Abstract
The role of metals in forming the primary cross-links in slug glue was investigated. Several metal-binding proteins were identified in the defensive glue produced by the slug Arion subfuscus. Notably, the C-lectins that are unique to the glue are iron-binding proteins. This is unusual for C-lectins. Dissociating these proteins from iron does not affect the glue's stiffness. Similarly, several proteins that can bind to zinc were identified, but dissociating the proteins from zinc did not weaken the glue. These results suggest that metal coordination is not involved in the primary cross-links of this hydrogel glue. The stable cross-links that provide stiffness are more likely to be created by a catalytic event involving protein oxidation. Cross-linking was unexpectedly difficult to prevent. Collecting the glue into a large volume of ice-cold buffer with reagents aimed at inhibiting oxidative cross-linking caused a slight loss of cross-linking, as demonstrated by the appearance of uncross-linked proteins in native gel electrophoresis. Notable among these was a protein that is normally heavily oxidized (asmp165). Nevertheless, this effect was not large, suggesting that the primary cross-links form before secretion.
Keywords: C-lectin, hydrogel, adhesive, iron, transition metals, protein oxidation
1. Background
The slug Arion subfuscus produces an unusual glue [1]. Like most slugs, it produces a lubricating mucus and a slightly tacky pedal mucus for transient adhesion during locomotion [2]. In addition, A. subfuscus can release a strongly adhesive defensive secretion from its back. When touched, the slug secretes a large volume of this glue as a dilute, watery gel over the entire dorsal surface. This secretion immediately adheres to any surface that contacts it and sets within seconds into a tough, seemingly elastomeric material [3].
The mechanics of the glue have been described, showing that the glue gains its toughness from a double network mechanism [3,4]. Like most forms of molluscan mucus, the glue contains long, entangled polyanions that provide a highly deformable network. This deformable network allows the glue to be stretched extensively before failure. In addition, there is a second, interpenetrating network of cross-linked proteins that provide stiffness [4]. Thus, the glue is stiff, but must be stretched extensively before failure, making it difficult to fracture. During this deformation, the cross-links rupture, acting as sacrificial bonds to dissipate energy [3].
The nature of these sacrificial cross-links is important, as they play such a central role in the glue's performance. They appear to form rapidly, and they are strong and numerous enough to create a high stiffness despite the fact that the glue is roughly 97% water [1]. The primary cross-links are stable, with an estimated bond lifetime greater than 10 min [3]. The bonds also appear capable of reforming after being ruptured [3]. Their rapid formation, stability and ability to reform makes them ideal sacrificial bonds. Understanding the nature of these bonds would be valuable for the design of similar, bioinspired materials.
There are several types of bonds connecting the polymers in the slug's glue, but it is unclear which are the main sacrificial bonds. The most likely candidates are metal-dependent [5]. There are abundant metal ions in the glue, especially calcium and magnesium (10–40 mM) as well as transition metals such as iron, zinc and copper (0.1–1 mM) [6,7]. In addition, most of the proteins in the glue have metal-binding domains [8]. There are matrilin-like proteins (asmp44, asmp57a, asmp61, asmp114 and asmp165) with multiple von Willebrand factor A (VWA) domains and epidermal growth factor (EGF) repeats [8], both of which are known to bind divalent ions such as calcium [9,10]. There are also C-lectins (asmp15a,b,c,f), which typically use calcium to bind to ligands [8]. Additionally, a form of catalase is unusually abundant, which typically uses an iron-binding haem group in its catalytic domain [11], and haemocyanin is present, which is known to bind copper. One of the matrilins (asmp57a) has an extensive histidine-rich repeat, which makes it likely to bind to zinc. Nevertheless, although there are abundant metal-binding domains, it is unknown if they bind to metals and if this plays a role in the mechanics of the glue.
Removal of metal ions weakens or helps solubilize the glue [7,12], suggesting that these ions contribute directly. In this case, the cross-links could arise from a metal ion interacting with ligands on two different proteins, thus holding the two proteins together. This type of interaction is important in several other biological adhesive systems. Caddisfly larvae and tube-building marine worms of the genus Sabellaria use magnesium and calcium to link phosphate-rich proteins into a cement [13,14]. Mussels use several different types of coordinate covalent bonds between metals and proteins. For example, iron–DOPA interactions cross-link the protective coating on the byssal threads [15], iron and calcium link plaque proteins [16] and zinc and copper link the collagenous thread fibres [17]. Zinc has been shown to harden other biomaterials such as the teeth of nereid worms [18]. In the development of synthetic materials, these kind of coordinate covalent interactions are highly effective in creating tough gels [19].
In slug glue, however, there is evidence that the primary sacrificial bonds do not consist of metal ions that directly link proteins together. Calcium and magnesium diffuse out of the glue easily, making it unlikely that they constitute the stable cross-links that stiffen the glue [3]. Instead, calcium and magnesium may serve primarily to balance the charge on the polyanionic carbohydrates, allowing them to take a much more compact conformation. This would be essential for the carbohydrates to provide the hidden length and extensibility necessary for the double network mechanism [3]. Additionally, while metal removal weakens the glue, it does not appear to disrupt the main cross-links that hold the proteins in large complexes [4], and attempts at iron chelation with deferoxamine have no effect on the glue after it sets [6]. Nevertheless, these experiments did not rule out strongly bound zinc or iron as the primary cross-link.
The primary alternative to direct involvement of metal ions in the cross-links is that the metal ions may catalyse cross-link formation. Some metal ions can catalyse oxidation of specific functional groups, converting them into groups that readily form stable covalent bonds. In the byssal apparatus of mussels, oxidation of the amino acid dopa creates dopa quinones that can form a number of different cross-links to solidify the byssus [17,20]. Similarly, insect cuticle hardening is catalysed by oxidation of phenolic groups to quinones using the copper enzyme laccase 2 [21], and the final solidification of tubeworm cement is triggered by the action of the copper enzyme catechol oxidase [14]. Finally, barnacle cement has been shown to contain the copper enzyme lysyl oxidase, and one of the cement proteins is oxidized [22]. While most of the focus on oxidation reactions in biological glues involves catechol moieties, many other groups can be oxidized [23]. The side chain of lysine is a common substrate for oxidation to a carbonyl group [24], which readily forms cross-links with nucleophilic functional groups. Several other amino acid side chains can be similarly oxidized [23].
