Abstract
Background:
Dermatoparasitic infestations due to the mites Demodex spp. and Sarcoptes scabie are prevalent dermatological disorders worldwide.
Methods:
Referral patients from the Departments of Dermatology, Infectious Diseases, and from the psychologists, in some cases, to the laboratory of Medical Helminthology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran were examined and documented for demodicosis and scabies from March 2009 to December 2020. All patients’ data were collected and then analyzed statistically by SDATA version 14, using the Chi-square test.
Results:
Out of 494-suspected patients suffering from dermal disorders, 99 patients (20.04%) and 20 cases (4.04%) were found infested with demodicosis and scabies, respectively. Most demodicosis cases belonged to the 46–60 year age group while the infestation rate of scabies was higher in the age group under 5 years (P=<0.0001). Demodicosis was seen more prevalent in women than men, and scabies were higher in men (P =0.15). The cases of demodicosis in fall and scabies in winter and spring were more frequent. Demodicosis picked up in 2015 and 2017 (P=0.03), while the prevalent year for scabies was in 2016 (P=0.77). Both current ectoparasites declined dramatically by Covid-19 pandemic.
Conclusion:
Demodicosis and scabies have been found correlated with age, and no statistical association was seen between the gender and seasonal factors. Besides, the obvious decline of demodicosis and scabies infestation rates during the Covid-19 outbreak can mention that social distance and hygiene standards have negative effects on dermatoparasites transmission.
Keywords: Dermatoparasitoses, Sarcoptes scabiei, Demodex, Iran
Introduction
Humans’ infestations by ectoparasites such as head lice, ticks and mites belonging to Arthropod species that normally live on or inside their host skin, are often assumed as a problem in less developed countries (1). About 80% - 90% of people during their life are afflicted by Demodex, mostly reported in females (2). D. folliculorum and D. brevis live within hair follicles and skin sebaceous gland respectively, are 2 main species that have been found in human skin (3). Demodex spp. with a density of < 5 D/cm2 is a saprophyte agent. When the host skin is a suitable microenvironment, transforming them into a pathogenic phase will be facilitated (4).
Pityriasis folliculorum (5) papulopustular rosacea (PPR) (6), granulomatous rosacea (7), inflammatory papule (8), folliculitis (9), hyperpigmentation (10) and blepharitis (11) are skin disorders associated with Demodex mites activity (12). Significant severe forms of rosacea can manifest atypical conditions making persistent skin lesions. Sometimes in heavy infestation, there is no clear distinction between a pathognomonic butterfly rash of Lupus erythematosus and demodicosis (13).
As Demodex mites commonly show tolerance with most antiseptic agents, such as 75% alcohol, 10% povidoneiodine, as well as erythromycin, documented experiences suggest using tea tree oil products as an effective treatment in chronic demodicosis (14–18).
Sarcoptes scabei var. hominis is an obligate mite causing human scabies, transmitted in close skin-to-skin or sexual contact (19). Scabies has been recorded highly in island countries of the Pacific with a higher rate in children. Besides, Panama, parts of Brazil, and indigenous communities of northern Australia are known as regions with a high burden scabies in the human population (20). Hospitals, nursing homes, prisons, or kindergartens are known as high-risk places for contamination where out-breaks of S. scabiei could be frequently experienced by residents (21). A study in the central prison of Hamadan city in Iran in 2013 showed that 2.6% of prisoners had scabies (22). The prevalence of scabies in Ghezel Hesar prison in Karaj and in Kerman central prison was also estimated at 2.2 and 1.2%, respectively (23, 24). Scabies was diagnosed in 57% of prisoners complaining of skin disorders in Bandar Abbas prison in Hormozgan (25). A descriptive study conducted in the Khorasan Province of Iran, recorded 18.7% of scabies contamination (26). In the patterns of skin diseases in Hormozgan, scabies was introduced as a frequent recurrent skin infection in his region (27). Scabies is more prevalent in humid regions of Iran, and the prevalence of scabies is the highest in (Hormozgan, Golestan, Mazandaran, and Gilan) with an incidence rate of 1 to 5 cases per 1000 people (28, 29).
