Abstract
Background:
Cardiac autonomic neuropathy is a frequent complication of type 2 diabetes mellitus. Cardiac autonomic neuropathy, in which sympathetic tone predominates over parasympathetic activity, increases both cardiovascular morbidity and mortality and unfortunately has no definitive treatment. Sodium-glucose cotransporter-2 inhibitors have been suggested to reduce sympathetic nervous system activity, based on the results from previous studies. In this study, we aimed to investigate the effect of 24-week treatment with dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, on cardiac autonomic function measures in patients with type 2 diabetes mellitus and cardiac autonomic neuropathy.
Methods:
Dapagliflozin 10 mg/day (n = 42) or non-sodium-glucose cotransporter-2 inhibitor oral antidiabetic(s) (n = 38) was added to the treatment of patients whose glycemic control could not be achieved with existing treatments. The patients with definite or confirmed cardiac autonomic neuropathy diagnosed by cardiovascular autonomic reflex tests underwent 24-hour Holter-electrocardiogram recordings to obtain heart rate variability and heart rate turbulence parameters before starting additional medication and after a 24-week treatment period.
Results:
In-group analyses showed that dapagliflozin 10 mg/day for 24 weeks improved heart rate variability and heart rate turbulence parameters and decreased the frequency of ventricular premature beats relative to their baseline values. No such findings were observed in the control group despite similar glycemic control. Comparisons between dapagliflozin group and the control group showed that these effects of dapagliflozin were significantly better than non-sodium-glucose cotransporter-2 inhibitor oral antidiabetics.
Conclusion:
Dapagliflozin improves measures of cardiac autonomic function compared to the control group in type 2 diabetic patients with cardiac autonomic neuropathy. This intergroup benefit, demonstrated for the first time, may be promising for the regression of cardiac autonomic neuropathy with sodium-glucose cotransporter-2 inhibitors.
Keywords: Cardiac autonomic neuropathy, dapagliflozin, heart rate variability, SGLT2 inhibitors, turbulence
Highlights
24-week dapagliflozin treatment improves heart rate variability and turbulence in patients with cardiac autonomic neuropathy.
Also, it decreases heart rate turbulence category (lower is better) and frequency of premature ventricular beats.
These beneficial effects were detected both within the dapagliflozin group and in comparison with the control group.
In comparison with a control group, the ameliorative effects of the sodium-glucose cotransporter-2 inhibition on heart rate variability and turbulence were demonstrated for the first time.
Introduction
Relatively new oral antidiabetic (OAD) drugs named sodium-glucose cotransporter-2 (SGLT2) inhibitors have cardiovascular benefits that go beyond their blood glucose control effects, such as reducing heart failure hospitalizations, and both cardiovascular and all-cause mortality.1,2 Cardiac autonomic neuropathy (CAN) is a frequent complication of type 2 diabetes mellitus (T2DM), and its incidence is expected to increase further as diabetes becomes an epidemic and occurs at earlier ages.3 Cardiac autonomic neuropathy, in which sympathetic activity is dominant due to the deterioration of the balance between the parasympathetic and sympathetic innervation of the heart, has effects that increase cardiovascular morbidity and mortality by causing QT interval prolongation, impaired heart rate variability (HRV), arrhythmias, blood pressure dysregulations such as orthostatic hypotension and reverse-dipping pattern, and silent myocardial ischemia and infarction.3,4 So far, there are several preventive and therapeutic strategies available to prevent its development or to address its clinical manifestations, but there is no definitive treatment that regresses CAN.3
The absence of compensatory heart rate increase in response to the decrease in blood pressure observed in studies examining the cardiovascular effects of SGLT2 inhibitors suggests that they may reduce sympathetic nervous system activity.1,2,5 Indeed, preclinical studies in mice have shown that dapagliflozin, an SGLT2 inhibitor, reduces the markers of sympathetic nervous system activity such as tyrosine hydroxylase and noradrenaline in heart and kidney cells.6,7 Based on these findings, SGLT2 inhibitors were hypothesized to be a promising treatment option for CAN, and the effects of this group of drugs on cardiac autonomic function parameters, including HRV and heart rate turbulence (HRT), were investigated in T2DM patients with different characteristics. Among them, EMPA-HEART CardioLink-6 Holter analysis study8 and Ang et al's9 study did not show a significant effect of empagliflozin and dapagliflozin on HRV parameters, respectively, whereas the EMBODY study revealed that empagliflozin improved HRV and HRT parameters in patients with T2DM who had a recent myocardial infarction.10 Inconsistencies between these results can be attributed to differences in study duration and whether the study subjects had stable or unstable coronary artery disease. In addition, in 2 of these studies, cardiovascular autonomic reflex tests (CARTs), which are the gold standard in the diagnosis of CAN, were not used.11 The clinical use of HRV obtained from 24-hour Holter-electrocardiogram (ECG) recordings in addition to CARTs has been suggested for CAN screening and diagnosis by the American Association of Clinical Endocrinology and the American College of Endocrinology.12 Similarly, HRT parameters may provide additional benefits in the diagnosis of CAN.13
In this study, we aimed to investigate the effect of 24-week dapagliflozin treatment on HRV and HRT parameters in T2DM patients who had a definite or confirmed diagnosis of CAN by CARTs.
Methods
Study Population
This study was carried out in the endocrinology and cardiology outpatient clinics of our hospital. Eligible patients for the study were determined as T2DM patients naïve to SGLT2 inhibitors and who were planned to add dapagliflozin 10 mg/day or other OAD drug(s) (except glucagon-like peptide-1 analogs or other SGLT2 inhibitors) to their treatment due to suboptimal glycemic control with their existing OAD medications. At baseline, almost all patients were using metformin, and approximately half were using additional OAD drugs, such as dipeptidyl peptidase-4 inhibitors, sulfonylureas, and/or thiazolidinediones groups. The choice of additional oral agent(s) was left to the discretion of the endocrinology physician according to patients’ clinical characteristics.
