Abstract
Background
Opioids can be effective analgesics, but long-term use may be associated with harms. In 2013, the first national, comprehensive, evidence-based pain management guideline was published, from the Scottish Intercollegiate Guideline Network (SIGN 136: Management of Chronic Pain) with key recommendations on analgesic prescribing. This study aimed to examine the potential impact on national opioid prescribing rates in Scotland.
Methods
Trends in national and regional community opioid prescribing data for Scotland were analysed from quarter one (Q1) 2005 to Q2 2020. Interrupted time series regression examined the association of SIGN 136 publication with prescribing rates for opioid-containing drugs. Gabapentinoid prescribing was used as a comparison drug.
Results
After a positive prescribing trend pre-publication, the timing of SIGN 136 publication was associated with a negative change in the trend of opioid prescribing rates (−2.82 items per 1000 population per quarter [PTPPQ]; P < 0.01). By Q2 2020, the relative reduction in the opioid prescribing rate was −20.67% (95% CI: −23.61, −17.76). This persisted after correcting for gabapentinoid prescribing and was mainly driven by the reduction in weak opioids, whereas strong opioid prescribing rates continued to rise. Gabapentinoid prescribing showed a significant rise in level (8.00 items per 1000 population; P = 0.01) and trend (0.27 items PTPPQ; P = 0.01) following SIGN 136 publication.
Conclusions
The publication of SIGN 136 was associated with a reduction in opioid prescribing rates. This suggests that changes in clinical policy through evidence-based national clinical guidelines may affect community opioid prescribing, though this may be partially replaced by gabapentinoids, and other factors may also contribute.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13012-022-01251-2.
Keywords: Opioids, Prescribing rates, Interrupted time series analysis, Clinical guideline, Chronic pain
Contributions to the literature.
In 2013, the Scottish Intercollegiate Guideline Network (SIGN) published the first national clinical guideline for the management of chronic pain (SIGN 136). This included key recommendations on safe and effective prescribing of opioids and warnings of the potential risk of harm.
However, there is a paucity of data on the effectiveness of clinical guidelines, especially in relation to opioid prescribing practices.
This study demonstrates the positive impact of changes in clinical and government policy on opioid prescribing rates and in primary care. It also highlights the continued importance of providing robust clinical guidelines based on reliable and up-to-date evidence.
Introduction
Chronic pain is a common and complex problem, with a debilitating impact on quality of life [1]. Whilst there is often no cure, opioids have been commonly used to treat patients with the disorder [2]. There is good evidence for their efficacy in acute and cancer-related pain, but very limited high-quality evidence for effectiveness in the long-term management of chronic non-malignant pain [3]. Inadequate pain relief with opioids can lead to dose escalation and tolerance, with risks of major adverse events such as dependence, addiction, overdose and death [4].
Opioid use has been increasing steadily worldwide, with the World Drug Report 2021 estimating that nearly 62 million people (aged 15–64) used opioids globally in 2019 [5]. In particular, the increase in prescribing of opioids in the USA from the late 1990s to the early 2010s has been well documented [6]. Possible explanations for this increase include an ageing population at greater risk of developing chronic pain conditions, the publication of clinical guidelines recommending opioids for chronic non-cancer pain, despite insufficient good quality evidence, (non-evidence-based) changes in recommendations on use from professional bodies and effective marketing from pharmaceutical companies [7]. This increase in opioid use has given rise to the “opioid epidemic” in the USA and there are concerns a similar situation could be happening in the UK, including Scotland [8, 9].
In December 2013, the Scottish Intercollegiate Guideline Network (SIGN), in consultation with the National Chronic Pain Steering Group of the Scottish Government and key stakeholders, published the first comprehensive evidence-based guideline for the assessment and management of chronic, non-malignant pain in adults (SIGN 136) [10]. SIGN 136 identified a research gap around understanding rates and effects of opioid prescribing in Scotland [11]. A resulting investigation of Scottish data revealed that prescribing rates of strong opioids doubled in the 10-year period leading up to the publication of SIGN 136 (2003–2012) [12]. One aspiration of SIGN 136 was to influence the safe and appropriate use of opioids in chronic pain, reducing unnecessary and potentially harmful prescribing. Although clinical guidelines can be important tools to support decision-making and reduce economic burden, there is a well-established gap between the publication of research evidence and changes to clinical practice, especially in the management of pain [13]. Popular estimates suggest that it takes 17 years for evidenced-based guidelines to be translated into clinical practice. In low back pain, it has been reported that adherence to clinical guidelines is only 40–67% [14, 15]. The barriers to clinical practice are complex and can include a lack of awareness of the most up-to-date guidelines and differing beliefs and attitudes about the best treatment pathway available. There is also evidence that dissemination and implementation activities tend to decrease over time, further limiting any potential positive impact [16]. Therefore, strategies to improve implementation are crucial and evidence suggests that using “active” rather than “passive” approaches, such as educational strategies, feedback on compliance and reminders [17], can cut the time taken to implement clinical guidelines to 3 years [18, 19]. However, a particular strategy may not work in all circumstances. Because of this, an important component of implementation research is evaluating the impact of interventions, in order to establish which approaches are most effective and if necessary make changes to implementation strategies. In this respect, interrupted time series analysis is a valuable tool for examining a repeated measure before and after a particular event to test for differences in immediate and gradual changes [20]. Furthermore, whilst it is recognised that clinical practice guidelines have the potential to increase the quality of care, systematic analysis of their impact is not common [21].
Gabapentinoids (mainly gabapentin and pregabalin) are licensed for the treatment of peripheral and central neuropathic pain, with strong evidence for their effectiveness [22, 23]. In contrast to opioids, SIGN 136 key recommendations did not caution about risks of harm related to gabapentinoid misuse, as, at the time of publication, there was little evidence of this.
Opioid prescribing rates have now become one of National Health Service (NHS) Scotland’s key National Therapeutic indicators, a set of prescribing indicators in specific therapeutic areas that can be used to compare prescribing behaviours against established guidelines [24]. These indicators clearly show that opioid prescribing rates are beginning to fall across Scotland. However, to the best of our knowledge, the potential impact of SIGN 136 on opioid prescribing rates has not been investigated. Therefore, this study aimed to analyse opioid prescribing rates in Scotland before and after SIGN 136 publication and to compare these to gabapentinoids.
Methods
We followed the Framework for Enhanced Reporting of Interrupted Time Series Studies (FERITS) statement [25], an adaptation of the Transparent Reporting of Evaluations with Nonrandomised Designs (TREND) statement [26], for the reporting of this study (Supplementary Table S1).
Study design
This study was an interrupted time series analysis of national-level prescribing data on opioid analgesics prescribed in primary care and dispensed by community pharmacies, to test the hypothesis that SIGN 136 is associated with a significant change in opioid prescribing trend. Gabapentinoid prescribing data were also obtained and used to correct for potential confounding from other interventions. The study period was from January 2005 to June 2020 and incorporated the SIGN 136 publication date (December 2013).
