Abstract
Ferroptosis is a novel kind of regulated cell death distinct from autophagy, apoptosis, and necrosis; it is predominantly caused by the iron-dependent lipid peroxidation. According to studies, numerous conventional signaling pathways and biological processes are implicated in the process of ferroptosis. In recent years, researchers have shown that ferroptosis plays an important role in the genesis, development, and metastasis of malignancies, including ovarian cancer. Several studies have revealed that ferroptosis has synergistic effects with chemotherapy, radiotherapy, and immunotherapy in inhibiting the growth of ovarian cancer cells. This suggests that ferroptosis is important in ovarian cancer treatment and may be a new target. In this review, we summarize the features of ferroptosis, including its underlying basis and function in ovarian cancer, as well as its potential applications in the treatment of ovarian cancer.
Keywords: ferroptosis, ovarian cancer, target, therapy
Introduction
Ovarian cancer (OC) is the primary cause of mortality from female reproductive cancers around the world 1. Moreover, because ovarian cancer is frequently diagnosed at an advanced stage, it presents a significant clinical challenge 2. Although overall survival at all stages of ovarian cancer has improved with advances in surgery, chemotherapy, and new immunotherapies, the 5-year overall survival rate for these patients remains at about 30% 3. Additionally, reliable biomarkers and therapeutic targets for the diagnosis of ovarian cancer are still lacking.
Ferroptosis is a recently identified form of regulated cell death driven by the iron-caused accumulation of lipid peroxide, and distinct from autophagy, apoptosis, and necrosis in morphology, biochemistry 4. Recent evidence demonstrates that ferroptosis modulates tumor genesis, development, and metastasis; thus, targeting ferroptosis may be a viable method for the treatment of ovarian cancer 5. In addition, several studies have revealed that ferroptosis has synergistic effects with chemotherapy, radiotherapy, and immunotherapy in inhibiting the growth of ovarian cancer cells. Chemotherapy is one of the standard treatments for ovarian cancer, which can effectively reduce the number of cancer cells and improve the prognosis of patients. However, some patients present with drug resistance or produce many side effects after treatment. At present, many studies have found that ferroptosis can synergize with chemotherapy to enhance the anticancer effect 6. Radiotherapy is a useful strategy for recurrent or refractory ovarian cancer. At present, many studies have found that ferroptosis can synergize with radiotherapy to enhance the anticancer effect 7. Immunotherapy's effectiveness in treating cancer has been widely recognized. However, immunotherapy is restricted since only one-third of ovarian cancer patients react to these agents. Recently, more than one kind of cell death mechanism has been shown to communicate with antitumor immunity. Wang et al. 8 found that the combination of ferroptosis induction and immunotherapy increased anticancer efficacy synergistically. Mechanistically, CD8+ T cells stimulated by immunotherapy induce ferroptosis in tumor cells. Ferroptotic cancer cells release high-mobility group box-1 (HMGB1) in an autophagy-dependent manner 9. As a significant damage-associated molecular pattern (DAMP), HMGB1 is an essential protein for the immunogenic cell death (ICD) of cancer cells 10. Ferroptotic cancer cells may trigger potent immune responses and enhance antitumor immunity through ICD. Therefore, ferroptosis plays an important role in the treatment of ovarian cancer. Further understanding of the related mechanism and clarifying the target of action are expected to provide a new method for the treatment of ovarian cancer.
In this review, we summarize the features of ferroptosis based on its underlying basis and function in ovarian cancer, as well as its potential applications in the treatment of ovarian cancer. Consequently, targeting ferroptosis might be a novel and promising method for eliminating ovarian cancer.
The characteristics of ferroptosis
Morphologically, ferroptotic cells showed decreased mitochondrial volume, increased mitochondrial bilayer membrane density, decreased or absent mitochondrial cristae, and disruption of the outer mitochondrial membrane (OMM)4. Unlike necrosis, ferroptosis does not display swelling of the cytoplasm and organelles or breakdown of the plasma membrane. Unlike apoptosis, ferroptosis does not display agglutination of chromatin or the formation of apoptotic bodies. Unlike autophagy, ferroptosis does not display the formation of classical autophagosomes 11. In addition, microscopically recognizing ferroptotic cells is possible thanks to a unique phenotypic known as the ballooning phenotype, which is characterized by the development of a clear, spherical cell composed mostly of empty cytosol 12.
The Mechanism of Ferroptosis
Iron overload induces ferroptosis
Iron is usually found as ferrous (Fe2+) and ferric (Fe3+). Food iron primarily exists as Fe3+, which is insoluble and needs to be converted to Fe2+ for absorption. Fe3+ binds to transferrin (TF) in the serum and then is subsequently identified by the transferrin receptor (TFRC) in the cell membrane, which facilitates Fe3+ entrance into the endosome. The STEAP3 metalloreductase is an endosomal enzyme that reduces Fe3+ to Fe2+. Finally, Fe2+ is released from the endosomes to the cytosol and stored in the labile iron pool by solute carrier family 11 member 2 (SLC11A2/DMT1). Excess Fe2+ can be stored in the iron-storage protein ferritin, including ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1), or excreted from the cell via the solute carrier family 40 member 1 (SLC40A1/ferroportin1/FPN) 13. In addition, excess iron can activate iron-containing enzymes (such as lipoxygenase) and produce reactive oxygen species (ROS) via the Fenton reaction, thereby promoting lipid peroxidation and ferroptosis 14. Therefore, intracellular iron plays a crucial role in the maintenance of cellular homeostasis.
