Key Points
Question
What is the association between Klotho protein levels in cerebrospinal fluid (CSF) and plasma, heterozygosity status of the KL-VS haplotype, and amyloid and tau burden among cognitively healthy controls and patients with Alzheimer disease (AD)?
Findings
In this cross-sectional study of 243 patients with AD and controls, CSF Klotho levels were significantly higher among controls and individuals with mild cognitive impairment due to AD compared with individuals with dementia due to AD. Furthermore, CSF Klotho levels were significantly associated with lower levels of CSF AD core biomarkers independent of clinical, KL-VS heterozygosity, or APOE4 status.
Meaning
These findings suggest that Klotho protein levels, but not KL-VS heterozygosity status, differ in clinical stages of AD and are associated with amyloid and tau burden.
Abstract
Importance
Identification of proteins and genetic factors that reduce Alzheimer disease (AD) pathology is of importance when searching for novel AD treatments. Heterozygosity of the KL-VS haplotype has been associated with reduced amyloid and tau burden. Whether this association is mediated by the Klotho protein remains unclear.
Objectives
To assess concentrations of Klotho in cerebrospinal fluid (CSF) and plasma among cognitively healthy controls and patients with AD and to correlate these findings with KL-VS heterozygosity status and amyloid and tau burden.
Design, Setting, and Participants
This case-control study combined 2 independent case-control AD cohorts consisting of 243 referred patients with AD and volunteer controls recruited from January 1, 2009, to December 31, 2018. Klotho levels were measured in CSF and plasma and correlated with KL-VS heterozygosity status and levels of CSF amyloid-β 42 (Aβ42), total tau, and phosphorylated tau. Statistical analysis was performed from January 1, 2021, to March 1, 2022.
Main Outcomes and Measures
Associations of Klotho levels in CSF and plasma with levels of CSF biomarkers were analyzed using linear regression. Association analyses were stratified separately by clinical groups, APOE4 status, and KL-VS heterozygosity. Pearson correlation was used to assess the correlation between CSF and plasma Klotho levels.
Results
A total of 243 participants were included: 117 controls (45 men [38.5%]; median age, 65 years [range, 41-84 years]), 102 patients with mild cognitive impairment due to AD (AD-MCI; 59 men [57.8%]; median age, 66 years [range, 46-80 years]), and 24 patients with dementia due to AD (AD-dementia; 12 men [50.0%]; median age, 64.5 years [range, 54-75 years]). Median CSF Klotho levels were higher in controls (1236.4 pg/mL [range, 20.4-1726.3 pg/mL]; β = 0.103; 95% CI, 0.023-0.183; P = .01) and patients with AD-MCI (1188.1 pg/mL [range, 756.3-1810.3 pg/mL]; β = 0.095; 95% CI, 0.018-0.172; P = .02) compared with patients with AD-dementia (1073.3 pg/mL [range, 698.2-1661.4 pg/mL]). Higher levels of CSF Klotho were associated with lower CSF Aβ42 burden (β = 0.519; 95% CI, 0.201-0.836; P < .001) and tau burden (CSF total tau levels: β = −0.884; 95% CI, 0.223 to −0.395; P < .001; CSF phosphorylated tau levels: β = −0.672; 95% CI, −1.022 to −0.321; P < .001) independent of clinical, KL-VS heterozygosity, or APOE4 status. There was a weak correlation between Klotho CSF and plasma levels among the entire cohort (Pearson correlation r = 0.377; P < .001).
Conclusions and Relevance
The findings of this case-control study suggest that Klotho protein levels were associated with clinical stages of AD, cognitive decline, and amyloid and tau burden and that these outcomes were more clearly mediated by the protein directly rather than the KL-VS heterozygosity variant. When selecting individuals at risk for clinical trials, the Klotho protein level and not only the genetic profile should be considered.
This case-control study assesses concentrations of Klotho protein in cerebrospinal fluid and plasma among cognitively healthy controls and patients with Alzheimer disease (AD) and correlates these findings with KL-VS heterozygosity status and amyloid and tau burden.
Introduction
Klotho is a transmembrane glycoprotein expressed mainly in the kidney and choroid plexus of the brain. The function of Klotho in the brain was initially derived from klotho (OMIM 604824) knockout mice that demonstrated cognitive impairment, premature death, and synaptic loss.1,2 Conversely, overexpression of klotho extends the lifespan, improves cognitive function, and reduces age-associated phenotypes in mice.3,4 A common haplotype consisting of 6 missense variants in the human Klotho gene is termed KL-VS.5 Heterozygosity for KL-VS is associated with higher levels of Klotho, longevity, greater cortical volume, and better global cognition in older adults, suggesting a possible protective role of Klotho in normal aging.6,7,8,9 Recent studies have shown an association between KL-VS heterozygosity status and reduced amyloid burden and risk of Alzheimer disease (AD) among people carrying the APOE4 allele.10,11,12 Moreover, KL-VS heterozygosity has been associated with lower amyloid-related tau pathology among elderly individuals at risk for AD13 and with a possible protective effect against an age-related increase in tau burden.14
To our knowledge, few studies have measured concentrations of Klotho in patients with AD. In cerebrospinal fluid (CSF), Klotho levels decreased with age and were higher in healthy elderly individuals compared with patients with AD.15 Low plasma Klotho levels have been associated with lower Mini-Mental State Examination (MMSE)16 scores and an increased risk of vascular dementia, but not with late-onset AD.17 No study has so far correlated Klotho levels with the KL-VS haplotype in patients with AD, to our knowledge.
The aim of this study was to assess concentrations of Klotho in CSF and plasma and to correlate these findings with KL-VS heterozygosity status to explore a possible association with amyloid and tau burden among a control group of cognitively healthy elderly individuals and patients with AD.