Generalized itchy symptoms originates from a hypersensitivity reaction to mite antigens, which exacerbates at night, leading to sleeplessness of patients spoiling the quality of their life (30).
This retrospective descriptive study aimed to estimate the prevalence of ectoparasites (D. scabies) in dermatological patients referred to the Laboratory of Helminthology, Tehran University of Medical Sciences in Iran. We believe that regular monitoring of ectoparasites in different regions might help researchers for control and management of skin disorders.
Materials and Methods
Since the patients who were suspected of having other ectoparasites such as head lice and/or have been annoyed by bed bugs were referred to the Entomology Lab, we mainly focused on the cases of demodicosis and scabies referred to the Laboratory of Helminthology. Demographic information (age, sex, date, and drug use), seasonal distribution, and the obtained skin test results of 494 patients who were suspected of demodicosis and scabies with skin symptoms such as itching, redness, and allergic inflammation referred to the Laboratory of Helminthology, School of Public Health, Tehran University of Medical Sciences, Iran were recorded during March 2009 to December 2020. The studied samples were received from dermatological, neuropsychological, and pathological clinics.
The information recorded during these 12 years was collected and imported into an excel file analyzed by SDATA version 14, using the Chi-square test. P value less than 0.05 was considered significant.
Sampling
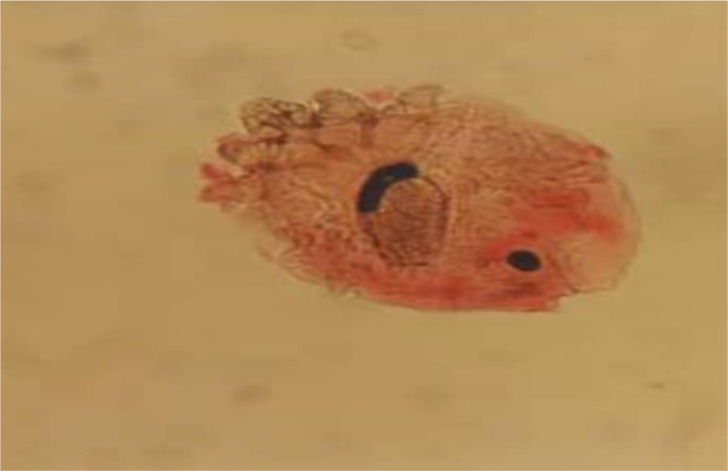
Dermal scraping was the practical method of choice used in our laboratory. Along with routine skin sampling, the non-invasive scotch tape test was also applied for young children and those with sensitive skin. To achieve a reliable sample, scalpel blades for skin scraping were used for cases with skin lesions. Samples become transparent enough in lacto phenol solution for further microscopic detection. Direct parasitological identification was implemented, then photography did by a camera-equipped microscope for each. The patients have been referred to the laboratory of Medical Helminthology by authorized physicians from the Medical Council of Iran. Sarcoptes scabiei (Fig. 1).
Fig. 1:
Scraping containing. Sarcoptes scabiei (10X) Demodex spp. (Fig. 2). Demodex spp. (Fig.3) are of sampling specimens kept in the archive of the late Dr. Iraj Mobedi.