Lipid Peroxidation induces ferroptosis
Polyunsaturated fatty acids (PUFAs) are prone to lipid peroxidation and are necessary for ferroptosis. As one of the PUFAs, arachidonic acid or adrenic acid (AA/AdA) is the primary substrate of lipid peroxidation in ferroptosis 15. Acyl-CoA synthetase long chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are essential in PUFA synthesis and remodeling 16. ACSL4 catalyzes the combination of free AA/AdA and CoA to create AA/AdA-CoA, while LPCAT3 stimulates the esterification of AA/AdA-CoA to membrane phosphatidylethanolamine (PE) to generate AA/AdA-PE. Different lipoxygenases can mediate lipid peroxidation, resulting in the hydroperoxides AA/AdA-PE-OOH and promoting ferroptosis 17. When the genes of ACSL4 or LPCAT3 are knocked out, lipid peroxidation decreases, inhibiting ferroptosis. Multiple membrane electron transport proteins, in particular, POR 18 and the NADPH oxidases (NOXs) 4, also contribute to ROS generation for lipid peroxidation in ferroptosis.
Dysregulation of antioxidant defence induces ferroptosis
System Xc- is an amino acid antiporter formed by the functional subunit SLC7A11 and the regulatory subunit SLC3A2. It is an essential antioxidant system in cells and maintains the production of glutathione (GSH) by exchanging extracellular cystine with intracellular glutamate 4. Some small molecule compounds or drugs (such as erastin, sorafenib, and glutamate) can inhibit SLC7A11, leading to GSH depletion and triggering ferroptosis 19. In addition, some cells can promote the synthesis of GSH through the transsulfuration pathway (a pathway that bypasses the system Xc- and produces cysteine via methionine). Consequently, system Xc- inhibitors could not cause ferroptosis in these cells 20.
GSH is a necessary cofactor of the glutathione peroxidase 4(GPX4) antioxidant response and can cycle between oxidized (GSSG) and reduced (GSH) forms, allowing it to engage in redox biochemical reactions 21. Consequently, the targeted regulation of GSH is an essential mechanism of ferroptosis.
GPX4 can eliminate phospholipid peroxides and exerts a critical role in preventing the accumulation of ROS and maintaining lipid homeostasis, ultimately blocking ferroptosis 4. Consequently, the targeted regulation of GPX4 is an essential mechanism of ferroptosis.
AIFM2-CoQ10 22, GCH1-BH4 23 and ESCRT-III membrane repair systems 24 are non-GPx4 pathways and also play an important role in protecting against oxidative damage during ferroptosis. AIFM2 not only controls the generation of reduced CoQ10 but also activates the ESCRT-III membrane repair system 25.
Ferroptosis in Ovarian Cancer-Associated Signaling Pathways
MicroRNA (miRNA) induces ferroptosis
MiRNA is a non-coding RNA that plays a crucial role in posttranscriptional gene regulation 26. Emerging evidence indicates that alternations in miRNAs are identified to be related to various human cancers and regulation of miRNAs can render cancer cells vulnerable to ferroptosis 27.
Ma et al. 28 showed that miR-424-5p negatively regulates ferroptosis in ovarian cancer cells by targeting ACSL4. Downregulation of miR-424-5p increased ACSL4 expression, a positive mediator of ferroptosis, which sensitized ovarian cancer cells to erastin and RSL3-induced ferroptosis. Interestingly, they found that ACSL4 is remarkably overexpressed in ovarian cancer tissues and is positively correlated with the aggressive phenotypes of ovarian cancer. This may suggest that ACSL4 is required for maintaining the aggressive phenotypes of malignant cells in normal conditions, but it facilitates triggering ferroptosis following erastin treatment.
Cai et al. 29 found that long non-coding RNA (lncRNA) ADAMTS9-AS1 prevents ferroptosis by targeting miR-587/SLC7A11 in the ovarian cancer cell line OVCAR3/CAOV-3. LncRNA is a gene expression regulator, involved in the progression of several cancers, including ovarian cancer 30. LncRNAs can attenuate miRNA-mediated inhibition of gene expression by sequestering miRNAs 31. In the study of Cai, inhibition of ADAMTS9-AS1 promoted the expression of miR-587, and overexpression of miR-587 enhanced the inhibition of SLC7A11 expression, ultimately promoted ferroptosis, and inhibited the proliferation and migration of ovarian cancer cells 29.
Lidocaine is widely used in clinics as a local anesthetic. However, recent studies have found that lidocaine can influence the development of a variety of tumors, including ovarian cancer 32. Some studies have even revealed that lidocaine can prevent tumor progression by regulating miRNA 33. Sun et al. 34 found that lidocaine induced ferroptosis in ovarian cancer cells by targeting miR-382-5p. Mechanistically, lidocaine upregulated miR-382-5p, which in turn suppressed SLC7A11 expression and triggered ferroptosis in ovarian cancer cells. Also, it was shown that lidocaine could stop SKOV-3 ovarian cancer cells from growing in mice. In tumor tissues, the expression of miR-382-5p was higher while the expression of SLC7A11 was lower.
