Abstract
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease. COPD is associated with accelerated lung aging. Circadian clock is believed to play important roles in COPD. Although the circadian molecular clock regulates cellular senescence, there is no information available regarding the impact of COPD. The aim of this study is to investigate the role of the circadian clock protein BMAL1 and CLOCK in cellular senescence in order to understand the cellular mechanisms of accelerated aging of COPD. Bmal1 and Clock levels were assessed in the plasma samples of non-smokers, smokers, and patients with COPD. The regulation of ciracadian clock expression and cell senescence by cigarette smoke extract (CSE) was studied in vitro, and small interfering RNA (siRNA) and overexpression of Bmal1 or Clock were employed to investigate the role of circadian clock on cell senescence. Herein, patients with COPD showed lower Bmal1 and Clock expression in the plasma. Interestingly, CSE exposure contributed to the increased cell senescence, decreased Clock and Bmal1 in human bronchial epithelial cells (Beas-2B cells). We found that knockdown of Clock or Bmal1 lead to upregulation of cell senescence in Beas-2B cells, while overexpression of Clock or Bmal1 inhibited cell senescence in Beas-2B cells, which is through the MAPK pathways. Therefore, our findings indicated that Bmal1 or Clock deficiency may be a significant factor to increase cellular senescence of the lung to develop COPD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-022-02237-y.
Keywords: Circadian clock, Chronic obstructive Pulmonary Disease (COPD), Smoke, Senescence
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease and is considered to be the most important respiratory disease worldwide [1]. Humans will live longer by 2050, 21% of the world’s population will be 60 years or older and the incidence of age-related chronic diseases is also increasing, such as COPD [2–6]. The incidence of COPD was five-fold higher in people aged 65 years than in people aged less than 40 years [7]. The pathogenesis of COPD is still remain unclear, and the main treatment method is to improve the symptoms of COPD patients, but it cannot delay disease progression and reduce mortality. Therefore, new perceptions into the pathogenesis of COPD are required.
The biological clock is a 24-hour internal timekeeping system that affects the rhythms of body’s behavior, physiology, and gene expression [8]. A central pacemaker located in the anterior suprachiasmatic nucleus (SCN) of the hypothalamus, controls the rhythms of behavior and physiology and synchronizes internal time with the external environment in mammals [9]. In addition to the central clock, peripheral tissues (lung, liver and heart) also contain autonomous circadian oscillators that regulate cellular functions and responses to environmental exposure [10–12]. The expression of biological clock genes are regulated by two transcription factors, the positive feedback loops including BMAL1 and CLOCK, and the negative feedback loops including PER and CRY. Activation of the BMAL1/CLOCK transcriptional complex induces the protein level of CRY and PER, which in turn inhibit their own transcription by interacting with BMAL1/CLOCK heterodimers. Circadian clock disruption was found in chronic lung disease, such as Obstructive sleep apnea (OSA) and COPD. This process might be regulated by the subunit α of hypoxia-inducible factor (HIF-1), as its increased level is associated with circadian clock overexpression in OSA [13]. In patients with acute exacerbation of COPD, lung function peaks in the early morning, and FVC, FEV1, and PEF decrease significantly in the night [14, 15]. This may be because cigarette exposure causes changes in circadian clock genes, hormone levels, pulmonary surfactants and mucus hypersecretion, leading to inflammatory responses and a reduction in lung function [16–18].
Cigarette smoking,a major risk factor for COPD, whereas, only 10–15% of smokers develop COPD. This implies there are other factors influence certain individuals. In the process of natural aging, lung function gradually declines and airflow limitation gradually increases. Hence, COPD is a disease associated with accelerated aging [19, 20]. Aging, a gradual decline in homeostasis, leading to an increased incidence of disease [21]. Compared with healthy adults, characteristics of lung aging in COPD patients including declined lung function, enlarged airspace, lossed elasticity and increased cellular senescence. The feature of senescence is morphologic changes, decreased proliferative capacity, and the expression of proteins known as senescence biomarkers [22, 23]. The effects of aging on circadian behavior and metabolism have been clearly documented [24]. Recently, Kondratov et al. have shown that BMAL1−/− mice leads to a significantly shorted lifespan and a premature aging manifestation [25]. Based on the results of Park et al., BMAL1 is associated with telomere length in both zebrafish and mice, and is characterized by rhythmical binding to the ends of chromosomes. [26] However, whether circadian rhythms and aging are associated with COPD and invovled mechanisms remains unclear.
