Abstract
Many challenges exist in the precise diagnosis and clinical management of secondary progressive multiple sclerosis (SPMS) because of the lack of definitive clinical, imaging, immunologic, or pathologic criteria that demarcate the transition from relapsing-remitting MS to SPMS. This review provides an overview of the diagnostic criteria/definition and the heterogeneity associated with different SPMS patient populations; it also emphasizes the importance of available prospective/retrospective tools to identify patients with SPMS earlier in the disease course so that approved disease-modifying therapies and nonpharmacological strategies will translate into better outcomes. Delivery of such interventions necessitates an evolving patient-clinician dialog within the context of a multidisciplinary team.
Over the past 2 decades, multiple attempts have been made to reach a consensus on the definition of secondary progressive multiple sclerosis (SPMS), which is characterized by insidious worsening of disability over time, independent of relapses.1,2,e1,e2 SPMS is present in a sizeable proportion of the multiple sclerosis (MS) population and has a high disease burden. The prevalence of SPMS varies globally (1–58 per 100,000 general population).3,4 In the European Union (EU), SPMS prevalence ranges from 3–50 per 100,000 whereas in the United States (US), the prevalence is estimated at 27–45 per 100,000.3
Although the onset of SPMS is identified as a “key turning point” in the MS disease continuum, SPMS is always diagnosed retrospectively by the subjective judgment of the clinician,e3-e5 i.e., after evidence of irreversible disability accrual on the Expanded Disability Status Scale (EDSS) becomes noticeably apparent, a process that can take up to 3 or more years. The inherent uncertainty as to whether disability in patients with relapsing MS is permanent or will resolve leads to a period of diagnostic uncertainty termed as the “transition phase” that delays the SPMS diagnosis.2,5,6,e6 In the last years, there is increasing awareness of the fact that progression independent of relapse activity (PIRA) may occur from the very beginning in MS and constitutes around half of the disability worsening experienced in relapsing-remitting MS (RRMS). In SPMS, the vast majority of disease worsening is driven by PIRA although a small amount of relapse-related worsening is still seen.e7,e8 Clinicians encounter challenges in diagnosis because of the lack of an generally accepted definition, heterogeneous manifestation of the disease, indistinct clinical features of progression, and lack of imaging or biomarkers that demarcate the relapsing-remitting and secondary progressive stages.1,7,8,e1,e5,e9,e10 Identifying the precise timing of transition across phenotypes can be difficult because of subjective symptom recall.9 Moreover, clinicians tend to be conservative in establishing a SPMS diagnosis because of the limited availability of treatment options explicitly approved for SPMS in most countries and the mental/emotional strain on the patient of having a confirmed SPMS diagnosis. To address these issues, disease phenotypes defined by underlying pathology are needed to identify the patients who are most likely to benefit from specific therapeutic interventions.10
Since treatment options for SPMS are emerging with recent approvals of oral disease-modifying therapies (DMTs) such as siponimod,e11 clinical management of SPMS will require an improved understanding of the transition phase as well as differences in patients' characteristics. Efforts toward the early detection of SPMS progression have been made with the use of modern tools, algorithms, and biomarkers. In this context, this review article aims to provide an overview of (1) the characteristics of SPMS cohorts from the phase 3 clinical trials, registries, and observational evidence; (2) tools and biomarkers that may help to detect SPMS progression earlier in the disease course; and (3) available treatments and symptom management for SPMS.
SPMS Population Heterogeneity
Patients With SPMS From Phase 3 Studies
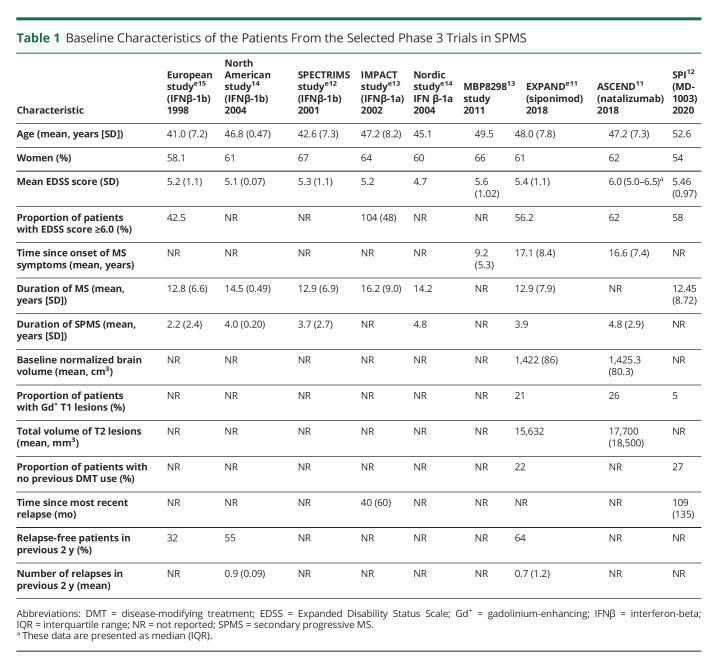
An overview of the baseline characteristics of patients with SPMS from the pivotal phase 3 studies (EXPAND, ASCEND, North American [NA]-SPMS, European Union [EU]-SPMS, SPECTRIMS, IMPACT, SPI2, and MBP8298) is provided in Table 1.11-14,e11-e15 As evidenced by these characteristics, the patient populations across the studies were heterogeneous with between-trial differences identified for age, duration of MS, relapse history, duration of SPMS, and proportion of patients with EDSS ≥6.0.
Table 1.
Baseline Characteristics of the Patients From the Selected Phase 3 Trials in SPMS
Multiple randomized studies assessed the efficacy and safety of interferon-beta-1a (IFN-β-1a) and IFN-β-1b in comparison with placebo in patients with SPMS.14,e12-e15 Some studies assessed the efficacy and safety of dirucotide, MD1003 (a biotin), siponimod, and natalizumab in comparison with placebo.11-13,e11 The inclusion/exclusion criteria of all the studies are outlined in eTable 1 (http://links.lww.com/NXI/A765).
