Abstract
A hallmark of gas gangrene (clostridial myonecrosis) pathology is a paucity of leukocytes infiltrating the necrotic tissue. The cause of this paucity most likely relates to the observation of leukocyte aggregates at the border of the area of tissue necrosis, often within the microvasculature itself. Infecting mice with genetically manipulated strains of Clostridium perfringens type A (deficient in either alpha-toxin or theta-toxin production) resulted in significantly reduced leukocyte aggregation when alpha-toxin was absent and complete abrogation of leukocyte aggregation when theta-toxin was absent. Thus, both alpha-toxin and theta-toxin are necessary for the characteristic vascular leukostasis observed in clostridial myonecrosis.
Clostridial myonecrosis is characterized by rapidly spreading edema and necrosis associated with bacterial proliferation and exotoxin production (8). Very few leukocytes are observed to infiltrate the myonecrotic tissue, although small aggregates of leukocytes are observed at the borders of the region showing necrosis, both in the extracellular space and within the lumina of small blood vessels (4, 8, 10). This suggests that the host response to Clostridium perfringens type A infection is defective; even multiple infections fail to provide protection against infection (13). Whether this defect results from the myonecrotic properties of the bacterial exotoxins or from exotoxin-mediated functional modulation of the inflammatory cascade remains to be elucidated.
The latter hypothesis is supported by studies in which exposure to purified alpha-toxin was shown to induce upregulation of intercellular adherence molecule (ICAM-1), E-selectin, and P-selectin expression as well as increased platelet aggregating factor (PAF) and interleukin 8 (IL-8) production (4, 6). Furthermore, exposure to purified theta-toxin has been shown to induce upregulation of the β2-integrin Mac-1 (CD11b/CD18) on the surfaces of leukocytes and ICAM-1 expression and PAF production by endothelial cells (4, 5, 14). Since all of these molecules are important for leukocyte migration across the endothelium during inflammation, their functional upregulation could cause excessively strong leukocyte-endothelium (and leukocyte-leukocyte) binding, thereby slowing or halting transendothelial migration.
The functional relevance of these exotoxin-mediated effects to the in vivo infection, however, is yet to be definitively established. In one study, purified alpha-toxin was injected into mice and indeed caused leukocyte accumulation, but the accumulation occurred in the extravascular space, not within the blood vessels, and therefore is unlikely to have been due to the proinflammatory changes to endothelial cells described in that study (6).
To address this issue further, we have employed isogenic sets of genetically manipulated strains of C. perfringens type A, the bacterium associated with over 80% of clinical cases of gas gangrene (7, 9, 13). The strains, derivatives of the type A strain 13, are unable to produce either alpha-toxin or theta-toxin (2, 3, 12). To prepare an inoculum for the mouse myonecrosis model a single colony, grown on the appropriate selective medium, was subcultured into Trypticase peptone glucose (TPG) broth and grown for 4 to 5 h at 37°C. The cells were then pelleted by centrifugation at 4°C (3,000 × g, 10 min), washed twice in phosphate-buffered saline (PBS), and diluted to 1010 CFU per ml, based on a previously determined standard plot of turbidity (measured at 650 nm) against the number of CFU per milliliter. For all experiments, the phenotype of each strain was verified (pre- and postinjection) by growth on horse blood agar and egg yolk agar to test for theta-toxin production and alpha-toxin production, respectively. The viable bacterial count of the inoculum was verified for each experiment. For heat-killed inocula, a 4-h wild-type C. perfringens TPG culture was autoclaved at 121°C for 30 min before the PBS washing steps (which removed preformed exotoxin). Samples were taken prior to heat treatment for viable counts and confirmation of toxin phenotype.
Clostridial myonecrosis animal model.
All animal studies (performed in duplicate) were undertaken in accordance with protocols approved by the Monash Medical School Animal Experimentation Ethics Committee. For each C. perfringens strain, 20 BALB/c mice 6 to 8 weeks of age were injected intramuscularly in the right thigh with 100 μl of the inoculum described above (109 CFU; this amount was previously determined to be optimal for producing typical gas gangrene pathology). Four mice were randomly chosen and killed at each time point (2, 4, 8, 12 and 24 h postinjection). The gross pathology of the infected limb was noted, and tissue samples were taken. Half of the samples collected at each time point were formalin fixed overnight and paraffin embedded for standard histology; the remaining samples were snap-frozen in OCT embedding compound (Tissue-Tek) by using liquid nitrogen. The frozen tissues were stored at −70°C for use in the immunohistological studies.