Fig. 2:
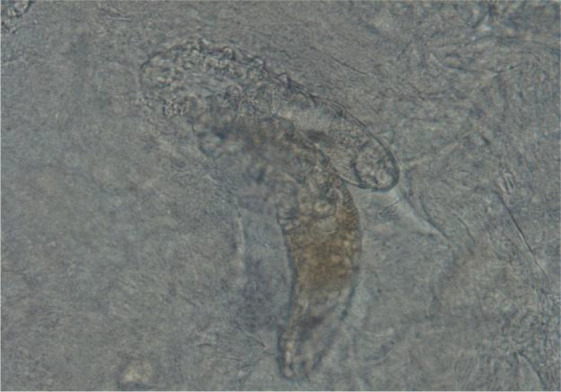
Scraping containing Demodex mites (40X)
Fig. 3:
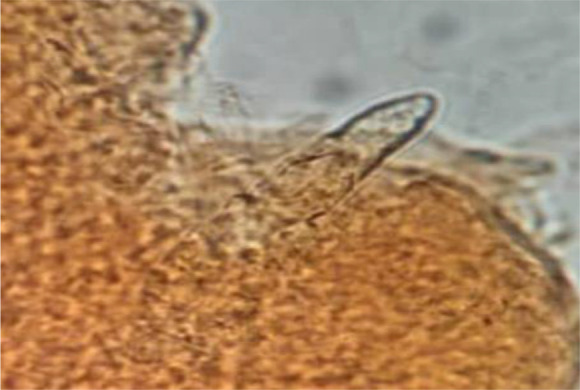
Scraping containing Demodex mites (40X)
Results
Out of 494 submitted patients (280 females and 214 males) 119 cases (24.08%) were infested by at least one ectoparasite. Demodex spp. and S. scabiei were causative agents in 99 (20.04%) and 20 cases (4.04%), respectively. As shown in Table 1, the cases were in different age groups ranging from 2 months to the oldest about 83 years old. Demodicosis was observed more prevalent in women than men, and scabies was highest in men. Most Demodex infestation rates were among the 46–60 years age group. Most scabies infestation rate belongs to the under 5-year age group. There is a significant correlation between infestation rate and age (P < 0.0001). There is no significant association between infestation rate and gender (P= 0.150) (Table 1).
Table 1:
Result of demodicosis and scabies final diagnosis based on age and gender
| Group | Description | Frequency | Proportion (%) | Scabies Infestation Rate (%) | Demodicosis Infestation Rate (%) | P-value |
|---|---|---|---|---|---|---|
| Age (yr) | <5 | 23 | 4.74 | 5/23 (21.73) | 1/23(4.34) | <0.0001 |
| 5–15 | 27 | 5.56 | 1/27 (3.70) | 3/27(11.11) | ||
| 16–30 | 120 | 24.74 | 7/120 (5.83) | 20/120(16.66) | ||
| 31–45 | 144 | 29.69 | 4/144 (2.77) | 26/144(18.05) | ||
| 46–60 | 107 | 22.06 | 0 | 32/107(29.90) | ||
| >60 | 64 | 13.19 | 3/64 (4.68) | 17/64(26.56) | ||
| Gender | Male | 214 | 43.3 | 12/214(5.60) | 42/214(19.62) | 0.150 |
| Female | 280 | 56.7 | 8/280(2.85) | 57/280(20.3) |
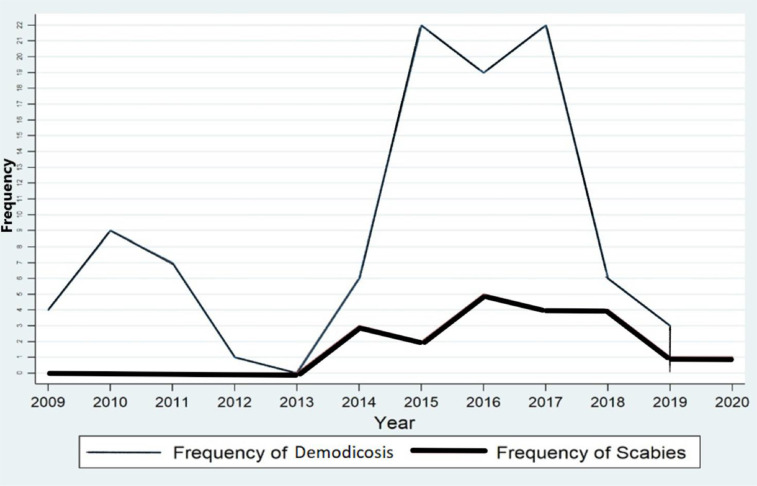
Most patients with demodicosis (n=22) were observed in the years 2015 and 2017. Meanwhile, Scabies positive cases in 2016 were highest with five patients. Our study indicated a significant decline in the incidence of Demodex and S. scabiei mites after the Covid-19 pandemic. The prevalence of demodicosis was seen significantly correlated with the year (P =0.03). Scabies cases were highest in 2016 with no statistical significance (P =0.77) (Fig.4).