HIPPO induces ferroptosis
Hippo is an evolutionarily conserved pathway that can sense and regulate the density-dependent phenotypes of cancer cells. It converges into two transcriptional co-activators, YAP (Yes-associated protein 1) and TAZ (transcriptional coactivator with PDZ-binding motif) 35. Yang et al. 36 found that cell density in ovarian cancer cells controls how sensitive they are to ferroptosis. They then looked into how YAP and TAZ affect ferroptosis in ovarian cancer cells.
Yang et al. 36 found that TAZ regulates CAOV2 ovarian cancer cells' sensitivity to erastin-induced ferroptosis. TAZ knockdown confers ferroptosis resistance, while TAZ overexpression sensitizes cells to ferroptosis. Integrated genomic analysis revealed that TAZ regulates the target gene ANGPTL4 directly to activate NOX2 and sensitizes ferroptosis. It is known that NOX generates ROS (the hallmark of ferroptosis) 37. They verified that knockdown of TAZ, ANGPTL4, or NOX2 reduced erastin-induced lipid peroxidation by measuring the lipid-based ROS. Thus, ferroptosis-inducing therapies may be especially effective against several TAZ-activated cancers.
A recent investigation has suggested that YAP plays a crucial role in regulating ferroptosis. Yang et al. 38 investigated the YAP-regulated ferroptosis in breast cancer cell line, renal cancer cell line, and lung cancer cell line. They found that overexpression of YAP makes cancer cells more susceptible to ferroptosis, whereas its knockdown gives ferroptosis resistance. Through integrated genomic approach, they identified that YAP regulates ferroptosis through regulating S-phase kinase-associated protein 2 (SKP2). Cells were protected from ferroptosis when SKP2 was repressed after YAP was knockdown. Therefore, the elevation of YAP expression can promote ferroptosis via SKP2. In addition, they also found that SKP2 was repressed after YAP was knocked down in ovarian cancer cells. Whether the pathway of YAP-SKP2-ferroptosis exists in ovarian cancer has not been reported yet, and it needs to be further studied. We speculate that YAP can also promote ferroptosis via SKP2 in ovarian cancer.
Stearoyl CoA desaturase 1 (SCD1) induces ferroptosis
SCD1 is a rate-limiting enzyme that catalyzes the conversion of saturated fatty acids (SFAs) to monounsaturated fatty acids (MUFAs) and that is upregulated in numerous malignancies, including prostate, liver, and breast cancer 39. It has been shown that lipid metabolism is a crucial mediator of ferroptosis. Specifically, to trigger ferroptosis, increased amounts of phosphatidylethanolamine (PE) containing oxidized PUFAs are required 16. Given how important lipid peroxidation is to ferroptosis, regulating the lipid composition of cells is likely to perturb ferroptosis.
Tesfay et al. found 40 that SCD1 can protect ovarian cancer cells from ferroptosis. In the study, they found that SCD1 mRNA and protein was significantly increase in the genetic model of ovarian cancer stem cells, ovarian cancer cell lines, and tissue. Upon SCD1 inhibition, cells underwent both ferroptosis and apoptosis: inhibition of SCD1 decreased CoQ10(an endogenous membrane antioxidant that has been implicated in protection from ferroptosis) 41, While long-chain saturated ceramides increased and unsaturated fatty acyl chains in membrane phospholipids decreased, these changes are associated with apoptosis promotion. Since two death pathways are activated simultaneously, SCD1 inhibition may be an effective part of anti-tumor therapy. In addition, blocking SCD1 sensitizes ovarian cancer cells to ferroptosis inducers in vitro and vivo 40. Combining SCD1 inhibitors and ferroptosis inducers as a treatment for ovarian cancer is an area that needs more research.
Xuan et al 42 reported a novel cellular antioxidant system that SCD1/FADS2 could regulate GPX4 and the GSH/GSSG ratio to prevent excess ROS-mediated oxidative stress and ferroptosis. SCD1/FADS2 are the key iron-containing enzymes whose enzymatic activities are executed by binding to iron43. When SCD1/FADS2 is inhibited, its iron-binding capacity declines, leading to an accumulation of cellular iron. Experiments also revealed that inhibition of SCD1/FADS2 activities led to an increase in Fe2+ levels, which in turn resulted in ROS buildup and lipid peroxidation 42.
Kato et al. found 44 that MI-463(a Menin‑mixed‑lineage leukemia inhibitor) induces ovarian cancer cell line death through the induction of ferroptosis, which may be due at least in part to the inhibition of SCD1 activity. Liu et al 40 reported that Agrimonolide (the main bioactive polyphenol isolated from Agrimonia pilosa Ledeb) inhibits cancer progression and induces apoptosis and ferroptosis by targeting inhibition of SCD1 in A2780 and SKOV-3 ovarian cancer cell lines.
p53 induces ferroptosis
p53 regulates cell cycle checkpoints, DNA repair, and apoptosis as an important tumor suppressor 45. Previous research has confirmed that p53 can promote ferroptosis by down-regulating SLC7A11 expression, thereby inhibiting the proliferation of lung cancer and breast cancer cell lines 46. However, there are few studies examining whether p53 inhibits ovarian cancer cells by ferroptosis.