Protein oxidation has been implicated in slug glue. Several proteins in slug glue are oxidized, with asmp165 being very heavily oxidized [25]. Slug glue is weakened by reagents that compete for carbonyls and thus disrupt the bonds created by protein oxidation [4,7]. Iron chelation also prevents the non-specific gel-stiffening activity of glue-specific proteins [6]. Thus, there is good evidence that metal-catalysed oxidation contributes to slug glue cross-linking, but it is still uncertain whether oxidation is responsible for the primary cross-links that form sacrificial bonds, or whether oxidation creates secondary links with other roles, and direct metal bridges involving metal coordination form the primary cross-links. Additionally, previous work analysed homogenized glue that had been diluted by a factor of 30 or used strong treatments that would be likely to have secondary effects on glue structure in addition to targeting specific bonds.
The goal of this paper is to identify the metal-binding proteins in the glue and determine if they play a direct role in cross-linking. It will analyse intact glue and use targeted treatments to identify which type of cross-link forms the primary sacrificial bonds responsible for the slug glue's stiffness.
2. Methods
Samples of slug glue were collected and stored as described previously [3]. Briefly, the slug's dorsal surface was rubbed with a thin metal spatula, causing the animal to release copious amounts of the defensive secretion. This was collected on the spatula and stored at −80°C in a microcentrifuge tube. Each sample represented the glue from one individual.
2.1. Identification of metal-binding proteins
Zinc-binding proteins in the glue were identified using Hi Trap IMAC FF (Immobilized Metal Affinity Chromatography, Fast Flow) beads (Cytiva). One millilitre columns were charged with 0.1 M ZnCl2 following the manufacturer's instructions. The column was then equilibrated with five column volumes of loading/binding buffer. In the first trials a denaturing loading buffer was used (8 M urea, 500 mM NaCl, 20 mM phosphate buffered saline, pH 7.5) while later experiments used the same buffer without urea. Glue was dissolved in loading buffer using a rotor-stator homogenizer followed by brief sonication on ice (three pulses of 10 s each). The dissolved samples were centrifuged at 14 000g for 10 min and the cleared extract was loaded on the column. The column was washed with five column volumes of loading buffer, then eluted with loading buffer containing 0.2 M imidazole. Column elution profiles were monitored by UV absorbance. UV-absorbing fractions were assayed with discontinuous sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) [26]. Most gels used a standard Tris–Cl buffer system in the gel with a sample loading buffer of 0.125 M Tris–Cl, pH 6.8, 4% SDS, 10% 2-mercaptoethanol, 10% glycerol and total acrylamide concentrations of 7.5% or 12.5%. For 15% gels, a Tris–Tricine buffer system was used. Gels were stained with Coomassie Blue R-250.
Iron-binding proteins were identified using Phos-select iron affinity beads (Sigma Aldrich). These beads use bound Fe3+ and thus would detect proteins with a high affinity for Fe3+. These columns may also detect Fe2+-binding proteins, but such proteins may be more likely to bind to the Zn2+ columns, since both Zn2+ and Fe2+ are considered borderline metal ions in the hard-soft Lewis acid classification and they share the same ligands. Also, it should be noted that proteins that already have a strongly bound metal ion in their binding centre might not bind to the metal on the column. This may be the case for an enzyme such as catalase. Nevertheless, this experiment was only looking for proteins involved in cross-links, and if a metal ion was involved in a cross-link, at least one polymer in the glue must be able to bind to it.
For the iron-binding experiments, a batch protocol was used rather than columns, due to the tendency for the proteins to clog iron-charged columns. In the batch procedure, beads were washed with buffer or sample, then centrifuged to allow removal of buffer from the sedimented beads. Each wash consisted of the addition of roughly two volumes of buffer to the beads, brief vortexing, mixing for 2 min with repeated inversion on a rotator, then centrifuging at 700g for 30 s and removing the supernatant. Before use, the beads were washed this way three times using 0.15 M acetic acid. Slug glue was dissolved in 0.15 M acetic acid and the extract added to the beads, vortexed and incubated with repeated inversion for 15 min. The beads with bound proteins were then washed three more times with 0.15 M acetic acid and the bound proteins were then released with 0.33 ml of elution buffer. Initial experiments used an elution buffer of ammonium hydroxide (400 mM, pH 11.3). Follow-up experiments tested a range of pH values. In these, the beads were eluted with sequentially increasing pH values of 8.1, 9.0, 10.1 and 11.3 (two washes at each pH). These trials were performed with both 400 and 40 mM ammonium-Cl buffers. Similar experiments were performed with Tris–Cl buffers (20 mM, pH 7.4, 8.0 and 9.0). After all experiments, some beads were washed with SDS–PAGE sample buffer to determine if there were any proteins that still bound after elution with high pH.
The main bands on SDS–PAGE containing iron-binding proteins were excised and identified by tandem mass spectrometry (Taplin mass spectrometry facility, Harvard University). There, in-gel tryptic digests and microcapillary liquid chromatography followed by tandem mass spectrometry (LC–MS/MS) were performed (see electronic supplementary material for details). The identified peptides were used to search the database of translated polypeptides generated from a previously created transcriptome for this species [8]. Most of the matches were for proteins that had been previously characterized [8]. Spectral counts were used to distinguish the primary proteins in each band from minor contaminants.
2.2. Measurement of zinc binding at different pH values
The zinc-binding ability of the polymers in the glue was characterized by measuring the amount of zinc released from the glue at different pH values [3]. Glue samples were cut into seven pieces of roughly 2 × 2 × 2 mm. For each sample, one of the pieces was soaked in 0.7% nitric acid to determine the total zinc concentration, while the others were soaked in sodium acetate buffers at different pH values, to determine how much of the zinc was removed at that pH. The starting mass of each was recorded and each sample was soaked for 1 h with gentle agitation in 1 ml of 50 mM sodium acetate buffer at a specific pH (ranging from 2.9 to 7.4) or 0.7% nitric acid. After 1 h, the solid glue was removed and the bathing solution, containing any ions that dissociated, was diluted into 0.7% nitric acid. The zinc concentration of this bathing solution was measured using an atomic absorption spectrometer (Shimadzu AA-6300). The dilutions and original mass of the piece of glue were used to calculate the amount of zinc removed from the glue relative to the total. The spectrometer was calibrated with a zinc standard (Agilent, ICP-030), and blanks containing only the buffers diluted in 0.7% nitric acid were measured.
