Abstract
Mycobacterium avium, the most common opportunistic pathogen in patients with AIDS, is frequently isolated from a variety of environmental sources, but rarely can these environmental isolates be epidemiologically linked with isolates known to cause human disease. Using a number of in vitro tissue culture assays, we found significant pathogenic differences between a serotype 4 human clinical M. avium isolate and a serotype 2 veterinary isolate. Cell association of the patient strain with a human intestinal cell line was 1.7 times that of the veterinary strain. Growth of this clinical strain in human peripheral blood mononuclear cell-derived macrophages increased from 12-fold higher than that of the veterinary isolate after 2 days to 200-fold higher after 4 days. By the conclusion of each experiment, lysis of all examined host cell types and accumulation of cell debris were observed in infections with the human isolate, but monolayers remained relatively intact in the presence of the animal isolate. The two strains also differed in the ability to stimulate human immunodeficiency virus replication in coinfected host cells, with p24 antigen levels after 6 days threefold higher in the cells coinfected with the clinical strain than in those infected with the veterinary strain. If the genetic differences responsible for the phenotypes observed in these assays can be identified and characterized, it may be possible to determine which M. avium strains in the environment are potential human pathogens.
Disease caused by Mycobacterium avium has long been recognized, occurring worldwide and endemic in certain geographic areas (19). However, reported cases of disseminated disease were historically rare, with fewer than 50 cases reported (1) before the emergence of AIDS in 1981. Human immunodeficiency virus (HIV) infection is now the most significant risk factor for M. avium-caused disease (19), which is estimated to occur in 50 to 60% of AIDS patients (2, 23). M. avium bacteria have been isolated from soil, plants, house dust (19, 27), and many natural sources of water. The bacteria are often found in large municipal water supplies and have been isolated from water systems of hospitals (9, 10, 19). Although all M. avium serotypes are found in the environment, certain serotypes are prevalent among patient isolates. In the United States, the predominant serotypes isolated from AIDS patients are 4, 8, and 1. A study of U.S. medical centers from 1982 to 1987 showed that 66% of M. avium patients were infected with these three serotypes, while only 2% were infected with serotype 2 (27), a classical bird isolate rarely associated with human disease. The fact that strains most frequently isolated from AIDS patients with disseminated disease are not the strains commonly found in stool specimens from healthy individuals suggests that there may be specific genetic determinants which confer virulence to particular disease-causing strains (15).
While much is still unknown about these virulence factors, studies have found a number of virulence-associated phenotypes. M. avium bacteria grown on solid medium yield morphologically distinguishable colonial variants; the smooth, flat, transparent variant has been found to be more pathogenic in animals than the smooth, domed, opaque colony types (24). Primary isolates from bacteremic patients typically are of the flat, transparent colony type and are able to grow in human macrophages, while the domed, opaque variants, which appear after subculture, do not (8, 21). Crawford and Bates found small plasmids in 26 strains isolated from AIDS patients and suggested that these plasmids may play a role in virulence (6). Hellyer et al. likewise found a higher rate of plasmid carriage in U.S. AIDS patients but no difference in carriage between AIDS and non-AIDS patients in the United Kingdom (16). The function of the plasmid encoded genes has yet to be determined.
Current clinical evidence suggests the gastrointestinal tract as the most likely route of M. avium infection (18, 19), with the respiratory tract as a secondary and less frequent pathway. It is also known that interaction with macrophages within the gastrointestinal and lung tissues and elsewhere usually determines the outcome of the infection. Research has defined some of the mechanisms by which M. avium bacilli survive within the macrophage (7, 12, 22, 25, 26), but interaction with human epithelial cells is less well characterized. Bermudez and Young compared the interactions of several strains of M. avium with intestinal epithelial cells and found some differences between clinical and nonclinical strains in the ability to bind to host cells (3). Mapother and Songer found differences in uptake of three animal isolates by another intestinal cell line (20). Much is still unknown, however, about the early events in the interaction between M. avium and gastrointestinal cells and whether lung epithelial cells are involved in M. avium pathogenesis. We have compared the interactions of a common serotype 4 human clinical isolate and a serotype 2 chicken isolate of M. avium with human peripheral blood macrophages and with human intestinal and human type II pneumocyte cell lines. We observed qualitative and quantitative differences between the serotype 4 and serotype 2 strains in their interactions with these host cell types in several in vitro assays. These assay systems and the observed phenotypic differences will be useful in ultimately identifying the associated virulence factors and may lead to a better understanding of which factors make strains associated with human disease significantly more pathogenic in immunocompromised, particularly AIDS, populations.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. avium serotype 4 strain PL47, a human clinical isolate from an AIDS patient (14) (provided by Gale Newman, Morehouse School of Medicine, Atlanta, Ga.), and M. avium ATCC 35713 serotype 2, subspecies avium Chester, a chicken isolate (American Type Culture Collection, Rockville, Md.), were grown in Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with albumin, dextrose, and catalase (BBL, Cockeysville, Md.) and 0.2% glycerol. Stock cultures were stored at −70°C in growth medium plus 20% glycerol. Strains were plated for viable counts on Middlebrook 7H11 agar with oleic acid, albumin, dextrose, and catalase enrichment (BBL).