Data source
Data on all opioids and gabapentinoids, prescribed through primary care (in the community) by general practitioners (GPs) and non-medical prescribers and dispensed by community pharmacists in Scotland, were obtained from Public Health Scotland (PHS). This was based on aggregated and publicly available data [27]. PHS (https://www.publichealthscotland.scot/) is part of NHS Scotland and holds individual-level prescribing data through the Prescribing Information System (PIS), which is a national data system, set up in 2009, that captures all NHS prescriptions dispensed and reimbursed in the community (https://www.ndc.scot.nhs.uk/National-Datasets/data.asp?SubID=9). In the UK, healthcare policy is devolved to the individual nations and, in Scotland, community prescriptions are free at the point of delivery. Pharmacists are reimbursed for the prescriptions they dispense. The PIS covers the population of Scotland (approximately 5.3 million), with GPs accounting for approximately 95% of community prescribing, and a capture rate of 98.7% from prescription forms [28]. Also included in the data request were annual mid-year population estimates for Scotland as of 30th June each year.
Formal ethical approval was not required as the study used publicly available data which contained no patient or prescriber identifiable information.
Publication of SIGN 136
The Scottish Intercollegiate Guideline Network (SIGN) was established in 1993 by the Scottish Medical Royal Colleges and is now part of Healthcare Improvement Scotland, part of NHS Scotland. It produces evidence-based clinical practice guidelines for use across NHS Scotland, with accredited methodology [29], and is a member organisation of the Guidelines International Network (https://g-i-n.net/). SIGN 136 was published in December 2013 and, after a systematic review of the evidence, included key recommendations and best practice statements on safe and effective opioid prescribing (Supplementary Box S1). The Scottish Government requires Health Boards to identify areas of concern where they are not meeting SIGN’s key recommendations, so they become important benchmark standards for care. The Scottish Government also provides regular feedback of opioid prescribing data to individual GP Practices as National Therapeutic Indicators, to ensure the implementation of SIGN 136. Therefore, it has formed the basis of pain service provision and improvement in Scotland since its publication. The guideline is aimed at all healthcare professionals involved in the assessment and management of adult patients with chronic non-malignant pain in non-specialist settings. At the time of publication, hard copies were disseminated to all primary care practices across Scotland and the guideline is available for download from SIGN’s website (https://www.sign.ac.uk/assets/sign136.pdf). A patient version was also available [30]. The opioids section of this guideline (section 5.3 “Opioids”) was subsequently updated in August 2019 [31]. However, for this study, we only considered the original 2013 publication as the “intervention”.
To correct for any potential unforeseen confounders acting in Scotland, such as changes in the prevalence of chronic pain or changes in policy involving the use of pharmacological interventions for chronic pain in general, we decided to use gabapentinoid prescribing as a comparison. SIGN 136 included guidance for gabapentinoids (gabapentin and pregabalin), though in contrast to opioids there were no specific recommendations warning of the potential for abuse, addiction or other side effects (Supplementary Box S2). Therefore, it was hypothesised that gabapentinoid prescribing rates would not have reduced as a result of SIGN 136 publication.
Outcome
The primary outcome was the number of opioid prescription items dispensed per 1000 population per quarter (PTPPQ). A prescription item refers to a single supply of a medicine on a prescription form and is a measure of prescribing activity. A prescription may contain multiple pharmaceutical products. If a prescription form includes three medicines, it is counted as three prescription items. The number of gabapentinoid (gabapentin and pregabalin) items dispensed PTPPQ was used as a comparison outcome. Quarters were defined as January to March (Q1), April to June (Q2), July to September (Q3) and October to December (Q4), inclusive. A list of all relevant opioid-containing drugs included in the study is given in Supplementary Table S2. These include single and compound analgesics found in chapter 4.7.2 (“Opioid analgesics”) of the British National Formulary (BNF) [32] as well as additional combination products of opioids (e.g. co-codamol) found elsewhere. Gabapentin and pregabalin are detailed in chapter 4.8.1 (“Control of epilepsy”) of the BNF. The dataset includes all items prescribed through the NHS in primary care in Scotland (which provides the first point of contact for patients in the healthcare system, usually through GP practices), dispensed in the community in the UK and submitted for reimbursement. Data on items prescribed but not subsequently submitted for dispensing by the patient (estimated to be ~6%) [28] or dispensed but not submitted for reimbursement by the pharmacy are not currently held by PHS. The small number of private prescriptions, hospital and direct supply of medicines to patients (e.g. prescriptions supplied through specialist clinics) were not included.
Statistical analysis
Linear regression was used to analyse the impact of SIGN 136 on opioid and gabapentinoid prescribing trends nationally. The model for the analysis is provided in Table 1. As part of this process, plots of each time series were studied to check that the assumption of linearity was appropriate in each analysis.
Table 1.
Model for a controlled interrupted time series analysis
| Y = β0 + β1 * Time + β2 * Intervention status + β3 * Intervention status * Time + β4 * Cohort status + β5 * Cohort status * Time + β6 * Cohort status * Intervention status + β7 * Cohort status * Intervention status * Time | |
| Y is the outcome (prescribing rate). | |
| β0–3 are coefficients representing the control cohort (e.g. gabapentinoid series) where β0 is the intercept or value of the outcome at the start of the study period, β1 is the change in outcome per unit time (trend) before the intervention, β2 is the immediate step change in level following the intervention and β3 is the change in trend following the intervention (relative to the trend before the intervention – β1). β1 and β3 can therefore be summed to provide the trend following the intervention. β0–3 can also be used in isolation as a standalone model for a single interrupted time series analysis. | |
| β4–7 are coefficients representing the difference between the case series (e.g. opioid series) and the control series. β4 is the difference in intercept level, β5 is the difference in trend before the intervention, β6 is the difference in immediate change in level following the intervention and β7 is the difference in change in trend following the intervention. β5 and β7 can be summed to provide the difference in trend following the intervention. | |
| Time, intervention status and cohort status relate to variables in the dataset representing the time elapsed since the start of the study period, the pre- or post-intervention period and the time series assignment (e.g. opioids or gabapentinoids). |
The analysis of opioid prescribing trends was stratified according to opioid strength (weak or strong) and recipients’ age (0–29, 30–49, 50–69 and 70+ years) and gender. The rationale for the stratification of drugs by strength was based on the recommendation within SIGN 136 that strong opioids should be considered as an option for patients with chronic low back pain and osteoarthritis and that patients taking strong opioids should be assessed for signs of abuse and addiction (Supplementary Box S1). The classification of each drug was based on their status in SIGN 136 with codeine, dihydrocodeine, meptazinol and tramadol considered weak opioids and buprenorphine, diamorphine, dipipanone, fentanyl, hydromorphone, methadone, morphine, oxycodone, papaveretum, pentazocine, pethidine and tapentadol considered strong opioids. Compound drugs were classified according to the parent opioid (Supplementary Table S2).
Previous studies have shown that the prevalence of opioid use increases with age and is more common in females compared to males [33]. Similarly, age and gender are also associated with chronic pain [34]. Given these findings, we hypothesised that opioid prescribing trends may also differ between these demographics.
Level and slope change models were used to test the hypothesised immediate and longer-term impact on prescribing behaviour that the publication of SIGN 136 would have had.
A controlled interrupted time series approach was used to compare opioid prescribing trends to gabapentinoid prescribing trends, weak opioid vs strong opioid prescribing and prescribing between men and women.
Buprenorphine (often in combination with naloxone) and methadone are used as opioid replacement therapy in the management of opioid dependence, as well as to treat chronic pain. Because of this, it is expected that prescribing behaviours will be different for these drugs in relation to SIGN 136 publication, compared to other opioids. To examine this effect, a sensitivity analysis was conducted excluding these drugs.