Hong et al. 47 found that PARP inhibitors promote lipid peroxidation and ferroptosis in ovarian cancer cells by downregulating SLC7A11 in a p53-dependent manner, thereby inhibiting tumor cancer cell growth.
Zhang et al. 48 found that SPIO-Serum (an iron-based nanomaterial) can induce lipid peroxidation and generate a large amount of ROS in ovarian cancer cells by downregulating GPX4 and system Xc-, ultimately leading to ferroptosis. Expression of p53 can facilitate SPIO-serum-induced ferroptosis by inhibiting system Xc- to promote lipid peroxidation and ROS accumulation.
SNAI2 induces ferroptosis
SNAI2 plays a multifunctional role in cancer progression, including the induction of tumorigenesis, invasion, and metastasis 49. Previous studies have found that SNAI2 is activated in ovarian cancer cells and has the potential to promote lymphovascular diffusion 50. However, the potential mechanism between ferroptosis and SNAI2 in ovarian cancer remains unclear.
Jin et al. 51 observed elevated SNAI2 expression in ovarian cancer cells, especially in SKOV3 cells. SNAI2 knockdown significantly suppressed cell viability, migration, invasion, and accelerated cell apoptosis by inducing ferroptosis in SKOV3 cells. In addition, SNAI2 knockdown significantly inhibited SLC7A11 protein expression. So, SNAI2 knockdown may induce ferroptosis and inhibit the progression of ovarian cancer by downregulating SLC7A11.
Nuclear factor erythroid 2-related factor 2 (Nrf2) induces ferroptosis
Overexpression of Nrf2 has been linked to decreased response to anticancer treatments and a worse prognosis for patients 52. Recently, Nrf2 has been shown to be an antioxidant transcription factor that protects tumor cells against ferroptosis 53. However, further research is required to determine whether ferroptosis is involved in Nrf2-induced resistance to anticancer therapies and a worse prognosis.
Liu et al. 54 found that Nrf2 could activate the transsulfuration pathway by upregulating Cystathionine-β-synthase (CBS), a rate-limiting enzyme of the transsulfuration pathway 55, resulting in resistance of ovarian cancer cells to erastin-induced ferroptosis. Therefore, activation of Nrf2/CBS is a novel anti-ferroptosis mechanism. Inhibition of the transsulfuration pathway by down-regulating Nrf2 can increase the sensitivity of ovarian cancer to ferroptosis.
Gai et al. 56 found that carboxymethylated pachyman (a carboxymethylated derivative of pachyman derived from Poria cocos) can induce ferroptosis by downregulating Nrf2 mRNA, and reducing Nrf2, HO-1, GPX4 protein levels in ovarian cancer cells. Therefore, the Nrf2 gene is expected to be a potential therapeutic target for ovarian cancer.
Ferroptosis in Ovarian Cancer Therapy
Chemotherapy and ferroptosis
When it comes to female gynecological cancers, ovarian cancer is by far the most lethal. Although chemotherapy can effectively reduce the number of cancer cells, residual tumors still persist 57. Importantly, residual tumors are accompanied by decreased apoptotic response, enhanced antioxidant defence, and enhanced efflux mechanisms, which result in the development of drug resistance 58, 59. Due to the shortcomings of chemotherapy, other regulatory death pathways, such as ferroptosis, have been investigated to improve cancer treatments. It has been shown that ferroptosis plays a crucial role in the chemotherapy of ovarian cancer and enhances the anticancer impact of cisplatin in the treatment of ovarian cancer.
Cheng et al. 6 showed that cisplatin triggered numerous forms of cell death in the ovarian cancer cell, including ferroptosis. Erastin elevated ROS levels and induced ferroptosis to enhance the cytotoxicity of cisplatin. Combination treatment with cisplatin and erastin appears to increase therapeutic results while reducing adverse effects in vitro and vivo models of ovarian cancer.
Wang et al. 60 observed that the expression of Frizzled 7 (FZD7) was elevated in platinum-tolerant ovarian cancer cells. The overexpression of FZD7 could decrease the sensitivity to platinum. Mechanistically, FZD7 overexpression activated the oncogenic factor Tp63, driving the upregulation of GPX4 and protecting cancer cells from chemotherapy-induced oxidative stress. Interestingly, inhibition of GPX4 caused these platinum-tolerant (Pt-T) ovarian cancer cells to become more sensitive to platinum and undergo ferroptosis. This suggests that targeting FZD is a novel treatment for platinum-tolerant cancer cells.
Multidrug resistance (MDR) is connected with overexpression of drug efflux transport ATP-binding cassette subfamily B member 1 (ABCB1) in cancer cells 61. In the study of ovarian cancer, Zhou et al. 62 found that erastin can overcome docetaxel resistance and enhance docetaxel's antitumor efficacy by inhibiting ABCB1. Whether ferroptosis is involved in erastin's enhancement of docetaxel's efficacy in ovarian cancer remains to be determined.