Methods
Participants
In total, 243 participants were selected from 2 Norwegian cohorts from January 1, 2009, to December 31, 2018; demographic and associated data are shown in Table 1. Controls (n = 54) and patients with mild cognitive impairment due to AD (AD-MCI; n = 30) or dementia due to AD (AD-dementia) (n = 19), defined according to National Institute on Aging–Alzheimer’s Association (NIA-AA) criteria,18,19 were recruited from the Department of Neurology, St Olavs Hospital, Trondheim, Norway (Trønderbrain cohort).20 Controls (n = 63) and participants with AD-MCI (n = 72) or AD-dementia (n = 5), defined according to NIA-AA criteria,18,19 were recruited from the Norwegian multicenter Dementia Disease Initiation (DDI) study.21 Exclusion criteria for both cohorts were applied as described elsewhere.20,21 All patients had pathologic concentrations of amyloid-β 42 (Aβ42) in CSF, and their disease was considered part of the AD continuum.18,19,22 The cutoff for Aβ42 in CSF in the Trønderbrain cohort was less than 630 pg/mL, calculated by maximizing the Youden index.23 The cutoff for Aβ42 in CSF in the DDI study was less than 708 pg/mL, determined by a positron emission tomographic [18F]-flutemetamol uptake study based on the same cohort.24 Controls were assessed as being cognitively healthy without signs of any neurologic disorders and with normal concentrations of Aβ42 in CSF. Written informed consent was obtained from all patients or suitable proxies, as well as from all control participants. The study was approved by the Regional Committees for Medical Research Ethics in Central and South-East Norway. This case-control study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Table 1. Demographic Data of the Clinical Groups in the DDI Study and Trønderbrain Cohorts.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Control group | AD group | AD-MCI group | AD-dementia group | |
| DDI study cohort | ||||
| No. | 63 | 77 | 72 | 5 |
| Age, median (range), y | 62 (41-77) | 68 (46-80)a | 68 (46-80)a | 73 (58-75) |
| Sex | ||||
| Male | 27 (42.9) | 47 (61.0)a | 44 (61.1)a | 3 (60.0) |
| Female | 36 (57.1) | 30 (39.0)a | 28 (38.9)a | 2 (40.0) |
| APOE4 allele | 25 (39.7) | 56 (72.7)a | 53 (73.6)a | 3 (60.0) |
| MMSE score, median (range) | 30 (27-30) | 27 (18-30)a | 27 (21-30)a | 25 (18-28)a |
| Trønderbrain cohort | ||||
| No. | 54 | 49 | 30 | 19 |
| Age, median (range), y | 68 (57-84) | 64 (54-72)a | 64 (54-72)a | 64 (54-69)a |
| Sex | ||||
| Male | 18 (33.3) | 24 (49.0) | 15 (50.0) | 9 (47.4) |
| Female | 36 (66.7) | 25 (51.0) | 15 (50.0) | 10 (52.6) |
| APOE4 allele | 20 (37.0) | 42 (85.7) | 24 (80.0)a | 18 (94.7)a |
| MMSE score, median (range) | 30 (28-30) | 26 (16-30)a | 28 (23-30)a | 24 (16-27)a,b |
Abbreviations: AD, Alzheimer disease; AD-MCI, AD with mild cognitive impairment; DDI, Dementia Disease Initiation; MMSE, Mini-Mental State Examination.
P < .01 compared with control group.
P < .01 compared with AD-MCI group.
Neurologic examination, blood screening, CSF collection, and cognitive tests, including the MMSE, were performed for all participants. Genotyping of APOE was performed as described elsewhere.20,21,25
Assessment of KL-VS Heterozygosity
Genotyping was conducted at deCODE Genetics (Reykjavik, Iceland). DNA was extracted from whole blood and genotyped with the Human Omni Express-24 version 1.1 (Illumina Inc) in accordance with the standard Illumina protocol, as described elsewhere.26 A total of 20 participants were excluded from the study cohort because of KL-VS homozygosity.
Sampling and Analysis of CSF and Plasma
Samples of CSF and plasma were collected from all participants following procedures described elsewhere.20,27 Commercial enzyme-linked immunosorbent assays (ELISAs; Fujirebio) were used to measure CSF levels of Aβ42, total tau (T-tau), and phosphorylated tau (P-tau). All samples were analyzed at Akershus University Hospital, Lørenskog, Norway, except those from the controls in Trønderbrain, which were analyzed at the neurobiological laboratory in Trondheim, Norway. Because of minor differences in the measurements between the 2 sites, control samples in Trønderbrain were excluded from the correlation analyses between CSF and plasma Klotho levels and levels of core CSF biomarkers.
Analysis of Klotho Concentrations
Klotho levels were analyzed in CSF and plasma samples using the commercially available human soluble α-Klotho Assay ELISA kit (Immuno-Biological Laboratories Co Ltd). Plasma samples were diluted 1:2, whereas CSF samples were analyzed undiluted. Klotho measurements in plasma were missing for 12 participants (controls, 11; AD-MCI group, 1) and Klotho measurements in CSF were missing for 9 control participants, as samples had been exhausted.
Statistical Analysis
Statistical analyses were performed from January 1, 2021, to March 1, 2022, using SPSS, version 28 (IBM Corp). Normality was assessed through the inspection of Q-Q plots, histograms, and the Shapiro-Wilks test of normality. Cerebrospinal fluid AD biomarkers had nonnormal distributions, and group comparisons were performed using the Mann-Whitney and Kruskal-Wallis tests. Group comparisons of CSF and plasma Klotho levels were performed by log-transforming Klotho levels, applying multivariable linear regression adjusting for age, sex, and APOE4 and KL-VS heterozygosity status. The association of age and sex with log Klotho levels was examined separately by univariable linear regression. Associations between CSF Klotho levels and plasma Klotho levels with levels of core CSF AD biomarkers were analyzed using univariable linear regression and stratified separately by clinical groups, APOE4 status, and KL-VS heterozygosity. Pearson correlation was used to assess the correlation between log levels of CSF Klotho and plasma Klotho. Analyses and associations were considered significant at a 2-sided P < .05.
Results
The demographic and clinical characteristics of the participants in this case-control study across 2 AD cohorts are summarized in Table 1. A total of 243 participants were included: 117 controls (45 men [38.5%] and 72 women [61.5%]; median age, 65 years [range, 41-84 years]), 102 patients with AD-MCI (59 men [57.8%] and 43 women [42.2%]; median age, 66 years [range, 46-80 years]), and 24 patients with AD-dementia (12 men [50.0%] and 12 women [50.0%]; median age, 64.5 years [range, 54-75 years]).