Exclusion criteria were as follows: any acute coronary syndrome in the last 1 year, known heart failure or having signs and symptoms of heart failure, cardiomyopathies, moderate or severe valvular heart disease, hyper/hypothyroidism, non-sinus rhythm, and use of any anti-arrhythmic drugs that may affect HRV and HRT indices, including beta-blockers, verapamil, and diltiazem. Since glucagon-like peptide-1 agonists may influence HRV parameters, patients who were to be added to a drug from this group were not included in the study.14 Patients using or planning to start insulin were also excluded from the trial.
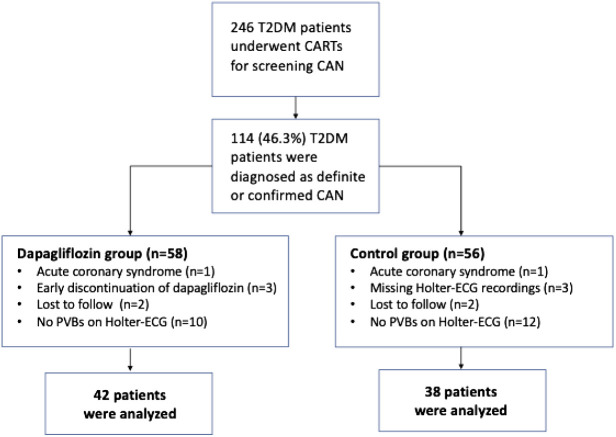
A total of 246 patients between the ages of 18 and 80 who met the above conditions underwent CARTs for the presence of CAN as recommended by the Toronto Consensus panel.11 How these tests are performed is described elsewhere.4 According to the results of these tests, 114 patients with at least 2 heart rate test abnormalities were diagnosed as definite or confirmed CAN and were included in the study population.15 These patients underwent anthropometric measurements, laboratory tests, 24-hour Holter-ECG recordings, and transthoracic echocardiographic examinations before starting additional medication and after a 24-week of treatment period. Patients who developed acute coronary syndrome during the study period or who could not continue the added antidiabetic drug(s) due to the side effects or intolerance were excluded from the study. In addition, patients who did not complete baseline and 24th-week Holter-ECG monitoring and/or whose recordings were not of sufficient quality for the evaluation were also excluded. The absence of premature ventricular beats (PVBs), which are required for HRT evaluation, in Holter-ECG recordings was another reason for exclusion. After all exclusions, 42 patients who were prescribed an additional dapagliflozin 10 mg/day regimen constituted the dapagliflozin group and 38 patients who were prescribed other OAD drug(s) as described above formed the control group (Figure 1).
Figure 1.
A total of 246 patients with type 2 diabetes mellitus (T2DM) met the inclusion criteria and underwent cardiovascular reflex tests (CARTs). Of these, 114 had definite or confirmed cardiac autonomic neuropathy (CAN) and were included in the study. Sixteen patients in the dapagliflozin group and 18 patients in the control group were excluded due to exclusion criteria. The remaining 80 patients (42 in the dapagliflozin group and 38 in the control group) completed 24-week study period. PVBs, premature ventricular beats.
The study was designed and conducted in accordance with the principles set out in the Declaration of Helsinki and was approved by the Local Medical Research Ethics Committee (approval date: January 21, 2022; approval number: 13). All subjects gave informed consent before enrollment.
Laboratory Tests, Anthropometric, and Blood Pressure Measurements
Venous blood samples were collected after at least 8 hours of fasting. Thyroid and kidney function tests, aspartate aminotransferase, alanine aminotransferase, fasting plasma glucose, HbA1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were measured in all subjects at baseline and after 24 weeks of additional treatment. Anthropometric data were obtained by well-trained medical staff using the same methods and instruments for all patients at baseline and after 24 weeks. The office blood pressure of subjects was measured using the traditional auscultation method with a sphygmomanometer in the Holter-ECG room before starting Holter recordings according to the relevant guidelines of the European Society of Cardiology.16
Holter-Electrocardiogram Examinations
The 24-hour Holter-ECG recordings were obtained with 3-channel digital recorders (CardioDay® for Windows Version 2.5, GE Healthcare, GETEMED Medizin- und Informationstechnik AG/Germany) and were reviewed by a reader who was unaware of the treatments received by the patients and the period of the study. While the frequency of PVBs was calculated by dividing the total number of PVBs by the total recording time in hours, their percentage was calculated by dividing the total number of PVBs by the total number of beats during the recording period. Time-domain HRV and HRT parameters were measured using the automatic features of the software. Defined as the physiological cyclical fluctuations in the time interval between sequential heartbeats, HRV includes the following parameters: standard deviation (SD) of normal to normal intervals (SDNN), SD of the average NN intervals for each 5-minute segment of the 24-hour recording (SDANN), mean of the 5-minute SD of the NN interval calculated over 24 hours (SDNN index), root mean square of successive RR interval differences (RMSSD), and the percentage of successive NN intervals that differ by >50 milliseconds (pNN50).17 In addition, mean RR interval was noted. The HRT is a baroreflex-mediated biphasic reaction of heart rate in response to PVBs.18 Instantaneous changes in heart rate after isolated PVBs can be detected by Holter-ECG as first shortening and then lengthening in sinus cycle length. These acceleration and deceleration phases are quantified by 2 numeric descriptors, turbulence onset (TO) and turbulence slope (TS). A TO value below 0% indicates early sinus acceleration and is considered normal, while a TS value above the 2.5 ms/RR range shows the normal expected late deceleration. Another HRT parameter, HRT category (HRTc), is calculated according to whether the TO and TS values are normal or not. If both TO and TS values are normal, it means HRTc is 0. If both values are abnormal, it means HRTc is 2. A HRTc 1 means either TO or TS is abnormal.18 All evaluations and measurements were made in accordance with the standards set by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.17,18
Sample Size Estimation
Sample size was calculated for SDNN, one of the most used HRV parameters. The mean difference in SDNN from baseline to 24 weeks between the dapagliflozin group and control group was estimated to be 15 ms and SD of 20 ms based on a previous study result using a similar approach.8 Accordingly, a sample size of 79 was calculated for 80% power with a 2-sided type I error level for group comparisons. We planned to enroll approximately 120 patients with CAN assuming 30% loss due to compelling exclusion criteria such as the absence of PVBs in Holter-ECG recordings. Hence, 246 patients who met the inclusion criteria were screened until the planned number of patients was reached. Of these, 114 patients with definite or confirmed CAN according to CARTs were included in the study.