A post hoc analysis15 investigated and observed differences in study results between the SPMS study conducted in the EU, in which IFN-β-1b significantly slowed the disease progressione15 and the NA-SPMS study conducted in the United States and Canada, in which this benefit was not observed.14 This analysis highlighted significant differences in the patient characteristics (i.e., the EU-SPMS patient population had early onset and more active disease than the NA-SPMS population). In the EU-SPMS study, the progression rate as measured by EDSS was 46% and annualized relapse rate (ARR) was 0.63 in the placebo group while NA-SPMS study participants had a progression rate of 34% and ARR of 0.28. The Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon beta-1a in MS (SPECTRIMS) study tested 2 doses of IFN-β-1b in patients with SPMS. The results showed that with a dose of 44 µg, the time to confirmed progression in disability was not significantly affected by treatment (hazard ratio, 0.83; 95% CI, 0.65; 1.07; p = 0.146 vs placebo). However, the relapse rate was significantly reduced to 0.50 per year (p < 0.001 for both doses).e12 In the International MS Secondary Progressive Avonex Controlled Trial (IMPACT) study, the median MS Functional Composite (MSFC) Z-score decreased by 40.4% in IFN-β-1a participants (−0.096 vs −0.161 in placebo participants, p = 0.033), the Nine-Hole Peg Test (9-HPT), and the Paced Auditory Serial Addition Test being the key contributors for this change. Furthermore, IFN-β-1a participants had 33% fewer relapses (p = 0.008), and IFN-β-1a was shown to reduce new or enlarging T2-hyperintense brain MRI lesions and gadolinium-enhancing (Gd+) lesions at months 12 and 24 (both p < 0.001).e13 However, no benefit in EDSS score was seen. Another study examined the benefit of low-dose IFN-β-1a (22 µg); patients treated with the low dose of IFN-β-1a vs placebo did not have a beneficial effect on either disability or relapse outcomes.e14
A Clinical Study of the Efficacy of Natalizumab on Reducing Disability Progression in Participants With Secondary Progressive Multiple Sclerosis (ASCEND), a phase 3, randomized, double-blind, placebo-controlled trial of natalizumab included a population with advanced disease (EDSS of 6–6.5; 63% requiring walking aid) and was more similar to the NA-SPMS study than the EU-SPMS study. Patients treated with natalizumab in the ASCEND study showed a progression rate of 44% and ARR of 0.08. This study did not meet the primary end point (disability outcome) at 2 years in the SPMS population.11 Furthermore, a phase 3 study of MBP8298 (myelin basic protein) did not show a clinical benefit compared with placebo in an SPMS population (n = 612) which expressed human leukocyte antigen haplotype DR2 or DR4 (progression rate [31%]; ARR [0.13]).13 Other studies that evaluated the potential of mitoxantrone (NCT00146159), dimethyl fumarate (NCT02430532), and cyclophosphamide vs methylprednisolone (NCT00241254) in SPMS were terminated early; therefore, no data are available for comparison.
In the EXploring the efficacy and safety of siponimod in PAtients with secoNDary progressive multiple sclerosis (EXPAND) phase 3 study, the SPMS population had high disability with a median EDSS score of 6.0 (range: 3.0–6.5), 56% required a walking aid, 21% had Gd+ lesions, and 36% had relapses in the past 2 years; the progression rate was 26%, and ARR was 0.07.e11 By contrast, the SPI2 study12 specifically recruited only participants with nonrelapsing progressive MS; potential participants with relapses in the prior 2 years were excluded and as a likely consequence, only 5% of participants had Gd+ lesions at baseline.
In general, the baseline characteristics from these phase 3 studies underscore the variability in SPMS trial methodology16 and highlight the heterogeneity of the enrolled patient populations such as presence/absence of relapses, age, and disease duration.e16,e17
Patients With SPMS From the Registries and Real-World Evidence
SPMS represents a challenge for current registries and real-world evidence efforts because patients with SPMS may be underrepresented due to delayed diagnosis and unrecognized disease progression. Progression in functional domains, not captured adequately by EDSS such as visual or cognitive symptoms, may not affect the total EDSS score in patients with limited ambulation because the patient will appear as clinically stable in EDSS terms. Moreover, challenges in the assessment of other disability functions (e.g., cognition, arm function, balance, bowel, and bladder function) in patients with SPMS with EDSS >4 have widely been recognized.17,e18 Clinicians may want to consider deterioration in any single-functional domain as an indicator of clinical progression.
Collective efforts from the SPMS research collaboration network (RCN) of 8 European MS registries are currently generating data on ∼40,000 patients with SPMS to (1) measure variability in SPMS prevalence as a function of diagnostic criteria and (2) describe characteristics and treatment patterns of patients with SPMS in routine clinical practice.e19 According to the latest results from the RCN group which included 3 registries, application of a decision tree classifier (RRMS/SPMS patients reclassification) increased the SPMS proportion from 16.6% to 26.2% in Germany, from 13.8% to 35.6% in United Kingdom, and from 24.5% to 25.4% in Sweden compared with clinically assigned SPMS proportion, indicating that underdiagnosis of SPMS is a common issue.18
An ongoing noninterventional real-world evidence study impAct of Mayzent [siponimod] on secondAry progressive multiple Sclerosis patients in a long-term non-Interventional study in GermAny (AMASIA) aims to analyze the effects of siponimod on SPMS patients with active disease (n = 435 patients enrolled as of July 15, 2021) over a 3-year observational phase.19,e20 Compared with the active SPMS subgroup population from the EXPAND study, the real-world population of AMASIA is older (55 years) with a longer overall disease history (mean 17 years), equally advanced disability (EDSS 6.0) but a higher rate (50%) of relapse activity within the past 2 years19 In the PANGAEA 2.0 EVOLUTION study20,e21 (n = 658 recruited), the interim analysis (data cutoff: January 28, 2021) results reported that patients with SPMS were older (53. 6 vs 49.5 years), had a longer disease history (17.2 vs 13.8 years), and higher EDSS score (5.1 vs 4.2) compared with those at high risk for SPMS.