Concentrations of CSF Klotho in Clinical Groups
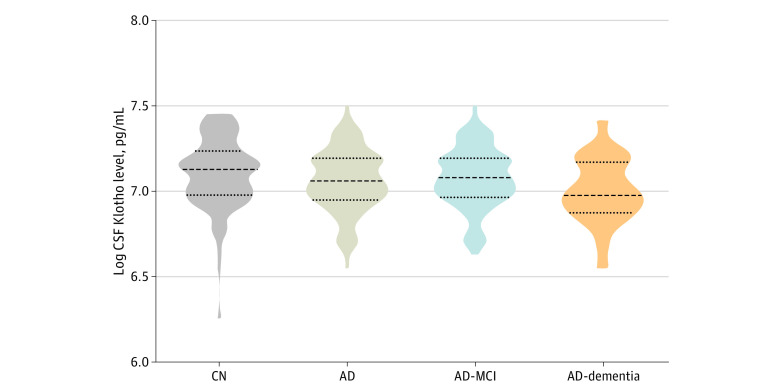
Figure 1 shows CSF levels of Klotho in the clinical groups. The control group had the highest median CSF Klotho concentration vs the total AD group (1236.4 pg/mL [range, 20.4-1726.3 pg/mL] vs 1162.6 pg/mL [range, 698.2-1810.3 pg/mL]). Within the AD group, the median concentration of Klotho in CSF decreased with disease severity, from 1188.1 pg/mL (range, 756.3-1810.3 pg/mL) in the AD-MCI group to 1073.3 pg/mL (range, 698.2-1661.4 pg/mL) in the AD-dementia group. The median concentration of CSF Klotho was significantly higher in the control group (β = 0.103; 95% CI, 0.023-0.183; P = .01) and AD-MCI group (β = 0.095; 95% CI, 0.018-0.172; P = .02) compared with the AD-dementia group, using multivariable linear regression adjusting for age, sex, KL-VS heterozygosity, and APOE4 status. There were no significant differences in median CSF Klotho concentrations between the control group and the AD-MCI group or between the control group and the entire AD group.
Figure 1. Cerebrospinal Fluid (CSF) Klotho Levels in the Clinical Groups.
This violin plot shows concentrations of Klotho in CSF measured by commercially available human soluble α-Klotho assay enzyme-linked immunosorbent assy kit in different clinical groups. AD indicates Alzheimer disease; AD-MCI, AD with mild cognitive impairment; and CN, cognitively healthy controls.
Concentrations of CSF Klotho and Age
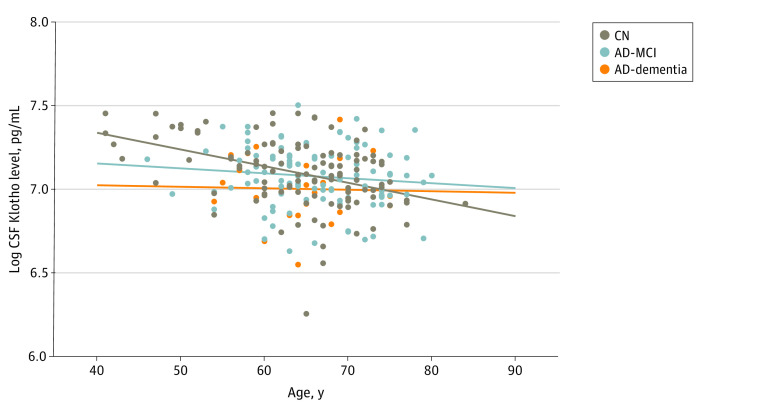
Analysis showed that CSF Klotho levels were weakly, but significantly, correlated with age in the entire cohort (R2 = 0.070; P < .001). When stratifying the analysis by sex, the result remained significant (women, R2 = 0.098; P < .001; men, R2 = 0.039; P = .04). The correlation significantly differed between the clinical groups and was associated with the control group (R2 = 0.168; P < .001) but not the other groups (Figure 2). Analysis showed significantly lower median CSF Klotho levels in the AD-dementia group compared with the control group among participants younger than 65 years (AD-dementia, 1057 pg/mL [range, 698.18-1411.9 pg/mL]; controls, 1313.1 pg/mL [range 846.5-1726.3 pg/mL]; P = .003). The same was not found in the older group (AD-dementia group: median, 1097.5 pg/mL [range, 888.7-1661.4 pg/mL]; controls: median, 1154 pg/mL [range, 520.4-1684.7 pg/mL]; P = .71).
Figure 2. Scatterplot of Cerebrospinal Fluid (CSF) Klotho Levels and Age in the Clinical Groups.
This scatterplot shows logarithmic-transformed concentrations of Klotho in CSF and age in different clinical groups. AD-MCI indicates Alzheimer disease with mild cognitive impairment; and CN, cognitively healthy controls.
Correlations of CSF Klotho Levels and MMSE Score
There was a weak but significantly positive association between MMSE score and CSF Klotho levels in the entire cohort (R2 = 0.025; P = .02). Subgroup analysis showed that CSF Klotho levels were significantly associated with MMSE score in the entire AD group (R2 = 0.048; P = .01) but not the control, AD-MCI, and AD-dementia groups.
Concentrations of CSF Klotho Stratified by APOE4 Status and Sex
There was no significant difference in CSF Klotho concentrations stratified by APOE4 status in the entire study population, the controls, or the combined AD-MCI and AD-dementia groups. Individuals in the AD-dementia group carrying APOE4 had significantly lower median concentrations of Klotho than noncarriers (APOE4 carriers, 1050.8 pg/mL [range, 698.1-1661.4 pg/mL]; noncarriers, 1378.6 pg/mL [range, 1357.7-1411.0]; P < .001). This result might be due to low sample size, as only 4 of 25 patients in this group were APOE4 noncarriers. There were no significant differences in concentrations of Klotho in CSF based on sex in the study population or at any group level.