All analyses used data from Q1 2005 to Q2 2020 inclusive, apart from the analyses involving age and gender, where data were only available between Q1 2010 and Q2 2020. The publication of SIGN 136 (i.e. the “intervention”) was defined as Q4, 2013, providing 36 data points before the publication (16 data points for the age and gender analyses) and 26 data points after the publication.
The effect of the publication was presented as the relative percentage change in prescribing rate at Q2 2020 compared to the predicted value at the same time point had pre-publication trends continued (the counterfactual, calculated from the model coefficient estimates). The 95% confidence intervals (CI) were calculated using model bootstrapping approaches [35]. All models were checked for autocorrelation using the Durbin-Watson statistic. A range of 1.50–2.50 was used to indicate an acceptable level of autocorrelation. Models outside this range were corrected using Newey-West standard errors.
All analyses were carried out using the statistical software R (version 4.0.3) [36].
Results
Summary statistics
The mid-year population estimates from 2005 to 2020 for the whole of Scotland are given in Supplementary Table S3. The estimated population size of Scotland was 5,110,200 in 2005 and 5,466,000 in 2020. A breakdown of the mid-year population estimates by age and gender is given in Supplementary Table S4 from 2010 to 2020.
Between Q1 2005 and Q2 2020, a total of 91,210,542 prescription items of the specified opioid or opioid-containing combination drugs included in this study were dispensed across Scotland. At the same time, a total of 12,036,499 prescription items of gabapentinoids were dispensed across Scotland. The total number of items of each drug prescribed is given in Supplementary Table S2.
Overall analysis
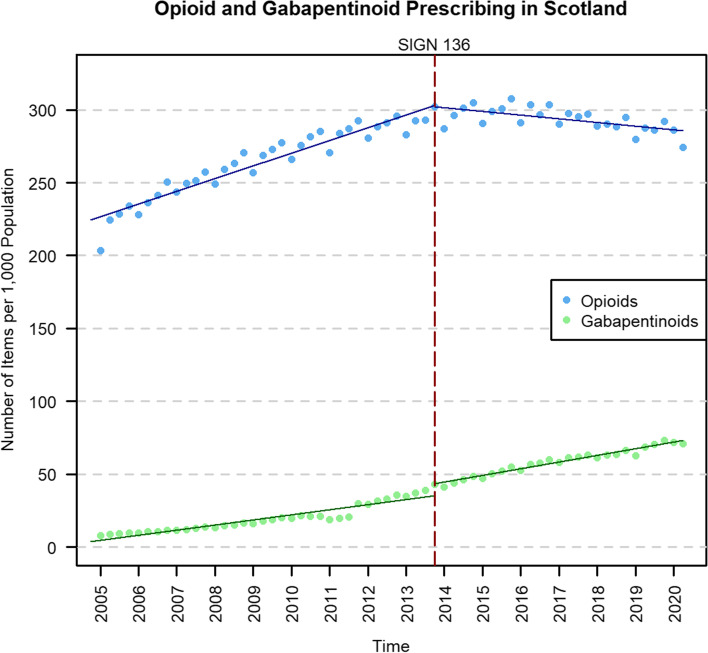
Across the whole of Scotland, the number of opioid prescription items rose from 1,040,276 in Q1 2005 to 1,608,984 in Q4 2013, an increase of 54.7%. Since the publication of SIGN 136 (Q4 2013), the number of opioid prescriptions has gradually fallen to 1,499,400 items in Q2 2020, a decrease of 6.8% (Fig. 1).
Fig. 1.
Opioid and gabapentinoid prescribing time series in Scotland before and after the publication of SIGN 136 in December 2013 (red dashed line). The solid lines represent the prescribing trend derived from the interrupted time series analysis
There was a significant positive trend in opioid prescribing rates pre-publication (2.19 items PTPPQ; P < 0.01), followed by no significant level change, and a significant change in trend following the publication (−2.82 items PTPPQ; P < 0.01). Opioid prescribing rates began to fall post-publication at −0.64 items PTPPQ, and at the end of the study period, the relative change was estimated to be −20.67% (95% CI: −23.61, −17.76) compared to the counterfactual (Table 2).
Table 2.
Single-group interrupted time series analysis of opioid and gabapentinoid prescribing in Scotland
| Opioids | Gabapentinoids | |||||
|---|---|---|---|---|---|---|
| Estimate (95% confidence interval) | Standard error | P value | Estimate (95% confidence interval) | Standard errora | P value | |
| Intercept, β0 | 224.46 (219.79, 229.12) | 2.33 | <0.01 | 3.62 (0.35, 6.90) | 1.64 | 0.03 |
| Pre-intervention trend, β1 | 2.19 (1.97, 2.41) | 0.11 | <0.01 | 0.88 (0.67, 1.08) | 0.10 | <0.01 |
| Change in level, β2 | −1.09 (−8.21, 6.03) | 3.56 | 0.76 | 8.00 (2.27, 13.73) | 2.86 | 0.01 |
| Change in trend, β3 | −2.82 (−3.24, −2.40) | 0.21 | <0.01 | 0.27 (0.07, 0.47) | 0.10 | 0.01 |
| Post-intervention trend, β1+3 | −0.64 (−0.98, −0.29) | 0.17 | <0.01 | 1.15 (1.04, 1.26) | 0.05 | <0.01 |
| Relative change, %b | −20.67 (−23.61, −17.76) | n/a | n/a | 25.89 (15.25, 43.29) | n/a | n/a |
| Durbin-Watson statistic | 1.64 | n/a | n/a | 0.80 | n/a | n/a |
β0–3 are coefficients from the single-group interrupted time series analysis model; the intercept represents the outcome at the start of the study period; the relative change is calculated compared to the predicted value at the same time point had the pre-intervention trend continued
aNewey-West
bCalculated at quarter 2 2020. 95% confidence interval calculated using model bootstrapping
In comparison, there was a significant positive trend in gabapentinoid prescribing pre-publication (0.88 items PTPPQ; P < 0.01), followed by a significant increase in level (8.00 items per 1000 population [PTP]; P < 0.01), and a significant positive change in trend post-publication (0.27 items PTPPQ; P = 0.01). The interrupted time series analysis and prescribing rates for gabapentinoids are presented in Table 2 and Fig. 1.
When opioid prescribing was adjusted for gabapentinoid prescribing (Table 3), the significant change in trend post-publication was maintained (−3.09 items PTPPQ; P < 0.01). There was also a significant negative difference in level change compared to gabapentinoids (−9.09 items PTP; P=0.02). The adjusted publication effect on the opioid prescribing rate at the end of the study period was calculated to be −24.85% (95% CI: −28.13, −21.61).
Table 3.