Fig.4:
Linear graph of distribution demodicosis and scabies from 2009 to 2020
Nearly one-third of the demodicosis cases were diagnosed in autumn. However, the seasonal trend for scabies showed the highest rates in winter and spring and no statistically significant correlation have been observed between the prevalence of demodicosis and scabies infestation in different seasons (P >0.05).
Discussion
Here the occurrences of two medical important ectoparasites (Demodex spp. & S. scabiei) in the patients referred to our laboratory have been discussed. In general the (24.08%) of the total cases were seen as positive illustrating the status of ectoparasitoses in the studied period. Seasonal trends as well as age groups along with the distribution of two mentioned ectoparasites in different genders have also been regarded in this study. Similar works in neighboring countries and from remote geographical regions were, however, compared with the results that we did retrieve. As can conclude from our data, it was clearly shown that in the present analysis the incidence of demodicosis (20.04%) was predominantly higher comparing with scabies (4.04%).
In our study, demodicosis was higher in women than men with no significant statically association. In Iraq, on 220 people with skin disease, demodicosis was reported more in women than men. Nevertheless, there was no significant relationship between the prevalence of demodicosis and gender, which is similar to our study (31) and the study of Suleyman Durmaz et al in Turkey (32). It was rarely recorded that males showed more prevalence rate of infestation with Demodex spp. compared to females indicating the hypothesis of having androgenic hormones, like testosterone, stimulates the sebaceous glands in men facilitating mites proliferations (33). Different aspects have also been studied by researchers regarding demodicosis, like what has been done in Hungary examining the risk factor of cosmetic use in the prevalence of demodicosis (34). Accordingly, men were more likely to develop demodicosis than women. Their results were in contrast with our study and more other studies that mentioned before and reported demodicosis in women more common than in men. The use of cosmetics introduced a protective factor against demodicosis due to toxic compounds in these creams, and in addition, these substances prevent oxygen access to the parasite (34). In general, the obtained results indicate that gender is not counted as an important effective variant for demodicosis (35, 36).
In Iraq, the prevalence of demodicosis was obviously increased with age, showing the highest prevalence of demodicosis in 3.83% of people over 60 years of age (31). Similarly, the prevalence rate of demodicosis was increased with age reaching 77% at the age of over 70 years according to the Poland study (36). Regardless of the basic condition of the patients, the prevalence of Demodex spp. in metabolic syndrome cases in Turkey, was reported higher in the age group 40 to 49 years old that is more similar to our conclusion that 46–60 age group significantly shows higher infestation rate in comparison with other age groups (2) .In a study conducted on 370 people in the United States, the prevalence of demodicosis was significantly increased in the age group of 51–90 (66.7%) among the 370 individuals (37).This study is consistent with many studies in terms of the enhanced prevalence of demodicosis with age (38–41). In another study on patients with posterior blepharitis, mites were detected as highly prevalent after the age of 50 compared with young age groups (42).
Seasonal factors have also been studied in the present work in which nearly one-third of the positive demodicosis (n=30) occurs in autumn (30/99). In Iraq, the prevalence of demodicosis in winter was higher than in other seasons (31). In South China in 2021, the incidence of eye infections due to Demodex spp. was significantly higher in autumn and winter than in other seasons amongst 1,575 school-children (43). Apparently, there is consensus in the literature that Demodex spp. prefer cold weather. In China, a study of two Demodex species recorded a temperature of 16–20°C as the optimum temperature for the parasite to grow in vitro and claim that Demodex spp. more tolerate cold weather and survive longer in low temperature (44).