Association Between CSF Klotho Levels and Levels of CSF AD Biomarkers
The concentrations of Aβ42, T-tau, and P-tau in CSF in the clinical groups in the DDI study cohort and the Trønderbrain cohort are shown in Table 2. Because of differences in site procedures, the control group from Trønderbrain (n = 54) had to be removed from the analysis. The associations between CSF Klotho levels and levels of core CSF AD biomarkers in the clinical groups are shown in Figure 3. A significant association was found for all 3 CSF AD biomarkers in the entire cohort; CSF Klotho levels had a significant positive association with CSF Aβ42 levels (β = 0.519; 95% CI, 0.201-0.836; P < .001), a significant negative association with CSF T-tau levels (β = −0.884; 95% CI, 0.223 to −0.395; P < .001), and a significant negative association with CSF P-tau levels (β = −0.672; 95% CI, −1.022 to −0.321; P < .001). Neither stratifying the analysis by clinical stage nor based on APOE4 status and KL-VS heterozygosity status showed a significant difference in the associations between CSF Klotho levels and levels of any of the 3 AD CSF biomarkers (eTables 1 and 2 in the Supplement).
Table 2. Concentrations of Core AD Biomarkers in CSF at Baseline in the Clinical Groups.
| Characteristic | Median (range), pg/mL | |||
|---|---|---|---|---|
| Control group | AD group | AD-MCI group | AD-dementia group | |
| DDI study cohort | ||||
| No. | 63 | 77 | 72 | 5 |
| CSF | ||||
| Aβ42 | 1090.0 (720.0-1880.0) | 535.0 (250.0-706.0)a | 537.5 (250.0-706.0)a | 413.0 (370.0-600.0)a |
| T-tau | 294.0 (102.0-1050.0) | 520.0 (60.0-1670.0)a | 502.0 (60.0-1370.0)a | 1040.0 (507.0-1670.0)a,b |
| P-tau | 48.0 (27.0-134.0) | 75.7 (23.0-196.0)a | 74.0 (23.0-177.0)a | 134.0 (67.0-196.0)a |
| Trønderbrain cohort | ||||
| No. | 54 | 49 | 30 | 19 |
| CSF | ||||
| Aβ42 | 1054.6 (665.8-1674.1) | 447.4 (173.0-611.6)a | 433.0 (173.0-602.6)a | 476.8 (322.1-611.6)a |
| T-tau | 272.6 (137.5-665.9) | 515.5 (135.4-2325.3)a | 438.4 (135.4-2325.3)a | 624.2 (221.9-1391.0)a |
| P-tau | 51.9 (32.8-86.4) | 76.4 (15.9-168.8)a | 71.9 (13.9-168.8)a | 80.4 (35.9-156.9)a |
Abbreviations: Aβ42, amyloid-β 42; AD, Alzheimer disease; AD-MCI, AD with mild cognitive impairment; CSF, cerebrospinal fluid; DDI, Dementia Disease Initiation; P-tau, phosphorylated tau; T-tau, total tau.
P < .01 compared with control group.
P < .05 compared with AD-MCI group.
Figure 3. Scatterplot of Cerebrospinal Fluid (CSF) Klotho and Core Alzheimer Disease (AD) Biomarkers in CSF at Baseline in the Clinical Groups.
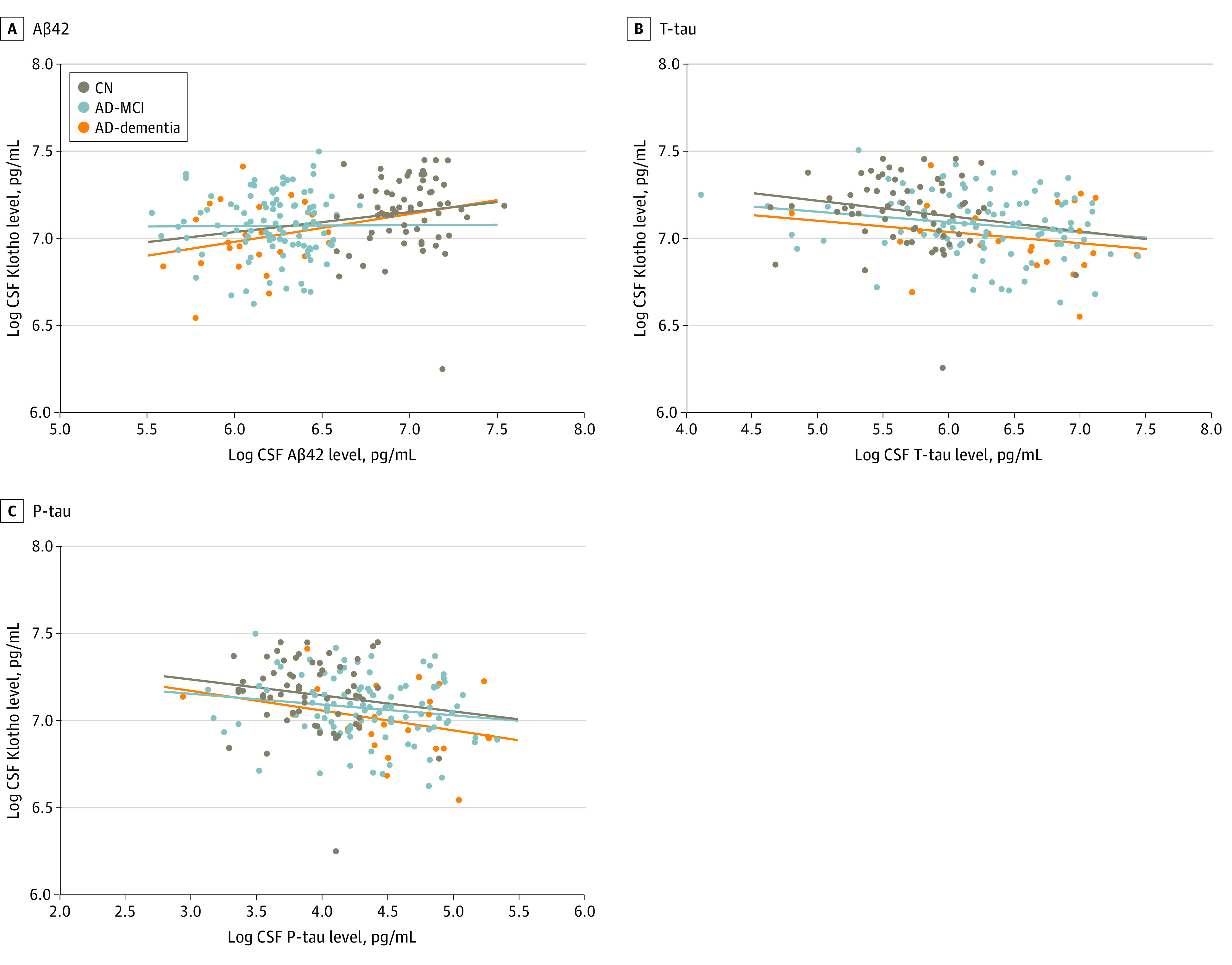
This scatterplot shows logarithmic-transformed concentrations of Klotho in CSF and core CSF AD biomarkers in different clinical groups. Aβ42 indicates amyloid-β 42; AD-MCI, AD with mild cognitive impairment; CN, cognitively healthy controls; P-tau, phosphorylated tau; and T-tau, total tau.