For the histological studies, 2 or 3 serial sections (4- to 5-μm thickness) were taken at approximately every 100 μm for each tissue sample and coded with an encrypted numbering system (to allow blind examination) prior to standard hematoxylin and eosin staining. The entire section was examined, focusing on the degree of muscle necrosis and leukocyte accumulation, as well as the status of any migrating leukocytes in relation to the position of the bacterial cells.
Sections from tissue samples taken 8 to 12 h postinjection were examined in further detail; every observable blood vessel was scored for the degree of leukocyte accumulation (Table 1) and for thrombosis (Table 2). These results were analyzed for statistical significance of differences from those for the toxin-negative control (tissues injected with heat-killed C. perfringens cells) and toxin-positive control (tissues injected with wild-type C. perfringens cells) by using the chi-square test. Photographs of fields which best represented as many histological features of each tissue sample as possible were taken (Fig. 1).
TABLE 1.
Frequency of leukocyte accumulation within blood vesselsa
| C. perfringens strain | Total no. of blood vessels | No. (%) of vessels with:
|
|
|---|---|---|---|
| Moderate leukocyte accumulation | Severe leukocyte accumulation | ||
| Wild-type heat-killed (JIR325)c | 201 | 9 (4.5) | 0 |
| Wild-type (JIR325)b | 478 | 46 (9.6) | 41 (8.6) |
| Theta-toxin-deficient (JIR4081)c | 485 | 27 (5.6) | 3 (0.6) |
| Alpha-toxin-deficient (JIR4120)bc | 466 | 72 (15.5) | 14 (3.0) |
Analysis of the frequency of leukocyte accumulation within blood vessels of tissue samples taken 8 to 12 h postinjection. Moderate leukocyte accumulation indicates that the vessel had a single layer of leukocytes lining the luminal surface of the blood vessel (an example of such a vessel is indicated in Fig. 1I). Severe leukocyte accumulation indicates multiple leukocyte layers (an example of such a vessel is indicated in Fig. 1E).
Differs significantly from tissues injected with heat-killed C. perfringens cells (P < 0.0001).
Differs significantly from tissues injected with wild-type C. perfringens cells (P < 0.0001).
TABLE 2.
Frequency of thrombotic blood vesselsa
| C. perfringens strain | Total no. of blood vessels | No. (%) of vessels with thrombosis |
|---|---|---|
| Wild-type heat-killed (JIR325)c | 201 | 0 |
| Wild-type (JIR325)b | 478 | 68 (14.2) |
| Theta-toxin-deficient (JIR4081)b | 485 | 63 (13.0) |
| Alpha-toxin deficient (JIR4120)c | 466 | 2 (0.4) |
Analysis of the frequency of thrombotic blood vessels in tissue samples taken 8 to 12 h postinjection. Thrombosis was determined by the presence of pale pink occlusions within hematoxylin and eosin-stained vessels, like those indicated in Fig. 1E and G, correlating to areas of platelet and fibrin deposition.
Differs significantly from tissues injected with heat-killed C. perfringens cells (P < 0.0001).
Differs significantly from tissues injected with wild-type C. perfringens cells (P < 0.0001).
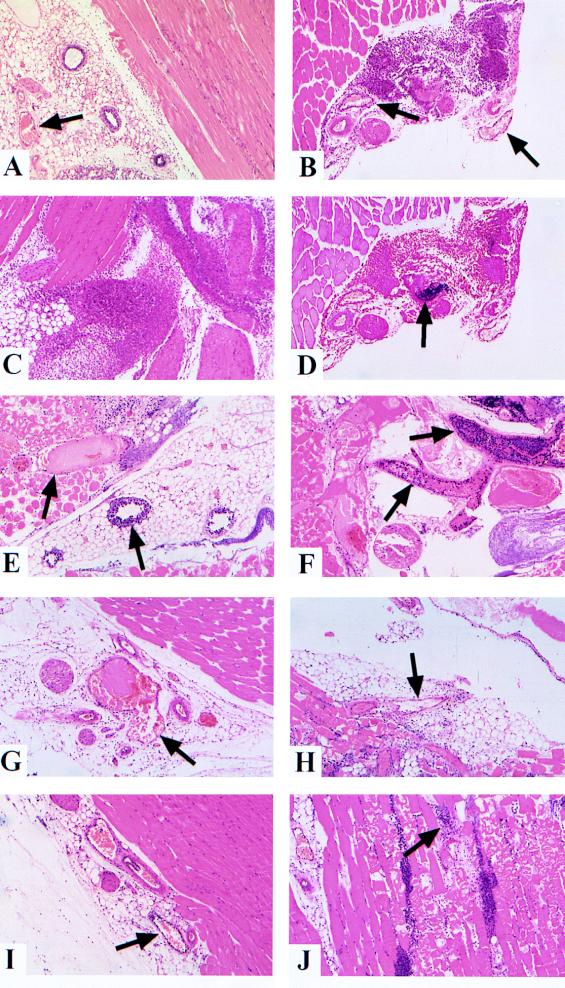
FIG. 1.