2.3. Measurement of glue stiffness at different pH values
The stiffness of the glue after incubation at different pH values was measured using a simple, custom-built uniaxial tensometer described previously [4]. Samples for mechanical testing were prepared as described previously [4]. Each sample was from a homogeneous mass of glue produced at one time from an individual slug and allowed to set into the rough shape of a ball. Because the glue is a very sticky, deformable hydrogel, test strips from these samples were cut while partially frozen. A pair of joined, parallel razor blades were used to cut the sample. The first cut created a 1.6 mm thick disc of glue. Two 1.6 × 1.6 mm strips were cut from this disc. After soaking, the samples were notably swollen and had a slippery exterior. Refreezing the strips before putting them in the clamps significantly improved the ability of the clamps to form a solid grip that was maintained after thawing. Previous work showed that freezing and thawing the samples did not detectably affect their mechanics [3]. The tensometer grips were also lined with superfine (600 grit) waterproof sandpaper to better hold the samples.
Samples were tested at pH values surrounding 9, because this is the pH at which the iron-binding proteins were found to dissociate. Previous work had already covered the range from 2.9 to 7.4 [3]. A total of nine glue samples were tested. Two test strips from each sample were compared; one was tested at pH 8 or 8.4 and the other at pH 9 or 10. The test strips were soaked for 10 min in buffer (40 mM Tris–Cl for pH 8 and 9, or 40 mM ammonium bicarbonate or ammonium hydroxide for pH 8.4 and 10). The buffer volume was roughly 100–300 times greater than the sample volume. The pH of the bathing buffer was checked after soaking most samples. This value was used in presenting the results because the samples' buffering capacity slightly reduced the pH of the pH 10 buffer.
The force required to reach a given extension was measured rather than the stress. This eliminated the effect of changes in cross-sectional area due to water uptake that can create a lower effective modulus without breaking cross-links. For data presentation, the forces were divided by the initial cross-sectional area, which was the same in every case. This gave a stress value that could be more readily compared between studies. Once the sample was clamped into the tensometer, the sample was extended until straight and the length between clamps was measured with calipers. Samples were then stretched to a strain of two to four (300–500% of original length) at a strain rate of roughly 0.3 s−1. The elastic modulus was calculated by regression on the linear region of the stress–strain curve.
2.4. Blocking catalytic cross-linking after secretion
To investigate whether catalytic cross-linking occurred after secretion of the glue, samples were collected directly into ice-cold buffer with inhibitors present in an attempt to block metalloenzymes. Glue was collected by finding undisturbed slugs and immediately immersing them into 5 ml of ice-cold buffer. The buffer consisted of Tris acetate (20 mM Tris, pH 7.5) and NaCl (200 mm) with one of several different treatments: hydroxylamine (0.15, 1.5, 15 or 150 mM), ethylenediaminetetraacetic acid (EDTA, 10 mM), deferoxamine (10 mM), some combination of these or no added treatments. The hydroxylamine was adjusted to pH 7.5 before mixing. As a control, some samples were collected into ambient temperature Tris acetate buffer with NaCl and no other reagents (roughly 20°C). At least three trials in each treatment were performed. Once the slug was in the tube, it was shaken to trigger secretion, and immediately returned to ice (except for the controls). Collecting and shaking the slugs led to massive release of glue, which mixed with the buffer and turned the entire volume into a loose gel. After roughly 15–20 min, all the samples were frozen and stored for analysis later that day or the next. For comparison, samples that had been allowed to set normally were also tested.
To see if proteins were or were not cross-linked, samples were analysed by native gel electrophoresis. Large, cross-linked complexes will not enter a non-denaturing gel, while uncross-linked protein may enter the gel and resolve as bands depending on the protein's structure. For electrophoresis, the samples were allowed to thaw partially so that a portion could be excised and mixed with an equal volume of 2× reducing native electrophoresis sample buffer (Tris acetate 80 mM, 10% 2-mercaptoethanol with glycerol and bromophenol blue). This was placed on ice and sonicated (4×, 10 s each) to break up polysaccharides, which otherwise create a loose gel. Native gel electrophoresis was performed with a Tris acetate buffer (40 mM Tris) at pH 7.5 on 10% polyacrylamide gels as previously described [4]. Under these conditions, only anionic proteins will run on the gel. Bovine serum albumin (0.1 mg ml−1) was run on most gels as a positive control to demonstrate clear band formation. In addition, some of each sample was reserved and urea was added to a concentration of 8 M in order to disrupt the secondary structure of fibrous proteins that might not normally enter a native gel even when they have not been cross-linked. Samples with urea were left overnight at room temperature before running on native gels. While gels were typically stained with Coomassie Blue R-250, several were stained with Stains-All [4] to see if any of the bands contained the sulfated glycosaminoglycans that make up a large fraction of the glue.
The identity of proteins in the gel bands was determined by second dimension SDS–PAGE following the native gel. Slices of unfixed native gel from the region containing bands (as determined by a Coomassie-stained guide lane run in parallel) were minced and mixed with 100 µl of 2× SDS–PAGE sample buffer, then heated with occasional vortexing at 60°C for 10 min. Then the extract from these was loaded onto a 12.5% SDS polyacrylamide gel. The gel was stained with Sypro Ruby. Several bands were also analysed by tandem mass spectrometry as described previously.
The mechanical properties of some gels collected directly into buffer were also measured. Glue samples collected directly into ice-cold Tris-acetate NaCl were compared with glue samples collected into ambient temperature Tris-acetate NaCl. The samples used for mechanical tests were not frozen and instead were collected and kept on ice for roughly 30 min before measurement. The mechanics were measured with a dynamic mechanical analyser (TA Instruments AR550) at 5% strain and 10 rad s−1. A Peltier plate was used to keep the temperature of all samples at 4°C during testing.