Eukaryotic cell lines.
INT 407 human intestinal cells (ATCC CCL6) were grown in Dulbecco’s modified Eagle medium with high glucose (0.45% wt/vol) (GibcoBRL, Grand Island, N.Y.) and 10% fetal bovine serum. Human type II alveolar pneumocytes (A549; ATCC CCL185) were grown in minimal essential medium (MEM; GibcoBRL) with 5% fetal bovine serum. Cell suspensions (5 × 104 cells/ml) were added to six-well tissue culture cluster plates (Costar Corp., Cambridge, Mass.) (3 ml/well). For microscopy, cells were seeded on glass coverslips in six-well dishes. Tissue culture dishes were incubated at 37°C in 5% CO2 until monolayers reached confluency (approximately 106 cells/well). THP-1 human monocyte cells (ATCC TIB202) were grown in suspension in RPMI 1640 (GibcoBRL) in T75 tissue culture flasks (Costar) at 37°C in 5% CO2.
Bacterial cell association and replication.
Prior to infection, the growth medium was removed from the wells containing the tissue culture monolayers and replaced with fresh medium (2 ml/well). Suspensions of bacteria were prepared in 7H9 broth, and the optical density at 600 nm was adjusted to 0.5 (∼108 CFU/ml); 10 μl of this suspension was added to each well, and the plates were incubated at 37°C in 5% CO2. On days 1, 3, and 5, the medium was removed from one infected well and the monolayer was washed three times with MEM (without serum); 1 ml MEM was then added to the well, and the monolayer was scraped from the surface with a cell scraper. The cell suspension was removed to a microcentrifuge tube and vortexed for 30 s. Dilutions were plated on 7H11 agar to determine viable counts of cell-associated bacteria (11).
Isolation of PBMCs.
Blood from purified protein derivative-negative, HIV-negative donors was collected in acid-citrate-dextrose anticoagulant. Erythrocytes were allowed to settle out by incubation with 0.6% dextran T70 (Pharmacia Biotech, Uppsala, Sweden) for 2 h at 37°C. The upper layer containing peripheral blood mononuclear cells (PBMCs) and platelets was removed, and cells were collected by centrifugation at 1,000 × g for 15 min. Cells were washed twice in Hanks’ balanced salt solution (HBSS) without Ca2+ or Mg2+. Mononuclear cells were isolated by density gradient centrifugation on Ficoll-Hypaque (Pharmacia). The cells were washed twice with three volumes of HBSS. After the final wash, the cells were resuspended in Iscove’s modified Dulbecco’s medium (IMDM; GibcoBRL) with 10% purified protein derivative-negative pooled human male serum. Cells were added to 6-well tissue culture dishes (2 × 106 cells/well), 24-well tissue culture dishes (2 × 105 cells/well), or T25 tissue culture flasks (8 × 106 cells/flask). Cells were incubated for 5 to 7 days, nonadherent cells were removed, cultures were washed twice with HBSS, and fresh IMDM with 10% human serum was added. Cells were incubated overnight before bacterial infection.
Infection of macrophages for viable counts.
Cell suspensions of the M. avium strains were added to the attached macrophages at a multiplicity of infection (MOI) of 1:10 (1 bacterium per 10 host cells). Each day, the infected macrophages were washed twice with HBSS and overlaid with fresh IMDM. Bacterial counts (two for each time point) were performed by lysing the macrophages by adding 0.1% Triton X-100, vigorously pipetting up and down several times, and plating serial dilutions of the lysate on Middlebrook 7H11 plates (13). Using light microscopy, we did not observe any clumping of intracellular bacteria.