Statistical Analysis
The Statistical Program for Social Sciences (IBM® SPSS Statistics for Windows, Version 22, IBM Corp., Armonk, NY, USA) and G*Power software (Version 3.1.9.6; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) were used for all statistical calculations. Nominal variables were presented as numbers and percentages, and continuous variables were presented as mean ± SD. Data were analyzed for normal distribution with the Kolmogorov–Smirnov test. For examining the differences between groups, the chi-square test was used for categorical variables and Student's t-test or Mann–Whitney U test (whichever was appropriate) for continuous variables. The objective of this trial was to compare the change in the HRV and HRT values from baseline to 24 weeks between dapagliflozin and control groups. To this end, the differences in HRV and HRT parameters between the 2 groups were compared by analysis of covariance. Statistical significance measures were presented as adjusted differences with corresponding 95% confidence interval (CI). In addition, to compare in-group differences between baseline and 24-week values, McNemar test was used for categorical variables, and paired samples t-test or Wilcoxon signed-rank test was used for continuous variables. A 2-sided P-value <.05 was considered statistically significant for all analyses.
Results
Baseline clinical, laboratory, physical examination, and echocardiographic findings of both groups are given in Table 1. Table 1 also presents information about patients' CART results and current medications. No significant difference was present between the 2 groups in terms of these characteristics. The average duration of T2DM was approximately 8.5 years, and mean HbA1c levels before additional therapy were above 7.5% in both groups.
Table 1.
Baseline Clinical Characteristics of the Study Population
| Dapagliflozin Group (n = 42) | Control Group (n = 38) | P | |
|---|---|---|---|
| Age, years | 60.5 ± 11.0 | 61.4 ± 9.8 | .680 |
| Gender, male/female, n (%) | 19/23 (45.2/54.8) | 15/23 (39.5/60.5) | .602 |
| Current smoking, n (%) | 10 (23.8) | 8 (21.1) | .768 |
| Hypertension, n (%) | 20 (47.6) | 17 (44.7) | .796 |
| Body weight, kg | 83.3 ± 13.8 | 81.7 ± 12.1 | .379 |
| Body mass index, kg/m2 | 29.6 ± 4.7 | 29.4 ± 5.0 | .587 |
| Duration of diabetes, years | 8.4 ± 3.1 | 8.6 ± 3.5 | .757 |
| Fasting plasma glucose, mg/dL | 154.5 ± 46.9 | 156.1 ± 42.0 | .877 |
| HbA1c, % | 7.81 ± 1.37 | 7.94 ± 1.31 | .672 |
| Total cholesterol, mg/dL | 192.1 ± 38.1 | 190.5 ± 39.5 | .852 |
| High-density lipoprotein cholesterol, mg/dL | 44.5 ± 12.0 | 45.5 ± 12.2 | .727 |
| Low-density lipoprotein cholesterol, mg/dL | 121.2 ± 29.9 | 116.1 ± 32.8 | .476 |
| Triglycerides, mg/dL | 131.9 ± 63.9 | 134.1 ± 68.6 | .880 |
| Systolic BP, mm Hg | 128.5 ± 19.9 | 129.3 ± 22.0 | .850 |
| Diastolic BP, mm Hg | 77.9 ± 11.8 | 79.3 ± 14.6 | .617 |
| Resting HR, beats/min | 75.2 ± 11.4 | 73.7 ± 10.6 | .541 |
| Left ventricular ejection fraction, % | 63.3 ± 5.7 | 62.4 ± 5.2 | .522 |
| Interventricular septum thickness, mm | 11.2 ± 1.9 | 10.9 ± 1.8 | .470 |
| Left ventricular posterior wall thickness, mm | 10.4 ± 1.6 | 10.2 ± 1.6 | .698 |
| Abnormal cardiovascular autonomic reflex tests | |||
| HR response to breathing, n (%) | 28 (66.7) | 22 (57.9) | .418 |
| HR response to standing, n (%) | 15 (35.7) | 18 (47.4) | .290 |
| HR response to Valsalva maneuver, n (%) | 19 (45.2) | 16 (42.1) | .778 |
| BP response to standing, n (%) | 9 (21.4) | 12 (31.6) | .303 |
| BP response to isometric exercise, n (%) | 5 (11.9) | 2 (5.3) | .436 |
| Current medical therapy | |||
| ACEI or ARB, n (%) | 17 (40.5) | 16 (42.1) | .882 |
| Dihydropyridine CCB | 18 (42.9) | 18 (47.4) | .685 |
| Diuretics, n (%) | 15 (35.7) | 12 (31.6) | .696 |
| Metformin, n (%) | 37 (88.1) | 34 (89.5) | .846 |
| DPP-4 inhibitors/sulfonylureas/thiazolidinediones, n (%) | 19 (45.2) | 20 (52.6) | .509 |
| Statins, n (%) | 23 (54.8) | 27 (71.1) | .133 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BP, blood pressure; CCB, calcium channel blockers; DPP-4, dipeptidyl peptidase-4; HR, heart rate; n, numbers.