Concentrations of CSF Klotho Stratified by KL-VS Heterozygosity
There were no significant differences in the distribution of individuals with KL-VS heterozygosity among the clinical groups (control group, 20.5% [24 of 117]; AD-MCI group, 28.4% [29 of 102]; AD-dementia group, 29.2% [7 of 24]). Median concentrations of Klotho in CSF were significantly higher among individuals carrying KL-VS heterozygosity compared with noncarriers in the AD group (KL-VS heterozygosity carriers, 1365.9 pg/mL [range, 935.8-1810.3 pg/mL]; noncarriers, 1119.1 pg/mL [range, 698.2-1592.7]; P < .001), the AD-MCI group (KL-VS heterozygosity carriers, 1401.1 pg/mL [range, 1064.1-1810.3]; noncarriers, 1127.5 pg/mL [range, 756.2-1592.7 pg/mL]; P < .001), and the control group (KL-VS heterozygosity carriers, 1312.9 pg/mL [range, 995.1-1726.2 pg/mL]; noncarriers, 1186.9 pg/mL [range, 520.4-1723.3 pg/mL]; P = .02), but not in the AD-dementia group. Noncarriers of KL-VS heterozygosity in the control group showed significantly higher median CSF Klotho levels compared with the AD group (control group, 1186.9 pg/mL [range, 520.4-1723.3 pg/mL]; AD group, 1119.1 pg/mL [range, 698.2-1592.7 pg/mL]; P = .01), whereas no significant difference was found among KL-VS heterozygosity carriers (eFigure 1 in the Supplement). Further analysis did not show any significant differences in CSF Klotho levels among KL-VS heterozygosity carriers in clinical subgroups. There was a significant association between KL-VS heterozygosity and CSF Klotho concentrations in the entire cohort (β = 0.157; 95% CI, 0.097-0.217; P < .001), but independent of clinical group and APOE4 status.
Associations Between KL-VS Heterozygosity and Concentrations of Amyloid and Tau in CSF
No significant associations were found between KL-VS heterozygosity and levels of any of the 3 CSF AD biomarkers in the entire cohort (Aβ42: β = 0.114; 95% CI, −0.258 to 0.030; P = .12; T-tau: β = −0.044; 95% CI, −0.248 to 0.160; P = .67; P-tau: β = 0.360; 95% CI, −0.127 to 0.199; P = .68) or when stratified by clinical groups or APOE4 status.
Correlations Between CSF Klotho and Plasma Klotho Concentrations
There was a significant but weak positive correlation between Klotho concentrations in CSF and plasma in the entire cohort (Pearson correlation r = 0.377; P < .001). Stratifying the result by clinical group showed significant correlations among controls (Pearson correlation r = 0.336; P < .001) and the AD group (Pearson correlation r = 0.427; P < .001). The positive correlation in the AD group was associated mainly with the AD-MCI group (Pearson correlation r = 0.465; P < .001) and was not found in the AD-dementia group (Pearson correlation r = 0.213; P = .32) (eFigure 2 in the Supplement).
Concentrations of Klotho in Plasma Based on APOE4 Status, Sex, and Age in the Clinical Groups
No significant differences were found in the concentrations of plasma Klotho between the clinical groups, nor when stratifying by APOE4 status and sex. Median plasma Klotho levels were significantly higher in men than women in the AD-dementia group (men, 871.4 pg/mL [range, 568.7-1027.9 pg/mL]; women, 711.9 pg/mL [range, 568.7-1027.9 pg/mL]; P = .04) but not in the other clinical groups. A significant negative association of age with plasma Klotho concentrations was seen in the control group (negative correlation, R2 = 0.147; P < .001) but not in other clinical groups (eFigure 3 in the Supplement).
Associations Between Plasma Klotho Levels and Levels of Core CSF AD Biomarkers
There was a significant negative association between plasma Klotho levels and CSF T-tau levels (β = −0.398; 95% CI, −0.719 to −0.077; P = .02) and CSF P-tau levels (β = −0.268; 95% CI, −0.526 to −0.010; P = .04), but not between plasma Klotho levels and CSF Aβ42 levels (β = 0.176; 95% CI, −0.054 to −0.406; P = .13). Further stratification of the analysis showed no significant differences in these associations (eTable 3 in the Supplement).