Previous research has demonstrated that glycolysis is up-regulated in numerous types of tumor cells while oxidative phosphorylation (OXPHOS) is down-regulated (OXPHOS) 63. Gentric et al. 64 found that high-OXPHOS metabolism can enhance the sensitivity of chemotherapy for ovarian cancer and improve the prognosis of patients by upregulating PML expression and reducing PGC-1 transcriptional activity to promote oxidative stress (including increased ROS and lipid peroxidation). Increased ROS content and lipid peroxidation are characteristics of ferroptosis 4. Ferroptosis may be involved in the enhanced sensitivity of chemotherapy by high-OXPHOS. Therefore, targeting high-OXPHOS and promoting ferroptosis may be a promising anti-tumor strategy.
Olaparib is a clinical PARP inhibitor that can induce DNA damage in BRCA-mutated ovarian cancer to produce anticancer effects. However, BRCA wild-type ovarian cancer can repair this DNA damage and cause olaparib resistance 65. Hong et al. 47 found that Olaparib can down-regulate SLC7A11 and induce ferroptosis, which resulted in anticancer effects in ovarian cancer cells (including BRCA wild-type and BRCA mutations). Inhibition of SLC7A11 induced ferroptosis significantly sensitized BRCA wild-type ovarian cancer cells to Olaparib.
Wang et al. 66 found that superparamagnetic iron oxide nanoparticles (SPIONs) (nucleic acid and drug carrier) could induce oxidative stress and ferroptosis in ovarian cancer cells and inhibit their proliferation, invasion, and drug resistance.
Chan et al. 67 established that MAP30 (a bioactive protein, isolated from bitter melon seeds) induces ferroptosis and strengthened the anticancer effect of cisplatin in vitro (OVCA433, HEY and HEYA8 ovarian cancer cell lines) and vivo.
Xuan et al. 42 found that inhibition of SCD1 and FADS2 could induce ferroptosis, reduce cisplatin resistance, and enhance cisplatin's anti-cancer effect; therefore, inhibition of SCD1 and FADS2 in combination with cisplatin was an effective treatment.
In conclusion, ferroptosis can enhance chemosensitivity and plays a crucial function in ovarian cancer chemotherapy. However, the genetic and metabolic factors of ovarian cancer cell sensitivity are still unclear, which limits the application of ferroptosis inducers. A recent study provided evidence that erastin fails to induce ferroptosis in a series of ovarian cancer cell lines 68. Interestingly, they found that the combination of erastin with ferlixit (a regularly used iron compound to treat anemia) can overcome ferroptosis resistance in ovarian cancer cell lines 68. This might be a way to overcome erastin-induced ferroptosis resistance.
Immunotherapy and ferroptosis
T cells, a crucial component of the adaptive immune system, can selectively eliminate pathogens and tumor cells and play an important role in cancer immunotherapy 69. Immunotherapy can boost anticancer effects by restoring and enhancing CD8+ T cell activity 70. Previous studies have revealed that immunotherapy can promote ferroptosis in ovarian cancer cells. Wang et al. 8 found that immunotherapy-activated CD8+ T cells can enhance the antitumor efficacy of immunotherapy by inducing ferroptosis in ovarian cancer cells. Mechanistically, activated CD8+ T cells could release interferon-gamma (IFN-γ), which inhibited cystine uptake by downregulating the expression of system Xc-, as a result, promoting lipid peroxidation accumulation and ferroptosis in ovarian cancer cells. Cyst(e)inase is an engineered synthetic therapeutic enzyme that can induce ferroptosis by degrading cysteine and cysteine 71, 72. Wang et al. 8 show that Cyst(e)inase in combination with PD-L1 blockade (an immune checkpoint inhibitor agent) can effectively inhibit the growth of the ID8 ovarian cancer cell line. This combination of drugs promotes lipid peroxidation and ferroptosis to a greater degree than Cyst(e)inase or PD-L1 blocker treatment alone. This might be a way to overcome immunotherapy resistance. In addition, Jiang et al. 73 found that Fe3O4-SAS@PLT (a biomimetic magnetic nanoparticle) can induce ferroptosis in the metastatic tumor, which significantly enhanced the immune efficacy of PD-1 inhibitors. Therefore, immunotherapy-induced ferroptosis in tumor cells is a unique anticancer mechanism that is anticipated to become a new form of cancer treatment.
Radiotherapy and ferroptosis
In the past, radiotherapy was often utilized to treat individuals with ovarian cancer. It was the backbone of adjuvant therapy for many years, but it was supplanted by cisplatin about three decades ago. Nevertheless, it remains a beneficial therapy for individuals with recurrent and refractory illnesses since it can result in a longer DFS 74. Radiotherapy can induce oxidative damage and lipid peroxidation in all cellular compartments 75. The accumulation of toxic lipid peroxidation is related to ferroptosis 4. However, there are few studies on the association between radiotherapy and ferroptosis. Lang et al. 7 revealed that radiotherapy can induce ferroptosis in ID8 ovarian cancer cells. Mechanistically, Radiotherapy suppressed the expression of SLC7A11 by activating the ataxia telangiectasia mutant gene (ATM), ultimately resulting in the accumulation of lipid peroxidation and ferroptosis in ovarian cancer cells. In order to explore whether inducing ferroptosis can enhance the effect of radiotherapy, they pretreated ID8 cells with ferroptosis inducers (such as erastin, FINs, etc.) and then irradiated these cells. The results showed that inducing ferroptosis can reduce cell survival compared to radiotherapy alone in ID8 cells. This indicates that inducing ferroptosis can enhance the effect of radiotherapy. The use of targeted ferroptosis and radiotherapy together could become a new way to treat cancer.