From the health points of view, poor sanitation in crowded regions will make the condition suitable to dispread these contagious agents, including scabies (45, 46). This is worth mentioning in the most crowded countries in the world like Bangladesh (47) with (90%) scabies infestation rate (48) followed by Thailand (87.3%) (49) and Sierra Leone (81.5%) have been recorded (46). In the present study, scabies in men exceeds women. Significantly, in children under 5 years old the highest rate compared to other age groups was seen, which is slightly similar to what has been conducted in Australia indicating the prevalence of the mites S. scabiei in children is higher than in adolescents and adults (50). The prevalence of scabies in Khorasan Province northeastern Iran was higher in men than women in 2014 and the age group of 10 to 19 years illustrated the highest incidence of scabies (26). Moreover, in Isfahan, Iran scabies was seen three times higher in men. Moreover, in this study, the prevalence of scabies in the age group of 15 to 39 years old was higher than in other age groups (28). Other studies were in agreement with our study and announced scabies prevalence was higher among the preschool and school age groups (51–54). In contrast, with so many previously published data, scabies in the U.K has been reported more prevalent in females than males, which could be presumably attributed to more concerns by females on medical issues leading to their more attendance to consult physician in this country (55).
Our data indicated that scabies in spring and winter is higher than in other seasons. The seasonal trend of scabies in South Korea indicated that by temperature increase from 5 °C to 25 °C, the number of patients who suffer from scabies gradually increased. They also stated that scabies outbreaks are correlated merely with the temperature with no seasonal associations and the optimum temperature at 14.5 °C was documented (56). In a long-term analysis of a large sample size, the British Dermatologists announced their findings showing a significantly higher rate of scabies in the cooler months of the year (57). It seems that reporting the highest outbreak in winter in some regions with a mean temperature about 14.5 °C and its autumn increasing prevalence in another geographical area with 7–15°C temperature sounds similar (58). The study conducted in Scotland shows the frequency of scabies higher in the first and third quarters of the year with no seasonal correlation (59).
Interestingly, in 2020 no positive demodicosis was recorded in our study. Moreover, scabies trend in this year manifests a significant decrease. Therefore, the Covid-19 pandemic situation might be attributed to this decline It is supposed that the current protocol used in preventing Covid-19 air-borne virus such as imposed social distancing and ventilation regulations might have been responsible for the recent reduction of ectoparasites such as mites. In this regard, the explosion of scabies cases in Turkey at the time of the Covid-19 pandemic compared with the past is in contrast with its trend in European countries and our present results. Turkey’s lifestyle such as its large household number as well as their preferences to stay in rural areas during the Covid-19 pandemic might have caused the shoot-up of scabies incident in this country. Furthermore, and referring to this article, in European countries, hospitals have been mainly focused on Covid patients while the non-urgent cases, including the scabies patients, were neglected. This clinical policy, which was also pursued in Iran, might be led to overlooking the actual number of dermatological disorders (60, 61).
Conclusion
Demodicosis and scabies can be regarded as multifactorial disorders affected by sex, age, environmental conditions, and the patient’s lifestyle. Besides, the apparent decline of demodicosis and scabies infestation rates during the Covid 19 outbreak can mention that social distance and hygiene standards improved in communities could be an obstacle to dermatoparasites transmission. The authors believe that taking advantage of a comprehensive and multidisciplinary approach could be a principal source of scientific information to diagnose the underlying cause of unknown skin disorders.
