Abstract
The differential diagnosis of bloody diarrhea is necessary to specify etiology and plan treatment. Misdiagnosis can lead to catastrophic results with the treatments to be given. In this case report, we present a case of schistosomal colitis mimicking ulcerative colitis in a 26-year-old Guinean male patient diagnosed in 2021.
Keywords: Ulcerative colitis, Inflammatory bowel disease, Schistosomal colitis, Turkey
Introduction
The causes of bloody diarrhea are acute infectious colitis (Shigella, Salmonella, Campylobacter, Yersinia, Amebic colitis, and other causes), ischemic colitis, antibiotic, drug-induced colitis, radiation colitis, diverticular associated colitis, diversion colitis, inflammatory bowel diseases (IBD), and malignancies (1). A correct diagnosis is key to planning a specific treatment and determining the prognosis.
Here we present a case with Schistosoma colitis who was diagnosed formerly as ulcerative colitis.
Case presentation
The case was a 26-year-old black male from Guinea, evaluated at Department of Gastroenterology, Umraniye Training and Research Hospital, Health Sciences University Istanbul, Turkey in 2021. He was not using any drugs or taking any herbal remedies and without an unremarkable medical history. He was not using any drugs or taking any herbal remedies. He suffered bloody diarrhea and abdominal pain 4–5 times a day for two months, beginning one month after visiting South Africa. During this period, he lost 5 kg. Followed by fever and fatigue which added to his previously complaints, he was examined in an external center. His detailed history, examination results, and stool microbiological tests did not reveal any infectious pathogens. The endoscopic and histopathological findings were consistent with ulcerative colitis with extensive involvement. Therefore, he was treated with mesalazine 3 gr/d (p.o) and mesalazine 4 gr/d enema for five weeks. Despite regular treatment, he still had abdominal pain and rectal bleeding but with significantly reduced diarrhea. Therefore, the patient applied to our clinic.
He had a body temperature of 36.6 °C and a heart rate of 79 beats/min. The abdominal and other system examinations did not reveal pathological findings. According to blood lab tests, white blood cell count: 7.8 103 u/L; C-reactive protein level 0.4 mg/dl (Upper limits of normal: 0.5 mg/dl); eosinophil count: 1.48 (103u/L) (Upper limits of normal: 0.5 103 u/L); hemoglobin: 14.0 g/dL. No pathogenic microorganism was detected in stool culture, serology (Entamoeba histolytica indirect hemagglutination) and direct microscopy, parasite egg in stool, and examination of Clostridium difficile toxin. In flexible sigmoidoscopy, no feature was detected, except mild hyperemia in the sigmoid colon and rectum (Fig. 1).
Fig. 1:
An endoscopic view of the diffuse hyperemia in the rectum
Biopsy was performed, and a sample of tissue (one from each segment) was taken from the left colon and rectum for histopathological evaluation. The biopsy revealed eosinophil-rich granulomas containing schistosome parasites at varying levels from the sub-superficial epithelium to the submucosa (Fig. 2–4). There was no histopathological chronicity that would corroborate the diagnosis of inflammatory bowel disease. The parasite was morphologically compatible with S. mansoni due to its lateral spin (Fig. 3). The patient received 40 mg/kg/d praziquantel (as a single dose). Eight weeks later, he was free of abdominal pain and rectal bleeding.