Future Perspective and Conclusion
Ferroptosis is a novel kind of regulated cell death distinct from autophagy, apoptosis, and necrosis. The precise mechanism of ferroptosis is still unknown. In its research, accumulating evidence shows that it involves many signal pathways and is closely related to the genesis, development, and metastasis of tumors. Its importance in tumor treatment has attracted extensive attention.
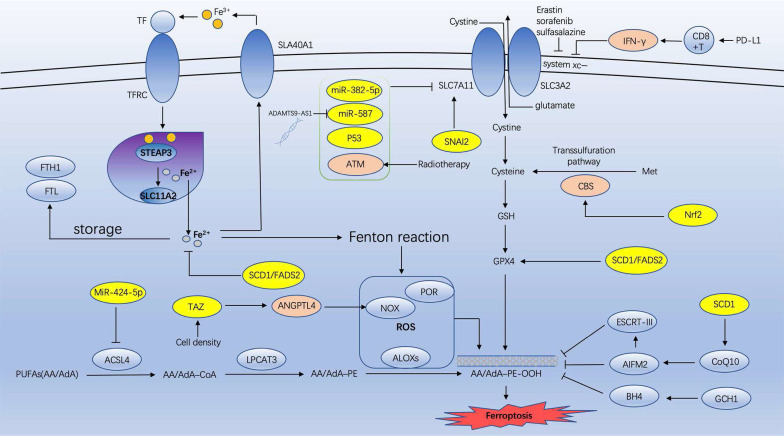
Ovarian cancer is among the most prevalent malignant tumors in females. It has the greatest mortality rate of all gynecological malignancies. Since Dixon proposed ferroptosis in 2012 4, numerous researchers have investigated its effects on various tumors, including ovarian cancer. However, the majority of research has been conducted on ovarian cancer cell lines, for instance, how ferroptosis inhibits the development of ovarian cancer cells. This article reviews the recent research on the association between ovarian cancer and ferroptosis, including the possible mechanism of targeting ferroptosis enhancing anti-ovarian cancer (Figure 1) and the mechanism of ferroptosis in various ovarian cancer cell lines (Table 1).
Figure 1.
The possible mechanism of targeting ferroptosis enhancing anti-ovarian cancer (IFN-γ: interferon-gamma; CBS: cystathionine-β-synthase; Nrf2: erythroid 2-related factor 2; SCD1: sterol CoA desaturase 1; GSH: glutathione; GPX4: glutathione peroxidase 4; ATM: Ataxia- Telangiectasia mutated gene; ACSL4: acyl-CoA synthetase long chain family member 4; LPCAT3: lysophosphatidylcholine acyltransferase 3; NOXs: NADPH oxidases; TAZ: transcriptional coactivator with PDZ-binding motif).
Table 1.
The mechanism of ferroptosis in various ovarian cancer cell lines.
| Author year | Ovarian cancer cell line | Mechanism | Refs |
|---|---|---|---|
| Ma et al., 2021 | HO8910/SKOV3 | MiR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4 | 28 |
| Cai et al., 2022 | OVCAR3/CAOV-3 | Long non-coding RNA ADAMTS9-AS1 attenuates ferroptosis by targeting microRNA-587/SLC7A11 | 29 |
| Sun et al., 2021 | SKOV-3 | Lidocaine promoted ferroptosis by targeting miR-382-5p /SLC7A11 axis in Ovarian cancer | 34 |
| Yang et al., 2020 | CAOV2 | A TAZ-ANGPTL4-NOX2 axis regulates ferroptosis and chemoresistance in EOC | 36 |
| Yang et al., 2021 | CAOV2 | The Hippo pathway effector YAP promotes ferroptosis via the E3 ligase SKP2 | 38 |
| Tesfay et al., 2019 | OVCAR-4/COV362 | SCD1 protects ovarian cancer cells from ferroptosis | 40 |
| Xuan et al., 2022 | OVCA433 | SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells | 42 |
| Hong et al., 2021 | HEY/A2780/SKOV3 | PARP inhibition promotes ferroptosis via suppressing SLC7A11 by P53 in ovarian cancer | 47 |
| Jin et al., 2022 | SKOV3/A2780/CAOV3 | SNAI2 promotes the development of ovarian cancer through regulating ferroptosis | 51 |
| Liu et al., 2020 | SKOV3/OVCA429 | Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance | 54 |
Presently, an increasing number of studies have demonstrated that ferroptosis can inhibit the progression of ovarian cancer cells, and ferroptosis in combination with chemotherapy, radiotherapy, and immunotherapy can enhance the tumor-inhibiting effect. In the future, targeting ferroptosis may become a novel treatment for ovarian cancer.