A more recent report from the Argentine MS registry (RelevarEM) described clinical and demographic characteristics of patients with SPMS.21 Registry patients had a median age of 53 years (interquartile range [IQR; 47–62]), 67% were women, the median EDSS was 6.5, and disease duration was 19.5 years (IQR 14–26) and with 48% in ongoing treatment. Furthermore, 86% had a disability certificate (allowing access to disability benefits), and only 23.7% were actively working. In addition, 35.6% of patients with SPMS had new MRI lesions, and 5% had clinical relapses in the last year of the registry entry.21
A recent real-world study of the Adelphi MS Disease-Specific Programme (DSP) identified 3580 patients with SPMS from a cohort of 37,318 patients with MS. Those with SPMS were further categorized as active SPMS (aSPMS) or nonactive SPMS (naSPMS) based on the presence or absence of 1 or more new MRI lesions or relapses in the previous 12 months, respectively. When comparing the active (n = 1889) and nonactive (n = 665) SPMS groups, the patients with aSPMS had a lower mean EDSS score (4.6 vs 5.2), a greater change in EDSS in the past 12 months (0.43 vs 0.02) and a lower proportion of moderate-to-severe disease (73.5 vs 87.8).22
The Adelphi DSP study also showed that 45.1% of patients with naSPMS receive no treatment, compared with 23.4% with aSPMS. Given the paucity of epidemiologic data exclusively for SPMS, more data coming from the registries could potentially provide clinicians with a better understanding of the treatment patterns/switches and off-label use of drugs along with real-time observations on the safety and efficacy of treatments.1,e22
According to natural history cohort studies, most of the patients with RRMS ultimately transition to SPMS over the course of the disease.23,e5,e23 In a natural history cohort, approximately 62% of patients with RRMS transitioned to SPMS by the age of 75 years (average age at onset: 45 years).23 In a cohort study of patients with MS (n = 1,099) followed for longer than 25 years, >90% had transitioned to the progressive phase.24 Another study in patients with RRMS who did not receive any treatment revealed that occurrence of a second clinical attack is typical within the first 2 years, and it takes approximately 15 years to convert to SPMS from disease onset.e24 Longitudinal data from the MSBase registry indicated that the median time to SPMS were 32.4 years from disease onset. This was further confirmed in a subcohort followed prospectively for ≤10 years from disease onset (n = 11,926) which revealed that the proportion of patients with SPMS at 32.4 years was 60%.e25 In addition, findings in a DMT-treated cohort of 517 patients suggest that only 18.1% of patients with RRMS progress to SPMS after a median duration of 16.8 years from disease onset.e26
Diagnostic Uncertainty, Defining SPMS and Assessing Disease Activity and Disability
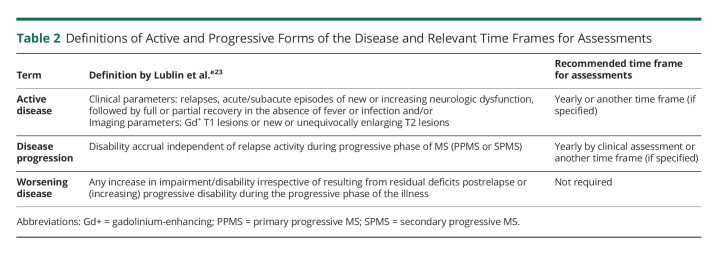
No “gold standard” definition of SPMS or clear clinical, imaging, immunologic, or pathologic criteria exist to confidently delineate patient progression from RRMS to SPMS.e7,e27 The most commonly used definition of SPMS coursee1 is based on the subjective judgment of the treating neurologist who retrospectively defines SPMS as a history of gradual progression after an initial RRMS course. This lack of a precise definition is largely due to the gradual nature of the transition rather without an identifiable tipping point. Indeed, the pathologic processes that result in secondary progression likely begin early in the relapsing phase of MS as evidenced by recent articles that describe confirmed and sustained progression without evidence of temporal relation to relapses in relapsing MS data sets.25 Many studies have examined the time required to confirm symptomatic disability progression because different time frames could be more or less successful in detecting progression. “Confirmed progression” was defined by an increase in neurologic dysfunction that persisted over a specified time period (e.g., 3, 6 or 12 months).e1 The sensitivity and specificity of various definitions considering confirmation time frame of 3, 6, 12, and 24 months were also evaluated.e3 The definition with the best performance involved 3-strata with a minimum EDSS score of 4, a pyramidal score ≥2, and a 3-month confirmation period without preceding relapse.e3 This definition could be applied to strengthen the study design and improve comparability of clinical trials and observational studies.
Nonetheless, this definition may not capture SPMS early enough and is more commonly used for clinical trials than in daily clinical practice. This suggests a need to develop a more objective and data-driven SPMS definition for better understanding of the disease course characterization among both clinicians and patients.8,e28
In Europe and the United States, recent marketing authorizations for DMTs (siponimod, ocrelizumab, and cladribine) used different definitions of activity: EU regulators defined activity as presence of relapses or imaging features of inflammatory activity, whereas US regulators limited the definition of activity to clinical relapses with no mention of MRI criteria.26,27,e29 Discrepancies in the use of clinical descriptors introduced by the regulatory agencies could potentially lead to confusion in clinical practice and future clinical trials; therefore, the clinical definitions for active disease, progression and worsening of the disease (Table 2), along with time frames for better clinical decision-making were recently reiterated.e28 Active inflammation was defined as a clinical relapse or MRI activity evidenced by new/enlarging T2 lesions or Gd+ lesions during the previous 5 years.e25,e28 Clear criteria to differentiate active vs nonactive forms of SPMS would be helpful for conducting clinical trials and for including patients in registries and observational studies, which may in turn harmonize regulatory decisions and allow drug development in the underserved naSPMS cohort. However, findings from the Adelphi MS DSP suggest that this may be challenging: When investigating how activity in SPMS was detected, activity was much more commonly found through MRI only (59.1%) than by relapse only (12.6%) or by both relapse and MRI combined (28.3%).22 Given that, in a 12-month period, patients with naSPMS are less likely to receive an MRI (58.7%) vs aSPMS (87.7%), the chance to miss activity and misclassify patients with SPMS with activity as nonactive is a real possibility. The results from the EXPAND trial showed that over half of patients deemed nonactive at baseline (no relapses in the previous 2 years and no T1 lesions at baseline) had renewed activity on placebo. Thus, defining aSPMS and naSPMS reliably is difficult, and more studies are needed to characterize how SPMS populations evolve over time.
Table 2.
Definitions of Active and Progressive Forms of the Disease and Relevant Time Frames for Assessments
Recently published observational data from the French population–based MS registry (Registre Lorrain des Scléroses en Plaques)28 investigated the frequency of active inflammation among 833 patients with SPMS who had at least 1 episode of clinical and/or radiologic activity during the 15 years after onset of progression. During the initial 5 years of the SPMS phase, approximately 10%–15% of patients experienced a clinical relapse, while the proportion of patients with active inflammation rose to 12%–24% after applying the clinical and radiologic assessments.28 Patients were more likely to have “disease activity” (evidenced by clinical relapse, MRI activity, or both) if they had experienced either a relapse or MRI activity in the previous 5 years. Conversely, the likelihood of disease activity was inversely related to age, level of disability, and DMT use.28,e30 Such population-based observational studies provide essential guidance to treating neurologists to identify any ongoing inflammation in patients with SPMS and to closely observe the patient characteristics suggestive of possible inflammation in their patients.e30
Another key aspect is that typically in SPMS trials, disability progression is measured as a change in an ordinal and predominantly ambulation-based EDSS (≥4), which alone is not a sufficient measure to precisely detect disability progression to SPMS.29,e3 Furthermore, certain psychometric limitations of the EDSS (low sensitivity and responsiveness especially at upper levels) are well described.17 Consequently, assessment of other disability functions (e.g., cognition, arm function, bowel, and bladder function) may therefore become difficult in patients with EDSS >4.0. These functions can, at least in part, be measured by tools commonly used in clinical trials such as the symbol digit modality test that assesses cognitive processing speede32 and the 9-HPT that assesses arm and hand dexterity.e32 Notably, well-validated tools for assessing bowel and bladder dysfunction in MS are lacking.30
Latest efforts7 at defining the clinical predictors of evolution to SPMS confirmed that disability worsening without a relapse (nr-CDW) poses a greater risk of progression to SPMS vs disability worsening due to incomplete recovery after a relapse (r-CDW) in patients with higher EDSS scores (>3). This highlights involvement of 2 pathologic processes underpinning the disease course: r-CDW likely reflects inflammation, whereas nr-CDW captures the neurodegenerative aspect of the disease.7 In this context, an initial CDW identified as nr-CDW can serve as a proxy for clinicians, warning them about the patient's possible progression to the SPMS phenotype and hints at identifying the “turning point” along the disease continuum. MS-treating neurologists in the United States who participated in a cross-sectional study rated “patient's clinical history in the past 1 year,” “neurologic examination” and “most recent MRI” as important clinical predictors for detecting progression from RRMS to SPMS during a clinical encounter.e33 The findings of this survey further substantiated the results of another global cross-sectional quantitative study in which patient history and gradual worsening of symptoms were viewed as predictors of progression to SPMS.31
Overall, the above factors emphasize the need for continued education and training of neurologists regarding diagnostic criteria improvements that may lead to earlier diagnosis in SPMS.e8,e28,e33,e34
Tools to Identify SPMS Earlier or Predict SPMS Progression
Currently, sensitive measures are required to predict SPMS progression earlier in the disease course. Different tools are in various stages of development, with some already being in the clinic.