3. Results
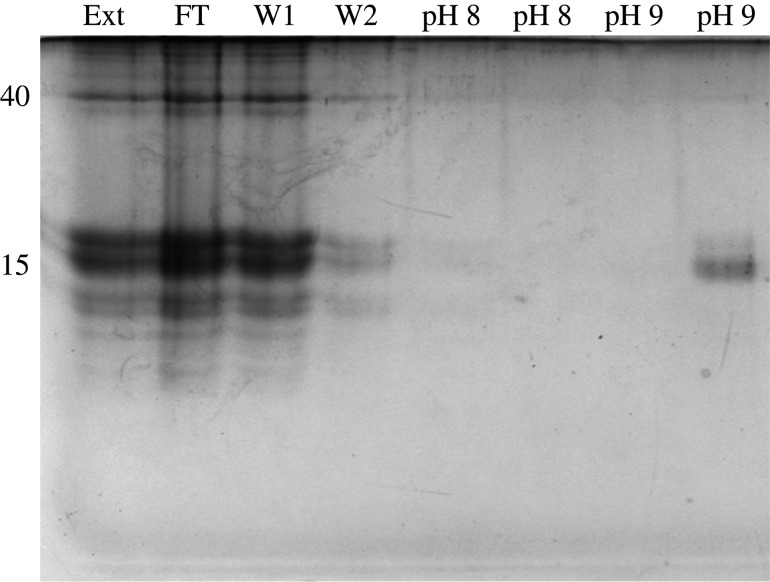
A group of proteins of 15–16 kDa bound to iron (Fe3+) on the Phos-select beads (figure 1). These proteins consistently dissociated at a pH of 9. No additional proteins eluted with higher pH washes or with SDS. The band containing the iron-binding proteins was excised after two separate experiments and the proteins were identified by LC–MS/MS using a transcriptome that had been previously generated [8]. The iron-binding proteins were the C-lectins that had been identified in previous work. Based on peptide counts, the most abundant proteins in this band, in order, were asmp15b, asmp15c, asmp15a, asmp15f and a previously unidentified protein that, like these other proteins, consisted entirely of a C-lectin domain (electronic supplementary material). The total peptide counts for each of these proteins ranged from 77 to 280. The next most abundant protein was asmp15d (total peptide count = 24), which is a C1q domain-containing protein in the same size range. None of the matrilin-like proteins bound to iron.
Figure 1.
Identification of iron-binding proteins by immobilized metal affinity chromatography. The lanes are Ext (original dissolved extract), FT (flow through, unbound material), W1 and W2 (first two washes), then two washes each at pH 8 and 9. The numbers on the left identify the molecular mass of two prominent bands.
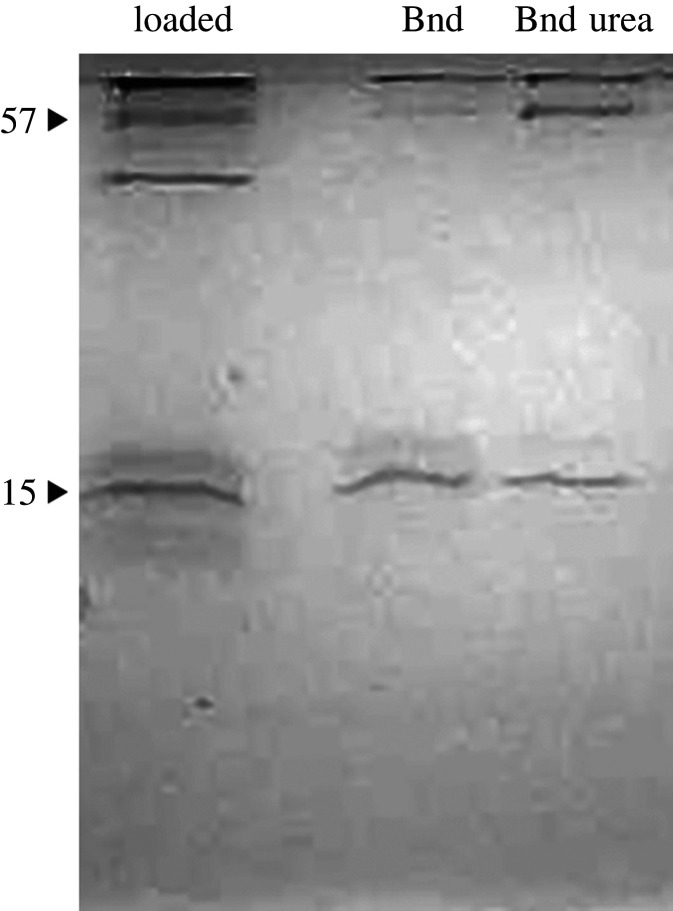
There were several different zinc-binding proteins (figure 2). One had an apparent mass of 57 kDa, though it was not clear whether this was asmp57a (a glycine- and histidine-rich matrilin-like protein), or asmp57b (catalase). In addition, the iron-binding C-lectins may also bind to zinc. Finally, asmp203 (haemocyanin-like) bound to the zinc beads.
Figure 2.
Identification of zinc-binding proteins by immobilized metal affinity chromatography. The left-most lane shows the original extract that was loaded. The two lanes on the right show the proteins that bound to the zinc in regular loading buffer, and loading buffer with 8 M urea.
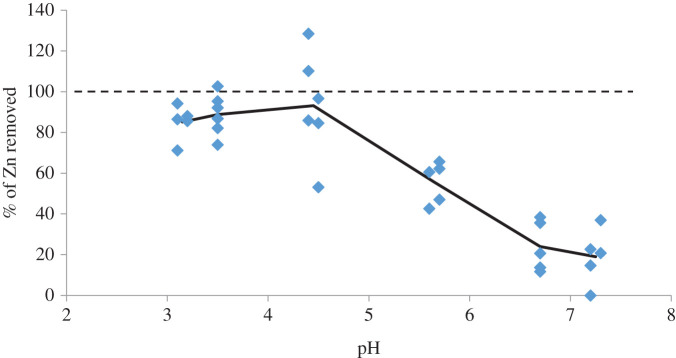
Because zinc is more abundant than iron in the glue, it was possible to measure the conditions under which zinc dissociates from intact glue using atomic absorption spectroscopy (figure 3). Most of the zinc remained bound to the glue at pH values of 6.7 and 7.2. At a pH of 5.7, however, roughly half of the zinc dissociated and diffused out of the glue within an hour. Essentially all the zinc dissociated and diffused out of the glue over the same time span at pH values of 4.5 and lower.
Figure 3.
The effect of pH on zinc binding in the glue. The amount of zinc dissociating from the glue relative to the original amount was measured using atomic absorption spectroscopy.
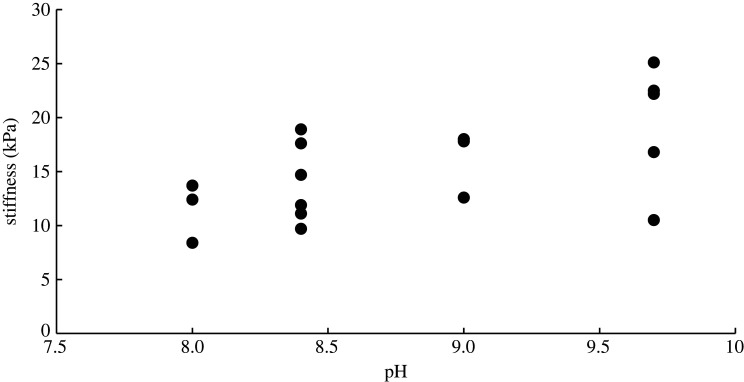
Knowing the pH at which the metal-binding proteins and metals dissociate, it was possible to test their importance to the glue's mechanics. Previously published work showed that slug glue is not weakened as the pH changes from 7.4 to 4.4 [3]. The present study showed that these are the pH values where the zinc-binding proteins go from fully bound to fully dissociated (figure 3). Similarly, the present work found that the modulus of the glue did not change across the pH range where iron changed from fully bound (pH 8) to fully dissociated (pH 10) (figure 4). The average stiffnesses at each pH value were 11.5 ± 2.8 kPa at pH 8 (N = 3), 14.0 ± 3.7 kPa at pH 8.4 (N = 6), 16.1 ± 3.1 kPa at pH 9 (N = 3) and 19.4 ± 5.8 kPa at pH 9.7 (N = 5). There was no significant difference among these average values (ANOVA, p = 0.10). The glue is clearly not becoming weaker as the iron-binding proteins dissociate at higher pH.