Acknowledgments
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Hongjiang Zhao and Yuan Xu. The first draft of the manuscript was edited by Hongkai Shang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Abbreviations
- OC
ovarian cancer
- OS
overall survival
- TF
transferrin
- TFRC
transferrin receptor
- FTL
ferritin light chain
- FTH1
ferritin heavy chain 1
- ROS
reactive oxygen species
- PUFAs
polyunsaturated fatty acids
- AA
arachidonic acid
- AdA
adrenic acid
- ACSL4
acyl-CoA synthetase long chain family member 4
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- NOXs
NADPH oxidases
- GSH
Glutathione
- GPX4
glutathione peroxidase 4
- YAP
yes-associated protein 1
- TAZ
transcriptional coactivator with PDZ-binding motif
- SKP2
S-phase kinase-associated protein 2
- SCD1
Sterol CoA desaturase 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- CBS
Cystathionine-β-synthase
- FZD7
Wnt receptor Frizzled 7
- ABCB1
ATP binding cassette subfamily B member 1
- IFN-γ
interferon-gamma
- ATM
Ataxia- Telangiectasia mutated gene
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–88. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015;309:C444–56. doi: 10.1152/ajpcell.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31:e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Q, Bao L, Li M, Chang K, Yi X. Erastin synergizes with cisplatin via ferroptosis to inhibit ovarian cancer growth in vitro and in vivo. J Obstet Gynaecol Res. 2021;47:2481–91. doi: 10.1111/jog.14779. [DOI] [PubMed] [Google Scholar]
- 7.Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L. et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9:1673–85. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK. et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 10.Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM. et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N. et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agmon E, Solon J, Bassereau P, Stockwell BR. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci Rep. 2018;8:5155. doi: 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agmon E, Stockwell BR. Lipid homeostasis and regulated cell death. Curr Opin Chem Biol. 2017;39:83–9. doi: 10.1016/j.cbpa.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–75. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 18.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W. et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–9. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–8. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19:e1800311. doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 22.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F. et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai E, Meng L, Kang R, Wang X, Tang D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun. 2020;522:415–21. doi: 10.1016/j.bbrc.2019.11.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun. 2020;523:966–71. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 26.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64:311–36. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma LL, Liang L, Zhou D, Wang SW. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68:165–73. doi: 10.4149/neo_2020_200707N705. [DOI] [PubMed] [Google Scholar]
- 29.Cai L, Hu X, Ye L, Bai P, Jie Y, Shu K. Long non-coding RNA ADAMTS9-AS1 attenuates ferroptosis by Targeting microRNA-587/solute carrier family 7 member 11 axis in epithelial ovarian cancer. Bioengineered. 2022;13:8226–39. doi: 10.1080/21655979.2022.2049470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Jia G, Zhang H, Wang L, Cong Y, Lv M. et al. LncRNA HOXB-AS3 promotes growth, invasion and migration of epithelial ovarian cancer by altering glycolysis. Life Sci. 2021;264:118636. doi: 10.1016/j.lfs.2020.118636. [DOI] [PubMed] [Google Scholar]
- 31.Zhu W, Xiao X, Chen J. Silencing of the long noncoding RNA LINC01132 alleviates the oncogenicity of epithelial ovarian cancer by regulating the microRNA-431-5p/SOX9 axis. Int J Mol Med. 2021. 48. [DOI] [PMC free article] [PubMed]
- 32.Liu C, Yu M, Li Y, Wang H, Xu C, Zhang X. et al. Lidocaine inhibits the metastatic potential of ovarian cancer by blocking Na(V) 1.5-mediated EMT and FAK/Paxillin signaling pathway. Cancer Med. 2021;10:337–49. doi: 10.1002/cam4.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui H, Lou A, Li Z, Yang J. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC Cancer. 2019;19:233. doi: 10.1186/s12885-019-5431-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sun D, Li YC, Zhang XY. Lidocaine Promoted Ferroptosis by Targeting miR-382-5p /SLC7A11 Axis in Ovarian and Breast Cancer. Front Pharmacol. 2021;12:681223. doi: 10.3389/fphar.2021.681223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori M, Triboulet R, Mohseni M, Schlegelmilch K, Shrestha K, Camargo FD. et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell. 2014;156:893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol Cancer Res. 2020;18:79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 38.Yang WH, Lin CC, Wu J, Chao PY, Chen K, Chen PH. et al. The Hippo Pathway Effector YAP Promotes Ferroptosis via the E3 Ligase SKP2. Mol Cancer Res. 2021;19:1005–14. doi: 10.1158/1541-7786.MCR-20-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igal RA. Stearoyl CoA desaturase-1: New insights into a central regulator of cancer metabolism. Biochim Biophys Acta. 2016;1861:1865–80. doi: 10.1016/j.bbalip.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J. et al. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019;79:5355–66. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ. et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xuan Y, Wang H, Yung MM, Chen F, Chan WS, Chan YS. et al. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics. 2022;12:3534–52. doi: 10.7150/thno.70194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rockfield S, Chhabra R, Robertson M, Rehman N, Bisht R, Nanjundan M. Links Between Iron and Lipids: Implications in Some Major Human Diseases. Pharmaceuticals (Basel) 2018. 11. [DOI] [PMC free article] [PubMed]