Prospective Approaches
Considering the heterogeneity of the SPMS clinical course, the use of multiple clinical markers is crucial for the assessment of disability progression in SPMS.e35 In addition to the EDSS factors, the neurologic and clinical history of the patient or an MSFCe36 assessment, which characterizes progression using functional tests, are valuable resources in detecting impairments during the progressive phase of the disease course. Of key importance in SPMS is an objective assessment of the disease status involving any chronic or long-standing changes and the ability to tease apart any direct causality of such changes with the inflammatory disease activity.17
Screening tools are being developed to identify patients earlier in their SPMS transition. These newer tools such as the MS Prediction Score,e37 MS Progression Discussion Tool,32,e38 or the SPMS nomograme39 can assess subtle signs of progressive disease and their influence on daily activities. To collect long-term monitoring data, these tools can be integrated into electronic health records and used as part of routine clinical assessments. This would enable modeling of disease progression and treatment simulation for individual patients.e40
MRI is an established diagnostic tool for MS.33 Quantitative MRI techniques have improved the data quality, providing better tissue-specific assessments and more sensitive measurements of gray matter changes. Brain volumes and spinal cord areas show promise for monitoring neurodegeneration in patients with SPMS who are characterized by less inflammation than patients with RRMS.34,35,e41,e42 These tools could help to distinguish disease-related and treatment-related brain volume and spinal cord changes as well as mark the transition from RRMS to SPMS.
Among additional imaging biomarkers, leptomeningeal contrast enhancement, slowly expanding lesions or T2-lesion volume have significant associations with clinical and/or MRI measures of disease progression; however, further characterization of their histopathologic correlates is warranted to support their use in the clinical practice.33 In addition, paramagnetic rim+ lesions characterized by accumulation of iron have been reported as prognostic and diagnostic biomarkers in MS for disability prediction through their disruptions to the structural connectome than compared with rim lesions. They have been found to be less prevalent compared with central veins both at patient-level and lesion-level; however, they are clinically important owing to their specificity to MS and association with disease severity. Thus, they can be combined with other biomarkers to improve their usage in prognosis and diagnosis of MS.e43,e44
Other neuroimaging and laboratory biomarkers that identify progression in MS include normalized magnetization transfer ratio, cortical gray matter, and positron emission tomography (translocator protein, myelin tracers), which are described in detail elsewhere.5
Optical coherence tomography (OCT) of the retina has also been explored in detecting progression in MS and was tested in neuroprotective strategies.36,e45 OCT assesses the retinal fiber layer (RNFL) and macular ganglion cell layer. According to the clinical trials that tested RNFL thickness and macular volume in progressive MS, more RNFL thinning was seen in patients with SPMS and patients with primary progressive MS than in those with RRMS, particularly within quadrants of the peripapillary retina.e46 OCT was also evaluated as a measure for neurodegeneration. OCtiMS, a multicenter, longitudinal, 3-year study, evaluated changes in RNFL and ganglion cell layer in 332 patients with MS. These OCT measures were highly reproducible for monitoring disease progression and for quantifying neurodegeneration in the early disease course.37 The results from the Secondary and Primary Progressive Ibudilast NeuroNEXT Trial in MS (SPRINT-MS) study suggest that for a therapy (e.g., ibudilast) which has a large treatment effect, OCT implementation in progressive MS trials could prove to be beneficial for a variety of reasons.e47
Among prognostic tools, biomarkers such as neurofilament light (NfL), glial fibrillary acidic protein (GFAP), or a combination of both have made considerable progress.38 Serum NfL monitoring indicates future or ongoing disease activity especially when other clinical parameters may seem stable.e48,e49 A recent systematic review described the available evidence on NfL as a biomarker of neuroinflammation, future brain atrophy, and immunosuppressive treatment response at a group level in progressive MS.39 In another study, CSF NfL levels were associated with a risk of conversion from RRMS to SPMS.e50 Hence, serum neurofilament could assist in phenotyping progressive disease in the future.40,e51 Another possible advancement could be an MS biosignature that combines serum NfL, serum GFAP, and MRI markers to monitor disease progression instead of waiting for clinical worsening.41
In recent biomarker discovery, metabolomics has evolved as another measure for prognosis that can be used for identifying disease pathways underpinning clinical phenotypes such as RRMS or SPMS.42 Metabolomics comprises a detailed study of the metabolome in a biological sample including all low molecular weight (<1,500 Da) metabolites. It was developed as an Absolute IDQ-p400 test kit that could be used for quantifying targeted metabolites in the CSF. The test is known to be resistant to sample handling variations. In a previous study, an age-matched and sex-matched, cohort of patients with SPMS and controls were used to explore the differences in metabolite concentrations.e52
High-quality, disease-specific patient-reported outcome (PRO) measures need to be developed that can capture the true concerns of patients in real time and assess the impact of both clinical and nonclinical interventions on a variety of outcomes. One way to initiate this could be by exploring the use of information technology to collect patient-level data and develop multidisciplinary care protocols for the collection of PROs.43,e53 In addition, longitudinal monitoring of PROs and MS performance testing may also help to identify distinctive evolutionary patterns in the PROs and Timed-25 Foot Walk Test (T25FW) that may be too subtle to recognize with serial neurologic examinations in clinic for patients approaching or in the midst of SPMS progression.44
Disability progression can also have an impact on health economic outcomes such as higher utilization of societal resources and can potentially lead to a significant increase in the societal costs of MS. In contrast to RRMS, the substantially higher economic and humanistic burden associated with SPMS can be attributed to the greater symptomatic burden and higher disability (EDSS),e54 which culminates in a steady and gradual decrease in health-related quality of life, as well as higher costs.e55,e56 Pharmacoeconomic tools to identify progression-related costse56 are under way that apply a standardized longitudinal model to estimate the higher societal economic costs associated with progression independent of relapse activity or relapse-associated worsening in SPMS.45 Ideally, appropriate and early treatment would delay the time of conversion to SPMS, limiting both the human and economic costs of severe disability. The MS Health Resource Survey46 is an online tool to investigate resource utilization both in cross-sectional and longitudinal studies. This could allow transparent estimation of the health economic impact of clinical endpoints across multiple regions.