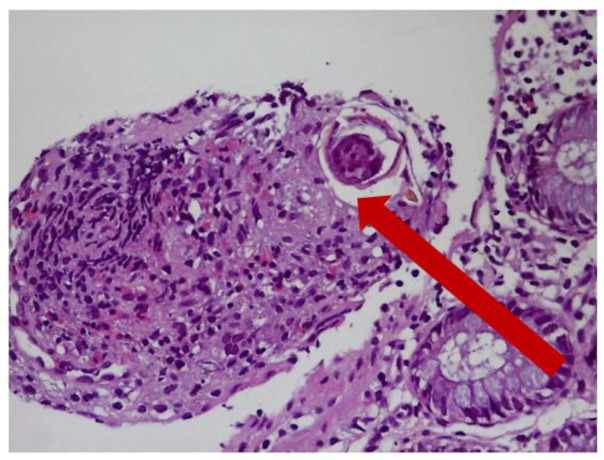
Fig. 2:
Schistosoma egg and structures eosinophil-rich granuloma are shown with the arrow (H&E X40) H&E: Hematoxylin Eosin X40: Microscope 40x Magnification
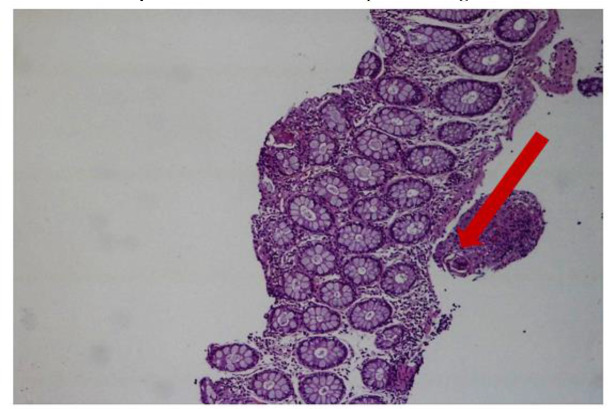
Fig. 4:
Schistosoma eggs and granuloma structures in the submucosa (H&E X100) H&E: Hematoxylin Eosin X100: Microscope 100x Magnification
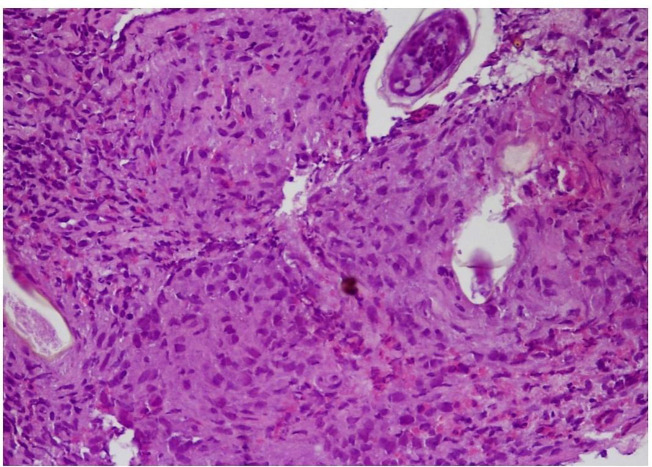
Fig. 3:
A granuloma structure with Schistosoma eggs under the epithelium in the lamina propria and Schistosoma eggs in the submucosa. The arrow indicates the lateral spine of Schistosoma mansoni. (H&E X40). H&E: Hematoxylin Eosin X40: Microscope 40x Magnification
Written informed consent was obtained from the patient in the case report. This case report was carried out with the approval of local Ethics Committee.
Discussion
Schistosoma parasites are found in freshwater snails in endemic regions. Totally 7 Schistosoma species can cause infection in humans. The three major species among these are S. mansoni (Africa and South America), S. haematobium (Africa and Middle East), and S. japonicum (East Asia). S. mansoni and S. japonicum cause intestinal tract disease, while S. haematobium causes genitourinary tract disease generally. The other four minor species are S. mekongi (Laos, Cambodia), the closely related S. malayensis, and the rare S. intercalatum and S. guineensis (West and Central Africa). Phylogenetically, the latter two are closely related to S. haematobium (2). All four are tropic for intestines and liver. S. mansoni infection occurs primarily among humans as well as some primates (3).
Metacercariae infect humans passing through the skin. Parasite eggs are transported to the liver, spleen, brain, spinal cord, peritoneal surfaces, colon, bladder, and skin via the splanchnic circulation (4,5). Schistosomal colitis is characterized by an inflammatory response of parasite eggs in the colon wall. Parasite secretes proteins and carbohydrates to protect themselves from the immune system and may be limited by the eosinophilic granulomatous reaction. On the other hand, they may cause erythema, edema, ulceration, hemorrhages, and scar tissue in the colon (4, 6).
Depending on the nonspecific symptoms of schistosomal infection, diagnosis may be difficult, especially in non-endemic areas. Symptoms in those with intestinal involvement are nausea, abdominal pain, rectal tenesmus, bloody diarrhea, and chronic active colitis with meteorism (6). Less frequently, schistosomal infections can be detected with the findings such as colonic polyps, formation of stricture, occult blood loss, and colorectal cancer (7).