Infection of macrophages and epithelial cells for light microscopy.
Glass cover slips were added to six-well tissue culture dishes before the PBMCs or epithelial cell suspensions were added as described above for each of these two cell types. Macrophages were infected with the M. avium strains at an MOI of 10:1 (∼106 bacteria/well) as previously described by Bermudez et al. (2, 4). After 48 h, the inoculum was removed, the monolayers were washed with HBSS, and fresh medium was added to each well. At 2, 4, 6, 7, and 10 days following infection, the medium was removed from one well, and the coverslip was washed with HBSS; the cells were then stained with Kinyoun’s acid-fast stain (BBL). A549 pneumocytes and INT 407 intestinal cells were infected at an MOI of 1:1 (∼106 bacteria/well) as described by Bermudez and Young (3). After 1, 3, or 5 days, the inoculum was removed and the coverslip was washed with phosphate-buffered saline before staining with Kinyoun’s acid-fast stain.
Infection for electron microscopy.
Macrophage or epithelial cell monolayers in T25 tissue culture flasks were infected with the M. avium strains at an MOI of 10:1 (∼5 × 106 CFU/flask) for 5 or 7 days. After 48 h, the macrophage monolayers were washed twice with HBSS and overlaid with fresh medium (IMDM with 10% human serum). After 5 or 7 days, the medium was removed and the cells were fixed with glutaraldehyde for 1 h, stored in collidine buffer at 4°C, and processed by standard procedures for electron microscopy (11).
In vitro HIV coinfection assay.
THP-1 human monocytes (2 × 106 cells) were incubated with the non-syncytium-inducing HIV strain BAL (MOI of 1 virus-particle per 1,000 cells) in a volume of 2 ml for 12 h, washed three times with fresh prewarmed RPMI 1640, and plated into 24-well dishes (Costar). A suspension of M. avium bacilli was added to yield an MOI of 1:1. Samples of 1 ml were removed at 2-day intervals and stored at −70°C, and 1 ml of fresh RPMI 1640 was added to each well. The viral growth of each sample was determined by using a p24 antigen-capture enzyme-linked immunosorbent assay ELISA (Coulter Immunology, Miami, Fla.) as instructed by the manufacturer. Results are expressed as picograms of p24 per milliliter.
RESULTS
Bacterial cell association and replication in human lung and intestinal cell lines.
Tissue culture monolayers of INT 407 intestinal epithelial cells were infected with M. avium bacilli at an MOI of 1:1 for 1, 3, or 5 days. Viable counts showed twice as many cell-associated bacilli of the serotype 4 strain than of the serotype 2 strain after 1 day (P = 0.005). Numbers of cell-associated bacteria increased over time for both strains, with a 24-fold increase in serotype 4 (P = 0.003) and a 28-fold increase in serotype 2 (P = 0.005) from 1 to 5 days following infection (Table 1). In broth cultures, only 10-fold increases in each strain were seen over the same 5-day period. Electron micrographs of serotype 4-infected INT 407 cells showed that approximately 30 to 40% of the cells were infected by day 5. However, there was evidence of much cell damage, with many bacilli seen in the extracellular debris. Micrographs of monolayers infected with the M. avium serotype 2 strain showed approximately 50% of the cells to be infected, with evidence of many bacteria within vacuoles. There was little evidence of cell damage, and few extracellular bacteria were observed. The cytoplasm in all host cells infected with either serotype was extensively vacuolated. There were no other readily discernible ultrastructural differences between cells infected with the two serotypes, nor were there observable differences in bacterial morphology between the two strains.
TABLE 1.
INT 407 cells infected with M. avium
| M. avium serotype | Inoculum (106 CFU) | Mean no. of attached and internalized bacteria ± SD at:
|
||
|---|---|---|---|---|
| 24 h | 72 h | 120 h | ||
| 4 | 1.8 | (1.9 ± 0.07) × 105 | (1.4 ± 0.19) × 106 | (4.6 ± 0.23) × 106 |
| 2 | 1.6 | (9.7 ± 1.2) × 104 | (8.0 ± 0.59) × 105 | (2.8 ± 0.20) × 106 |
In monolayers of A549 human type II pneumocyte cells infected with the two M. avium strains, viable counts of cell-associated bacteria were 10- to 100-fold less than in the INT 407 cells. There was also very little difference in cell association and growth between the two strains in the A549 cells and less than a fourfold increase in viable counts of either strain over 5 days. Examination by electron microscopy found no evidence of intracellular or extracellular bacilli.