Herein, we explore the role of the core circadian clock protein (BMAL1 and CLOCK) in cellular senescence for clarifying the mechanisms of COPD. In this study, to address this hypothesis, we therefore studied the expression of clock genes in the serum of healthy controls and COPD patients. In addition, we determined the role of circadian clock in cellular senescence in human bronchial epithelial.
Materials and methods
Ethics Statement
All patients signed the informed consents. The study protocol was approved by the Medical Ethical Committee of the First Affiliated Hospital of Wannan Medical College. All experiments were performed under the guidelines.
Human blood specimens
Seventy-six blood samples (30 non-smokers, 20 smokers, and 26 smokers with COPD) were obtained from the First Affiliated Hospital of Wannan Medical College. The plasma was isolated from blood samples and stored at -80 ℃ until further analysis.
Cell culture
Human bronchial epithelium (Beas-2B) cells were purchased from the Chinese Academy of Sciences on Type Culture Collection Cell Bank (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum and 1% streptomycin/penicillin/glutamate solution (Gibco) at 37 ℃ in 5% CO2.
Assessment of Senescence-associated β-Galactosidase staining
Beas-2B cells were seeded in a 6-well plate with 2 × 103cell/cm2. Cells were cultured after 2 days, discard the medium, rinsed cells once with PBS, add 1 ml of fixative to each well for 15 min and then rinsed three times with PBS. Then 1 ml per well of working solution of β-galactosidase with X-Gal was placed, and the plate was maintained at 37°C overnight (senescence-associated β-galactosidase staining kit from Beyotime, China). The cells were observed under an inverted microscope.
Preparation of Aqueous cigarette smoke extract
CSE was prepared using a modification to a previously modified method [27–29]. The total particulate matter (TPM) content of Marlboro Red cigarette was 10 mg/cigarette, tar (10 mg/cigarette), and nicotine (0.8 mg/cigarette). Preparing fresh CSE for each experiment before use.
Transfection with siRNA
Three sequences for Clock and Bmal1 siRNAs (GenePharma, Shanghai, China) was designed and a control siRNA.The siRNAs were transfected into Beas-2B cells using Lipofectamine 3000 (invitrogen) reagent for 48 h according to instructions.
Plasmids
Plasmids for expressing CLOCK (pcDNA-CLOCK) or BMAL1 (pcDNA-BMAL1) were purchased from GenePharma (Shanghai, China). For overexpression assays, 2.5 µg plasmids were transfected with Lipofectamine 3000 (invitrogen) reagent into the cells according to the reagent instructions.
Western blot analysis
Total cell protein was extracted by PMSF RIPA lysis method. Samples were separated by SDS/PAGE (Invitrogen, USA) and then transferred to nitrocellulose (NC) membranes. Membranes were blocked with 5% BSA for 1 h and then incubated with the indicated primary antibody overnight at 4 °C. Antibodies against: Clock (Cat# ab93804, Abcam), Bmal1 (Cat# ab93806, Abcam), pH2AX (Cat# ab81299, Abcam), phospho-ERK (Cat# 4370, CST), phospho-P38 MAPK (Cat# 4511, CST), ERK (Cat# 4695, CST), P38 MAPK (Cat# 8690, CST), and GAPDH (Cat# ab8245, Abcam). The gray values of protein bands were measured using Image Lab 2.0 software (Bio-Rad). GAPDH was used as the loading control.
RNA extraction, reverse transcription and real-time RT-PCR
Total RNA was isolated following the user’s manual using trizol reagent (Invitrogen). Quantitative PCRs were performed using the SYBR Green (Takara). The primers are provided in supplemental Table 1.
ELISA
Enzyme-linked Immune Sorbent Assay kit was used to assess serum protein concentrations: BMAL1and CLOCK (EIAab, Wuhan, China). BMAL1 and CLOCK were detected using ELISA kits according to the manufacturers’ instructions.