Acknowledgements
The authors greatly acknowledge the School of Public Health at Tehran University of Medical Sciences for the entire official and financial support.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.McNair CM. Ectoparasites of medical and veterinary importance: drug resistance and the need for alternative control methods. J Pharm Pharmacol. 2015;67(3):351–63. [DOI] [PubMed] [Google Scholar]
- 2.Enginyurt O, Karaman U, Cetin F, Ozer A. The prevalence of Demodex species and its relationship with the metabolic syndrome in women of Malatya province, Turkey. Jundishapur J Microbiol. 2015;8(10) :e24322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tehrani S, Tizmaghz A, Shabestanipour G. The Demodex mites and their relation with seborrheic and atopic Dermatitis. Asian Pac J Trop Med. 2014;7S1:S82–4. [DOI] [PubMed] [Google Scholar]
- 4.Lacey N, Raghallaigh SN, Powell FC. Demodex mites-commensals, parasites or mutualistic organisms? Dermatology. 2011;222(2):128. [DOI] [PubMed] [Google Scholar]
- 5.AYRES S. Pityriasis folliculorum (Demodex). Arch Derm Syphilol. 1930;21(1):19–24. [Google Scholar]
- 6.AYRES S, Anderson NP. Acne rosacea: response to local treatment for Demodex folliculorum. JAMA. 1933;100(9):645–7. [Google Scholar]
- 7.Ayres S, Jr. Rosacea and Rosacea-like Demodicldosis. Int J Dermatol. 1987;26(3):198–9. [DOI] [PubMed] [Google Scholar]
- 8.Seifert H. Demodex folliculorum als Ursache eines solitaren tuberkuloiden granuloms. Z Hautkr. 1978;53(15):540–2. [PubMed] [Google Scholar]
- 9.Purcell SM, Hayes TJ, Dixon SL. Pustular folliculitis associated with Demodex folliculorum. J Am Acad Dermatol. 1986;15(5 Pt 2):1159–62. [DOI] [PubMed] [Google Scholar]
- 10.Ayres S, Jr, Ayres S, 3rd. Demodectic eruptions (demodicidosis) in the human: 30 years’ experience with 2 commonly unrecognized entities: pityriasis folliculorum (Demodex) and acne rosacea (Demodex type). Arch Dermatol.1961;83:816–27. [DOI] [PubMed] [Google Scholar]
- 11.Alvarenga LS, Mannis MJ. Ocular rosacea. Ocul Surf. 2005;3(1):41–58. [DOI] [PubMed] [Google Scholar]
- 12.Forton F, Germaux M-A, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52(1):74–87. [DOI] [PubMed] [Google Scholar]
- 13.Shirzadeh E, Bagheri A, Abdizadeh MF, Kanavi MR. Severe Rosacea: A Case Report. J Ophthalmic Vis Res. 2017;12(4):429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielory L, Katelaris C, Lightman S, Naclerio RM. Treating the ocular component of allergic rhinoconjunctivitis and related eye disorders. MedGenMed. 2007;9(3):35. [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y-Y, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007;26(2):136–43. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Di Pascuale M, Li W, et al. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005;89(11):1468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tighe S, Gao Y-Y, Tseng SC. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl Vis Sci Technol. 2013;2(7): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom (Auckl). 2018;10:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora P, Rudnicka L, Sar-Pomian M, et al. Scabies: A comprehensive review and current perspectives. Dermatol Ther. 2020:e13746. [DOI] [PubMed] [Google Scholar]
- 20.Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15(8):960–7. [DOI] [PubMed] [Google Scholar]
- 21.Hengge UR, Currie BJ, Jäger G, Lupi O, Schwartz RA. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6(12):769–79. [DOI] [PubMed] [Google Scholar]
- 22.Nazari M, Moradi A, Anvari PM. Epidemiological survey of Scabies in the central prison of Hamadan in 2013. Pajouhan Scientific Journal 2015;13(3):1–7. [Google Scholar]