Concentrations of Plasma Klotho Stratified by KL-VS Heterozygosity
Among the entire cohort, carriers of KL-VS heterozygosity tended to have higher median plasma Klotho levels compared with noncarriers (carriers, 889.4 pg/mL [range, 501.7-1563.5 pg/mL]; noncarriers, 796.2 pg/mL [range, 351.9-2733.8 pg/mL]; P = .08). Although no significant difference was found in the control group, median plasma Klotho levels in the AD group were significantly higher among KL-VS heterozygosity carriers compared with noncarriers (carriers, 910.9 pg/mL [range, 501.7-1563.5 pg/mL]; noncarriers, 790.1 pg/mL [range, 351.9-2733.8 pg/mL]; P = .002). The significant increase in median Klotho levels was associated with the AD-MCI group (carriers, 936.6 pg/mL [range, 501.7-1563.5 pg/mL]; noncarriers, 790.1 pg/mL [range, 351.9-2733.8 pg/mL]; P = .006) and was not present in the AD-dementia group.
Discussion
To our knowledge, this is the first study to evaluate the interaction between the KL-VS heterozygosity haplotype and Klotho levels in CSF and plasma and to correlate them with amyloid and tau burden in clinical stages of AD. We found the lowest concentrations of CSF Klotho in individuals with AD-dementia. Klotho levels in CSF decreased significantly with age in the control group but not the AD-MCI or AD-dementia groups. Higher levels of CSF Klotho were associated with lower CSF amyloid and tau burden, independent of clinical, KL-VS heterozygosity, or APOE4 status. The same association was not found with the KL-VS heterozygosity haplotype, although it resulted in higher CSF Klotho levels in the control and AD-MCI groups, but not in the AD-dementia group. There was a weak correlation between Klotho levels in CSF and plasma, and although plasma Klotho levels had a positive association with tau burden, an association with amyloid burden was not found.
Our results indicate a disease stage–dependent positive association of CSF Klotho levels. Only 1 study has addressed CSF Klotho levels in patients with AD and found a similar decrease compared with controls.15 How Klotho is associated with progression of AD in the human brain remains unknown, but mouse studies suggest that Klotho modulates N-methyl-d-aspartate receptor function and activates microglia cells to promote cognitive function.28,29 We found significantly higher levels of CSF Klotho in KL-VS heterozygosity carriers in the control and AD-MCI groups but not the AD-dementia group. Our findings suggest that, with higher levels of Klotho, progression along the AD continuum is less likely and that the association of KL-VS heterozygosity status with higher Klotho levels is abolished in the final stages of the disease.
A previous study showed a negative correlation of CSF Klotho levels with age in a combined cohort of controls and patients with AD.15 We confirmed this finding for the control group, but in the AD group, this age correlation was lost. This finding indicates that the decrease of CSF Klotho levels occurs at a younger age in AD patients. Klotho expression is regulated by promoter methylation, and increased methylation with age results in downregulated expression of Klotho.30,31 Thus, our results suggest that this epigenetic mechanism might be dysregulated early in the pathogenesis of AD. An increased Klotho promoter methylation level has already been described in several malignant neoplasms, and epigenetic silencing of Klotho expression was associated with poor prognosis.31,32,33,34
In this study, we demonstrate a significant positive association of CSF Klotho levels with CSF Aβ42 levels independent of clinical, KL-VS heterozygosity, or APOE4 status, suggesting that CSF Klotho levels are associated with amyloid burden among all individuals. No study has explored the association of CSF Klotho levels with amyloid burden, to our knowledge. Several studies have shown that the KL-VS heterozygosity haplotype is associated with reduced amyloid burden in controls carrying APOE4,10,11,12 whereas 1 study could not confirm these results.35 We found no significant association between KL-VS heterozygosity and CSF Aβ42 levels, but this finding should be interpreted with caution owing to our somewhat limited sample size. This was evident in a recent study that had to double the sample size to identify a significant association between KL-VS heterozygosity and amyloid burden.13 Together, the association of the CSF Klotho protein levels, but not the genetic variant KL-VS heterozygosity, with levels of amyloid indicates that the results are mediated more robustly by the protein and not indirectly by the genotype that is correlated with higher protein levels. Thus, an association between CSF Klotho levels and amyloid burden is detectable, even in a cohort with low sample size. It is also suggested that KL-VS heterozygosity not only increases the concentration of Klotho but also changes its function, making the possible protective effect of CSF Klotho even more potent.36
A possible protective effect of KL-VS heterozygosity on tau accumulation has been explored to a lesser extent,13,14 and we are the first, to our knowledge, to report an association between CSF Klotho levels and CSF T-tau and P-tau levels in a clinical cohort with AD. This association was independent of clinical stage and KL-VS heterozygosity or APOE4 status. All our controls were amyloid negative, which indicates that higher levels of CSF Klotho may be associated with less formation of neurofibrillary tangles and may be associated with neuroprotective mechanisms in all individuals, independent of clinical or amyloid status. We could not find a significant association between the KL-VS heterozygosity haplotype and T-tau or P-tau levels, nor in the stratified analysis for APOE4 carriers and noncarriers, in contrast to 2 recent studies13,14 that demonstrated a protective association of KL-VS heterozygosity with tau burden. The deviation with our study might again be explained by low sample sizes in the groups when stratified by the KL-VS haplotype. The explanation as to how the Klotho level is associated with tau pathology is still unclear, but it has been linked to several biological processes, including calcium signaling,37 growth factor functions,38 insulin regulation,3 and reactive oxygen species regulation, thereby decreasing neuronal damage.39,40 It has also been speculated whether Klotho’s involvement in autophagy might be associated with the clearance of AD-related proteins.41,42
The significant association of CSF Klotho levels with lower tau burden suggests a potential association of Klotho levels with cognitive function. There was a significant but weak positive association between CSF Klotho levels and MMSE score in the AD group but not the control group, although the smaller variation in MMSE scores in the control group might have been associated with the result. This finding indicates that lower levels of CSF Klotho could be associated with greater cognitive decline among patients with AD. Two previous studies have demonstrated a similar positive correlation, but in heterogenous cohorts of patients with AD and other types of dementia, as well as controls.15,43 Cognitive decline and gray matter atrophy are closely associated with the progressive development of neurofibrillary tangles in the presence of amyloid pathology and are more likely associated with AD disease progression than amyloid burden.44,45,46 Taking this into account, the correlation between CSF Klotho levels and MMSE score in the AD group in our study might be mediated by the association of CSF Klotho levels and tau pathology.