In Table 2, 24-hour Holter-ECG findings are given. There was no difference in terms of the duration of Holter-ECG recordings, mean heart rate, mean RR interval, and frequency and percentage of PVBs. In addition, baseline HRV and HRT parameters were similar before additional OAD drugs were started.
Table 2.
Comparison of Baseline 24-Hour Holter-ECG Findings, Time-Domain Heart Rate Variability, and Heart Rate Turbulence Parameters Between Dapagliflozin and Control Groups
| Dapagliflozin Group (n = 42) | Control Group (n = 38) | P | |
|---|---|---|---|
| 24-hour Holter-ECG findings | |||
| Recording duration, hours | 23.2 ± 0.6 | 23.1 ± 0.5 | .767 |
| PVBs per hour, n | 15.9 ± 20.7 | 15.1 ± 21.1 | .877 |
| PVBs percent | 0.329 ± 0.424 | 0.315 ± 0.426 | .916 |
| Mean heart rate, beats/minute | 81.6 ± 6.0 | 81.0 ± 5.4 | .605 |
| Mean RR interval, ms | 746.1 ± 64.4 | 749.7 ± 65.7 | .808 |
| Heart rate variability parameters | |||
| SDNN, ms | 102.1 ± 25.1 | 100.3 ± 24.1 | .747 |
| SDANN, ms | 85.1 ± 22.4 | 82.9 ± 23.7 | .681 |
| SDNN index, ms | 31.2 ± 15.4 | 31.0 ± 13.2 | .952 |
| RMSSD, ms | 41.6 ± 28.3 | 39.2 ± 27.6 | .765 |
| pNN50, % | 4.02 ± 3.33 | 4.13 ± 2.96 | .680 |
| Heart rate turbulence parameters | |||
| Turbulence onset | −0.0454 ± 0.0995 | -0.0486 ± 0.0876 | .880 |
| Turbulence slope, ms/RR | 3.64 ± 4.77 | 3.97 ± 4.61 | .479 |
| HRTc 0, numbers (%) | 12 (28.6) | 14 (36.8) | .731 |
| HRTc 1, numbers (%) | 19 (45.2) | 15 (39.5) | |
| HRTc 2, numbers (%) | 11 (26.2) | 9 (23.7) | |
HRTc, heart rate turbulence category; n, numbers; pNN50, percentage of successive normal to normal (NN) intervals that differ by more than 50 ms; PVBs, premature ventricular beats; RMSSD, root mean square of successive RR interval difference; SDANN, standard deviation (SD) of the average NN intervals for each 5-minute segment of the 24-hour recording; SDNN, SD of NN intervals; SDNN index, mean of the 5-minute SD of the NN interval calculated over 24 hours; ECG, electrocardiogram.
Heart rate variability parameters of both groups at baseline and after 24 weeks of additional OAD treatment(s) are presented in Table 3. In-group analyses showed that 24 weeks of dapagliflozin add-on therapy improved HRV parameters relative to their baseline values. However, such an improvement was not observed in the control group despite similar glycemic control. The adjusted difference of HbA1c between the groups was −0.037% (95% CI: −0.197% to 0.122%, P = .645). However, intergroup comparisons revealed that adjunctive therapy with dapagliflozin resulted in an increase (improvement) of 8.79 ms in SDNN (P = .005), 6.11 ms in SDANN (P = .036), 4.16 ms in SDNN index (P = .010), 6.95 ms in RMSSD (P = .019), and 1.58% in pNN50 (P = .03) relative to the non-SGLT2 inhibitor antidiabetics (Table 3). Similarly, in-group comparisons pointed out that HRT parameters were found to be significantly improved only in dapagliflozin group. Intergroup analysis showed that add-on therapy with dapagliflozin for 24 weeks increased the TS values and decreased the HRTc and TO values compared to therapy without an SGLT2 inhibitor in type 2 diabetic patients with CAN (Table 3).
Table 3.