Histological analysis of tissue injected with various strains of C. perfringens type A. Sections were stained with hematoxylin and eosin unless otherwise stated. Arrows indicate the positions of blood vessels or bacterium-leukocyte aggregates. (A) Inoculum containing PBS only. There is little leukocyte infiltration into the tissue, and there is no leukocyte accumulation within small venules. (B, C, and D) Heat-killed C. perfringens inoculum. There is little or no leukocyte accumulation within blood vessels at 4 h postinjection (panel B). Extensive leukocyte infiltration into the infected tissue is apparent by 8 h (panel C). Infiltrating leukocytes are observed to surround and penetrate bacterial aggregates by 4 h (analyzed by using gram-stained serial section) (panel D). (E and F) Wild-type C. perfringens inoculum. Profound leukocyte accumulation and thrombosis are observed in vessels collected at 4 h (panel E) and 8 h (panel F), with very little leukocyte infiltration into surrounding tissue. (G and H) Theta-toxin-deficient C. perfringens inoculum. Little or no leukocyte accumulation is present within blood vessels at 4 h although blood vessel thrombosis is prominent (panel G). Significant leukocyte infiltration of surrounding tissue is observed at 8 h (panel H), although leukocytes rarely colocalize with bacterial aggregates. (I and J) Alpha-toxin-deficient C. perfringens inoculum. Occasional leukocyte accumulation is observed within blood vessels at 8 h (panel I); blood vessel thrombosis is rarely observed. Significant leukocyte infiltration of surrounding tissue is apparent by 12 h (panel J); penetration and clearance of the aggregates occur but to a reduced degree compared to that observed in tissues injected with heat-killed C. perfringens cells.
Heat-killed C. perfringens inoculum.
Heat-killed, washed wild-type C. perfringens inoculum induced tissue histopathology indicative of a normal inflammatory response. Large numbers of leukocytes infiltrated the tissue between 4 and 8 h postinjection and migrated to the site of injection. As expected, due to the absence of exotoxins, no muscle necrosis was observed. Furthermore, only 4.5% of blood vessels counted displayed any signs of leukocyte accumulation (Table 1). Marked leukocyte migration to the site of infection was observed as early as 4 h postinjection (Fig. 1B), and the migration increased dramatically by 8 to 12 h (Fig. 1C). Gram staining of serial sections demonstrated leukocytes penetrating the bacterial aggregates by 4 h postinjection (Fig. 1D). These bacterial aggregates were absent by 8 to 12 h postinjection, suggesting successful clearance of the bacterial cells.
Wild-type C. perfringens inoculum.
In mice injected with the viable wild-type C. perfringens cells (JIR325), extensive muscle necrosis was observed by 8 to 12 h postinjection. As with all of the C. perfringens strains, the bacterial cells were observed initially in the extracellular regions, particularly the fat and connective tissues. As the infection spread, the bacterial cells colonized myonecrotic tissue behind the advancing border of necrosis. Little leukocyte infiltrate was observed in the tissues at all time points, with the exception of small leukocyte aggregates (usually distant from necrotic areas) observed in both the blood vessels and the extracellular matrix. Leukocyte accumulation was observed within blood vessels at the borders of the necrotic area as early as 2 to 4 h postinjection, and the accumulation increased by 8 to 12 h (Fig. 1E and F). Of the blood vessels counted, 9.6 and 8.6% displayed moderate and severe leukocyte accumulation, respectively (Table 1); these amounts were significantly increased compared to levels observed in tissues injected with heat-killed C. perfringens cells (P < 0.0001). The severe accumulation appeared to involve multiple binding events (leukocyte to endothelium, leukocyte to leukocyte, and leukocyte to platelet binding), which resulted in several layers of leukocytes halting within the vessel and failing to migrate across the endothelial layer. Significant thrombosis was also observed (Fig. 1E and F), with 14.2% of vessels displaying signs of thrombotic occlusion (Table 2; P < 0.0001 compared to the level in tissues injected with heat-killed C. perfringens cells).