Retrospective Approaches
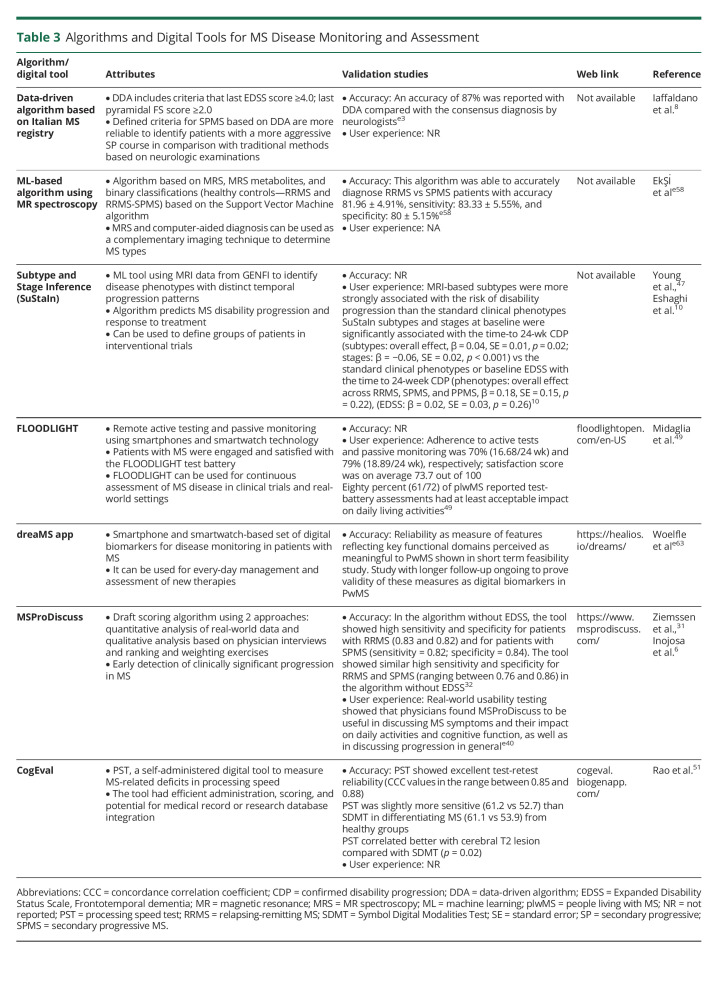
It was recently reported that a data-driven algorithm identifies more patients with aggressive and progressive SPMS by starting at a minimum EDSS of 4.0 at the time of conversion to SPMS, thus omitting the “progression events” which start at lower EDSS scores (Table 3).8 Machine learning algorithms may serve as a prognostic tool to predict SPMS disability progression without significant human intervention or burden.e57 Identification of patients with the highest progression risk has immediate application for inclusion in future SPMS trials and would reduce exposure of low-risk patients to investigational therapies. In another study, a support vector machine algorithm was used for automatic classification of healthy controls, patients with RRMS, and patients with SPMS by using mass resonance spectroscopy and machine learning methods. The results showed classification of RRMS and SPMS with 83.33% accuracy, 81.81% sensitivity, and 85.71% specificity.e58 An unsupervised machine learning algorithm—Subtype and Staging Inference (SuStaIn)—was also introduced to detect data-driven disease subtypes with distinct temporal progression patterns based on MRI scans.47 SuStaIn can be used to disentangle temporal and phenotypic heterogeneity algorithms. MRI-based subtypes defined using SuStaIn were able to predict MS disability progression and response to treatment.10
Table 3.
Algorithms and Digital Tools for MS Disease Monitoring and Assessment
Recently, a scoring algorithm that integrates data from ranking and weighting exercises, qualitative interviews, and a real-world observational study was developed.e59 This comprehensive approach could be applied to capture early signs of progression to SPMS. Based on this questionnaire, age, MS disease activity, and EDSS were the most significant physician-reported predictors of progression to SPMS, while patient-reported strongest predictors of progression to SPMS were age, mobility, and self-care using multiple logistic regression.
With the advent of new technical advances, digital tools48,e60 may present a convenient method for patients to self-assess and self-monitor outcomes (Table 3). FLOODLIGHT is a digital application used in clinical trials that combines active assessments and passive monitoring of movement to track MS symptoms. FLOODLIGHT sensor-based measures can be used in clinical trials and real-world settings to assess feasibility of remote active testing and passive monitoring using smartphones and smartwatch technology.49 The MSProDiscuss digital tool may be useful for early detection of clinically significant progression in MS, after a series of questions taking approximately 4 minutes to complete, a traffic light system helps to understand the likelihood of progression to SPMS.e61 The DreaMS app was developed to assess a smartphone and smartwatch-based set of digital biomarkers for disease monitoring in MS.e62,e63 Before implementation, validation of these digital concepts will be necessary with long-term cohort data matched with the clinical opinions of multidisciplinary teams.
Impairments in cognitive function can be an early identifier of disease progression because the deficits/worsening may be present in patients without physical disability.50,e64 In clinical practice, quantitative cognitive tests are not routinely administered by neurologists.e65 However, introduction of digital tools such as CogEval may aid neurologists in evaluating cognitive function in patients with MS.51 Cognitive impairment in MS remains therapeutically challenging. Possible approaches to address this unmet need involve cognitive rehabilitation and exercise training.50,e66
Treatments and Symptom Management for SPMS
Ultimately, early identification of SPMS will not be helpful if it is not linked to treatment with appropriate therapies. A harmonized definition of SPMS will also help in subsequent inclusion in SPMS trials. The role of DMTs in slowing SPMS progression and evolving treatments that exhibit immunomodulatory, neuroprotective/regenerative properties have been extensively discussed in many recent articles.52,53,e67-e72 Symptom management, however, also plays a crucial role in patient care.