Baseline Versus 24-Week Comparisons and Intergroup Analysis of 24-Hour Holter-ECG findings, Time-Domain Heart Rate Variability, and Heart Rate Turbulence Parameters Between Dapagliflozin and Control Groups
| Dapagliflozin Group (n = 42) | Control Group (n = 38) | Adjusted Difference Between Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 24 Weeks | P | Baseline | 24 Weeks | P | Mean (95% CI) | P | |
| 24-hour Holter-ECG findings | ||||||||
| PVBs per hour, n | 15.9 ± 20.7 | 11.3 ± 17.6 | <.001 | 15.1 ± 21.1 | 14.4 ± 20.1 | .519 | −3.89 (−7.61, −0.16) | .041 |
| PVBs percent | 0.329 ± 0.424 | 0.233 ± 0.351 | <.001 | 0.315 ± 0.426 | 0.304 ± 0.423 | .280 | −0.084 (−0.158, −0.011) | .025 |
| Mean heart rate, beats/minute | 81.6 ± 6.0 | 82.4 ± 6.2 | .414 | 81.0 ± 5.4 | 81.6 ± 5.9 | .417 | 0.083 (−2.244, 2.409) | .944 |
| Mean RR interval, ms | 746.1 ± 64.4 | 748.9 ± 65.2 | .785 | 749.7 ± 65.7 | 751.0 ± 66.8 | .933 | 1.45 (−34.24, 37.14) | .936 |
| Heart rate variability parameters | ||||||||
| SDNN, ms | 102.1 ± 25.1 | 113.1 ± 29.5 | <.001 | 100.3 ± 24.1 | 102.6 ± 23.4 | .067 | 8.79 (2.76, 14.82) | .005 |
| SDANN, ms | 85.1 ± 22.4 | 92.3 ± 27.4 | .006 | 82.9 ± 23.7 | 84.1 ± 23.3 | .380 | 6.11 (0.41, 11.81) | .036 |
| SDNN index, ms | 31.2 ± 15.4 | 35.9 ± 17.4 | .003 | 31.0 ± 13.2 | 31.5 ± 12.6 | .141 | 4.16 (1.01, 7.32) | .010 |
| RMSSD, ms | 41.6 ± 28.3 | 49.9 ± 28.4 | .001 | 39.2 ± 27.6 | 40.6 ± 26.2 | .099 | 6.95 (1.19, 12.70) | .019 |
| pNN50, % | 4.02 ± 3.33 | 5.8 ± 4.59 | <.001 | 4.13 ± 2.96 | 4.34 ± 2.67 | .174 | 1.58 (0.57, 2.58) | .003 |
| Heart rate turbulence parameters | ||||||||
| Turbulence onset | −0.0454 ± 0.0995 | −0.0673 ± 0.1044 | <.001 | −0.0486 ± 0.0876 | −0.0458 ± 0.0903 | .104 | −0.025 (−0.039, −0.010) | .001 |
| Turbulence slope, ms/RR | 3.64 ± 4.77 | 5.22 ± 5.56 | <.001 | 3.97 ± 4.61 | 4.21 ± 5.14 | .129 | 1.348 (0.705, 1.991) | <.001 |
| HRTc 0, n (%) | 12 (28.6) | 22 (52.4) |
.001 |
14 (36.8) | 16 (42.1) |
.223 |
N/A |
<.001 |
| HRTc 1, n (%) | 19 (45.2) | 13 (31) | 15 (39.5) | 14 (36.8) | ||||
| HRTc 2, n (%) | 11 (26.2) | 7 (16.6) | 9 (23.7) | 8 (21.1) | ||||
| Clinical and laboratory findings | ||||||||
| HbA1c, % | 7.81 ± 1.37 | 7.41 ± 1.17 | <.001 | 7.94 ± 1.31 | 7.5 ± 1.08 | <0.001 | −0.037 (−0.197, 0.122) | .645 |
| Body weight, kg | 83.3 ± 13.8 | 81.0 ± 12.3 | <.001 | 81.7 ± 12.1 | 82.3 ± 11.9 | 0.195 | −2.92 (−4.18, −1.65) | <.001 |
| Body mass index, kg/m2 | 29.6 ± 4.7 | 28.9 ± 4.3 | <.001 | 29.4 ± 5.0 | 29.6 ± 5.2 | 0.212 | −1.03 (−1.50, −0.56) | <.001 |
| Systolic BP, mm Hg | 128.5 ± 19.9 | 122.7 ± 18.2 | <.001 | 129.3 ± 22.0 | 127.9 ± 18.5 | 0.394 | −5.7 (−7.8, −0.7) | .020 |
| Diastolic BP, mm Hg | 77.9 ± 11.8 | 75.0 ± 11.4 | <.001 | 79.3 ± 14.6 | 78.8 ± 10.7 | 0.805 | −2.9 (−4.7, −1.0) | .086 |
| Resting heart rate, beats/minute | 75.2 ± 11.4 | 76.1 ± 10.2 | .569 | 73.7 ± 10.6 | 72.9 ± 9.5 | 0.760 | 1.6 (−3.8, 7.1) | .349 |
Data presented as mean ± standard deviation. Statistically significant P-values are given in bold.
BP, blood pressure; HRTc, heart rate turbulence category; n, numbers; N/A, not available; pNN50, percentage of successive normal to normal (NN) intervals that differ by more than 50 milliseconds; PVBs, premature ventricular beats; RMSSD, root mean square of successive RR interval difference; SDANN, standard deviation (SD) of the average NN intervals for each 5-minute segment of the 24-hour recording; SDNN, SD of NN intervals; SDNN index, mean of the 5-minute SD of the NN interval calculated over 24 hours; ECG, electrocardiogram.
In terms of frequency and percentage of PVBs, both groups were similar at baseline. In the 24th-week Holter-ECG recordings, the frequency of PVBs in the dapagliflozin group significantly decreased compared to baseline values; although there was a numerical decrease in the control group, it was not significant. Intergroup comparisons showed that patients receiving dapagliflozin had 3.89 fewer PVBs per hour than patients who did not (Table 3).
After treatment for 24 weeks, body weight and body mass index decreased significantly in the dapagliflozin group compared to the control group. Adjusted difference between the 2 groups was −2.92 kg (95% CI, −4.18 to −1.65, P < .001) for body weight and −1.03 kg/m2 (95% CI, −1.50 to −0.56 kg/m2, P < .001) for body mass index (Table 3). Similarly, 24 weeks of dapagliflozin add-on therapy resulted in a reduction of 5.7 mm Hg and 2.9 mm Hg in systolic and diastolic blood pressure, respectively, compared to treatments without an SGLT2 inhibitor. However, despite this decrease, neither the mean heart rate on 24-hour Holter-ECG recordings nor the resting heart rate on physical examination was significantly increased in the dapagliflozin group. Mean heart rate at baseline and at the end of 24 weeks was not different within and between groups.