- 23.Nasiri Km, Sharifi I, Khajeh Kam, Pourlashkari M. Prevalence of infectious skin diseases in the central prison of Kerman. Iran J Dermatol. 2000;1(13):19–25. [Google Scholar]
- 24.Rahmati Rm, Malekzad F, Rahmati Rs. Prevalence of scabies and pediculosis in Ghezel Hesar prison. Iranian Journal of Clinical Infection Disease. 2007;2(2):87–90. [Google Scholar]
- 25.Poudat A, Nasirian H. Prevalence of pediculosis and scabies in the prisoners of Bandar Abbas, Hormozgan province, Iran. Pak J Biol Sci. 2007;10(21):3967–9. [DOI] [PubMed] [Google Scholar]
- 26.Berenji F, Marvi-Moghadam N, Naghibozakerin Meibodi P. A retrospective study of ectoparasitosis in patients referred to Imam Reza Hospital of Mashhad, Iran. Biomed Res Int. 2014;2014:104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baghestani S, Zare S, Mahboobi AA. Skin disease patterns in Hormozgan. Int J Dermatol. 2005;44(8):641–5. [DOI] [PubMed] [Google Scholar]
- 28.Dehghani R, Ghannaee Arani M, Zarghi I. Scabies contamination status in Iran: A review. Int J Epidemiol. 2016;3(1):86–94. [Google Scholar]
- 29.Hosseini-Shokouh SJ, Rahimi-Dehgolan S, Noorifard M, Dabbagh-Moghaddam A, Barati M, Tabibian E. The assessment of epidemiologic aspects of scabies in Iran’s Army during 2004 to 2010. Annals of Military and Health Sciences Research. 2014;12(4):163–167. [Google Scholar]
- 30.Cohen PR. Classic and non-classic (surrepticius) scabies: diagnostic and treatment considerations. Cureus. 2020;12(3):e7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedair BH, Salman SD, Abtan AF. A Survey of Human Face Mites Demodex (Acari, Demodicidae) in Patients with Dermatological Symptoms in Baghdad, Iraq. Int J Surg. 2021:3827–33. [Google Scholar]
- 32.Durmaz S, Yula E, Aycan Kaya O, et al. Sociodemographic characteristics of patients with Demodex brevis and Demodex folliculorum infestation and its association with rosacea and Behçet’s disease. Biomed Res. 2015;26(3):549–55. [Google Scholar]
- 33.Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7(5):583–9. [DOI] [PubMed] [Google Scholar]
- 34.Horváth A, Neubrandt D, Ghidán Á, Nagy K. Risk factors and prevalence of Demodex mites in young adults. Acta Microbiol Immunol Hung. 2011;58(2):145–55. [DOI] [PubMed] [Google Scholar]
- 35.Aycan OM, Otlu GH, Karaman U, Daldal N, Atambay M. Frequency of the appearance of Demodex sp. in various patient and age groups. Turkiye Parazitol Derg. 2007;31(2):115–8. [PubMed] [Google Scholar]
- 36.Sędzikowska A, Osęka M, Skopiński P. The impact of age, sex, blepharitis, rosacea and rheumatoid arthritis on Demodex mite infection. Arch Med Sci. 2018;14(2):353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengbusch H, Hauswirth J. Prevalence of hair follicle mites, Demodex folliculorum and D. brevis (Acari: Demodicidae), in a selected human population in western New York, USA. J Med Entomol. 1986;23(4):384–8. [DOI] [PubMed] [Google Scholar]
- 38.Roth A. Demodex folliculorum in hair follicles of eyelid skin. Ann Ophthalmol. 1979;11(1):37–40. [PubMed] [Google Scholar]
- 39.Riechers R, Kopf AW. Cutaneous infestation with Demodex folliculorum in man: a quantitative approach based on dermal-epidermal separation. J Invest Dermatol. 1969;52(1):103–6. [DOI] [PubMed] [Google Scholar]