Figure 4.
The effect of pH on the stiffness of the glue. Stiffness was measured on intact strips soaked in buffer of different pH values using a uniaxial tensometer. The iron-binding proteins dissociate from iron at pH 9.
Collecting the glue directly into ice-cold Tris acetate buffer did not lead to a change in mechanical properties relative to collecting into room temperature buffer. The four samples tested after ambient temperature collection had a storage modulus of 12.5 Pa ± 8.2 and loss modulus of 4.3 ± 2.7 (average ± s.d.) while those collected into ice-cold buffer had a storage modulus of 11.5 ± 1.9 and a loss modulus of 4.0 ± 3.0 (t-test, N = 4, p = 0.41 for storage modulus). In both cases, the material was a highly deformable gel but was notably more elastic than viscous. The material could stretch extensively but would not break and returned to its original configuration with little visible hysteresis once the stress was removed. This is noteworthy given that the slugs typically produce 50–100 mg of glue when stimulated and collecting this secretion into 5 ml represents a 50- to 100-fold dilution.
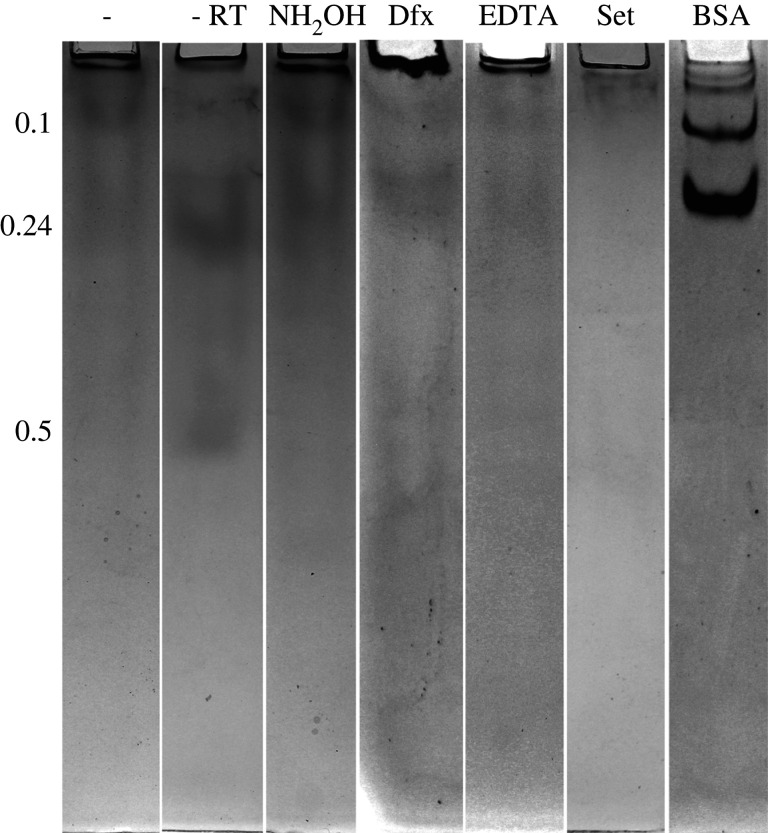
Glue collected directly into a relatively large volume of buffer was slightly less cross-linked than glue that had been allowed to set after collecting normally (figure 5). None of the treatments, however, were able to prevent cross-linking sufficiently to lead to consistently clear, dark bands on the native gel. In most experiments, several faint, distorted bands were visible on the native gel, but the intensity of staining varied considerably even within treatments. The bands were at relative mobilities of roughly 0.1, 0.24 and 0.5 (distance relative to the bottom of the gel). The highest hydroxylamine concentration (150 mM) led to the appearance of notably darker, but still blurred bands and a few sharp but faint bands just above the band at 0.24 (data not shown). Lower hydroxylamine concentrations, EDTA and deferoxamine all led to the presence of the same bands, but not to a substantially greater extent than controls without these reagents or glue collected into ambient temperature buffer (figure 5). In one trial with 10 mM deferoxamine, however, there were clear dark bands similar in clarity and intensity to the BSA control (electronic supplementary material, figure S1). This result was the only one of 16 trials with deferoxamine that gave such clear bands. When 8 M urea was used to disrupt secondary structure, the primary difference was the appearance of a band with very low mobility near the top of the gel. This band was equally present in all samples treated with urea. BSA always ran as a series of sharp, dark bands corresponding to the monomer and oligomers of this protein.
Figure 5.
Native gel electrophoresis of samples collected and rapidly dispersed directly into Tris-acetate NaCl buffer with different treatments. All samples were collected into ice-cold buffer except as noted. The relative mobilities of bands that consistently appeared are shown on the left. Samples in lanes 1, 3 and 5 were treated with 8 M urea before loading on the gel. Lane 1 is the negative control (with urea), lane 2 is the negative control in warm buffer, lane 3 also had 15 mM NH2OH (with urea), lane 4 had 10 mM deferoxamine, lane 5 had 10 mM EDTA (with urea), lane 6 had glue that was allowed to set into a solid, then dissolved in the buffer, lane 7 is BSA.
Second dimension SDS–PAGE identified several proteins that were able to enter the native gel when collected into ice-cold buffer (figure 6). The most consistent was asmp165, which always showed up in a distinct region encompassing several millimetres near the top of the gel, but only rarely formed a band. When it did, it was the band at a relative mobility of roughly 0.1. Asmp40 was also often present in the gel but did not correspond to any clear bands and was also present on the native gel when glue that had already been set was analysed. The region containing the larger, heavily distorted band at a relative mobility of roughly 0.24 contained several proteins in the 57–61 kDa size range, a band in the 15 kDa size range and some other faint bands, but none of these were particularly strong or consistent. The region in the middle of the gel where the final blurred band showed up in some samples had no proteins detected in the second dimension SDS–PAGE. All of the negatively charged proteins were highly abundant at the top boundary of the gel, showing that most failed to enter the gel (figure 6). It was not possible to clearly differentiate the contents of the urea-dependent band from the material at the top of the gel. Stains-All staining confirmed that none of the bands on the native gel contained polyanionic polysaccharides, which were always found at the top of the lane, failing to penetrate into the gel.