Theta-toxin-deficient C. perfringens inoculum.
Muscle from tissues injected with the theta-toxin-deficient strain JIR4081 (1) displayed a delayed spread of muscle necrosis; extensive necrosis was not observed until 12 to 16 h postinjection (in contrast to 8 to 12 h for the wild-type strain). The amount of leukocyte infiltrate was increased markedly compared to that in wild-type C. perfringens-infected tissues, but it was never increased to the degree observed in tissues injected with heat-killed C. perfringens cells. Leukocyte accumulation within the blood vessels did not differ significantly from that in tissues injected with heat-killed C. perfringens cells (Table 1; 5.6% of blood vessels displayed moderate leukocyte accumulation) despite the extensive muscle necrosis present. However, many of the blood vessels (13.0%; P < 0.0001 compared to the percentage of vessels in tissues injected with heat-killed C. perfringens cells) (Table 2) did appear thrombotic (Fig. 1G and H). Indeed, the frequency of thrombosis in tissues infected with the theta-toxin-deficient strain was not significantly different from that observed for wild-type strain infection (P = 0.58). Numerous leukocytes were able to penetrate areas of myonecrosis (Fig. 1H) but appeared unable to penetrate the bacterial aggregates to the degree observed for heat-killed C. perfringens-injected tissues).
Alpha-toxin-deficient C. perfringens inoculum.
Muscle tissue from mice injected with the alpha-toxin-deficient strain JIR4120 (1) appeared relatively healthy at all time points studied and displayed a marked decrease in the severity of muscle necrosis; only a few small sites of necrosis appeared at later time points (Fig. 1J). There was also a reduction in the frequency of severe leukocyte accumulation within blood vessels (3.0%) compared to that (8.6%) for the wild-type strain (Table 1); however, 15.5% of blood vessels (Table 1) were observed to have a moderate build-up of leukocytes (Fig. 1I). Thus, while the overall severity of the leukocyte accumulation was significantly reduced (Table 1; P < 0.0001 compared to tissues injected with wild-type C. perfringens cells), the total percentages of vessels displaying some degree of leukocyte accumulation were almost identical for the alpha-toxin deficient (18.5%) and wild-type (18.2%) strains. Despite the significant leukocyte accumulation (Table 1; P < 0.0001 compared to tissues injected with heat-killed C. perfringens cells), thrombosis of the vessels was not observed (Table 2, 0.4%). Numerous leukocytes were present throughout the infected tissue, including the sites of myonecrosis and bacterial aggregates (Fig. 1J). However, bacterial aggregates persisted at all time points, suggesting that clearance of the infection was retarded (compared to observations of heat-killed C. perfringens-injected tissues).
Relevance of animal model results to clinical disease.
There are two general mechanisms by which the exotoxins of C. perfringens may effectively paralyze the host inflammatory response. First, the toxins may exert a purely cytotoxic effect on the leukocytes or endothelium. Second, a genuine modulation of the inflammatory cascade may occur, resulting in leukocyte aggregation within the blood vessels and the extracellular matrix, preventing effective migration toward, and clearance of, the bacteria themselves.
Previous studies have demonstrated a modulation of leukocyte and endothelial adhesion molecule expression after exposure to alpha-toxin and theta-toxin, supporting the hypothesis that a genuine modulation and inhibition of the inflammatory response allows the infection to persist. In particular, purified theta-toxin and alpha-toxin enhanced Mac-1, ICAM-1, P-selectin, and E-selectin expression and increased PAF and IL-8 production (4–6). In vivo, these events could lead to discordant binding between the leukocytes and the endothelium or extracellular matrix, inhibiting rapid leukocyte infiltration and clearance of the pathogen. Indeed, this prediction correlates with the observed pathology of clostridial myonecrosis (4, 8, 10).
Infection with a theta-toxin-deficient C. perfringens strain produced no detectable leukocyte aggregation within the blood vessels or the extracellular matrix, despite the extensive muscle necrosis observed. However, thrombosis was present in many blood vessels, possibly attributable to the effects of alpha-toxin, either due to its reported ability to stimulate PAF release by endothelial cells (6) or simply due to platelet deposition stimulated by endothelial cell damage. This result not only establishes the importance of theta-toxin in the vascular accumulation of leukocytes but also provides evidence that the leukocyte paucity is not purely due to a cytotoxic effect, as the thrombotic occlusions in these tissues were completely free of leukocyte accumulation.