Statistical analyses
Statistical analyses were carried out with SPSS 25.0 software and GraphPad Prism 6.0. Normality of data distribution was assessed prior to the application of parametric tests. The results are shown as the mean ± SD. Data were analyzed by one-way analysis of variance between multiple groups and t test between two groups. The level of significance was set at p < 0.05.
Results
Decreased clock and Bmal1 level in the serum of COPD patients
Based on the patients’ lung function and smoking history, we divided the 76 subjects into three groups: non-smokers, smokers and smokers with COPD group. We summarizes the clinical characteristics of the subjects in Supplementary Table 2. The three groups were similar in terms of age and body mass index. The levels of Clock and Bmal1 in the serum of COPD patients were significantly lower than those of healthy controls (Fig. 1 A and B). As previously reported, disturbances in the circadian rhythm are involved in the development of COPD.
Fig. 1.
Decreased Bmal1 and Clock protein level were observed in the plasma of COPD patients
A.Plasma were collected from non-smokers (n = 30), smokers (n = 20), and COPD (n = 26). A Bmal1 protein level. B Clock protein level. #p < 0.05, *p < 0.05, ***p < 0.001 vs. non-smokers
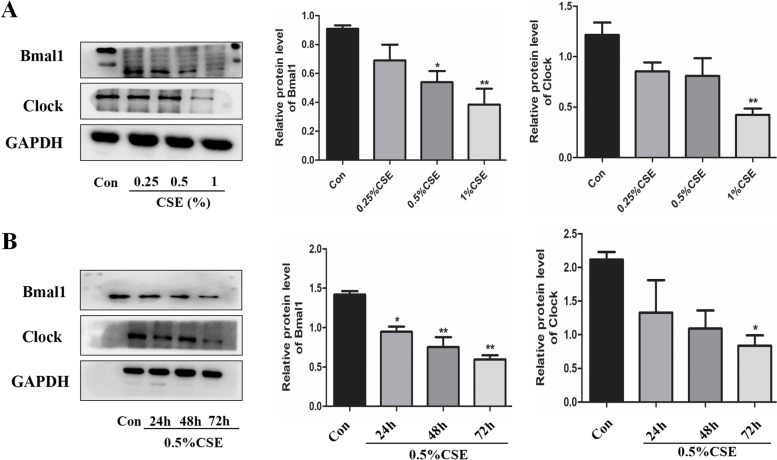
Cigarette smoke down-regulate Bmal1 and clock expression in bronchial epithelial cells
Following a different concentration of CSE stimulated, the expression of Bmal1 and Clock were decreased significantly at concentration of 1%CSE (Fig. 2A). To explore the association between clock genes and CSE in COPD, we also treated Beas-2B cells with 0.5%CSE for 24 h, 48 and 72 h. We observed a decreased Bmal1 and Clock in Beas-2B cells after 72 h of CSE (Fig. 2B).
Fig. 2.
CSE decreased the expression of Bmal1 and Clock in Beas-2B cells
Higher levels of cellular senescence in CSE-induced bronchial epithelial cells
Cigarette Smoke leads to accelerated aging in Beas-2B cells. CSE (0.25%, 0.5% and 1%) stimulated Beas-2B cells for 24 h, respectively. As shown in Fig. 3A, phosphorylated H2AX (pH2AX) was increased in a concentration-dependent manner. Phosphorylated H2AX is a marker of cellular senescence and DNA double-strand breaks (DSB) [30, 31]. And the mRNA expression of p16 and p21 were also increased after 24 h of CSE (Fig. 3B and C). Cells were seeded in six-well plates and then stimulated with CSE (0.25%, 0.5% and 1%) for 24 h and cellular senescence was detected the next day. An increase of cell senescence was showed by the senescence associated-gal staining analysis (Fig. 3D).
Fig. 3.