Once patients transition to SPMS, mobility, and other physical aspects are typically more impaired than in RRMS.4,54 Symptoms including spasticity, pain, fatigue, cognitive impairment, bladder and bowel issues, gait dysfunction, mood dysregulation, and sleep disturbance require attention.e73 Management of a patient's specific constellation of symptoms and complex psychosocial needs by using a combination of pharmacologic and nonpharmacologic approaches may need to be considered for improving quality of life.55,e74 A recent study explored the usability of a mobile app for real-time assessment of fatigue and associated symptoms in patients with MS.e75 SPMS is associated with broad and complex comorbidities and symptoms and an increased likelihood of a minority patients with SPMS will eventually require palliative care.e76,e77 Multidisciplinary teams, therefore, involving a neurologist; primary care physician; physical, occupational, and speech therapists; psychologist; urologist; and specialists in physical medicine and rehabilitation, pain management, and infectious diseases, can offer comprehensive support for effective management of SPMS.e77 This multidisciplinary approach provides a holistic view of factors along the patient journey (e.g., diagnosis, disease course and evolution over time, treatment patterns across cohorts, perspectives from the patients, care providers, and physicians, etc.) to identify overarching challenges encountered by all stakeholders involved in the management of MS.56,57,e79,e80 It is also imperative for clinicians to improve collaboration and referral pathways while managing patients with SPMS.e80
The Managing the Transition to SPMS (ManTra)58 study from Italy and Germany evaluated the experiences of patients recently diagnosed with SPMS. According to the report, >40% of recently diagnosed patients with SPMS were unaware of their disease, highlighting a gap in the patient-physician communication and information exchange that needs to be addressed, despite a period of diagnostic uncertainty. Furthermore, the study also documented certain patient needs such as access to the “physiotherapy and exercise programs” and more “patient active involvement in health care.”
Amid the diagnostic uncertainty between the RRMS and SPMS phenotypes, the perception of MS as “one disease” has undergone a paradigm shift over time. The current emphasis of the medical and scientific community is, therefore, on the timely detection of progressive elements within the MS disease continuum to identify an early window of opportunity for effective treatment to modify the disease trajectory. Despite challenges in the clinical management of SPMS, including ambiguities associated with the definition of SPMS and active vs nonactive forms of the disease, a combination of prospective and retrospective tools/approaches, and enhanced awareness of the heterogeneity of different patient populations included in registries, real-world cohorts, randomized controlled trials and their extensions, are expected to optimize care for patients with SPMS. Such care should not be restricted to pharmacologic interventions but include nonpharmacological strategies based on collaborative efforts in multidisciplinary teams.
Acknowledgment
All named authors meet the criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors are responsible for intellectual content and data accuracy. Financial support only for medical editorial assistance was provided by Novartis AG. All authors had full control of the content and made independently the final decision for all aspects of this article.
Glossary
- ARR
annualized relapse rate
- CDW
confirmed disability worsening
- DMTs
disease-modifying therapies
- DSP
Disease-Specific Programme
- EDSS
Expanded Disability Status Scale
- EU
European Union
- GFAP
glial fibrillary acidic protein
- IFN-β
interferon-beta
- IQR
interquartile range
- MS
multiple sclerosis
- MSFC
MS functional composite
- NA
North American
- OCT
optical coherence tomography
- PIRA
progression independent of relapse activity
- PRO
patient-reported outcome
- RCN
research collaboration network
- RNFL
retinal fiber layer
- RRMS
relapsing-remitting MS
- SPMS
secondary progressive MS
Appendix. Authors
Study Funding
This study was funded by Novartis Pharma AG, Switzerland.
Disclosure
T. Ziemssen has received speaking honoraria and financial support for research activities from Almirall, Biogen, Celgene, Novartis, Roche, Teva, and Sanofi. V. Bhan has received honoraria and consulting fees from Biogen, EMD Serono, Genzyme, Novartis, Roche, Sanofi, and Teva Neurosciences. J. Chataway in the last 3 years has received support from the Efficacy and Evaluation Programme, a Medical Research Council and National Institute for Health Research (NIHR) partnership and the Health Technology Assessment Programme, the UK MS Society, the US National MS Society, and the Rosetrees Trust. He is supported in part by the NIHR, University College London Hospitals, Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by the Canadian MS society and local principal investigator for commercial trials funded by Actelion, Biogen, Novartis, and Roche. He has received an investigator grant from Novartis and has taken part in advisory boards/consultancy for Azadyne, Biogen, Celgene, Janssen, MedDay, Merck, NervGen, Novartis, and Roche. P. Labauge has received fees and honoraria from Merck, Sanofi Genzyme, Novartis, Roche, and Teva. T. Chitnis has received research funding from Serono, Novartis, and Verily and has participated as a consultant or advisor for Biogen Idec, Novartis, and Sanofi Genzyme. B.A.C. Cree has received personal compensation for consulting from Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Neuron23, Novartis, Sanofi, TG Therapeutics, and Therini and received research support from Genentech. M. Trojano has served on Scientific Advisory Boards for Biogen, Novartis, Roche, Merck, BMS Celgene, and Janssen; has received speaker honoraria from Biogen, Sanofi, Merck, Roche, and Novartis; and has received research grants for her Department from Biogen, Merck, Roche, and Novartis. E.K. Havrdova has received honoraria/research support from Biogen, Merck Serono, Novartis, Roche, and Teva and has served as a member of advisory boards for Actelion, Biogen, Celgene, Merck Serono, Novartis, and Sanofi Genzyme. L. Kappos's institution (University Hospital Basel) has received in the last 3 years and used exclusively for research support at the Department: steering committee, advisory board, and consultancy fees and support of educational activities from Actelion, Allergan, Almirall, Baxalta, Bayer, Biogen, Celgene/Receptos, CSL-Behring, Desitin, Excemed, Eisai, Genzyme, Japan Tobacco, Merck, Minoryx, Novartis, Pfizer, F. Hoffmann-La Roche Ltd, Sanofi Aventis, Santhera, and Teva and license fees for Neurostatus-UHB products. The Research of the MS Center in Basel has been supported by grants from Bayer, Biogen, Novartis, the Swiss MS Society, the Swiss National Research Foundation, Inno-Suisse, the European Union, and Roche Research Foundations. J. Nakahara has received speaking honoraria from AbbVie, Alexion, Astellas, Biogen, Chugai, CSL-Behring, Daiichi-Sankyo, Eisai, Fujimoto Pharma, JB, Mitsubishi-Tanabe, Novartis, Otsuka, Sanofi, Sumitomo Dainippon, and Takeda; consultancy fees from Alexion, Biogen, Chugai, Mitsubishi-Tanabe, and Novartis; and received research funds from AbbVie, Biogen, Böehringer-Ingelheim, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, JB, Kyowa-Kirin, Mitsubishi-Tanabe, MSD, Otsuka, Pfizer, Shionogi, Sumitomo Dainippon, Takeda, and Tsumura. C. Oreja-Guevara has received speaker and consulting fees from Biogen, Celgene, Merck KGaA (Darmstadt, Germany), Novartis, Roche, Sanofi Genzyme, and Teva. J. Palace has received support for scientific meetings and honorariums for advisory work from Merck Serono, Novartis, Chugai, Alexion, Roche, Medimmune, Argenx, UCB, Mitsubishi, Amplo, Janssen, Sanofi. Grants from Alexion, Roche, Medimmune, Amplo biotechnology, and UCB. Patent ref P37347WO and license agreement Numares multimarker MS diagnostics Shares in AstraZeneca. She acknowledges partial funding by highly specialized services NHS England. B. Singer has received research grant support from AbbVie, Alkermes, Biogen, Greenwich Biosciences, MedImmune, Novartis, Roche, and Sanofi Genzyme and consulting and/or speaking fees from AbbVie, Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Janssen, Genentech, Greenwich Biosciences, Novartis, Roche, Sanofi Genzyme, and TG Therapeutics. A. Patil is an employee of Novartis Healthcare Pvt. Ltd. India. B. Rauser is an employee of Novartis Pharma GmbH, Nuremberg, Germany. T. Hach is an employee of Novartis Pharma AG, Basel, Switzerland.