Tissue samples from the thigh muscle of mice infected with an alpha-toxin-deficient strain displayed significant leukocyte accumulation within numerous blood vessels, but the accumulation was reduced in severity compared to that for tissues injected with the wild-type strain. Additionally, numerous leukocytes infiltrated the infected tissue. Thus, alpha-toxin also contributes to the leukocyte accumulation observed in wild-type C. perfringens infections—the ability to produce theta-toxin is insufficient to completely inhibit leukocyte transendothelial migration and mass infiltration to the site of infection. The results also show that alpha-toxin is essential for thrombosis formation, which was absent in muscles infected with the alpha-toxin-deficient strain. Thrombosis is an important factor in the pathology of gas gangrene, as the resulting blood vessel occlusion should reduce oxygen tension in the tissue, enhancing conditions for the anaerobic growth of C. perfringens.
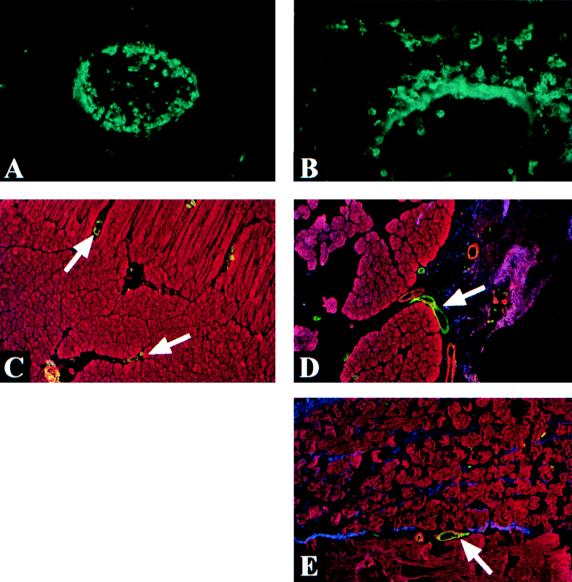
We therefore conclude that both alpha-toxin production and theta-toxin production are required for the formation of leukocyte aggregates to the degree observed within the blood vessels and the extracellular matrix of tissues injected with wild-type C. perfringens cells. The histological results suggest that the phenomenon of vascular leukostasis involves both a cytotoxic component, due to alpha-toxin, and a modulatory component, due to theta-toxin. However, precise in vivo evidence for the cellular and molecular mechanisms underlying the phenomenon remains elusive. Our studies on adhesion molecule and cytokine expression in vivo have not yet revealed any changes which would explain the accumulation of leukocytes within the blood vessels of tissues injected with the wild-type strain and, to a lesser extent, the alpha-toxin mutant strain. In particular, compared to tissues injected with heat-killed C. perfringens cells, no increase in Mac-1 or ICAM-1 staining was observed in tissues injected with any of the live C. perfringens strains (Fig. 2). Indeed, the intensity of Mac-1 staining remained constant regardless of the inoculum content (including the PBS alone control injection). However, Mac-1 staining clearly illustrated the difference between unhindered, directional leukocyte migration across the endothelial layer (Fig. 2A) and the leukocyte aggregation and inhibition of transendothelial migration observed in response to wild-type C. perfringens (Fig. 2B).
FIG. 2.
Immunofluorescence staining of Mac-1 and ICAM-1. (A and B) Mac-1 staining. Accumulation of leukocytes within blood vessels is marked for tissue injected with wild-type C. perfringens inocula (panel A) compared to blood vessels in tissue injected with heat-killed C. perfringens inocula (panel B), which remain free of leukocyte build-up. Polarized staining of the blood vessel wall observed in the heat-killed C. perfringens-injected tissue (panel B) is most likely attributable to shedding of Mac-1 by leukocytes during direct transendothelial migration. (C, D, and E) ICAM-1 staining. The apical surface of endothelial cells stained strongly for ICAM-1 (green) in tissues injected with PBS alone (panel C) or with heat-killed (panel D) and wild-type (panel E) C. perfringens cells. C. perfringens cells (blue) and muscle fibers (red) were also visualized in these tissues by using antibody conjugates. The intensity of ICAM-1 staining in tissue injected with heat-killed or wild-type C. perfringens cells was observed to be consistently brighter than that in tissue injected with PBS alone, although the difference was not highly significant. No difference in ICAM-1 staining intensities between tissue samples injected with either live or heat-killed C. perfringens cells was observed.