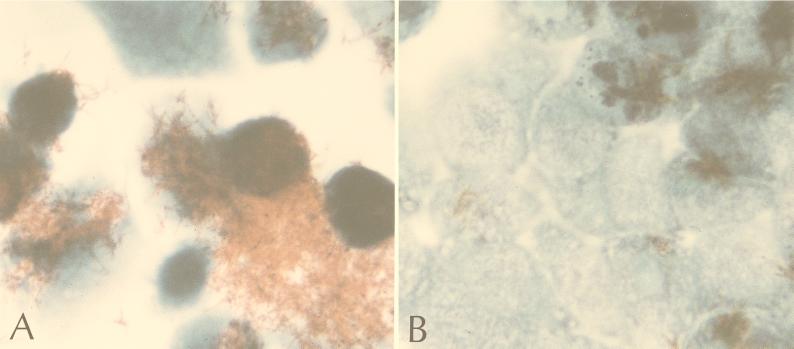
Staining of infected INT 407 cells with Kinyoun’s acid-fast stain revealed clear quantitative and phenotypic differences between monolayers infected with the two strains after 3 days: large clumps of serotype 4 bacilli associated with more than 50% of the host cells, while much smaller clusters of serotype 2 associated with less than 25% of the host cells. After 5 days this difference was more dramatic: masses of bacilli were present and much of the host cell monolayer was destroyed in the serotype 4 infection, while small clusters of bacilli and a relatively intact monolayer remained in the serotype 2 infection (Fig. 1).
FIG. 1.
INT 407 intestinal cells 5 days after infection with the M. avium serotype 4 strain (A) or M. avium serotype 2 strain (B) at an MOI of 1:1 (magnification, ×1,000).
Bacterial cell association and replication in macrophages.
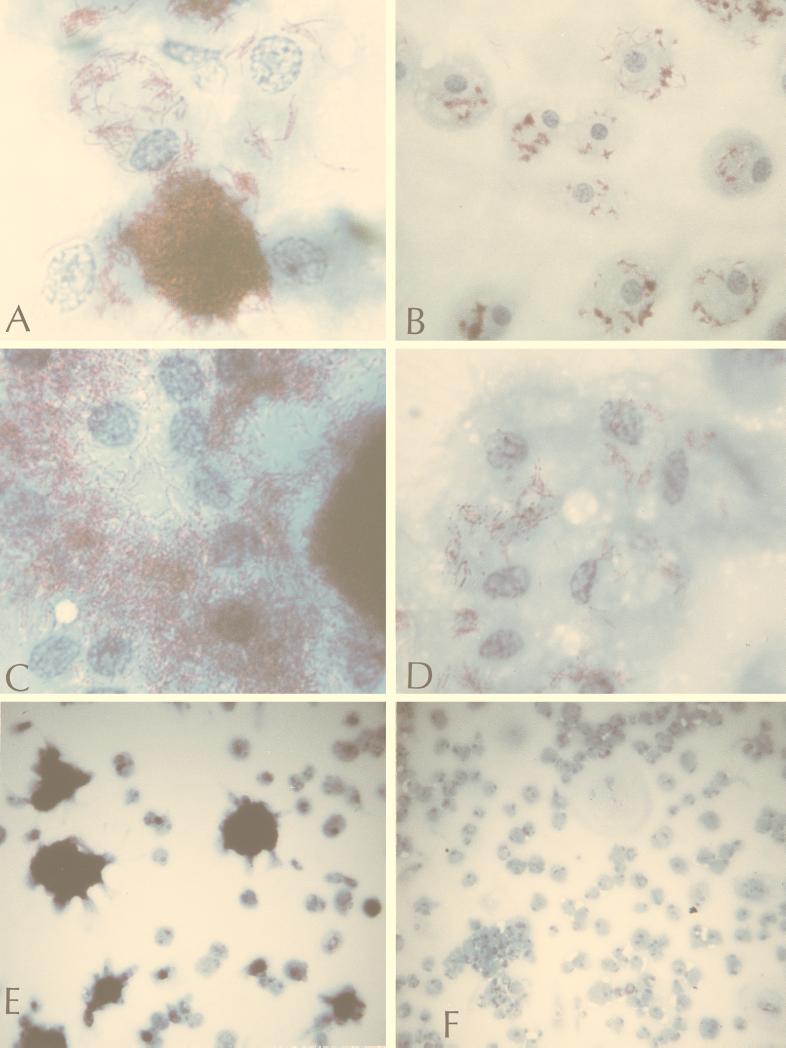
Microscopic examination of infected macrophage monolayers showed similar numbers of cell-associated acid-fast serotype 4 and 2 bacilli at 6 and 24 h postinfection, with small clusters of bacteria attached to more than 50% of the host cells. After 48 h, bacilli of both serotypes were seen in clusters attached to 90 to 100% of the cells in the monolayer. However, in the serotype 4 infections there were more attached clusters of bacilli per cell and more bacilli per cluster. At 96 h, large masses of serotype 4 bacilli were seen attached to many host cells, with evidence of some host cell lysis, while the serotype 2 bacilli remained in smaller clusters, often appearing to be contained within vacuoles. After 