Figure 6.
Second dimension SDS–PAGE of bands that appeared on native gels after glue was collected directly into cold buffer. The numbers across the top show the relative mobilities of bands on the native gel that were analysed by 2D PAGE. The left-most lane (Top) is taken from a strip less than 1 mm thick (0.01 relative mobility) shaved from the top of the gel (bottom of the loading well). The numbers down the left side show the mass in kDa of selected proteins on SDS–PAGE.
Tandem mass spectrometry (electronic supplementary material) confirmed that the bands with a relative mobility of roughly 0.1 contained mostly asmp165 and also asmp57b (catalase). By contrast, the blurred, distorted band at a relative mobility near 0.24 contained relatively few peptides corresponding to any sequences in the transcriptome. The most abundant protein identified in this band was a peritrophin-domain-containing protein (predicted 21 kDa), but the peptide counts were low (34) and not notably higher than those from unrelated proteins in this band. For comparison there were 606 total peptides in the band containing Asmp165. Finally, in some experiments, there were a series of faint bands that formed a ladder-like pattern in the bottom half of the gel starting at a relative mobility of 0.5, spaced at equal intervals with similar staining intensity. Three of the faint bands on the bottom half of the gel were analysed by MS/MS and all consisted of previously unidentified EF-hand-containing proteins (11.5–11.8 kDa). These were moderately abundant (60–122 total peptides mapped).
4. Discussion
Although many proteins in slug glue have metal-binding domains, the only iron-binding proteins were the C-lectins (asmp15a–c,f). C-lectins as a whole are not known for iron binding [27], so this study demonstrates an interesting variation of this type of protein. These particular lectins appear to have been modified to bind to iron as part of their function in the glue. That function, however, does not appear to be as part of the cross-linked network. If iron-binding did form the primary cross-links, then at pH values where the C-lectins dissociate from iron, the modulus would be expected to drop drastically. Previous work demonstrated that breaking the primary cross-links can lead to a decrease in modulus of greater than 90% [3]. Nevertheless, at pH values where the C-lectins dissociate from iron, the glue does not become significantly weaker. Thus, coordinate covalent cross-linking by iron does not appear to play a central role in the cohesive strength of the glue. This is consistent with the relatively low abundance of iron (approx. 0.1 mM). Furthermore, the C-lectins are surface-binding adhesive proteins [28] that are not part of the cross-linked network [4]. The iron is not involved in adhesive interactions, since EDTA does not affect the ability of the C-lectins to bind to surfaces [28]. Given the fact that EDTA helps dissociate the bulk glue proteins from the adhesive proteins [28], it seems likely that the iron is involved in linking the adhesive proteins to the bulk of the glue.
There are multiple zinc-binding proteins in the glue including the C-lectins, haemocyanin and asmp57 (either a or b), but zinc does not form the main cross-links in the glue. Previously published mechanical tests from neutral to acidic pH found that at pH 7.4, 6.4, 5.5 and 4.4, the modulus was 8.2, 9.7, 11.6 and 8.1 kPa, respectively [3]. The atomic absorption spectroscopy experiments in the present work showed that most of the zinc would be bound at pH 7.4, roughly half would be dissociated at pH 5.5 and essentially all would be dissociated at pH 4.4, the lack of a mechanical effect when the zinc goes from fully bound to fully dissociated makes it unlikely that zinc forms the primary cross-links. Because calcium and magnesium have also been ruled out as the main cross-links [3], it appears that the primary sacrificial bonds in slug glue are not coordinate covalent bonds involving any of these ions. These ions will presumably have some effect on gel mechanics, because of their abundance and charge, but it does not seem to be as the main cross-link. Performing further dynamic mechanical testing across a range of strain rates under different conditions may provide insight into the nature of these interactions. Previous work found that the elastic component of the glue's modulus was substantially greater than the viscous component when tested across a wide range of strain rates (0.01–50 rad s−1) [3], but further work on freshly collected glue could provide deeper insight.
Having ruled out coordinate interactions, the most likely type of bond to form the primary cross-links are dynamic covalent bonds resulting from protein oxidation [25]. Nevertheless, this study was not able to demonstrate unequivocally that such bonds form the primary cross-links. Attempting to block cross-linking was only partially successful, leading to the presence of some faint, distorted bands. All of the treatments aimed at blocking catalytic cross-linking were able to do so to some extent but not reliably more than controls collected similarly. On the other hand, this rapid dispersal in buffer did lead to a reduction in cross-links relative to glue that had already set. With glue that had already set, few proteins were able to enter the native gel and no clear bands were formed, whereas glue collected directly into buffer often led to blurry band formation and several proteins entering the native gel.
In a few cases, these bands were quite clear (electronic supplementary material, figure S1), but this was not consistent. Higher concentrations of hydroxylamine (150 mM) also led to clearer, darker banding, but this concentration is high enough that it could be acting as a competitor that disrupts existing bonds rather than preventing their formation. Since hydroxylamine inhibits catalase and other haem enzymes at concentrations well below 1 mM [29], it is unlikely that the effect of 150 mM hydroxylamine is due to enzyme inhibition when 15 mM hydroxylamine did not have this effect. An effect of hydroxylamine as a competitor, disrupting existing bonds, would be consistent with previous work [4]. Hydroxylamine is a potent nucleophile that would readily bind to carbonyl groups that result from protein oxidation [25]. As Schiff bases formed from these carbonyls periodically dissociate, hydroxylamine could displace the normal ligand and prevent bond reformation.
Notably, one of the bands that appeared when glue was collected directly into buffer contained asmp165. The fact that this protein was typically present in a well-defined area of the gel without always forming bands could be explained by incomplete dissociation from other proteins, partial disruption of fibrous secondary structure and/or variation in extent of oxidation. Since most oxidation events replace positively charged lysine residues with uncharged carbonyls [24], variation in the extent of oxidation would create variation in charge and thus blurring of the band on a native gel. These effects would not arise on a denaturing gel because SDS would provide uniform negative charge and completely disrupt secondary structure and other interactions. Notably, in the one trial where near complete failure to cross-link occurred (electronic supplementary material, figure S1), this band at a relative mobility of 0.1 containing asmp165 was the clearest and darkest band.