CSE increased cellular senescence in Beas-2B cells
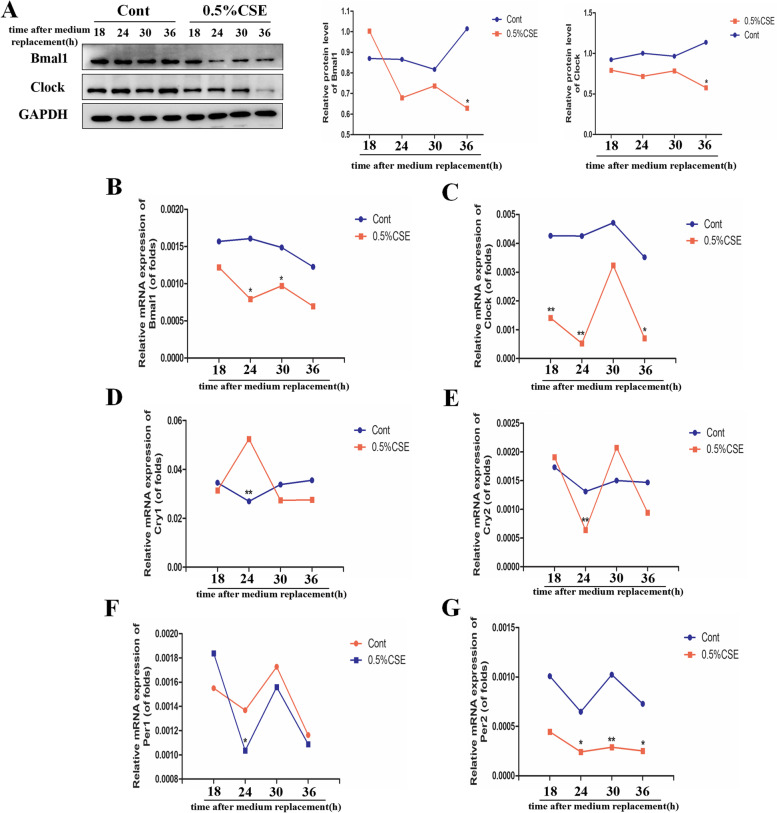
Cigarette smoke triggered circadian disturbance in bronchial epithelial cells
To further investigate the effect of CSE on the circadian rhythms in COPD, we stimulated Beas-2B cells with CSE for 24 h. In our study, we found Bmal1 and Clock showed a regular rhythmic expression in the control group in 24 h cycle. However, 0.5%CSE significantly disturbed the circadian rhythms (Fig. 4A). Then, we detected the mRNA expression of other core clock genes (Fig. 4B-G). We observed that CSE disturbed the mRNA expression of Clock, Bmal1, Per1, Per2, Cry1 and Cry2. We also found that the expression levels of Bmal1 and Clock are lowest at Zeitgeber time 24 (ZT24), nevertheless Cry1 peaked at ZT24. Cry1 mRNA expression seemed to be inversely correlated with Bmal1 and Clock mRNA level.
Fig. 4.
CSE induced circadian disturbance Beas-2B cells
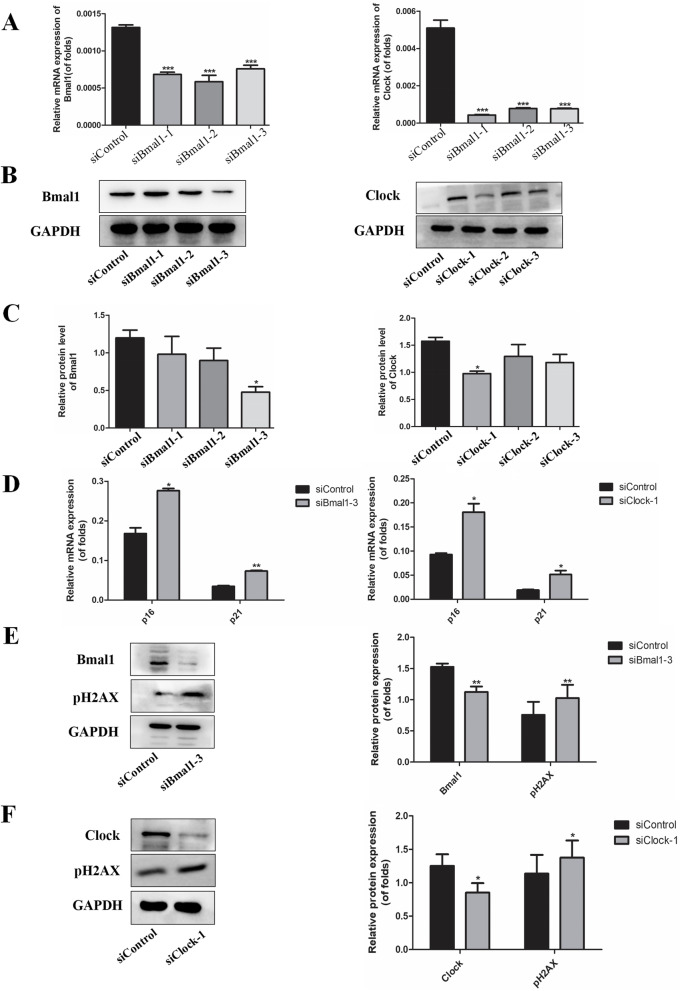
Knockdown of Bmal1 and clock increased cell senescence in bronchial epithelial cells
To study the association between circadian rhythms and cellular senescence, Beas-2B cells were transfected with Bmal1 siRNA (siBmal1-1, siBmal1-2, and siBmal1-3) or Clock siRNA (siClock-1, siClock-2, and siClock-3) for 48 