6, 7, and 10 days, phenotypic and numerical differences between the two strains became progressively more striking. Large masses of serotype 4 bacilli attached to all cells and were seen free in the surrounding medium, with considerable destruction of the host cell monolayer. In serotype 2 infections, small clusters of bacteria were seen attached to 90 to 100% of the host cells; however, no bacteria were seen in the surrounding medium, and there appeared to be very little host cell damage (Fig. 2).
FIG. 2.
Human macrophages infected with the M. avium serotype 4 strain (A, C, and E) or M. avium serotype 2 strain (B, D, and F) after 7 (A and B) or 10 (C to F) days of infection at an MOI of 10:1. Magnification, ×1,000 (A to D) and ×100 (E and F).
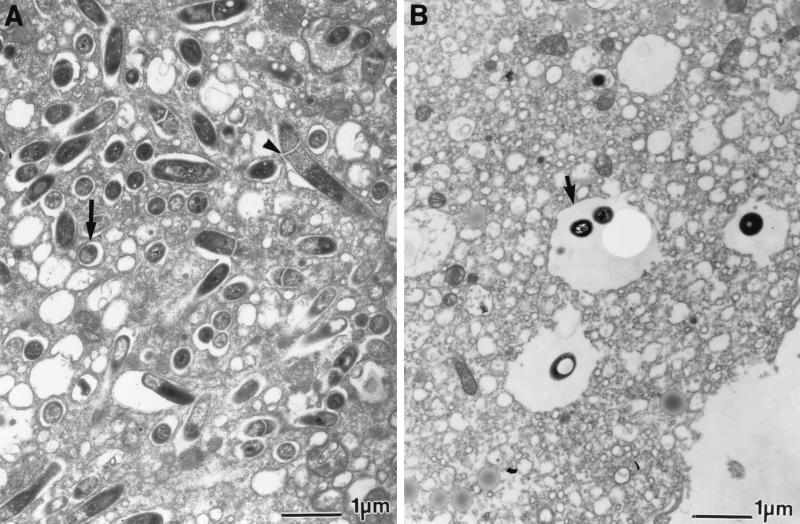
Electron micrographs taken 5 or 7 days after infection showed that macrophages infected with the serotype 4 strain contained 100 to 200 vacuoles per cell, with each vacuole containing a single bacterium (Fig. 3A). In many of the vacuoles the bacterium appeared to be divided by a septum, suggesting that bacteria may have been replicating within the vacuole. In contrast, after 5 or 7 days of infection with the serotype 2 strain, macrophages were observed to contain only one to three vacuoles each containing one or two bacteria (Fig. 3B).
FIG. 3.
Electron micrographs of human macrophages infected for 5 days with the M. avium serotype 4 strain (A) or M. avium serotype 2 strain (B) at an MOI of 10:1. Note the individual bacteria within vacuoles (arrows) and the septa dividing several individual bacilli (arrowheads). Magnification, ×13,500.