The identification of asmp165 in these experiments suggests a possible cross-linking role for this protein. This is consistent with the fact that it is the most heavily oxidized protein in the glue; it is eight times more oxidized than any other protein [25]. It is also one of the proteins that has not been assigned to an adhesive function. Of the other matrilin-like proteins, asmp44, asmp61 and asmp114 are all adhesive. These proteins adhere strongly to a wide variety of surfaces [28], making it unsurprising that they were not resolved by the native gel. The predicted structure of asmp165 matches what we would expect for a cross-linked protein. Previous research found that this protein had three regions, an N-terminal region with no known homology, a middle region with two VWA domains, and a C-terminal region with multiple EGF repeats [8]. Looking further into this with BLAST [30], the middle third of the protein has greatest similarity to collagen VI (35% identity to a region of that large protein). The C-terminal third has greatest similarity to fibrillin 1 or 2 (52% identity to a region of that large protein). These are common components of the extracellular matrix. Fibrillin is particularly interesting because it typically forms the structural component of microfibres [31]. These fibrillin microfibres are often associated with elastin to form elastic fibres [31]. Collagen VI can form microfibres as well [32].
The lack of a dramatic effect of collecting the glue into ice with reagents aimed to inhibit cross-linking suggests that the key reactions may happen before secretion. Rather than secreting precursors that are then enzymatically modified once in place, it is possible that the glue becomes cross-linked before it is secreted. Alternatively, key oxidative reactions may occur immediately before or right at the time of secretion, but cross-link formation involving the newly created functional groups may then form after, even at cold temperature. In such a scenario, it may be possible to inhibit some cross-linking, but very difficult or rare to block all of the cross-linking. Arguing against this alternative is the relative lack of effect of low concentrations of hydroxylamine during secretion, which should aggressively interact with carbonyls before they form cross-links. While there was one trial where cross-linking appeared to be completely blocked (electronic supplementary material, figure S1), the weight of evidence suggests that this was due to a relatively rare set of conditions. It is plausible that this trial was due to an animal that was not fully prepared to secrete the glue. It was noted that the slugs appear to become darker before secretion, and this could reflect an oxidation within the gland. Nevertheless, forming the primary cross-links in advance of secretion would seem to complicate the process of glue formation, since most traditional glues start out in liquid form and only set once they are in place. These findings are intriguing and will be explored further in a follow-up paper analysing the secretory system and the timing of cross-linking.
Although zinc is more abundant than iron in the glue, it is still unclear what its function is, since its removal had no mechanical effect. It is possible that the C-lectins and haemocyanin only bind available zinc as a side effect of their other metal-binding ability. The proteins identified as zinc binding may have good metal-binding domains that are designed for calcium, iron or copper, but may also be capable of binding to zinc if it is present. For example, both Fe2+ and Zn2+ are borderline metal ions in the hard-soft classification of Lewis acids. A protein that bound to Fe2+ would probably also bind to Zn2+. Thus, the C-lectins may have an affinity for Fe2+ and Zn2+ as well as Fe3+. This could explain why a mix of unrelated proteins bound to zinc, rather than just one single protein. By contrast, the glycine- and histidine-rich portion of asmp57a looks well-designed to bind to zinc, but again there is no known function for it. The relatively high concentration of zinc (approx. 1 mM) does imply some function, but at present that is subject only to speculation. Further work will be necessary to determine the function of zinc in the glue, and to confirm the identity of the proteins that bind to zinc using LC–MS/MS.
5. Conclusion
The primary sacrificial bonds that cross-link slug glue do not appear to involve metal coordination. Instead, there is a rapid cross-linking event that may happen before secretion and that is difficult to block. This event probably involves protein oxidation. Interestingly, the C-lectins in slug glue bind to iron and this iron may be involved in linking the C-lectins, which serve an adhesive function, to the cross-linked matrix of the glue. The co-option of these C-lectins for use in an adhesive and their modification to become iron-binding is an interesting development. The C-lectins in slug glue have been previously identified as the most common proteins that are unique to the glue as opposed to the mucus used in locomotion [33]. Their ability to form heterotrimers and bind to a wide range of surfaces [28] combined with their iron-binding ability and its possible use in linking components of the glue, highlights the versatility and power of these proteins for use in biomaterials. Finally, this study identifies a protein, asmp165, that may be an important part of the cross-linked network in the glue.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material [34].
Authors' contributions
C.C.: data curation, formal analysis, investigation, writing—original draft; B.F.: investigation, writing—original draft; M.Z.: investigation, writing—original draft; H.H.: data curation, investigation; A.M.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This work was supported by internal funding sources at Ithaca College.