References
- 1.Boyko A, Therapontos C, Horakova D, et al. Approaches and challenges in the diagnosis and management of secondary progressive multiple sclerosis: a Central Eastern European perspective from healthcare professionals. Mult Scler Relat Disord. 2021;50:102778. [DOI] [PubMed] [Google Scholar]
- 2.Katz Sand I, Krieger S, Farrell C, Miller AE. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler J. 2014;20(12):1654-1657. [DOI] [PubMed] [Google Scholar]
- 3.Khurana V, Sharma H, Medin J. Estimated prevalence of secondary progressive multiple sclerosis in the USA and Europe: results from a systematic literature search (P2. 380). Neurology 2018:90(15 suppl):P2.380. [Google Scholar]
- 4.Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. Neuropsychiatr Dis Treat. 2017;13:1349-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippi M, Preziosa P, Langdon D, et al. Identifying progression in multiple sclerosis: new perspectives. Ann Neurol. 2020;88(3):438-452. [DOI] [PubMed] [Google Scholar]
- 6.Inojosa H, Ziemssen T. How to reduce the delay of diagnosing secondary progression in multiple sclerosis. Mult Scler J. 2021;27(4):646-647. [DOI] [PubMed] [Google Scholar]
- 7.Carotenuto A, Signoriello E, Lanzillo R, et al. Unraveling diagnostic uncertainty in transition phase from relapsing-remitting to secondary progressive multiple sclerosis. Mult Scler Relat Disord. 2020;43:102211. [DOI] [PubMed] [Google Scholar]
- 8.Iaffaldano P, Lucisano G, Patti F, et al. Transition to secondary progression in relapsing-onset multiple sclerosis: definitions and risk factors. Mult Scler. 2021;27(3):430-438. [DOI] [PubMed] [Google Scholar]
- 9.Davies F, Wood F, Brain KE, et al. The transition to secondary progressive multiple sclerosis: an exploratory qualitative study of health professionals' experiences. Int J MS Care. 2016;18:257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eshaghi A, Young AL, Wijeratne PA, et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat Commun. 2021;12(1):2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor R, Ho P-R, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415. [DOI] [PubMed] [Google Scholar]
- 12.Cree BAC, Cutter G, Wolinsky JS, et al. Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):988-997. [DOI] [PubMed] [Google Scholar]
- 13.Freedman MS, Bar-Or A, Oger J, et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology. 2011;77(16):1551-1560. [DOI] [PubMed] [Google Scholar]
- 14.The North American Study Group on Interferon Beta. Interferon beta-1b in secondary progressive MS. Neurology. 2004;63(10):1788-1795. [DOI] [PubMed] [Google Scholar]
- 15.Kappos L, Weinshenker B, Pozzilli C, et al. Interferon beta-1b in secondary progressive MS: a combined analysis of the two trials. Neurology. 2004;63:1779-1787. [DOI] [PubMed] [Google Scholar]
- 16.McAdams M, Stankiewicz JM, Weiner HL, Chitnis T. Review of phase III clinical trials outcomes in patients with secondary progressive multiple sclerosis. Mult Scler Relat Disord. 2021;54:103086. [DOI] [PubMed] [Google Scholar]
- 17.Inojosa H, Schriefer D, Ziemssen T. Clinical outcome measures in multiple sclerosis: a review. Autoimmun Rev. 2020;19(5):102512. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg L, Stahmann A, Middleton R, et al. Comparison of the proportions of secondary progressive multiple sclerosis between three registries within the SPMS research collaboration network. Neurology. 2020;94(15 suppl):3977. [Google Scholar]
- 19.Klotz L, Weber MS, Schreiber H, et al. The AMASIA study: real world insights on siponimod treated patients with secondary progressive multiple sclerosis in Germany. Poster presented at the DGN Congress 2021; IP025. Accessed April 5, 2022. dgnvirtualmeeting.org/#!events/359/eposters/order=primary_ref&resourcetype_ids=3,4&event_ids=359&contenttype_ids=null&page=1&query=AMASIA.