Results of the controlled interrupted time series analysis of opioid prescribing rates adjusted for gabapentinoid prescribing in Scotland
| Estimate (95% confidence interval) | Standard error | P value | |
|---|---|---|---|
| Difference in intercept, β4 | 220.83 (215.85, 225.81) | 2.51 | <0.01 |
| Difference in pre-intervention trend, β5 | 1.31 (1.07, 1.54) | 0.12 | <0.01 |
| Difference in change in level, β6 | −9.09 (−16.69, −1.49) | 3.84 | 0.02 |
| Difference in change in trend, β7 | −3.09 (−3.54, −2.64) | 0.23 | <0.01 |
| Difference in post-intervention trend, β5+7 | −1.78 (−2.13, −1.43) | 0.17 | <0.01 |
| Adjusted relative change, %a | −24.85 (−28.13, −21.61) | n/a | n/a |
| Durbin-Watson statistic | 1.53 | n/a | n/a |
β4–7 are coefficients from the controlled interrupted time series analysis model; the intercept represents the outcome at the start of the study period; the relative change is calculated compared to the predicted value at the same time point had the pre-intervention trend continued
aCalculated at quarter 2 2020. 95% confidence interval calculated using model bootstrapping
Stratified analysis
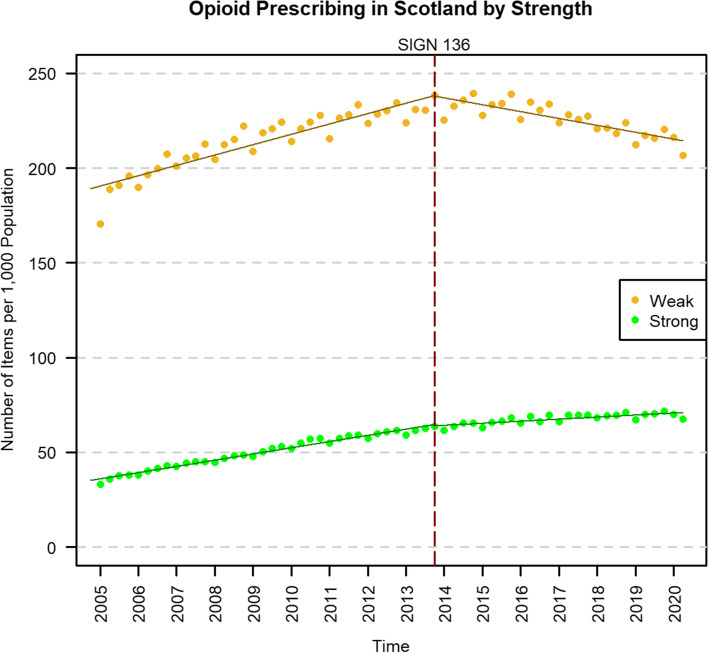
When stratifying opioid prescribing by strength (Fig. 2), both weak (−2.27 items PTPPQ; P < 0.01) and strong opioids (−0.55 items PTPPQ; P < 0.01) showed significant negative changes in trend post-publication, but non-significant changes in level (Table 4). However, there was a significantly greater negative change in the trend for weak opioids than for strong opioids in the adjusted analysis (−1.72 items PTPPQ; P < 0.01; Supplementary Table S5). Post-publication, weak opioid prescribing rates began to fall at −0.91 items PTPPQ, whereas strong opioid rates continued to rise at 0.27 items PTPPQ. The relative change was estimated to be −21.68% (95% CI: −24.73, −18.60) for weak opioids and −17.49% (95% CI: −20.11, −14.78) for strong opioids.
Fig. 2.
Prescribing time series of weak1 and strong2 opioids in Scotland, before and after the publication of SIGN 136 in December 2013 (red dashed line). The solid lines represent the prescribing trend derived from the interrupted time series analysis. 1Weak opioids are the following and their compounds: codeine, dihydrocodeine, meptazinol and tramadol. 2Strong opioids are the following and their compounds: buprenorphine, diamorphine, dipipanone, fentanyl, hydromorphone, methadone, morphine, oxycodone, papaveretum, pentazocine, pethidine and tapentadol
Table 4.
Results of the single interrupted time series analyses of weak and strong opioid prescribing rates in Scotland
| Weak1 | Strong2 | |||||
|---|---|---|---|---|---|---|
| Estimate (95% confidence interval) | Standard error | P value | Estimate (95% confidence interval) | Standard error | P value | |
| Intercept, β0 | 189.29 (185.48, 193.09) | 1.90 | <0.01 | 35.17 (34.15, 36.19) | 0.51 | <0.01 |
| Pre-intervention trend, β1 | 1.36 (1.18, 1.54) | 0.09 | <0.01 | 0.82 (0.77, 0.87) | 0.02 | <0.01 |
| Change in level, β2 | −0.34 (−6.15, 5.47) | 2.90 | 0.91 | −0.75 (−2.30, 0.80) | 0.77 | 0.34 |
| Change in trend, β3 | −2.27 (−2.61, −1.93) | 0.17 | <0.01 | −0.55 (−0.64, −0.46) | 0.05 | <0.01 |
| Post-intervention trend, β1+3 | −0.91 (−1.17, −0.64) | 0.13 | <0.01 | 0.27 (0.18, 0.36) | 0.04 | <0.01 |
| Relative change, %a | −21.68 (−24.73, −18.60) | n/a | n/a | −17.49 (−20.11, −14.78) | n/a | n/a |
| Durbin-Watson statistic | 1.63 | n/a | n/a | 1.57 | n/a | n/a |
β0–3 are coefficients from the single-group interrupted time series analysis model; the intercept represents the outcome at the start of the study period; the relative change is calculated compared to the predicted value at the same time point had the pre-intervention trend continued
1Weak opioids are the following and their compounds: codeine, dihydrocodeine, meptazinol and tramadol
2Strong opioids are the following and their compounds: buprenorphine, diamorphine, dipipanone, fentanyl, hydromorphone, methadone, morphine, oxycodone, papaveretum, pentazocine, pethidine and tapentadol
aCalculated at quarter 2 2020. 95% confidence interval calculated using model bootstrapping
In the gender analysis for opioids, both women (−3.26 items PTPPQ; P < 0.01) and men (−2.52 items PTPPQ; P < 0.01) showed significant negative changes in trend post-publication, but non-significant changes in level (Supplementary Fig. S1). Opioid prescribing rates began to fall post-publication for both women (−0.54 items PTPPQ) and men (−0.44 items PTPPQ; Supplementary Table S6). There were no significant differences between the genders in the adjusted analysis, in terms of the post-publication effects (Supplementary Table S7) and the relative change was estimated to be −19.11% (95% CI: −23.54, −13.17) for women and −21.54% (95% CI: −25.86, −15.51) for men.
Finally, in the age analysis (Supplementary Fig. S2), all groups showed non-significant changes in level except the >70 years old group (13.61 items PTP; P = 0.03) and all the groups showed significant negative changes in trend post-publication (0–29 years: −0.74; 30–49 years: −5.47; 50–69 years: −3.21; >70 years: −3.46 items PTPPQ respectively). All the age groups showed significant negative post-publication trends (0–29 years: −0.41; 30–49 years: −1.15; >70 years: −0.27 items PTPPQ respectively), except the 50–69 year group. The relative changes were estimated to be −36.13 (95% CI: −44.28, −22.53) in 0–29 years, −31.64 (95% CI: −35.33, −26.83) in 30–49 years, −15.29 (95% CI: −21.25, −6.53) in 50–69 years and −12.04 (95% CI: −16.20, −6.30) in >70 years (Supplementary Table S8).
Sensitivity analysis
A sensitivity analysis was conducted removing buprenorphine, buprenorphine and naloxone and methadone hydrochloride, which are used to treat opioid dependence as well as chronic pain.