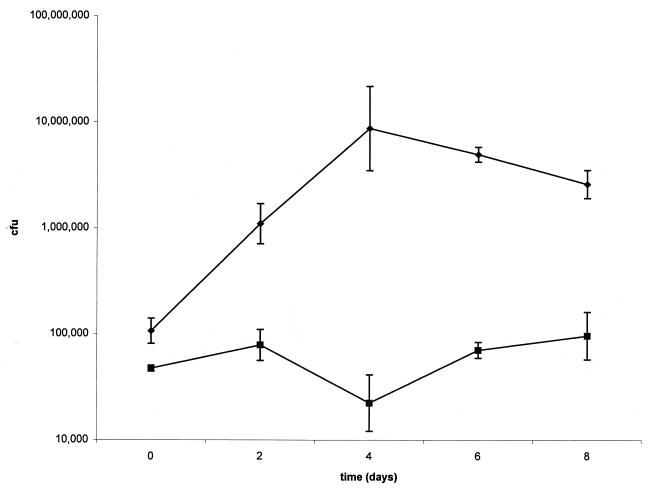
Viable counts (Fig. 4), which represent cell-associated bacteria, increased over the first 4 days and then declined slightly or leveled off over the next 4 days. Cell-associated bacilli of M. avium serotype 4 were 12-fold more numerous than the serotype 2 bacilli after the first 2 days, and this difference increased to as much as 200-fold by day 4.
FIG. 4.
M. avium growth in human macrophages infected at an MOI of 1:10. Growth curves show CFU of the M. avium serotype 4 strain (diamonds) and the M. avium serotype 2 strain (squares) per milliliter.
HIV coinfection assay.
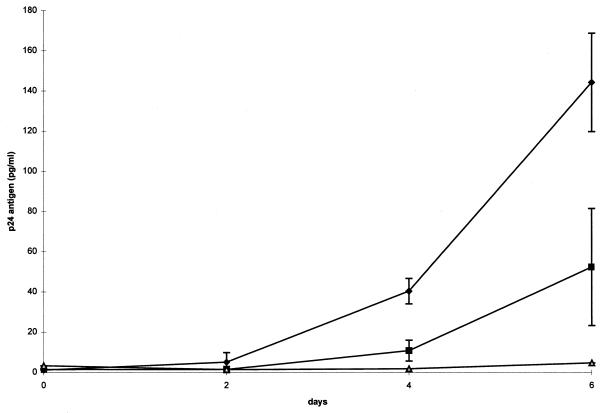
There were increases in detectable p24 antigen when HIV-infected THP-1 cells were coinfected with either strain of M. avium. Antigen levels in coinfections with the serotype 4 strain were 22 and 30 times greater than those in the control (HIV alone) after 4 and 6 days of infection (P = 0.001). Levels with the serotype 4 coinfection were 3.7 and 2.8 times those with the serotype 2 coinfection after 4 (P = 0.001) and 6 (P = 0.002) days, respectively. P values were determined by a two-sample t test (Fig. 5).
FIG. 5.
Levels of p24 antigen production following coinfection of THP-1 cells with M. avium and HIV. Data illustrate protein concentrations in cells infected with the M. avium serotype 4 strain (diamonds), the M. avium serotype 2 strain (squares), and HIV alone (triangles). Differences between serotype 4 and serotype 2 coinfections and between the serotype 4 coinfection and HIV alone at 4 and 6 days were statistically significant (P < 0.003) by the two-sample t test.
DISCUSSION
While much can be learned from the large body of mycobacterial research using mice, chicken, and other animal models, none of these models exhibit the full spectrum of human disease (5). The use of human cell model systems may be more relevant for attempts to uncover particular aspects of virulence mechanisms in human disease. Using human cells, we found quantifiable differences between a serotype 4 human clinical strain of M. avium and a serotype 2 veterinary strain that may be of significance in identifying virulence mechanisms in humans. The fact that both strains grew very well in association with human intestinal epithelial cells is consistent with the hypothesis that the gastrointestinal tract is the most likely port of entry. The higher numbers of the clinical serotype 4 strain associated with the intestinal cells after the first 24 h may give this strain an advantage over the veterinary serotype 2 strain in colonizing the intestinal epithelium and establishing an infection. The differences in observed cytotoxicity between cells infected for 5 days with the serotype 2 and 4 isolates may simply reflect the twofold lower number of serotype 2 bacilli in the assay at that time point. Alternatively, there could be an intrinsic cytotoxic difference between these two strains. Future research will determine which of these possibilities is correct.