- 20.Inojosa H, Rauser B, Ettle B, Ziemssen T. The transitional phase of multiple sclerosis: the concept of PANGAEA 2.0 evolution study. Mult Scler Relat Disord. 2020;46:102523. [DOI] [PubMed] [Google Scholar]
- 21.Miguez J, Pappolla A, Patrucco L, Cristiano E, Vrech C, Rojas J. Clinical and demographic aspects of secondary progressive multiple sclerosis in Argentina. Medicina 2020;80(6):606-610. [PubMed] [Google Scholar]
- 22.Giovannoni G, Houchen E, Sobisek L, et al. MRI activity versus relapses as markers of disease activity in SPMS: data from real world and pivotal clinical studies. Accessed November 11, 2022. Available at: https://ectrims2021.abstractserver.com/program/#/details/presentations/1220. [Google Scholar]
- 23.Tutuncu M, Tang J, Zeid NA, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler J. 2013;19(2):188-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice G. The natural history of secondary progressive multiple sclerosis: observations from the London study group. Mult Scler J. 2002;8(1):81-82. [DOI] [PubMed] [Google Scholar]
- 25.Graf J, Leussink VI, Soncin G, et al. Relapse-independent multiple sclerosis progression under natalizumab. Brain Commun. 2021;3(4):fcab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Food and Drug Administration (USA). Siponimod Summary Review. Accessed April 5, 2022. http://accessdata.fda.gov/drugsatfda_docs/nda/2019/209884Orig1s000SumR.pdf. [Google Scholar]
- 27.European Medicines Agency. Summary of Opinion—Ocrevus; 2017. Accessed January 20, 2022. ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-ocrevus.en.pdf. [Google Scholar]
- 28.Mathey G, Ancel T, Garot T, et al. Clinical and radiological activity of secondary progressive multiple sclerosis in a population-based cohort. Eur J Neurol. 2021;28(7):2238-2248. [DOI] [PubMed] [Google Scholar]
- 29.Koch MW, Mostert J, Repovic P, Bowen JD, Uitdehaag B, Cutter G. Reliability of outcome measures in clinical trials in secondary progressive multiple sclerosis. Neurology. 2021;96(1):e111-e120. [DOI] [PubMed] [Google Scholar]
- 30.Patel DP, Elliott SP, Stoffel JT, Brant WO, Hotaling JM, Myers JB. Patient reported outcomes measures in neurogenic bladder and bowel: a systematic review of the current literature. Neurourol Urodyn. 2016;35(1):8-14. [DOI] [PubMed] [Google Scholar]
- 31.Ziemssen T, Tolley C, Bennett B, et al. A mixed methods approach towards understanding key disease characteristics associated with the progression from RRMS to SPMS: physicians' and patients' views. Mult Scler Relat Disord. 2020;38:101861. [DOI] [PubMed] [Google Scholar]
- 32.Ziemssen T, Piani-Meier D, Bennett B, et al. A physician-completed digital tool for evaluating disease progression (multiple sclerosis progression discussion tool): validation study. J Med Internet Res. 2020;22(2):e16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavazzi E, Zivadinov R, Dwyer MG, et al. MRI biomarkers of disease progression and conversion to secondary-progressive multiple sclerosis. Expert Rev Neurother. 2020;20(8):821-834. [DOI] [PubMed] [Google Scholar]
- 34.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5(5):256-266. [DOI] [PubMed] [Google Scholar]
- 35.Masek M, Vaneckova M, Krasensky J, et al. Secondary-progressive form of multiple sclerosis: MRI changes versus clinical status. Neuroendocrinol Lett. 2008;29(4):461. [PubMed] [Google Scholar]
- 36.Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9:921-932. [DOI] [PubMed] [Google Scholar]
- 37.Paul F, Calabresi PA, Barkhof F, et al. Optical coherence tomography in multiple sclerosis: a 3-year prospective multicenter study. Ann Clin Transl Neurol. 2021;8(12):2235-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams T, Zetterberg H, Chataway J. Neurofilaments in progressive multiple sclerosis: a systematic review. J Neurol. 2021;268(9):3212-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapoor R, Smith KE, Allegretta M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology. 2020;95(10):436-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhle J, Kropshofer H, Maceski AM, et al. Plasma glial fibrillary acidic protein correlates with characteristics of advanced disease and treatment response in secondary progressive multiple sclerosis. Neurology. 2020;94(15 suppl):1782. [Google Scholar]
- 42.Yeo T, Sealey M, Zhou Y, et al. A blood-based metabolomics test to distinguish relapsing-remitting and secondary progressive multiple sclerosis: addressing practical considerations for clinical application. Sci Rep. 2020;10(1):12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Amico E, Haase R, Ziemssen T. Patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord. 2019;33:61-66. [DOI] [PubMed] [Google Scholar]
- 44.Conway DS, Thompson NR, Meng X, Johnson K, Fox RJ. Patient reported outcomes and performance metrics at diagnosis of secondary progressive multiple sclerosis. Mult Scler J. 2021;27(5):742-754. [DOI] [PubMed] [Google Scholar]
- 45.Ness N-H, Schriefer D, Haase R, Ettle B, Cornelissen C, Ziemssen T. Differentiating societal costs of disability worsening in multiple sclerosis. J Neurol. 2020;267(4):1035-1042. [DOI] [PubMed] [Google Scholar]
- 46.Ness N-H, Haase R, Kern R, et al. The multiple sclerosis health resource utilization survey (MS-HRS): development and validation study. J Med Internet Res. 2020;22(3):e17921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young AL, Marinescu RV, Oxtoby NP, et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat Commun. 2018;9(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maillart E, Labauge P, Cohen M, et al. MSCopilot, a new multiple sclerosis self-assessment digital solution: results of a comparative study versus standard tests. Eur J Neurol. 2020;27(3):429-436. [DOI] [PubMed] [Google Scholar]
- 49.Midaglia L, Mulero P, Montalban X, et al. Adherence and satisfaction of smartphone-and smartwatch-based remote active testing and passive monitoring in people with multiple sclerosis: nonrandomized interventional feasibility study. J Med Internet Res. 2019;21(8):e14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLuca J, Chiaravalloti ND, Sandroff BM. Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat Rev Neurol. 2020;16(6):319-332. [DOI] [PubMed] [Google Scholar]
- 51.Rao SM, Losinski G, Mourany L, et al. Processing speed test: validation of a self-administered, iPad((R))-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler. 2017;23(4):1929-1937. [DOI] [PubMed] [Google Scholar]
- 52.Samjoo IA, Worthington E, Haltner A, et al. Matching-adjusted indirect treatment comparison of siponimod and other disease modifying treatments in secondary progressive multiple sclerosis. Curr Med Res Opin. 2020;36(7):1157-1166. [DOI] [PubMed] [Google Scholar]
- 53.Yong HYF, Yong VW. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat Rev Neurol. 2022;18(1):40-55. [DOI] [PubMed] [Google Scholar]
- 54.Chataway J, Murphy N, Khurana V, Schofield H, Findlay J, Adlard N. Secondary progressive multiple sclerosis: a systematic review of costs and health state utilities. Curr Med Res Opin. 2021;37(6):995-1004. [DOI] [PubMed] [Google Scholar]
- 55.Rommer PS, Eichstädt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler J. 2019;25(12):1641-1652. [DOI] [PubMed] [Google Scholar]
- 56.Giovannetti AM, Pietrolongo E, Borreani C, et al. Conversion to secondary progressive multiple sclerosis: multistakeholder experiences and needs in Italy. PloS One. 2020;15(2):e0228587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meek C, Topcu G, Moghaddam N, das Nair R. Experiences of adjustment to secondary progressive multiple sclerosis: a meta-ethnographic systematic review. Disabil Rehabil. 2020;43(2):3135-3146. [DOI] [PubMed] [Google Scholar]
- 58.Solari A, Giovannetti AM, Giordano A, et al. Conversion to secondary progressive multiple sclerosis: patient awareness and needs. Results from an online survey in Italy and Germany. Front Neurol. 2019;10:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- eReferences e1-e80 are available at http://links.lww.com/NXI/A765.