In the analysis of opioids overall, there was no change in the statistical significance status of the change in level or change in trend post-publication, compared to the main analysis (Supplementary Table S9). However, in comparison with gabapentinoids, the difference in change in level changed to being non-significant (−6.51 items PTP; P = 0.07). Nonetheless, the adjusted relative change in the sensitivity analysis was similar to the main analysis (Supplementary Table S10; Supplementary Fig. S3).
The sensitivity analysis of strong opioids produced a significant positive change in level (1.83 items PTP; P < 0.01) and a reduced but still significant negative change in trend (−0.24 items PTPPQ; P < 0.01). This led to a reduced relative change of −8.96% (95% CI: −11.24, −6.13), compared to −17.49% in the main analysis (Supplementary Table S11). There were no changes in the controlled model with weak opioids (Supplementary Table S12; Supplementary Fig. S4).
The analysis of opioids by gender and age showed no divergences from the main results (Supplementary Tables S13-S15; Supplementary Figs. S5-S6).
Discussion
Summary
In this study, the publication of a national clinical guideline on the management of chronic pain (SIGN 136) in 2013 was associated with a significant negative change in the trend in primary care opioid prescribing in Scotland that resulted in a relative reduction of 21% by Q2 2020. This finding persisted when comparing to gabapentinoids, which was not associated with any similar changes in prescribing trend. Stratified analyses by opioid strength, age category and gender showed that SIGN 136 was associated with a significant negative change in trend in all groups. Despite this, prescribing rates of strong opioids continued to rise post-publication, albeit at a slower rate than pre-publication. To the best of our knowledge, this is the first study to analyse changes in opioid prescribing trends in Scottish primary care and to examine the association of prescribing rates with a specific intervention intended to influence these.
Interpretation
Increasing opioid prescribing in the UK and elsewhere has been well documented [37–40]. This is also the case in Scotland where prescribing of opioids has increased both locally and nationally [9, 12]. However, the time period covered by the Scottish studies was prior to the publication of SIGN 136. Similar increases have been identified more recently across the UK [41]. This continuing increase in opioid prescribing rate across the UK beyond 2013 appears to be in contrast with the results from the current study.
However, we found that this decrease in prescribing numbers and most of the change in trend associated with SIGN 136 was being driven by weak opioids, with strong opioid prescribing rates continuing to increase. This increase in strong opioid prescribing rates appears to be in line with a previous study in Wales [37]. This may reflect a marked change in prescribing behaviour for weak opioids, whilst changes in prescribing behaviour for strong opioids have been slower. However, weak opioids continued to be much more frequently prescribed than strong opioids overall.
The results from the sensitivity analysis removing buprenorphine, buprenorphine and naloxone and methadone hydrochloride, which are also used as opioid replacement therapies to treat opioid dependence, largely corroborated the findings from the main analyses.
In addition to the opioid findings, there was also a significant increase in gabapentinoid prescribing trend. A possible reason for this could be an increase in the number of neuropathic pain cases being diagnosed and treated, particularly as gabapentinoids are recommended first- or second-line treatment for neuropathic pain in national and international guidelines [42, 43]. However, this is unlikely to account for all of the increase in gabapentinoid prescribing rates [44]. Another potential explanation is that the publication of SIGN 136 has led to a swap of prescription of opioids for gabapentinoids. Gabapentinoids are licensed for the treatment of neuropathic pain, yet there is increasing evidence that they are being prescribed off-label for other forms of pain [45], despite limited evidence for their effectiveness for non-neuropathic pain [46, 47]. However, gabapentinoids have themselves recently been associated with increased rates of adverse outcomes and the increase in their use is a cause for concern [48, 49]. This increase has prompted the reclassification of gabapentin and pregabalin as class C controlled drugs in the UK (placing greater legal restrictions on their supply) and the complete removal of pregabalin from the formulary for neuropathic pain in Northern Ireland [50].
Strengths and weaknesses
Due to the epidemiological design of the study, the impact of other guidelines, policies and related interventions within Scotland cannot be ruled out. In the UK, these include the reclassification of tramadol in 2013 [51], chronic pain initiatives [52] and an online resource, “Opioids Aware”, to support prescribing of opioids for chronic pain [53]. Furthermore, initiatives outside of the UK such as the Helping to End Addiction Over the Long-term (HEAL) initiative in the USA [54] may have further influenced more recent prescribing practices in Scotland. Media coverage of the opioid epidemic in the USA is also likely to have raised awareness amongst the general public and patients may have gained a greater understanding of the risks of opioid treatments and other options available. The Centers for Disease Control and Prevention (CDC) also produced a guideline for prescribing opioids for chronic pain in the USA and this contained similar recommendations on assessing patients for ongoing pain relief and risk of harms as SIGN 136 [2]. However, these guidelines were published in 2016 and are therefore less likely to be confounding factors influencing opioid prescribing trends in Scotland in the years immediately following the publication of SIGN 136.
It should also be noted that even though SIGN 136 is targeted at the treatment of chronic non-malignant pain, the information available in the PIS did not include indications for each prescription and we were unable to obtain this information. Therefore, the dataset will have included patients with acute pain, as well as pain caused by cancer. It is not anticipated that prescribing for these groups would be influenced by the publication of SIGN 136 in the same way as those with chronic non-malignant pain. Therefore, the effect estimates for the association of SIGN 136 with change in level and trend are likely to be conservative.
This study used prescription items as a measure of prescribing activity. Whilst this approach is commonly used in studies investigating prescribing trends, it does not account for patient and clinician variations in the quantity of drug supplied per unit time, although the Department of Health and Social Care recommends that not more than 30 days’ supply should be given on one prescription. Additionally, it does not allow for different potencies which can be important considerations in analysing opioid use [38]. A common metric used to account for this is the morphine milligramme equivalents, which is correlated with opioid overdose misuse [55]. However, it was not possible to obtain information on opioid doses for the current study.
In contrast to the findings in this study of decreasing opioid prescribing rates since December 2013, it is interesting to note a previous study in which regulatory warnings about the cardiovascular safety of diclofenac in Scotland appeared to increase the rate of switching to opioids around the same time as SIGN 136 publication [56]. As a result, it is possible that without this influence, the negative change in opioid prescribing trend associated with SIGN 136 may have been greater.
A revised version of SIGN 136 was also published in August 2019, providing more up-to-date evidence-based guidance on opioid prescribing [31]. Through both this and the original version, SIGN 136 has been influential in driving UK and Scottish Government policy on chronic pain management and has been incorporated into clinical practice with the publication of the Scottish National Prescribing Strategy for Chronic Pain [57], the Royal College of Anaesthetists Quality Improvement Compendium [58] and the Medicines and Healthcare products Regulatory Agency guidance on the safe use of opioids [59]. The potential impact of the update has not yet been assessed and should be the focus of future studies. So too should the potential impact of the COVID-19 pandemic, which has had a major impact on emergency and specialist care services with concern around a potential increase in opioid prescribing rates as patients turn to primary care [60].
Regardless of the potential cause, the reduction in opioid prescribing trend described in this study demonstrates the important role that evidence-based clinical guidelines potentially play in prescribing behaviours. Previous studies have indicated that GPs’ beliefs about whether or not opioids are appropriate for chronic non-cancer pain are a driver in whether to prescribe them, and a lack of a consistent approach and effective alternatives are barriers to deprescribing opioids, despite clear concerns about potential harms such as addiction, dependence and misuse [61–63]. This supports the need for dedicated guidelines, based on strong evidence.