There was a weak correlation between CSF and plasma Klotho levels in our study population, but we could not confirm that plasma Klotho levels were associated with clinical stages of AD or amyloid burden, as seen with CSF Klotho levels. Plasma Klotho levels were negatively associated with tau burden independent of clinical stage, indicating that higher levels of Klotho in blood may be associated with less neurodegeneration in general, independent of AD-specific amyloid pathology. Studies have also shown an association between lower levels of plasma Klotho and lower MMSE scores in older age and a higher risk for vascular dementia17,47 but not with an increased risk of AD.48 As with CSF Klotho levels, plasma Klotho levels were negatively correlated with age in the control group but not the AD group, suggesting a tissue-independent decrease in Klotho expression at a younger age among individuals with AD. The kidney produces Klotho for the blood, and the choroid plexus in the brain produces Klotho for the CSF.49 It is therefore natural to assume that CSF Klotho has a greater association with brain function.
Limitations
This case-control study has some limitations. First, the need for CSF collection led to the low sample size, especially in the AD-dementia group. Second, the cross-sectional design did not allow any causal interpretations. Third, all controls had Aβ42 CSF levels below the pathologic threshold, eliminating the possibility to study the association of Klotho levels with preclinical AD.
Conclusions
Our findings suggest that the Klotho protein, but not the KL-VS heterozygosity haplotype, differs in clinical stages of AD and is associated with cognitive decline and amyloid and tau burden. This may be explained by an accelerated decrease in Klotho during aging in patients with AD. The mechanisms causing the favorable effects of Klotho and methods for increasing the level of Klotho should be further explored.
eFigure 1. CSF Klotho by KL-VS Heterozygosity in the Clinical Groups
eFigure 2. Correlation Between CSF and Plasma Levels of Klotho
eFigure 3. Scatterplot of Plasma Klotho and Age in the Clinical Groups
eTable 1. Associations Between CSF Klotho and Core CSF AD Biomarkers in Clinical Groups
eTable 2. Associations Between CSF Klotho and AD Core CSF Biomarkers in Subgroups Based on APOE4+ and KL-VSHET+ Status
eTable 3. Associations Between Plasma Klotho and Core CSF AD Biomarkers
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45-51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 2.Shiozaki M, Yoshimura K, Shibata M, et al. Morphological and biochemical signs of age-related neurodegenerative changes in Klotho mutant mice. Neuroscience. 2008;152(4):924-941. doi: 10.1016/j.neuroscience.2008.01.032 [DOI] [PubMed] [Google Scholar]
- 3.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829-1833. doi: 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laszczyk AM, Fox-Quick S, Vo HT, et al. Klotho regulates postnatal neurogenesis and protects against age-related spatial memory loss. Neurobiol Aging. 2017;59:41-54. doi: 10.1016/j.neurobiolaging.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arking DE, Krebsova A, Macek M Sr, et al. Association of human aging with a functional variant of Klotho. Proc Natl Acad Sci U S A. 2002;99(2):856-861. doi: 10.1073/pnas.022484299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries CF, Staff RT, Harris SE, et al. Klotho, APOEε4, cognitive ability, brain size, atrophy, and survival: a study in the Aberdeen Birth Cohort of 1936. Neurobiol Aging. 2017;55:91-98. doi: 10.1016/j.neurobiolaging.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama JS, Sturm VE, Bonham LW, et al. Variation in longevity gene KLOTHO is associated with greater cortical volumes. Ann Clin Transl Neurol. 2015;2(3):215-230. doi: 10.1002/acn3.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubal DB, Yokoyama JS, Zhu L, et al. Life extension factor Klotho enhances cognition. Cell Rep. 2014;7(4):1065-1076. doi: 10.1016/j.celrep.2014.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama JS, Marx G, Brown JA, et al. Systemic Klotho is associated with KLOTHO variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav. 2017;11(2):391-400. doi: 10.1007/s11682-016-9598-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson CM, Schultz SA, Oh JM, et al. KLOTHO heterozygosity attenuates APOE4-related amyloid burden in preclinical AD. Neurology. 2019;92(16):e1878-e1889. doi: 10.1212/WNL.0000000000007323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belloy ME, Eger SJ, Le Guen Y, et al. ; A4 Study Team; Insight 46 Study Team; Australian Imaging Biomarkers and Lifestyle (AIBL) Study; Alzheimer’s Disease Neuroimaging Initiative . KL∗VS heterozygosity reduces brain amyloid in asymptomatic at-risk APOE∗4 carriers. Neurobiol Aging. 2021;101:123-129. doi: 10.1016/j.neurobiolaging.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD; Alzheimer’s Disease Neuroimaging Initiative . Association of Klotho-VS heterozygosity with risk of Alzheimer disease in individuals who carry APOE4. JAMA Neurol. 2020;77(7):849-862. doi: 10.1001/jamaneurol.2020.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neitzel J, Franzmeier N, Rubinski A, et al. ; Alzheimer’s Disease Neuroimaging Initiative (ADNI) . KL-VS heterozygosity is associated with lower amyloid-dependent tau accumulation and memory impairment in Alzheimer’s disease. Nat Commun. 2021;12(1):3825. doi: 10.1038/s41467-021-23755-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driscoll I, Ma Y, Gallagher CL, et al. Age-related tau burden and cognitive deficits are attenuated in KLOTHO KL-VS heterozygotes. J Alzheimers Dis. 2021;79(3):1297-1305.Published correction appears in J Alzheimers Dis. 2021;82(3):1369-1370. doi: 10.3233/JAD-200944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semba RD, Moghekar AR, Hu J, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett. 2014;558:37-40. doi: 10.1016/j.neulet.2013.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 17.Brombo G, Bonetti F, Ortolani B, et al. Lower plasma Klotho concentrations are associated with vascular dementia but not late-onset Alzheimer’s disease. Gerontology. 2018;64(5):414-421. doi: 10.1159/000488318 [DOI] [PubMed] [Google Scholar]