- 40.Zhong J, Tan Y, Li S, et al. The prevalence of Demodex folliculorum and Demodex brevis in cylindrical dandruff patients. J Ophthalmol. 2019;2019: 8949683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutiérrez B, Soto R, Catalán A, Araya JE, Fuentes M, González J. Demodex folliculorum (Trombidiformes: Demodicidae) and Demodex brevis Prevalence in an Extreme Environment of Chile. J Med Entomol. 2021;58(6):2067–74. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Ponce D, Zuazo F, Cartes C, et al. High prevalence of Demodex spp. infestation among patients with posterior blepharitis: correlation with age and cylindrical dandruff. Arch Soc Esp Oftalmol. 2017;92(9):412–8. [DOI] [PubMed] [Google Scholar]
- 43.Zhang N, Liu Y, Wen K, et al. Prevalence of Ocular Demodex Infestation in Children: An Epidemiological Survey in South China. Eye Contact Lens. 2021;47(1):60–4. [DOI] [PubMed] [Google Scholar]
- 44.Zhao YE, Guo N, Wu LP. The effect of temperature on the viability of Demodex folliculorum and Demodex brevis. Parasitol Res. 2009;105(6):1623–8. [DOI] [PubMed] [Google Scholar]
- 45.Walker GJ, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007;(3):CD000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terry B, Kanjah F, Sahr F, Kortequee S, Dukulay I, Gbakima A. Sarcoptes scabiei infestation among children in a displacement camp in Sierra Leone. Public Health. 2001;115(3):208–11. [DOI] [PubMed] [Google Scholar]
- 47.Karim SA, Anwar K, Khan M, et al. Socio-demographic characteristics of children infested with scabies in densely populated communities of residential madrashas (Islamic education institutes) in Dhaka, Bangladesh. Public Health. 2007;121(12):923–34. [DOI] [PubMed] [Google Scholar]
- 48.Hayee M, Akhtar N, Ahsan S, Ara R. The scabies problem in a village of Bangladesh. Health Today. 1998;3:68–70. [Google Scholar]
- 49.Pruksachatkunakorn C, Wongthanee A, Kasiwat V. Scabies in Thai orphanages. Pediatr Int 2003;45(6):724–7. [DOI] [PubMed] [Google Scholar]
- 50.Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373(24):2305–13. [DOI] [PubMed] [Google Scholar]
- 51.Heukelbach J, Wilcke T, Winter B, Feldmeier H. Epidemiology and morbidity of scabies and pediculosis capitis in resource-poor communities in Brazil. Br J Dermatol. 2005;153(1):150–6. [DOI] [PubMed] [Google Scholar]
- 52.Christophersen J. The epidemiology of scabies in Denmark, 1900 to 1975. Arch Dermatol.1978;114(5):747–50. [PubMed] [Google Scholar]
- 53.Shrank AB, Alexander SL. More cases of scabies. Br Med J.1968;1(5589):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savin J. Scabies in Edinburgh from 1815 to 2000. J R Soc Med. 2005;98(3):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lassa S, Campbell M, Bennett C. Epidemiology of scabies prevalence in the UK from general practice records. Br J Dermatol. 2011;164(6):1329–34. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, Cheong HK. Epidemiologic trends and seasonality of scabies in South Korea, 2010–2017. Korean J Parasitol. 2019;57(4):399–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mimouni D, Ankol O, Davidovitch N, Gdalevich M, Zangvil E, Grotto I. Seasonality trends of scabies in a young adult population: a 20-year follow-up. Br J Dermatol. 2003;149(1):157–9. [DOI] [PubMed] [Google Scholar]
- 58.Park SY, Hong JS, Roh JY, et al. Epidemiological and clinical study of scabies in Korea: multicenter retrospective study. Korean J Parasitol. 2013;51(9):678–84. [Google Scholar]
- 59.Schofield C. Seasonsal variations in the reported incidence of sexually transmitted diseases in Scotland (1972–76). Br J Vener Dis. 1979;55(3):218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kutlu Ö, Aktaş H. The explosion in scabies cases during COVID-19 pandemic. Dermatol Ther. 2020;33(5):e13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porsuk AÖ, Cerit Ç . Status of Scabies Cases in COVID-19 Pandemic Days. Iran J Parasitol. 2021;16(3):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]