This study also highlights interrupted time series analysis as a potential tool for assessing the impact of clinical guidelines. A previous study that used a similar approach found that the reclassification of tramadol as a schedule 3 controlled substance in June 2014 was significantly associated with a reduction in tramadol utilisation in England and Wales [51]. Despite its advantages in analysing potential intervention impact, interrupted time series analysis cannot help to explain why an intervention has (or has not) had an impact. To answer this question requires qualitative approaches, something that should be explored in future studies.
Despite focussing on prescribing rates in relation to SIGN 136, we were unable to assess other opioid-related outcomes, such as abuse, misuse and overdose. Since a key aim of SIGN 136 is to improve patient quality of life, it would be interesting to see if incidence rates for these outcomes have fallen in line with opioid prescribing since the publication of SIGN 136, as would be expected given their close association [64]. Recent data from Scotland show opioid-related death rates are continuing to rise, though this could be due to illicit use as well as iatrogenic use [65].
Conclusion
In conclusion, opioid prescribing rates have been falling in Scotland since 2013. Whilst this effect cannot be definitively linked to the publication of SIGN 136, it at least suggests that changes in Scottish clinical and government policy relating to chronic pain management, most of which have been inspired by its publication, are having a positive effect on opioid prescribing practices in primary care. This highlights the importance of providing continued robust clinical advice, based on up-to-date evidence, for the safe and effective treatment for chronic pain.
Supplementary Information
Additional file 1: Supplementary Box S1. Summary of the original SIGN 136 guideline (2013 edition) intervention for use of opioids in the management of adults with chronic non-malignant pain in non-specialist settings. Supplementary Box S2. Summary of the original SIGN 136 guideline (2013 edition) recommendations for use of gabapentinoids (gabapentin and pregabalin) in the management of adults with chronic non-malignant pain in non-specialist settings. Supplementary Table S1. Framework for Enhanced Reporting of Interrupted Time Series Studies (FERITS) Statement. Supplementary Table S2. List of opioids (BNF chapter 4.7.2), opioid-containing combination analgesics and gabapentinoid drugs included in the study and the number of items prescribed in Scotland between Q1 2005 and Q2 2020. Supplementary Table S3. Mid-year population estimates for Scotland. Supplementary Table S4. Mid-year population estimates for age and gender demographics in Scotland. Supplementary Table S5. Results of the controlled interrupted time series analysis of weak compared to strong opioid prescribing rates in Scotland. Supplementary Table S6. Results of the single interrupted time series analyses of female and male opioid prescribing rates in Scotland. Supplementary Table S7. Results of the controlled interrupted time series analysis of females compared to male opioid prescribing rates in Scotland. Supplementary Table S8. Results of the single interrupted time series analyses of opioid prescribing rates by age category in Scotland. Supplementary Table S9. Single-group interrupted time series analysis of opioids in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S10. Results of the controlled interrupted time series analysis of opioid prescribing rates adjusted for gabapentinoid prescribing in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S11. Results of the single interrupted time series analyses of female and male opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S12. Results of the controlled interrupted time series analysis of females compared to male opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S13. Results of the single interrupted time series analyses of opioid prescribing rates by age category in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S14. Results of the single interrupted time series analyses of strong opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S15. Results of the controlled interrupted time series analysis of weak compared to strong opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Figure S1. Opioid prescribing time series across Scotland by gender, before and after the publication of SIGN 136 in December 2013. Supplementary Figure S2. Opioid prescribing time series across Scotland by age, before and after the publication of SIGN 136 in December 2013. Supplementary Figure S3. Opioid and gabapentinoid prescribing time series in Scotland (without opioid replacement therapies) before and after the publication of SIGN 136 in December 2013. Supplementary Figure S4. Prescribing time series of weak and strong opioids in Scotland (without opioid replacement therapies), before and after the publication of SIGN 136 in December 2013. Supplementary Figure S5. Opioid prescribing time series across Scotland by gender (without opioid replacement therapies), before and after the publication of SIGN 136 in December 2013. Supplementary Figure S6. Opioid prescribing time series across Scotland by age (without opioid replacement therapies), before and after the publication of SIGN 136 in December 2013.
Acknowledgements
We acknowledge the help and support of Public Health Scotland for managing and supplying the anonymised data.
Authors’ contributions
B.H.S. and L.A.C. conceived the study. H.L.H., D.R.M., N.T., B.H.S. and L.A.C contributed to the design. H.L.H. acquired the data and conducted the analysis. H.L.H., D.R.M., N.T., B.H.S. and L.A.C contributed to interpreting the results. H.L.H. wrote the initial draft of the manuscript and D.R.M., N.T., B.H.S. and L.A.C revised the article for important intellectual content. H.L.H., D.R.M., N.T., B.H.S. and L.A.C approved the final version and agreed to be accountable for the findings.
Funding
No external financial support was received for this study.
Availability of data and materials
Summary data used in this study are available from the corresponding author, upon reasonable request. Requests for community prescribing data in Scotland can be made to Public Health Scotland.
Declarations
Ethics approval and consent to participate
Formal ethical approval and consent were not required as the study used aggregated and publicly available data which contained no patient or prescriber identifiable information.
Consent for publication
Not applicable.
Competing interests
L.A.C. chaired the Guideline Development Group for the Scottish Intercollegiate Guideline Network publication, “Management of Chronic Pain”, to which this paper refers, proposed an update due to changing evidence, and contributed to the update of the opioids section. She has been Vice Chair of SIGN since October 2020 and was a member of the Scottish Government group that developed the National Prescribing Strategy for Chronic Pain.
B.H.S. was a member of the Guideline Development Group for the Scottish Intercollegiate Guideline Network publication, “Management of Chronic Pain”, to which this paper refers and contributed to the update of the opioids section. He was the Scottish Government’s National Lead Clinician for Chronic Pain (2014 to 2021) and was a member of the Scottish Government group that developed the National Prescribing Strategy for Chronic Pain.
D.R.M. is supported by a Wellcome Trust Clinical Research Development Fellowship (Grant 214588/Z/18/Z) and reports grants from the Chief Scientist Office (CSO), Health Data Research UK (HDR-UK) and the National Institute of Health Research (NIHR), outside of the submitted work.
H.L.H. and N.T. declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Blair H. Smith and Lesley A. Colvin are joint senior authors.
References
- 1.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872–882. doi: 10.1001/jama.2018.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth. 2018;120(6):1335–1344. doi: 10.1016/j.bja.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Office on Drugs and Crime. World Drug Report 2021. New York; United Nations; 2021. Available from: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html.
- 6.Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- 7.Smith BH, Fletcher EH, Colvin LA. Opioid prescribing is rising in many countries. BMJ. 2019;367:l5823. doi: 10.1136/bmj.l5823. [DOI] [PubMed] [Google Scholar]
- 8.Kimber J, Hickman M, Strang J, Thomas K, Hutchinson S. Rising opioid-related deaths in England and Scotland must be recognised as a public health crisis. Lancet Psychiatry. 2019;6(8):639–640. doi: 10.1016/S2215-0366(19)30209-3. [DOI] [PubMed] [Google Scholar]
- 9.Ruscitto A, Smith BH, Guthrie B. Changes in opioid and other analgesic use 1995-2010: repeated cross-sectional analysis of dispensed prescribing for a large geographical population in Scotland. Eur J Pain. 2015;19(1):59–66. doi: 10.1002/ejp.520. [DOI] [PubMed] [Google Scholar]
- 10.Scottish Intercollegiate Guideline Network. SIGN 136: management of chronic pain: a national clinical guideline. Edinburgh: SIGN; 2013. Available from: https://www.sign.ac.uk/assets/sign136.pdf.