h, and then mRNA and protein expression was detected. The cells transfected with Bmal1 siRNA and Clock siRNA showed a lower expression of two genes (Fig. 5 A-C). Based on the results, we used siBmal1-3 and siClock-1 in the subsequent experiments. After knocking down Bmal1 and Clock, we found that the mRNA levels of p16 and p21 were significantly increased (Fig. 5D). Besides, phosphorylated H2AX was augmented significantly in Bmal1-KD cells and Clock-KD cells (Fig. 5E and F). The results suggest that the depletion of circadian clock accelerated aging in bronchial epithelial cells.
Fig. 5.
Knockdown of Bmal1 or Clock stimulated cellular senescence in Beas-2B cells
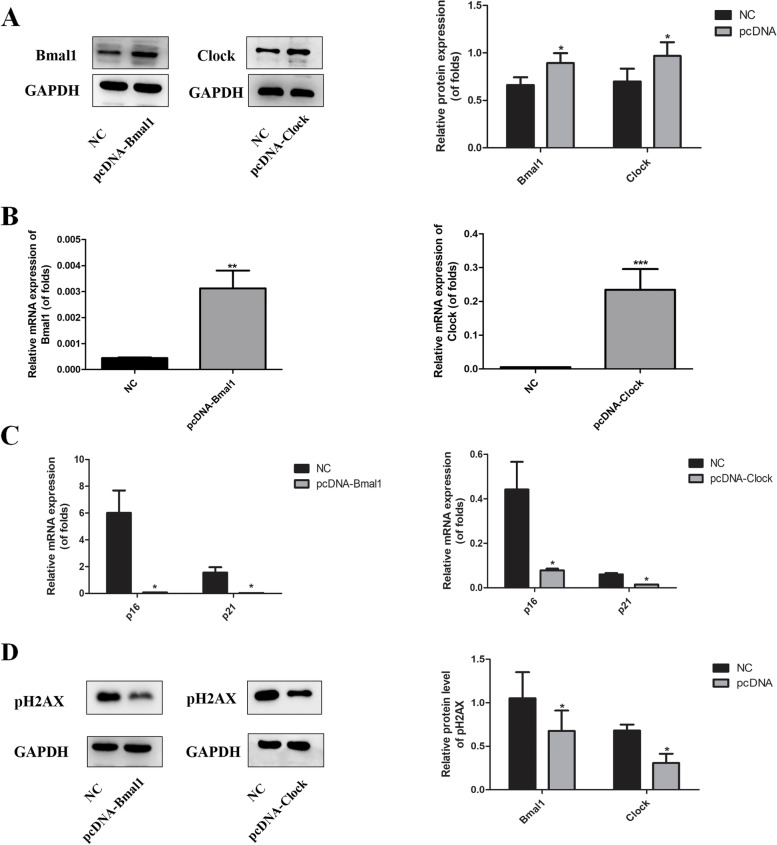
Overexpression of Bmal1 and clock repressed cell senescence in bronchial epithelial cells
To further examing the effect of Bmal1 and Clock on cell senescence in bronchial epithelial cells, cells overexpressing.
Bmal1 and Clock were established. Western blot and RT-PCR analyses confirmed the increase in Bmal1 and Clock protein expression, respectively (Fig. 6 A and B). As seen in Fig. 6 C, the mRNA levels of p16 and p21 were significantly repressed. Western blot analyses showed that overexpression of Bmal1 and Clock inhibited phosphorylated H2AX (Fig. 6D).