- 44.Kato I, Kasukabe T, Kumakura S. Menin-MLL inhibitors induce ferroptosis and enhance the anti-proliferative activity of auranofin in several types of cancer cells. Int J Oncol. 2020;57:1057–71. doi: 10.3892/ijo.2020.5116. [DOI] [PubMed] [Google Scholar]
- 45.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 46.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong T, Lei G, Chen X, Li H, Zhang X, Wu N. et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021;42:101928. doi: 10.1016/j.redox.2021.101928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Xia M, Zhou Z, Hu X, Wang J, Zhang M. et al. p53 Promoted Ferroptosis in Ovarian Cancer Cells Treated with Human Serum Incubated-Superparamagnetic Iron Oxides. Int J Nanomedicine. 2021;16:283–96. doi: 10.2147/IJN.S282489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan H, Wang X, Li W, Shen M, Wei Y, Zheng H. et al. ASB13 inhibits breast cancer metastasis through promoting SNAI2 degradation and relieving its transcriptional repression of YAP. Genes Dev. 2020;34:1359–72. doi: 10.1101/gad.339796.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Yang X, Xu S, Jin P, Li X, Wei X. et al. Reprogramming of stromal fibroblasts by SNAI2 contributes to tumor desmoplasia and ovarian cancer progression. Mol Cancer. 2017;16:163. doi: 10.1186/s12943-017-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin Y, Chen L, Li L, Huang G, Huang H, Tang C. SNAI2 promotes the development of ovarian cancer through regulating ferroptosis. Bioengineered. 2022;13:6451–63. doi: 10.1080/21655979.2021.2024319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y. et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–43. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu N, Lin X, Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br J Cancer. 2020;122:279–92. doi: 10.1038/s41416-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuhra K, Augsburger F, Majtan T, Szabo C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules. 2020. 10. [DOI] [PMC free article] [PubMed]
- 56.Jing T, Guo Y, Wei Y. Carboxymethylated pachyman induces ferroptosis in ovarian cancer by suppressing NRF1/HO-1 signaling. Oncol Lett. 2022;23:161. doi: 10.3892/ol.2022.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Cardenas H, Fang F, Condello S, Taverna P, Segar M. et al. Epigenetic targeting of ovarian cancer stem cells. Cancer Res. 2014;74:4922–36. doi: 10.1158/0008-5472.CAN-14-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16:1215–28. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Zhao G, Condello S, Huang H, Cardenas H, Tanner EJ. et al. Frizzled-7 Identifies Platinum-Tolerant Ovarian Cancer Cells Susceptible to Ferroptosis. Cancer Res. 2021;81:384–99. doi: 10.1158/0008-5472.CAN-20-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nanayakkara AK, Follit CA, Chen G, Williams NS, Vogel PD, Wise JG. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci Rep. 2018;8:967. doi: 10.1038/s41598-018-19325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou HH, Chen X, Cai LY, Nan XW, Chen JH, Chen XX. et al. Erastin Reverses ABCB1-Mediated Docetaxel Resistance in Ovarian Cancer. Front Oncol. 2019;9:1398. doi: 10.3389/fonc.2019.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R. et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017;7:716–35. doi: 10.1158/2159-8290.CD-16-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gentric G, Kieffer Y, Mieulet V, Goundiam O, Bonneau C, Nemati F. et al. PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers. Cell Metab. 2019;29:156–73.e10. doi: 10.1016/j.cmet.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pommier Y, O'Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8:362ps17. doi: 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 66.Huang Y, Lin J, Xiong Y, Chen J, Du X, Liu Q. et al. Superparamagnetic Iron Oxide Nanoparticles Induce Ferroptosis of Human Ovarian Cancer Stem Cells by Weakening Cellular Autophagy. J Biomed Nanotechnol. 2020;16:1612–22. doi: 10.1166/jbn.2020.2991. [DOI] [PubMed] [Google Scholar]
- 67.Chan DW, Yung MM, Chan YS, Xuan Y, Yang H, Xu D. et al. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol Res. 2020;161:105157. doi: 10.1016/j.phrs.2020.105157. [DOI] [PubMed] [Google Scholar]
- 68.Battaglia AM, Sacco A, Perrotta ID, Faniello MC, Scalise M, Torella D. et al. Iron Administration Overcomes Resistance to Erastin-Mediated Ferroptosis in Ovarian Cancer Cells. Front Oncol. 2022;12:868351. doi: 10.3389/fonc.2022.868351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spetz J, Presser AG, Sarosiek KA. T Cells and Regulated Cell Death: Kill or Be Killed. Int Rev Cell Mol Biol. 2019;342:27–71. doi: 10.1016/bs.ircmb.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–9. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W. et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23:120–7. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z. et al. Platelet Membrane-Camouflaged Magnetic Nanoparticles for Ferroptosis-Enhanced Cancer Immunotherapy. Small. 2020;16:e2001704. doi: 10.1002/smll.202001704. [DOI] [PubMed] [Google Scholar]
- 74.Flores-Balcázar CH, Urías-Arce DM. Radiotherapy in women with epithelial ovarian cancer: historical role, current advances, and indications. Chin Clin Oncol. 2020;9:49. doi: 10.21037/cco-20-10. [DOI] [PubMed] [Google Scholar]
- 75.Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]