- 11.Colvin LA, Stein A, Smith BH. Managing chronic pain: a clinical challenge: new SIGN guidelines provide a practical evidence-based approach and identify research gaps. Br J Anaesth. 2014;112(1):9–12. doi: 10.1093/bja/aet470. [DOI] [PubMed] [Google Scholar]
- 12.Torrance N, Mansoor R, Wang H, Gilbert S, Macfarlane GJ, Serpell M, et al. Association of opioid prescribing practices with chronic pain and benzodiazepine co-prescription: a primary care data linkage study. Br J Anaesth. 2018;120(6):1345–1355. doi: 10.1016/j.bja.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Chambers CT. From evidence to influence: dissemination and implementation of scientific knowledge for improved pain research and management. Pain. 2018;159(1):S56–S64. doi: 10.1097/j.pain.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 14.Fritz JM, Cleland JA, Brennan GP. Does adherence to the guideline recommendation for active treatments improve the quality of care for patients with acute low back pain delivered by physical therapists? Med Care. 2007;45(10):973–980. doi: 10.1097/MLR.0b013e318070c6cd. [DOI] [PubMed] [Google Scholar]
- 15.Rutten GM, Degen S, Hendriks EJ, Braspenning JC, Harting J, Oostendorp RA. Adherence to clinical practice guidelines for low back pain in physical therapy: do patients benefit? Phys Ther. 2010;90(8):1111–1122. doi: 10.2522/ptj.20090173. [DOI] [PubMed] [Google Scholar]
- 16.Kryworuchko J, Stacey D, Bai N, Graham ID. Twelve years of clinical practice guideline development, dissemination and evaluation in Canada (1994 to 2005) Implement Sci. 2009;4(1):1–11. doi: 10.1186/1748-5908-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomasone JR, Kauffeldt KD, Chaudhary R, Brouwers MC. Effectiveness of guideline dissemination and implementation strategies on health care professionals’ behaviour and patient outcomes in the cancer care context: a systematic review. Implement Sci. 2020;15(1):1–18. doi: 10.1186/s13012-020-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fixsen DL, Blasé KA, Timbers GD, Wolf MM. In search of program implementation: 792 replications of the Teaching-Family Model. Behav Anal Today. 2014;8(1):96. doi: 10.1037/h0100104. [DOI] [Google Scholar]
- 19.Morris ZS, wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taljaard M, McKenzie JE, Ramsay CR, Grimshaw JM. The use of segmented regression in analysing interrupted time series studies: an example in pre-hospital ambulance care. Implement Sci. 2014;9(1):1–4. doi: 10.1186/1748-5908-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lugtenberg M, Burgers JS, Westert GP. Effects of evidence-based clinical practice guidelines on quality of care: a systematic review. Qual Saf Health Care. 2009;18(5):385–392. doi: 10.1136/qshc.2008.028043. [DOI] [PubMed] [Google Scholar]
- 22.Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):CD007076. doi: 10.1002/14651858.CD007076.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938. doi: 10.1002/14651858.CD007938.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Public Health Scotland . National therapeutic indicators data visualisation. 2021. [Google Scholar]
- 25.The Equator Network . Reporting guidelines under development for observational studies. 2021. [Google Scholar]
- 26.Des Jarlais DC, Lyles C, Crepaz N, The Trend Group Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361–366. doi: 10.2105/AJPH.94.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Public Health Scotland . Prescriptions in the community. 2022. [Google Scholar]
- 28.Alvarez-Madrazo S, McTaggart S, Nangle C, Nicholson E, Bennie M. Data Resource Profile: The Scottish National Prescribing Information System (PIS) Int J Epidemiol. 2016;45(3):714F–715F. doi: 10.1093/ije/dyw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scottish Intercollegiate Guideline Network. SIGN 50: a guideline developer’s handbook. Edinburgh: SIGN; 2019. Available from: https://www.sign.ac.uk/media/1050/sign50_2019.pdf.
- 30.Scottish Intercollegiate Guidelines Network. Managing chronic pain. Edinburgh: SIGN; 2019. Available from: https://www.sign.ac.uk/media/1175/pat136_2019.pdf.
- 31.Scottish Intercollegiate Guideline Network. SIGN 136: management of chronic pain: a national clinical guideline [revised edition]. Edinburgh: SIGN; 2019. Available from: https://www.sign.ac.uk/media/1108/sign136_2019.pdf.
- 32.Joint Formulary Committee. British National Formulary. London: BMJ Group and Royal Pharmaceutical Society; 2020. Available from: http://www.medicinescomplete.com.
- 33.Macfarlane GJ, Beasley M, Jones GT, Stannard C. The epidemiology of regular opioid use and its association with mortality: prospective cohort study of 466 486 UK biobank participants. EClinicalMedicine. 2020;21:100321. doi: 10.1016/j.eclinm.2020.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143–148. doi: 10.1016/j.jclinepi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: a language and environment for statistical computing. Vienna; 2020. Available from: https://www.r-project.org/.
- 37.Davies E, Phillips C, Rance J, Sewell B. Examining patterns in opioid prescribing for non-cancer-related pain in Wales: preliminary data from a retrospective cross-sectional study using large datasets. Br J Pain. 2018;13(3):145–158. doi: 10.1177/2049463718800737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis HJ, Croker R, Walker AJ, Richards GC, Quinlan J, Goldacre B. Opioid prescribing trends and geographical variation in England, 1998-2018: a retrospective database study. Lancet Psychiatry. 2019;6(2):140–150. doi: 10.1016/S2215-0366(18)30471-1. [DOI] [PubMed] [Google Scholar]
- 39.Karanges EA, Blanch B, Buckley NA, Pearson SA. Twenty-five years of prescription opioid use in Australia: a whole-of-population analysis using pharmaceutical claims. Br J Clin Pharmacol. 2016;82(1):255–267. doi: 10.1111/bcp.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guy GP, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jani M, Birlie Yimer B, Sheppard T, Lunt M, Dixon WG. Time trends and prescribing patterns of opioid drugs in UK primary care patients with non-cancer pain: a retrospective cohort study. PLoS Med. 2020;17(10):e1003270. doi: 10.1371/journal.pmed.1003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulin DE, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328–335. doi: 10.1155/2014/754693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):406–407. doi: 10.3399/bjgp12X653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montastruc F, Loo SY, Renoux C. Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993-2017. JAMA. 2018;320(20):2149–2151. doi: 10.1001/jama.2018.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fallon M, Hoskin PJ, Colvin LA, Fleetwood-Walker SM, Adamson D, Byrne A, et al. Randomized double-blind trial of pregabalin versus placebo in conjunction with palliative radiotherapy for cancer-induced bone pain. J Clin Oncol. 2016;34(6):550–556. doi: 10.1200/JCO.2015.63.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peckham AM, Evoy KE, Ochs L, Covvey JR. Gabapentin for off-label use: evidence-based or cause for concern? Subst Abus. 2018;12:1178221818801311. doi: 10.1177/1178221818801311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evoy KE, Sadrameli S, Contreras J, Covvey JR, Peckham AM, Morrison MD. Abuse and misuse of pregabalin and gabapentin: a systematic review update. Drugs. 2021;81(1):125–156. doi: 10.1007/s40265-020-01432-7. [DOI] [PubMed] [Google Scholar]
- 49.Torrance N, Veluchamy A, Zhou Y, Fletcher EH, Moir E, Hebert HL, et al. Trends in gabapentinoid prescribing, co-prescribing of opioids and benzodiazepines, and associated deaths in Scotland. Br J Anaesth. 2020;125(2):159–167. doi: 10.1016/j.bja.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Health and Social Care Board. Northern Ireland Medicines Management Newsletter - July 2021 - Volume 12, Issue 7. Belfast: Health and Social Care Board; 2021. Available from: https://hscbusiness.hscni.net/pdf/MM Newsletter July 2021 260721.pdf.