Discussion
In this study, we investigated the effects of dapagliflozin, an SGLT2 inhibitor, on HRV and HRT parameters, which reflect sympathetic and parasympathetic nerve activities, in patients with CAN. For this purpose, dapagliflozin 10 mg/day or non-SGLT2 inhibitor oral antidiabetics (to provide similar glycemia) were added to the treatment of patients whose glycemic control could not be achieved with their existing OAD treatments. Before the start of additional therapy and after 24 weeks of treatment, HRV and HRT values obtained from 24-hour Holter-ECG recordings were compared within and between dapagliflozin and control groups. Our main findings are as follows: (1) dapagliflozin treatment for 24 weeks resulted in improvement in both HRV and HRT parameters and decrease in the frequency and percentage of PVBs in patients with definite or confirmed CAN diagnosed with CARTs; (2) such an improvement was not found in the control group, which achieved similar glycemic control with OAD drugs other than SGLT2 inhibitors; (3) intergroup analyses showed that dapagliflozin significantly increased SDNN, SDANN, SDNN index, RMSSD, pNN50, and TS values and significantly decreased TO, and HRT categories, and also frequency and percentage of PVBs compared to non-SGLT2 inhibitor oral antidiabetics; (4) within-group and between-group analyses confirmed the absence of reflex heart rate increase to SGLT2 inhibitor-induced blood pressure reduction found in previous studies. To the best of our knowledge, this study is the first to demonstrate that an SGLT2 inhibitor has ameliorative effects on HRV, and HRT parameters compared to a control group, in patients with definite or confirmed CAN.
Cardiac autonomic neuropathy may occur after the first year of T2DM and increases morbidity and cardiovascular mortality.3,4 However, no definitive treatment that regresses CAN has been reported to date.3 Since neuropathies affect the longest nerves first, the balance between parasympathetic and sympathetic tone is disturbed, due to the vagus nerve damage.4 As a result, a condition characterized by overactivity of the sympathetic nervous system occurs that leads to resting tachycardia and impaired HRV, even at subclinical stage of CAN.4 The fact that SGLT2 inhibitors regressed sympathetic nervous system activity markers such as tyrosine hydroxylase and noradrenaline in preclinical studies and decreased sympathetic nerve hyperactivity demonstrated by I-123 MIBG scintigraphy in clinical studies suggest that they may normalize or reduce sympathetic dominance in diabetic CAN.7,8,10,19 In addition, the lack of compensatory heart rate increase in response to the decrease in blood pressure caused by these drugs and the lower risk of atrial fibrillation, atrial flutter, and serious ventricular arrhythmias observed in clinical studies in favor of SGLT2 inhibitors supports that they may modulate the cardiovascular autonomic function by reducing sympathetic nervous system activity.1,2,5,20,21 In line with these results, SGLT2 inhibition seems to be a promising mechanism in search of the treatment to regress CAN.
Therefore, we planned to conduct our study in a T2DM population with a definite or confirmed diagnosis of CAN by CARTs, and we found improvement in HRV, and HRT parameters, which may indicate regression in CAN with appropriate duration of SGLT2 inhibition, perhaps at least 6 months. Because, in a similar but relatively smaller population study with CAN, there were no differences in any of the HRV parameters between 12-week of dapagliflozin (n = 26) versus 12-week glimepiride treatments (n = 19).9 In fact, a slight increase in the mean change of low-frequency (LF) to high-frequency (HF) power ratio was observed, which reflects cardiac sympathetic nerve activity, while on dapagliflozin, contrasting with a decline while on glimepiride from baseline to 12 weeks; however, the difference in the change of LF:HF ratio from baseline to 12 weeks between the 2 drugs was not significant (0.28, 95% CI: −0.24 to 0.80, P = .28).9 Considering the results achieved with 6 months of treatment in our study, we can speculate that 3 months of SGLT2 inhibition may not be a sufficient period to improve HRV parameters. However, in the EMPA-HEART CardioLink-6 study comparing the treatment of 6-month empagliflozin to placebo, empagliflozin did not improve HRV parameters in T2DM patients with stable coronary artery disease.8 Despite the same SGLT2 inhibition duration as in our study, the reason for the different results on HRV parameters may be that the patients were not homogeneous in terms of the presence and severity of CAN, since CARTs were not used.8 In addition, the degree of glycemic control may not be similar between groups, as baseline and end-of-study differences in HbA1c were not reported.8 In another empagliflozin versus placebo study, the EMBODY trial, patients with T2DM were randomized to empagliflozin 10 mg/day or placebo 15 days after an acute myocardial infarction.10 This study examined the effect of SGLT2 inhibition on HRT, which mainly reflects cardiac parasympathetic nerve activity, in addition to HRV parameters for the first time.10 After treatment for 24 weeks, significant improvements were observed in the empagliflozin group in terms of HRTc, time-domain HRV parameters except pNN50, and all frequency domain HRV parameters, while no significant changes were observed in these parameters in the placebo group, except for SDNN. However, intergroup comparisons showed no significant difference between the empagliflozin and placebo groups.10 In contrast, we found that the improvement in HRV and HRT parameters in dapagliflozin group was significantly more than in the control group. There could be several reasons for this result. First, it may be that the 2 study populations have different coronary artery disease characteristics. While the EMBODY study included patients with a recent acute myocardial infarction, we examined a stable patient population who did not have any acute coronary syndrome in the past 1 year and even excluded patients who developed an acute coronary syndrome during the study. Because HRV may be impaired up to 1 year after an acute myocardial infarction, these abnormalities cannot be expected to be at the same level for every patient.22 Second, it could be that patients in the EMBODY study were not homogeneous in terms of left ventricular function, because no information was given about the baseline values of left ventricular ejection fraction, which can also show dynamical changes within months after acute myocardial infarction.10,23 Since the relationship between heart failure and impaired HRV is well established, we excluded patients with known heart failure or having signs and symptoms of heart failure.24 Finally, the groups may not be similar in terms of the presence and severity of CAN, since patients in the EMBODY trial did not underwent CARTs.10 The intergroup benefit in HRV and HRT parameters not seen in the EMBODY study but observed in ours is likely due to the similarity in left ventricular function, coronary artery disease characteristics, and CAN severity between the dapagliflozin and control groups.