References
- 1.Smith AM. 2016. The biochemistry and mechanics of gastropod adhesive gels. In Biological adhesives (ed. Smith AM), pp. 177-192. Cham, Switzerland: Springer. [Google Scholar]
- 2.Denny MW. 1983. Molecular biomechanics of molluscan mucous secretions. In The Mollusca, vol. I (eds Wilbur K, Simkiss K, Hochachka PW), pp. 431-465. New York, NY: Academic Press. [Google Scholar]
- 3.Fung T-M, Gallego Lazo C, Smith AM. 2019. Elasticity and energy dissipation in the double network hydrogel adhesive of the slug Arion subfuscus. Phil. Trans. R. Soc. B 374, 1784. ( 10.1098/rstb.2019.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilks AM, Rabice SR, Garbacz HS, Harro CC, Smith AM. 2015. Double-network gels and the toughness of terrestrial slug glue. J. Exp. Biol. 218, 3128-3137. ( 10.1242/jeb.128991) [DOI] [PubMed] [Google Scholar]
- 5.Smith AM. 2013. Multiple metal-based cross-links: protein oxidation and metal coordination in a biological glue. In Biological and biomimetic adhesives: challenges and opportunities (eds Santos R, Aldred N, Gorb S, Flammang P), pp. 3-15. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
- 6.Werneke SW, Swann C, Farquharson L, Hamilton KS, Smith AM. 2007. The role of metals in molluscan adhesive gels. J. Exp. Biol. 210, 2137-2145. ( 10.1242/jeb.006098) [DOI] [PubMed] [Google Scholar]
- 7.Braun M, Menges M, Opoku F, Smith AM. 2013. The relative contribution of calcium, zinc and oxidation-based cross-links to the stiffness of Arion subfuscus glue. J. Exp. Biol. 216, 1475-1483. ( 10.1242/jeb.077149) [DOI] [PubMed] [Google Scholar]
- 8.Smith AM, Papaleo C, Reid CW, Bliss JM. 2017. RNA-Seq reveals a central role for lectin, C1q and von Willebrand factor A domains in the defensive glue of a terrestrial slug. Biofouling 33, 741-754. ( 10.1080/08927014.2017.1361413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao Z, Handford P, Mayhew M, Knott V, Brownlee GG, Stuart ZD. 1995. The structure of a Ca2+-binding epidermal growth factor-like domain: its role in protein–protein interactions. Cell 82, 131-141. ( 10.1016/0092-8674(95)90059-4) [DOI] [PubMed] [Google Scholar]
- 10.Whittaker CA, Hynes RO. 2002. Distribution and evolution of von Willebrand/Integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13, 3369-3387. ( 10.1091/mbc.E02-05-0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamocky M, Furtmuller PG, Obinger C. 2008. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal. 10, 1527-1548. ( 10.1089/ars.2008.2046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AM, Robinson TM, Salt MD, Hamilton KS, Silvia BE, Blasiak R. 2009. Robust cross-links in molluscan adhesive gels: testing for contributions from hydrophobic and electrostatic interactions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 152, 110-117. ( 10.1016/j.cbpb.2008.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashton NA, Roe DR, Weiss RB, Cheatham TE, Stewart RJ. 2013. Self-tensioning aquatic caddisfly silk: Ca2+-dependent structure, strength, and load cycle hysteresis. Biomacromolecules 14, 3668-3681. ( 10.1021/bm401036z) [DOI] [PubMed] [Google Scholar]
- 14.Stewart RJ, Wang CS, Song IT, Jones JP. 2017. The role of coacervation and phase transitions in the sandcastle worm adhesive system. Adv. Colloid Interface Sci. 239, 88-96. ( 10.1016/j.cis.2016.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington MJ, Masic A, Holten-Andersen N, Waite JH, Fratzl P. 2010. Iron-clad fibers: a metal-based biological strategy for hard flexible coatings. Science 328, 216-220. ( 10.1126/science.1181044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang DS, Zeng H, Masic A, Harrington MJ, Israelachvili JN, Waite JH. 2010. Protein- and metal-dependent interactions of a prominent protein in mussel adhesive plaques. J. Biol. Chem. 33, 25 850-25 858. ( 10.1074/jbc.m110.133157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite JH. 2017. Mussel adhesion – essential footwork. J. Exp. Biol. 220, 517-530. ( 10.1242/jeb.134056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broomell CC, Mattoni MA, Zok FW, Waite JH. 2006. Critical role of zinc in hardening of Nereis jaws. J. Exp. Biol. 209, 3219-3225. ( 10.1242/jeb.02373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degtyar E, Harrington MJ, Politi Y, Fratzl P. 2014. The mechanical role of metal ions in biogenic protein-based materials. Angew. Chem. 53, 12 026-12 044. ( 10.1002/anie.201404272) [DOI] [PubMed] [Google Scholar]
- 20.Sagert J, Sun C, Waite JH. 2006. Chemical subtleties of mussel and polychaete holdfasts. In Biological adhesives (eds Smith AM, Callow JA), pp. 125-143. Berlin, Germany: Springer. [Google Scholar]
- 21.Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ. 2005. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl Acad. Sci. USA 102, 11 337-11 342. ( 10.1073/pnas.0504982102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So CR, et al. 2017. Oxidase activity of the barnacle adhesive interface involves peroxide-dependent catechol oxidase and lysyl oxidase enzymes. Appl. Mater. Interfaces 9, 11 493-11 505. ( 10.1021/acsami.7b01185) [DOI] [PubMed] [Google Scholar]
- 23.Tanzer ML. 1973. Cross-linking of collagen. Science 180, 561-566. ( 10.1126/science.180.4086.561) [DOI] [PubMed] [Google Scholar]
- 24.Requena JR, Chao C-C, Levine RL, Stadtman ER. 2001. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc. Natl Acad. Sci. USA 98, 69-74. ( 10.1073/pnas.98.1.69) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradshaw A, Salt M, Bell A, Zeitler M, Litra N, Smith AM. 2011. Cross-linking by protein oxidation in the rapidly setting gel-based glues of slugs. J. Exp. Biol. 214, 1699-1706. ( 10.1242/jeb.051581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hames BD. 1990. One dimensional polyacrylamide gel electrophoresis. In Gel electrophoresis of proteins: a practical approach (eds Hames BD, Rickwood D), pp. 1-147. Oxford, UK: IRL Press. [Google Scholar]
- 27.Zelensky AN, Gready JE. 2005. The C-type lectin-like domain superfamily . FEBS J. 272, 6179-6217. 10.1111/j.1742-4658.2005.05031.x [DOI] [PubMed] [Google Scholar]
- 28.Smith AM, Huynh P, Griffin S, Baughn M, Monka P. 2021. Strong, non-specific adhesion using C-lectin heterotrimers in a molluscan defensive secretion. Integr. Comp. Biol. 61, 1440-1449. ( 10.1093/icb/icab100) [DOI] [PubMed] [Google Scholar]
- 29.Blaschko H. 1935. The mechanism of catalase inhibitions. Biochem. J. 29, 2303-2312. ( 10.1042/bj0292303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403-410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 31.Jensen SA, Robertson IB, Handford PA. 2012. Dissecting the fibrillin microfibril; structural insights into organization and function. Structure 20, 215-225. ( 10.1016/j.str.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 32.Cescon M, Gattazzo F, Chen P, Bonaldo P. 2015. Collagen VI at a glance. J. Cell Sci. 128, 3525-3531. ( 10.1242/jcs.169748) [DOI] [PubMed] [Google Scholar]
- 33.Pawlicki JM, Pease LB, Pierce CM, Startz TP, Zhang Y, Smith AM. 2004. The effect of molluscan glue proteins on gel mechanics. J. Exp. Biol. 207, 1127-1135. ( 10.1242/jeb.00859) [DOI] [PubMed] [Google Scholar]
- 34.Christoforo C, Fleming B, Zeitler M, Haws H, Smith AM. 2022. Data from: Metal-binding proteins and cross-linking in the defensive glue of the slug Arion subfuscus. Figshare. ( 10.6084/m9.figshare.c.6296340) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Christoforo C, Fleming B, Zeitler M, Haws H, Smith AM. 2022. Data from: Metal-binding proteins and cross-linking in the defensive glue of the slug Arion subfuscus. Figshare. ( 10.6084/m9.figshare.c.6296340) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material [34].