- 51.Chen TC, Chen LC, Knaggs RD. A 15-year overview of increasing tramadol utilisation and associated mortality and the impact of tramadol classification in the United Kingdom. Pharmacoepidemiol Drug Saf. 2018;27(5):487–494. doi: 10.1002/pds.4320. [DOI] [PubMed] [Google Scholar]
- 52.The National Institute for Health and Care Excellence . Medicines optimisation in chronic pain. 2017. [Google Scholar]
- 53.Faculty of Pain Medicine of the Royal College of Anaesthetists . Opioids Aware. 2016. [Google Scholar]
- 54.Collins FS, Koroshetz WJ, Volkow ND. Helping to end addiction over the long-term: the research plan for the NIH HEAL initiative. JAMA. 2018;320(2):129–130. doi: 10.1001/jama.2018.8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nam YH, Bilker WB, DeMayo FJ, Neuman MD, Hennessy S. Incidence rates of and risk factors for opioid overdose in new users of prescription opioids among US Medicaid enrollees: a cohort study. Pharmacoepidemiol Drug Saf. 2020;29(8):931–938. doi: 10.1002/pds.5067. [DOI] [PubMed] [Google Scholar]
- 56.Morales DR, Morant SV, MacDonald TM, Mackenzie IS, Doney ASF, Mitchell L, et al. Impact of EMA regulatory label changes on systemic diclofenac initiation, discontinuation, and switching to other pain medicines in Scotland, England, Denmark, and The Netherlands. Pharmacoepidemiol Drug Saf. 2020;29(3):296–305. doi: 10.1002/pds.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scottish Government Effective Prescribing and Therapeutics Branch. The Scottish chronic pain prescribing strategy. Edinburgh: Scottish Government; 2018. Available from: https://www.therapeutics.scot.nhs.uk/pain/.
- 58.The Royal College of Anaesthetists. Raising the standards: RCoA quality improvement compendium. London: The Royal College of Anaesthetists; 2020. Available from: https://rcoa.ac.uk/sites/default/files/documents/2020-%0A08/21075RCoAAuditRecipeBook_Combined_Final_25.08.2%0A020_0.pdf.
- 59.Medicines and Healthcare Products Regulatory Agency. Opioids: risk of dependence and addiction. London; 2020. Available from: https://www.gov.uk/drug-safety-update/opioids-risk-of-dependence-and-addiction.
- 60.Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesth. 2020;125(4):436–440. doi: 10.1016/j.bja.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutchinson K, Moreland AME, Amanda AC, Weinman J, Horne R. Exploring beliefs and practice of opioid prescribing for persistent non-cancer pain by general practitioners. Eur J Pain. 2007;11(1):93–98. doi: 10.1016/j.ejpain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 62.White R, Hayes C, Boyes AW, Chiu S, Paul CL. General practitioners and management of chronic noncancer pain: a cross-sectional survey of influences on opioid deprescribing. J Pain Res. 2019;12:467–475. doi: 10.2147/JPR.S168785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blake H, Leighton P, van der Walt G, Ravenscroft A. Prescribing opioid analgesics for chronic non-malignant pain in general practice – a survey of attitudes and practice. Br J Pain. 2015;9(4):225–232. doi: 10.1177/2049463715579284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomes T, Khuu W, Martins D, Tadrous M, Mamdani MM, Paterson JM, et al. Contributions of prescribed and non-prescribed opioids to opioid related deaths: population based cohort study in Ontario, Canada. BMJ. 2018;362:k3207. doi: 10.1136/bmj.k3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Box S1. Summary of the original SIGN 136 guideline (2013 edition) intervention for use of opioids in the management of adults with chronic non-malignant pain in non-specialist settings. Supplementary Box S2. Summary of the original SIGN 136 guideline (2013 edition) recommendations for use of gabapentinoids (gabapentin and pregabalin) in the management of adults with chronic non-malignant pain in non-specialist settings. Supplementary Table S1. Framework for Enhanced Reporting of Interrupted Time Series Studies (FERITS) Statement. Supplementary Table S2. List of opioids (BNF chapter 4.7.2), opioid-containing combination analgesics and gabapentinoid drugs included in the study and the number of items prescribed in Scotland between Q1 2005 and Q2 2020. Supplementary Table S3. Mid-year population estimates for Scotland. Supplementary Table S4. Mid-year population estimates for age and gender demographics in Scotland. Supplementary Table S5. Results of the controlled interrupted time series analysis of weak compared to strong opioid prescribing rates in Scotland. Supplementary Table S6. Results of the single interrupted time series analyses of female and male opioid prescribing rates in Scotland. Supplementary Table S7. Results of the controlled interrupted time series analysis of females compared to male opioid prescribing rates in Scotland. Supplementary Table S8. Results of the single interrupted time series analyses of opioid prescribing rates by age category in Scotland. Supplementary Table S9. Single-group interrupted time series analysis of opioids in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S10. Results of the controlled interrupted time series analysis of opioid prescribing rates adjusted for gabapentinoid prescribing in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S11. Results of the single interrupted time series analyses of female and male opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S12. Results of the controlled interrupted time series analysis of females compared to male opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S13. Results of the single interrupted time series analyses of opioid prescribing rates by age category in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S14. Results of the single interrupted time series analyses of strong opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Table S15. Results of the controlled interrupted time series analysis of weak compared to strong opioid prescribing rates in Scotland (without buprenorphine, buprenorphine and naloxone and methadone hydrochloride). Supplementary Figure S1. Opioid prescribing time series across Scotland by gender, before and after the publication of SIGN 136 in December 2013. Supplementary Figure S2. Opioid prescribing time series across Scotland by age, before and after the publication of SIGN 136 in December 2013. Supplementary Figure S3. Opioid and gabapentinoid prescribing time series in Scotland (without opioid replacement therapies) before and after the publication of SIGN 136 in December 2013. Supplementary Figure S4. Prescribing time series of weak and strong opioids in Scotland (without opioid replacement therapies), before and after the publication of SIGN 136 in December 2013. Supplementary Figure S5. Opioid prescribing time series across Scotland by gender (without opioid replacement therapies), before and after the publication of SIGN 136 in December 2013. Supplementary Figure S6. Opioid prescribing time series across Scotland by age (without opioid replacement therapies), before and after the publication of SIGN 136 in December 2013.
Data Availability Statement
Summary data used in this study are available from the corresponding author, upon reasonable request. Requests for community prescribing data in Scotland can be made to Public Health Scotland.