ICAM-1 staining increased slightly in tissues injected with heat-killed (Fig. 2D) or wild-type (Fig. 2E) C. perfringens cells compared to staining for tissues from control mice (Fig. 2C). However, there were no observable differences between tissues injected with heat-killed cells and those injected with wild-type cells. Thus, altered ICAM-1 expression is unlikely to be an underlying mechanism of leukocyte accumulation, since vascular leukostasis is not observed when heat-killed C. perfringens cells are injected.
The lack of increased Mac-1 and ICAM-1 staining levels contradicts previous studies by Bryant and coworkers, where increased expression of these cellular adhesion molecules was observed in response to purified toxins (4, 5). One likely explanation is that injecting individual, purified toxins may not accurately reflect an infection with live bacteria that secrete a range of exotoxins (reviewed in references 7 and 11), which may have synergistic effects. Alternatively, it is possible that immunofluorescence staining of the infected tissues is not sensitive enough to detect subtle changes in adhesion molecule expression that may be sufficient to disrupt the inflammatory cascade. Further studies are required to investigate whether the reported effects of alpha-toxin and theta-toxin on adhesion molecule expression have functional significance at the level of leukocyte migration across the endothelial layer and the relevance of this to clinical gas gangrene.
Acknowledgments
The work performed in the Department of Microbiology, Monash University, was supported by a grant to J. I. Rood from the Australian National Health and Medical Research Council.
REFERENCES
- 1.Awad M, Bryant A, Stevens D, Rood J. Virulence studies on chromosomal α-toxin and θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol. 1995;15:191–202. doi: 10.1111/j.1365-2958.1995.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 2.Awad M, Rood J. Isolation of α-toxin, θ-toxin and κ-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb Pathog. 1997;22:275–284. doi: 10.1006/mpat.1996.0115. [DOI] [PubMed] [Google Scholar]
- 3.Bannam T, Rood J. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid. 1992;229:233–235. doi: 10.1006/plas.1993.1025. [DOI] [PubMed] [Google Scholar]
- 4.Bryant A, Bergstrom R, Zimmerman G, Salyer J, Hill H, Tweten R, Sato H, Stevens D. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol Med Microbiol. 1993;7:321–336. doi: 10.1111/j.1574-695X.1993.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Bryant A, Stevens D. Phospholipase C and perfringolysin O from Clostridium perfringens upregulate endothelial cell-leukocyte adherence molecule 1 and intercellular leukocyte adherence molecule 1 expression and induce interleukin-8 synthesis in cultured human umbilical vein endothelial cells. Infect Immun. 1996;64:358–362. doi: 10.1128/iai.64.1.358-362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunting M, Lorant D, Bryant A, Zimmerman G, McIntyre T, Stevens D, Prescott S. Alpha toxin from Clostridium perfringens induces proinflammatory changes in endothelial cells. J Clin Investig. 1997;100:565–574. doi: 10.1172/JCI119566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonel J. Toxins of Clostridium perfringens type A, B, C, D, and E. In: Dorner F, Drews J, editors. Pharmacology of bacterial toxins. Oxford, United Kingdom: Pergamon Press; 1986. p. 477. [Google Scholar]
- 8.McNee J, Dunn J. The method of spread of gas gangrene into living muscle. BMJ. 1917;1:727–729. doi: 10.1136/bmj.1.2944.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onderdonk A B, Allen S D. Clostridium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 574–586. [Google Scholar]
- 10.Robb-Smith A. Tissue changes induced by Cl. welchii type A filtrates. Lancet. 1945;2:362–368. [Google Scholar]
- 11.Rood J, Cole S. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991;55:621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan J, Warner T, Scott P, Bannam T, Berryman D, Rood J. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid. 1992;27:207–219. doi: 10.1016/0147-619x(92)90023-4. [DOI] [PubMed] [Google Scholar]
- 13.Wells C, Wilkins T. Clostridia: spore-forming anaerobic bacilli. In: Brown S, editor. Medical microbiology. 3rd ed. New York, N.Y: Churchill Livingstone; 1991. pp. 279–296. [Google Scholar]
- 14.Whatley R, Nelson P, Zimmerman G, Stevens D, Parker C, McIntyre T, Prescott S. The regulation of platelet-activating factor production in endothelial cells. The role of calcium and protein kinase C. J Biol Chem. 1989;264:6325–6333. [PubMed] [Google Scholar]