- 18.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grøntvedt GR, Lauridsen C, Berge G, et al. The amyloid, tau, and neurodegeneration (A/T/N) classification applied to a clinical research cohort with long-term follow-up. J Alzheimers Dis. 2020;74(3):829-837. doi: 10.3233/JAD-191227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fladby T, Pålhaugen L, Selnes P, et al. Detecting at-risk Alzheimer’s disease cases. J Alzheimers Dis. 2017;60(1):97-105. doi: 10.3233/JAD-170231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. doi: [DOI] [PubMed] [Google Scholar]
- 24.Kalheim LF, Fladby T, Coello C, Bjørnerud A, Selnes P. [18F]-Flutemetamol uptake in cortex and white matter: comparison with cerebrospinal fluid biomarkers and [18F]-fludeoxyglucose. J Alzheimers Dis. 2018;62(4):1595-1607. doi: 10.3233/JAD-170582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berge G, Sando SB, Rongve A, Aarsland D, White LR. Apolipoprotein E ε2 genotype delays onset of dementia with Lewy bodies in a Norwegian cohort. J Neurol Neurosurg Psychiatry. 2014;85(11):1227-1231. doi: 10.1136/jnnp-2013-307228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rongve A, Witoelar A, Ruiz A, et al. Author correction: GBA and APOE ε4 associate with sporadic dementia with Lewy bodies in European genome wide association study. Sci Rep. 2019;9(1):15168. doi: 10.1038/s41598-019-51827-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fladby T, Pålhaugen L, Selnes P, et al. Detecting at-risk Alzheimer’s disease cases. J Alzheimers Dis. 2017;60(1):97-105. doi: 10.3233/JAD-170231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubal DB, Zhu L, Sanchez PE, et al. Life extension factor Klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci. 2015;35(6):2358-2371. doi: 10.1523/JNEUROSCI.5791-12.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng CY, Yang TT, Zhou HJ, et al. Lentiviral vector-mediated overexpression of Klotho in the brain improves Alzheimer’s disease–like pathology and cognitive deficits in mice. Neurobiol Aging. 2019;78:18-28. doi: 10.1016/j.neurobiolaging.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 30.King GD, Rosene DL, Abraham CR. Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age (Dordr). 2012;34(6):1405-1419. doi: 10.1007/s11357-011-9315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Jeong DJ, Kim J, et al. The anti-aging gene KLOTHO is a novel target for epigenetic silencing in human cervical carcinoma. Mol Cancer. 2010;9:109. doi: 10.1186/1476-4598-9-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinek T, Shulman M, Israeli S, et al. Epigenetic silencing of the tumor suppressor Klotho in human breast cancer. Breast Cancer Res Treat. 2012;133(2):649-657. doi: 10.1007/s10549-011-1824-4 [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Wang X, Wang X, et al. Klotho is silenced through promoter hypermethylation in gastric cancer. Am J Cancer Res. 2011;1(1):111-119. [PMC free article] [PubMed] [Google Scholar]
- 34.Xie B, Zhou J, Yuan L, et al. Epigenetic silencing of Klotho expression correlates with poor prognosis of human hepatocellular carcinoma. Hum Pathol. 2013;44(5):795-801. doi: 10.1016/j.humpath.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 35.Porter T, Burnham SC, Milicic L, et al. Klotho allele status is not associated with Aβ and APOE ε4–related cognitive decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2019;76:162-165. doi: 10.1016/j.neurobiolaging.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 36.Tucker Zhou TB, King GD, Chen C, Abraham CR. Biochemical and functional characterization of the Klotho-VS polymorphism implicated in aging and disease risk. J Biol Chem. 2013;288(51):36302-36311. doi: 10.1074/jbc.M113.490052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase Klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310(5747):490-493. doi: 10.1126/science.1114245 [DOI] [PubMed] [Google Scholar]
- 38.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770-774. doi: 10.1038/nature05315 [DOI] [PubMed] [Google Scholar]
- 39.Zeldich E, Chen CD, Colvin TA, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289(35):24700-24715. doi: 10.1074/jbc.M114.567321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou HJ, Zeng CY, Yang TT, Long FY, Kuang X, Du JR. Lentivirus-mediated Klotho up-regulation improves aging-related memory deficits and oxidative stress in senescence-accelerated mouse prone-8 mice. Life Sci. 2018;200:56-62. doi: 10.1016/j.lfs.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 41.Fernández ÁF, Sebti S, Wei Y, et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136-140. doi: 10.1038/s41586-018-0162-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uddin MS, Stachowiak A, Mamun AA, et al. Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci. 2018;10:04. doi: 10.3389/fnagi.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kundu P, Zimmerman B, Quinn JF, et al. Serum levels of α-Klotho are correlated with cerebrospinal fluid levels and predict measures of cognitive function. J Alzheimers Dis. 2022;86(3):1471-1481. doi: 10.3233/JAD-215719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020;12(524):eaau5732. doi: 10.1126/scitranslmed.aau5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison TM, La Joie R, Maass A, et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann Neurol. 2019;85(2):229-240. doi: 10.1002/ana.25406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki Y, Imura A, Urakawa I, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398(3):513-518. doi: 10.1016/j.bbrc.2010.06.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shardell M, Semba RD, Rosano C, et al. Plasma Klotho and cognitive decline in older adults: findings from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2016;71(5):677-682. doi: 10.1093/gerona/glv140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cararo-Lopes MM, Mazucanti CHY, Scavone C, Kawamoto EM, Berwick DC. The relevance of α-KLOTHO to the central nervous system: some key questions. Ageing Res Rev. 2017;36:137-148. doi: 10.1016/j.arr.2017.03.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. CSF Klotho by KL-VS Heterozygosity in the Clinical Groups
eFigure 2. Correlation Between CSF and Plasma Levels of Klotho
eFigure 3. Scatterplot of Plasma Klotho and Age in the Clinical Groups
eTable 1. Associations Between CSF Klotho and Core CSF AD Biomarkers in Clinical Groups
eTable 2. Associations Between CSF Klotho and AD Core CSF Biomarkers in Subgroups Based on APOE4+ and KL-VSHET+ Status
eTable 3. Associations Between Plasma Klotho and Core CSF AD Biomarkers