Many researchers have described the intracellular trafficking of virulent strains of M. avium in the macrophage and the persistence of the bacteria in nonacidified vacuoles that do not fuse with lysosomes (7, 12, 22, 25, 26). We clearly saw significant bacterial replication and host cell lysis in the serotype 4 strain-infected macrophages. Electron micrographs show some evidence of damage to cell membranes, suggesting that infected cells had lysed, releasing bacilli into the extracellular space. Although the M. avium serotype 2 strain grew over the first 4 days within the macrophages, bacterial numbers increased only threefold and then leveled off, suggesting that the macrophages were able to limit bacterial growth. Electron micrographs show only one to three vacuoles each containing one or two bacilli in each of the infected cells, while serotype 4-infected cells are filled with bacterium-containing vacuoles. The in vivo interaction with macrophages clearly plays a primary role in determining disease outcome, and so it is perhaps significant that the most dramatic difference between these two strains in vitro is in their growth in macrophages.
Correlation of colony morphology and virulence has been observed by a number of researchers. Schaefer et al. (24) compared colony variants in mice and chickens by using bacterial persistence in the mouse lung and survival time of infected chickens to assess virulence, while Meylan et al. (21) and Crowle et al. (8) assessed patient and laboratory strains on the basis of growth in macrophages; all found flat, transparent colonies to be more virulent. However, they also found wide differences in virulence among the transparent colony strains, and studies in our lab have shown little correlation between virulence in human macrophages or chicken embryos and colony type (19a). In this study, we detected no transparent colonies of the clinical serotype 4 or the nonhuman serotype 2 strains even when the strains were passaged through macrophages or through the transformed cell lines. All of our observed phenotypic differences were associated with the opaque colony type of each strain.
M. avium infection, occurring in 50 to 60% of AIDS patients, is the cause of substantial morbidity and shortened survival (17). Disseminated M. avium infection is most often seen in the late stages of AIDS when patients are severely immunocompromised with CD4 counts of <100/μl. As immunity to M. avium is primarily T cell dependent (8), these patients are incapable of mounting any significant immune response. Our results show enhanced HIV replication in human monocytes/macrophages with M. avium coinfection, and we have seen a similar trend in HIV replication in coinfected T cells (8a). The observed differences in cell-associated growth between the two strains likely do not contribute to the differences in HIV induction. As we have reported elsewhere (8a), in comparable experiments using equal numbers of heat-killed serotype 4 and 2 bacilli or in the presence of streptomycin, serotype 4 was observed to stimulate greater HIV replication than serotype 2. These increases in viral numbers may contribute to further progression of the disease. In turn, the advancing HIV infection may facilitate further dissemination by M. avium. That this enhanced HIV replication in macrophages is as much as three times higher in coinfection with the clinical serotype 4 strain than with the nonhuman serotype 2 strain (50 times higher with coinfected CD8 depleted PBMC [8a]) suggests that a mechanism exists by which particular strains of mycobacteria are capable of greater enhancement of HIV replication than others. Ultimately understanding of this mechanism may suggest treatments to block the synergistic enhancement of HIV and mycobacterial replication.
We have examined three additional environmental isolates and obtained preliminary results which confirm our observations. However, any definitive conclusions on the role of these observed phenotypes in mycobacterial virulence must await a more extensive evaluation of a broader range of isolates. We have focused this study on only two strains since there are demonstrable virulence-related phenotypic differences between them. If the genetic differences responsible for the observed phenotypic differences between the clinical serotype 4 strain and the serotype 2 veterinary isolate can be identified and characterized, it may ultimately be possible to detect which of the many M. avium strains in the environment may be potential human pathogens.
ACKNOWLEDGMENTS
We express our appreciation to Jack Crawford and Tom Shinnick for discussion, comments, and critical review of the manuscript and to Tim Green for assistance with statistical analyses.
W.E.S. was funded through a fellowship from the ASM/NCID Postdoctoral Program. Part of this study was funded through a grant from the CDC Opportunistic Infections Program.