There are few reported cases of colonic schistosomiasis in non-endemic regions (8). To our knowledge, there is no reported case in Turkey. The case in question was diagnosed with ulcerative colitis in an external center and admitted to our clinic due to the persistence of his symptoms. We re-evaluated the patient to confirm the diagnosis, due to developing symptoms after his visit to South Africa, and the presence of eosinophilia and persistent abdominal pain. Although, these are not specific features but are warning signs of infectious pathologies.
Intestinal schistosomiasis is often diagnosed by colonoscopy. Mucosal features are usually detected by colonoscopy. Pathological results of mucosal biopsy or microscopic examination are the main methods of diagnosing it (9). Microscopic examination of stool and urine remain the gold standard tests for the diagnosis of schistosomiasis, albeit with some limitations. In addition, rarely histopathological detection of parasite eggs in the colon supports the diagnosis of schistosomal colitis (10). The sigmoid colon and rectum are the most common involvement sites. Supporting the case we presented here literature demonstrates misdiagnosed schistosomal colitis cases as inflammatory bowel diseases. Ye et al evaluated 96 cases of colonic schistosomiasis for endoscopic findings and reported that a quarter of patients were misdiagnosed at initial evaluation (ulcerative colitis most commonly) (4). A biopsy had been performed on the patient in the center where he had been diagnosed with IBD. We evaluated those biopsy preparations for a second time but did not detect parasite eggs. Moreover, we did not detect significant chronicity that would corroborate the diagnosis of IBD, either. We performed an endoscopic evaluation and detected a large number of parasite eggs in the submucosal area of the samples.
Conclusion
Especially for patients with a history of travel to endemic regions, schistosomal colitis should be included in the differential diagnosis of IBD and a careful evaluation should be done for the presence of parasite eggs in histopathological samples.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interests.
References
- 1.Schlenker Christine, Sue C. Eng, Surawicz Christina M.. “Considerations in the differential diagnosis of colitis.” Inflammatory Bowel Disease: Translating basic science into clinical practice. Blackwell Publishing Ltd; 2010. p. 292–302. [Google Scholar]
- 2.Webster BL, Southgate VR, Littlewood DT. A revision of the interrelationships of Schistosoma including the recently described Schistosoma guineensis. Int J Parasitol 2006; 36:947–55. [DOI] [PubMed] [Google Scholar]
- 3.Rudge JW, Webster JP, Lu DB, et al. Identifying host species driving transmission of schistosomiasis japonica, a multihost parasite system, in China. Proc Natl Acad Sci U S A 2013; 110:11457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelwan ML. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr Ther Res Clin Exp. 2019;91:5–9. doi: 10.1016/j.curtheres.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szekeres C, Galletout P, Jaureguiberry S, et al. Neurological presentation of schistosomiasis. Lancet. 2013;381(9879):1788. doi: 10.1016/S0140-6736(13)60317-7 [DOI] [PubMed] [Google Scholar]
- 6.Ye C, Tan S, Jiang L, et al. Endoscopic characteristics and causes of misdiagnosis of intestinal schistosomiasis. Mol Med Rep. 2013;8(4):1089–1093. doi: 10.3892/mmr.2013.1648 [DOI] [PubMed] [Google Scholar]
- 7.Mu A, Fernandes I, Phillips D. A 57-Year-Old Woman With a Cecal Mass. Clin Infect Dis. 2016;63(5):703–705. doi: 10.1093/cid/ciw413 [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Lei W, Xiao-Li L, et al. [Clinical features of imported schistosomiasis mansoni in Beijing Citya report of 6 cases]. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2017;29(2):150–154. doi: 10.16250/j.32.1374.2016207 [Article in Chinese]. [DOI] [PubMed] [Google Scholar]
- 9.Qin X, Liu CY, Xiong YL, et al. The clinical features of chronic intestinal schistosomiasis-related intestinal lesions. BMC Gastroenterol. 2021;21(1):12. doi: 10.1186/s12876-020-01591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray DJ, Ross AG, Li YS, et al. Diagnosis and management of schistosomiasis. BMJ. 2011; 342:d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]