.CD005381.pub2 [DOI] [PubMed] [Google Scholar]
- 113.Hakkou F, Tazi A, Iraki L. Ramadan, health, and chronobiology. Chronobiol Int. (1994) 11:340–2. 10.3109/07420529409057250 [DOI] [PubMed] [Google Scholar]
- 114.El Moutawakil B, Hassounr S, Sibai M, Rafai MA, Fabrigoule C, Slassi I. N – 10 Impact du jeûn du Ramadan sur les fonctions attentionnelles. Rev. Neurol. (2007) 163:60. 10.1016/S0035-3787(07)90595-0 [DOI] [Google Scholar]
- 115.Blair C. Developmental science and executive function. Curr Dir Psychol Sci. (2016) 25:3–7. 10.1177/0963721415622634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harder-Lauridsen NM, Rosenberg A, Benatti FB, Damm JA, Thomsen C, Mortensen EL, et al. Ramadan model of intermittent fasting for 28 d had no major effect on body composition, glucose metabolism, or cognitive functions in healthy lean men. Nutr Burbank Los Angel Cty Calif. (2017) 37:92–103. 10.1016/j.nut.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 117.Agrigoroaei S, Lachman ME. Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. J Gerontol B Psychol Sci Soc Sci. (2011) 66(Suppl 1.):i130–40. 10.1093/geronb/gbr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weinstein AM, Voss MW, Prakash RS, Chaddock L, Szabo A, White SM, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. (2012) 26:811–9. 10.1016/j.bbi.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. (2013) 15:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiong J, Ye M, Wang L, Zheng G. Effects of physical exercise on executive function in cognitively healthy older adults: a systematic review and meta-analysis of randomized controlled trials: physical exercise for executive function. Int J Nurs Stud. (2021) 114:103810. 10.1016/j.ijnurstu.2020.103810 [DOI] [PubMed] [Google Scholar]
- 121.Ilieva IP, Hook CJ, Farah MJ. Prescription stimulants’ effects on healthy inhibitory control, working memory, and episodic memory: a Meta-analysis. J Cogn Neurosci. (2015) 27:1069–89. 10.1162/jocn_a_00776 [DOI] [PubMed] [Google Scholar]
- 122.Li L, Yu Q, Zhao W, Herold F, Cheval B, Kong Z, et al. Physical activity and inhibitory control: the mediating role of sleep quality and sleep efficiency. Brain Sci. (2021) 11:664. 10.3390/brainsci11050664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang CJ, Lin PC, Hung CL, Chang YK, Hung TM. Type of physical exercise and inhibitory function in older adults: an event-related potential study. Psychol Sport Exerc. (2014) 15:205–11. 10.1016/j.psychsport.2013.11.005 [DOI] [Google Scholar]
- 124.Canivet A, Albinet CT, Rodríguez-Ballesteros M, Chicherio C, Fagot D, André N, et al. Interaction between BDNF polymorphism and physical activity on inhibitory performance in the elderly without cognitive impairment. Front Hum Neurosci. (2017) 11:541. 10.3389/fnhum.2017.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marc A, Jaylene M. Reference Module in Neuroscience and Biobehavioral Psychology. Amsterdam: Elsevier; (2021). [Google Scholar]
- 126.Frederiksen KS, Verdelho A, Madureira S, Bazner H, O’Brien JT, Fazekas F, et al. Physical activity in the elderly is associated with improved executive function and processing speed: the Ladis study. Int J Geriatr Psychiatry. (2015) 30:744–50. 10.1002/gps.4220 [DOI] [PubMed] [Google Scholar]
- 127.Penke L, Maniega SM, Murray C, Gow AJ, Valdés Hernández MC, Clayden JD, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. (2010) 30:7569–74. 10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Edwards JD, Bart E, O’Connor ML, Cissell G. Ten years down the road: predictors of driving cessation. Gerontologist. (2010) 50:393–9. 10.1093/geront/gnp127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. (2007) 30:123–52. 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doniger GM, Simon ES, Zivotofsky AZ. Comprehensive computerized assessment of cognitive sequelae of a complete 12-16 hour fast. Behav Neurosci. (2006) 120:804–16. 10.1037/0735-7044.120.4.804 [DOI] [PubMed] [Google Scholar]
- 131.Dias GP, Murphy T, Stangl D, Ahmet S, Morisse B, Nix A, et al. Intermittent fasting enhances long-term memory consolidation, adult hippocampal neurogenesis, and expression of longevity gene klotho. Mol Psychiatry. (2021) 26:6365–79. 10.1038/s41380-021-01102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fabre C, Chamari K, Mucci P, Massé-Biron J, Préfaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. (2002) 23:415–21. 10.1055/s-2002-33735 [DOI] [PubMed] [Google Scholar]
- 133.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obes Silver Spring Md. (2006) 14:345–56. 10.1038/oby.2006.46 [DOI] [PubMed] [Google Scholar]
- 134.Gomez-Pinilla F. Collaborative effects of diet and exercise on cognitive enhancement. Nutr Health. (2011) 20:165–9. 10.1177/026010601102000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Åkerstedt T, Nisson KPM, Kecklund G. Sleep and recovery. In: Perrew PL, Ganster DC, Sonnentag S. editors. The Research in Occupational Stress and Well-Being: Current Perspectives on Job-Stress Recovery. (Vol. 7), Bingley: Emerald Group Publishing; (2009). p. 205–47. [Google Scholar]
- 136.Waheed W, Mirza N, Waheed MW, Malik A, Panagioti M. Developing and implementing guidelines on culturally adapting the Addenbrooke’s cognitive examination version III. (ACE-III): a qualitative illustration. BMC Psychiatry. (2020) 20:492. 10.1186/s12888-020-02893-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA. (1983) 249:2917–21. [PubMed] [Google Scholar]
- 138.Ortega RM, Requejo AM, Andrés P, López-Sobaler AM, Quintas ME, Redondo MR, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. (1997) 66:803–9. 10.1093/ajcn/66.4.803 [DOI] [PubMed] [Google Scholar]
- 139.Suhr JA, Hall J, Patterson SM, Niinistö RT. The relation of hydration status to cognitive performance in healthy older adults. Off J Int Organ Psychophysiol. (2004) 53:121–5. 10.1016/j.ijpsycho.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 140.Adan A. Cognitive performance and dehydration. J Am Coll Nutr. (2012) 31:71–8. 10.1080/07315724.2012.10720011 [DOI] [PubMed] [Google Scholar]
- 141.Tucker KL. Nutrient intake, nutritional status, and cognitive function with aging. Ann N Y Acad Sci. (2016) 1367:38–49. 10.1111/nyas.13062 [DOI] [PubMed] [Google Scholar]
- 142.Wittbrodt MT, Millard-Stafford M. Dehydration impairs cognitive performance: a meta-analysis. Med Sci Sports Exerc. (2018) 50:2360–8. 10.1249/MSS.0000000000001682 [DOI] [PubMed] [Google Scholar]
- 143.Philippou E, Constantinou M. The influence of glycemic index on cognitive functioning: a systematic review of the evidence. Adv Nutr Bethesda Md. (2014) 5:119–30. 10.3945/an.113.004960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Garfield V, Farmaki AE, Fatemifar G, Eastwood SV, Mathur R, Rentsch CT, et al. Relationship between glycemia and cognitive function, structural brain outcomes, and dementia: a mendelian randomization study in the UK biobank. Diabetes. (2021) 70:2313–21. 10.2337/db20-0895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.