REFERENCES
- 1.Bermudez L E, Inderlied C B, Young L S. Mycobacterium avium complex in AIDS. Curr Clin Top Infect Dis. 1992;12:257–281. [PubMed] [Google Scholar]
- 2.Bermudez L E, Parker A, Goodman J R. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect Immun. 1997;65:1916–1925. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Young L S. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect Immun. 1994;62:2021–2026. doi: 10.1128/iai.62.5.2021-2026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Young L S, Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991;59:1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark-Curtiss J E. Identification of virulence determinants in pathogenic mycobacteria. Curr Top Microbiol Immunol. 1998;225:57–79. doi: 10.1007/978-3-642-80451-9_4. [DOI] [PubMed] [Google Scholar]
- 6.Crawford J T, Bates J H. Analysis of plasmids in Mycobacterium avium-intracellulare isolates from persons with acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986;134:659–661. doi: 10.1164/arrd.1986.134.4.659. [DOI] [PubMed] [Google Scholar]
- 7.Crowle A J, Dahl R, Ross E, May M H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991;59:1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowle A J, Tsang A Y, Vatter A E, May M H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986;24:812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Dezzutti, C. S., et al. J. Infect. Dis., in press.
- 9.du Moulin G C, Stottmeier K D, Pelletier P A, Tsang A Y, Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA. 1988;260:1599–1601. doi: 10.1001/jama.260.11.1599. [DOI] [PubMed] [Google Scholar]
- 10.Falkinham J O., III Epidemiology of Mycobacterium avium infections in the pre- and post-HIV era. Res Microbiol. 1994;145:169–172. doi: 10.1016/0923-2508(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 11.Fischer L J, Quinn F D, White E H, King C H. Intracellular growth and cytotoxicity of Mycobacterium haemophilum in a human epithelial cell line (Hec-1-B) Infect Immun. 1996;64:269–276. doi: 10.1128/iai.64.1.269-276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frehel C, Offredo C, Dechastellier C. The phagosomal environment protects virulent Mycobacterium avium from killing and destruction by clarithromycin. Infect Immun. 1997;65:2792–2802. doi: 10.1128/iai.65.7.2792-2802.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan H, Newman G W, Remold H G. Human macrophages acquire a hyporesponsive state of tumor necrosis factor alpha production in response to successive Mycobacterium avium serovar 4 stimulation. Infect Immun. 1995;63:1921–1926. doi: 10.1128/iai.63.5.1921-1926.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampson S J, Portaels F, Thompson J, Green E P, Moss M T, Hermon-Taylor J, McFadden J J. DNA probes demonstrate a single highly conserved strain of Mycobacterium avium infecting AIDS patients. Lancet. 1989;i:65–68. doi: 10.1016/s0140-6736(89)91427-x. [DOI] [PubMed] [Google Scholar]
- 16.Hellyer T J, Brown I N, Dale J W, Easmon C S. Plasmid analysis of Mycobacterium avium-intracellulare (MAI) isolated in the United Kingdom from patients with and without AIDS. J Med Microbiol. 1991;34:225–231. doi: 10.1099/00222615-34-4-225. [DOI] [PubMed] [Google Scholar]
- 17.Horsburgh C R, Hanson D L, Jones J L, Thompson S E. Protection from Mycobacterium avium complex disease in human immunodeficiency virus-infected persons with a history of tuberculosis. J Infect Dis. 1996;174:1212–1217. doi: 10.1093/infdis/174.6.1212. [DOI] [PubMed] [Google Scholar]
- 18.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 19.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Long, E. G., et al. Unpublished observations.
- 20.Mapother M E, Songer J G. In vitro interaction of Mycobacterium avium with intestinal epithelial cells. Infect Immun. 1984;45:67–73. doi: 10.1128/iai.45.1.67-73.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meylan P R, Richman D D, Kornbluth R S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990;58:2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh Y K, Straubinger R M. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect Immun. 1996;64:319–325. doi: 10.1128/iai.64.1.319-325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pozniak A L, Uttley A H, Kent R J. Mycobacterium avium complex in AIDS: who, when, where, why and how? Soc Appl Bacteriol Symp Ser. 1996;25:40S–46S. [PubMed] [Google Scholar]
- 24.Schaefer W B, Davis C L, Cohn M L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970;102:499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- 25.Sturgill-Koszycki S, Schaible U E, Russell D G. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- 27.Yakrus M A, Good R C. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990;28:926–929. doi: 10.1128/jcm.28.5.926-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]