Consistent with the results of previous studies, the absence of compensatory heart rate increase in patients receiving dapagliflozin, despite a significant decrease in systolic and diastolic blood pressure values compared to baseline values, supports the decreasing modulation of sympathetic activity by SGLT2 inhibition. Another finding demonstrating the sympathoinhibitor effects of this group of drugs is that they reduce the ventricular extrasystole burden. In a trial that investigated the acute effects of dapagliflozin in patients with T2DM within a 2-week period, dapagliflozin reduced the percentage of PVBs compared to placebo (1.4% vs. 0.2%, P <.05).25 Similar to this short-term finding, the longer-term results in our study, which consisted of subjects who were relatively healthy in terms of cardiac functions and did not use drugs such as beta-blockers and non-dihydropyridine calcium channel blockers that could influence the frequency of PVBs, suggest that dapagliflozin may provide antiarrhythmic benefits in patients with CAN. However, it is known that significant daily variations in the frequency of PVBs may occur and that up to 6 days of Holter-ECG monitoring may be required to assess the maximum daily burden of PVBs.26 For this reason, we believe that our results should be supported by studies specifically focusing on this issue with longer Holter recordings even for up to 1 week.
An important issue regarding the conflicting results found in this and previous studies is whether the improvement in cardiac autonomic functions is directly from the sympathoinhibitor effects of SGLT2 inhibitors or is secondary to their slow-progressing beneficial metabolic effects that become noticeable in the long term, such as weight loss. Indeed, moderate weight loss has been shown to improve HRV in overweight and obese adults with T2DM.27 Mouridsen et al28 demonstrated that even an average weight loss of 3.9 kg in the overweight women was associated with increased HRV as indicated by SDNN. Therefore, in this study, the additional benefit of a mean weight loss of 2.3 kg after 24 weeks of dapagliflozin regimen may have overestimated the favorable effects of dapagliflozin on cardiac autonomic function by contributing to the improvement in HRV.
Cardiac autonomic neuropathy is an important public health problem, as it causes silent myocardial ischemia and infarction, arrhythmias, orthostatic hypotension, cardiomyopathy, and perioperative cardiovascular instability, resulting in increased morbidity and mortality.3,4 Therefore, it is important to find medications that will slow the development of CAN or regress the existing CAN. The SGLT2 inhibitors seem to be beneficial in the attenuation of CAN, for which a definitive treatment has not been demonstrated until now.
Study Limitations
Our study has several limitations. First, it was conducted on a relatively small number of patients. However, this limitation is also valid for the trials reported so far. Second, we defined “stable coronary artery disease” as the “absence of any acute coronary syndrome requiring hospitalization in the last 1 year.” However, there may be silent acute coronary syndrome patients among our diabetic subjects. The third limitation of our study is that CARTs were not repeated at the end of the study to check for a reduction in the number of abnormal tests. However, we planned our study to examine the changes in HRV and HRT parameters before and after the treatment period. This was because the measurements obtained from Holter-ECG recordings were independent of patient-factor compared to CARTs. Another limitation is that we examined only time-domain analyses of the Holter-based HRV tests; however, many time and frequency domain variables obtained during the 24-hour period are known to be highly correlated with each other.17 Another one is the difference in the groups in terms of body weight change between baseline and 24 weeks. Although there was no significant weight change in the control group, weight loss in the dapagliflozin group may have affected the study results in favor of dapagliflozin. Lastly, as we documented the ameliorative effects of dapaglifozin on HRV and HRT only in a small population of diabetic patients with a definite diagnosis of CAN, this benefit should not be generalized to all diabetic patients.
Conclusion
Dapagliflozin, an SGLT2 inhibitor, improved both HRV (decreased sympathetic and increased parasympathetic activities) and HRT (increased parasympathetic activity) compared to the control group in a relatively healthy T2DM population with definite or confirmed CAN. This finding is promising for the regression of CAN with SGLT2 inhibitors while providing blood glucose regulation in T2DM. However, larger randomized controlled studies with longer follow-ups are needed to examine the effects of SGLT2 inhibitors on cardiac autonomic function in patients with diabetic CAN.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Ethics Committee of Kahramanmaraş Sütçü İmam University (approval no: 2022/13).
Informed Consent: All authors gave final consent and agreed to be responsible for all aspects of this work, ensuring integrity and accuracy.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.S.B.; Design – A.S.B., M.Ş., A.Ç.A.; Supervision – A.S.B., E.A., A.Ç.A.; Materials – E.Ç., M.Ş.; Data Collection and/or Processing – E.Ç., K.G.; Analysis and/or Interpretation – E.A., E.Ç., K.G.; Literature Review – A.S.B., K.G.; Writing – A.S.B.; Critical review – M.Ş., E.A., A.Ç.A.
Acknowledgments: None.
Declaration of Interests: The authors have no conflicts of interest to declare.
Funding: This study received no funding.
References
- 1. Zinman B, Wanner C, Lachin JM.et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117 2128. 10.1056/NEJMoa1504720) [DOI] [PubMed] [Google Scholar]
- 2. Wiviott SD, Raz I, Bonaca MP.et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347 357. 10.1056/NEJMoa1812389) [DOI] [PubMed] [Google Scholar]
- 3. Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019;43(1):3 30. 10.4093/dmj.2018.0259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015;6(1):80 91. 10.4239/wjd.v6.i1.80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMurray JJV, Solomon SD, Inzucchi SE.et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995 2008. 10.1056/NEJMoa1911303) [DOI] [PubMed] [Google Scholar]
- 6. Matthews VB, Elliot RH, Rudnicka C, Hricova J, Herat L, Schlaich MP. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J Hypertens. 2017;35(10):2059 2068. 10.1097/HJH.0000000000001434) [DOI] [PubMed] [Google Scholar]
- 7. Herat LY, Magno AL, Rudnicka C.et al. SGLT2 inhibitor-induced sympathoinhibition: A novel mechanism for cardiorenal protection. JACC Basic Transl Sci. 2020;5(2):169 179. 10.1016/j.jacbts.2019.11.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg V, Verma S, Connelly KA.et al. Does empagliflozin modulate the autonomic nervous system among individuals with type 2 diabetes and coronary artery disease? The EMPA-HEART CardioLink-6 Holter analysis. Metabol Open. Metabol Open. 2020;7(7):100039. 10.1016/j.metop.2020.100039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ang L, Kidwell KM, Dillon B.et al. Dapagliflozin and measures of cardiovascular autonomic function in patients with type 2 diabetes (T2D). J Diabetes Complications. 2021;35(8):107949. 10.1016/j.jdiacomp.2021.107949) [DOI] [PubMed] [Google Scholar]
- 10. Shimizu W, Kubota Y, Hoshika Y.et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148. 10.1186/s12933-020-01127-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spallone V, Ziegler D, Freeman R.et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639 653. 10.1002/dmrr.1239) [DOI] [PubMed] [Google Scholar]
- 12. Vinik AI, Camacho PM, Davidson JA.et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on testing for autonomic and somatic nerve dysfunction. Endocr Pract. 2017;23(12):1472 1478. 10.4158/EP-2017-0053) [DOI] [PubMed] [Google Scholar]
- 13. Lin K, Wei L, Huang Z, Zeng Q. Combination of Ewing test, heart rate variability, and heart rate turbulence analysis for early diagnosis of diabetic cardiac autonomic neuropathy. Med (Baltim). 2017;96(45):e8296. 10.1097/MD.0000000000008296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakatani Y, Kawabe A, Matsumura M.et al. Effects of GLP-1 receptor agonists on heart rate and the autonomic nervous system using holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care. 2016;39(2):e22 e23. 10.2337/dc15-1437) [DOI] [PubMed] [Google Scholar]
- 15. Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491 498. 10.2337/diacare.8.5.491) [DOI] [PubMed] [Google Scholar]
- 16. Williams B, Mancia G, Spiering W.et al. ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953 2041. 10.1097/HJH.0000000000001940) [DOI] [PubMed] [Google Scholar]
- 17. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of pacing and electrophysiology. Eur Heart J. 1996;17(3):354 381. 10.1093/oxfordjournals.eurheartj.a014868) [DOI] [PubMed] [Google Scholar]
- 18. Bauer A, Malik M, Schmidt G.et al. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol. 2008;52(17):1353 1365. 10.1016/j.jacc.2008.07.041) [DOI] [PubMed] [Google Scholar]
- 19. Kiuchi S, Hisatake S, Kabuki T.et al. Long-term use of ipragliflozin improved cardiac sympathetic nerve activity in a patient with heart failure: A case report. Drug Discov Ther. 2018;12(1):51 54. 10.5582/ddt.2017.01069) [DOI] [PubMed] [Google Scholar]
- 20. Zelniker TA, Bonaca MP, Furtado RHM.et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141(15):1227 1234. 10.1161/CIRCULATIONAHA.119.044183) [DOI] [PubMed] [Google Scholar]
- 21. Curtain JP, Docherty KF, Jhund PS.et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42(36):3727 3738. 10.1093/eurheartj/ehab560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brateanu A. Heart rate variability after myocardial infarction: what we know and what we still need to find out. Curr Med Res Opin. 2015;31(10):1855 1860. 10.1185/03007995.2015.1086992) [DOI] [PubMed] [Google Scholar]
- 23. Chew DS, Heikki H, Schmidt G.et al. Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin Electrophysiol. 2018;4(5):672 682. 10.1016/j.jacep.2017.12.015) [DOI] [PubMed] [Google Scholar]
- 24. Guzzetti S, Magatelli R, Borroni E, Mezzetti S. Heart rate variability in chronic heart failure. Auton Neurosci. 2001;90(1-2):102 105. 10.1016/S1566-0702(01)00274-0) [DOI] [PubMed] [Google Scholar]
- 25. Ilyas F, Jones L, Tee SL.et al. Acute pleiotropic effects of dapagliflozin in type 2 diabetic patients with heart failure with reduced ejection fraction: a crossover trial. ESC Heart Fail. 2021;8(5):4346 4352. 10.1002/ehf2.13553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loring Z, Hanna P, Pellegrini CN. Longer ambulatory ECG monitoring increases identification of clinically significant ectopy. Pacing Clin Electrophysiol. 2016;39(6):592 597. 10.1111/pace.12852) [DOI] [PubMed] [Google Scholar]
- 27. Sjoberg N, Brinkworth GD, Wycherley TP, Noakes M, Saint DA. Moderate weight loss improves heart rate variability in overweight and obese adults with type 2 diabetes. J Appl Physiol (1985). 2011;110(4):1060 1064. 10.1152/japplphysiol.01329.2010) [DOI] [PubMed] [Google Scholar]
- 28. Mouridsen MR, Bendsen NT, Astrup A, Haugaard SB, Binici Z, Sajadieh A. Modest weight loss in moderately overweight postmenopausal women improves heart rate variability. Eur J Prev Cardiol. 2013;20(4):671 677. 10.1177/2047487312444367) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a