Fig. 6.
Overexpression of Bmal1 or Clock inhibited cellular senescence in Beas-2B cells
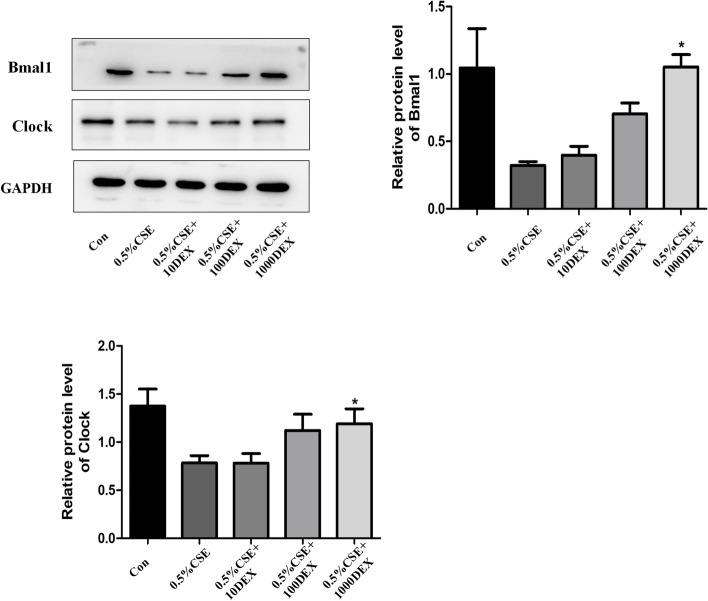
Dexamethasone influence the expression of Bmal1 and clock in bronchial epithelial cells
Dexamethasone (DEX), an agonist of the glucocorticoid receptor, is well known to reset cellular clocks [32]. Cells were pretreated with Dex (10, 100, and 1000 pM)for 1 h prior to incubation with 0.5% CSE for 24 h. We showed that Dex increased the expression of Bmal1 level in Beas-2B cells stimulated by CSE. In addition, Dex also significantly increased Clock protein level in Beas-2B cells (Fig. 7).
Fig. 7.
Effect of dexamethasone on the protein expression of Bmal1 and Clock in Beas-2B cells
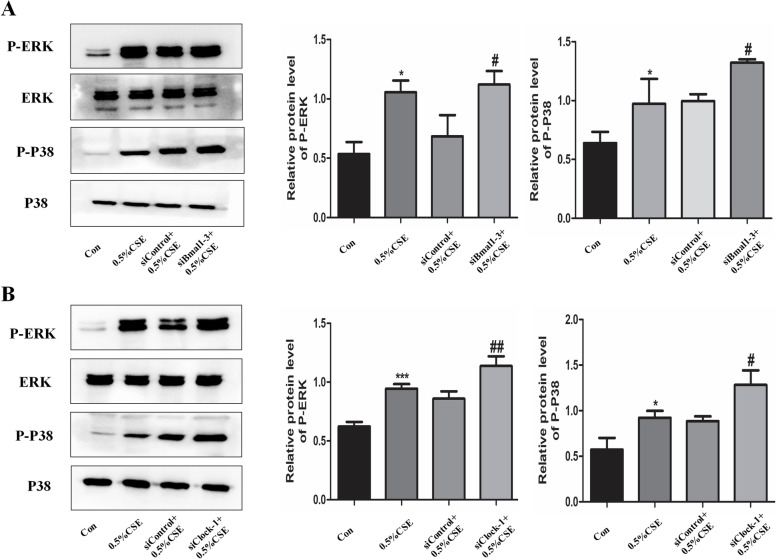
Circadian clock regulate cell senescence via MAPK pathways in bronchial epithelial cells
In previous studies, MAPK signaling pathways were closely related to the mechanism of COPD. In the present study, Beas-2B cells were stimulated with 0.5% CSE for 1 h and MAPK pathways were activated by CSE. Furthermore, we also established Bmal1 knockdown cells and Clock knockdown cells, then cells were incubated with 0.5% CSE for 1 h. Our results showed that CSE-induced P38 and ERK phosphorylation was also upregulated after knocking down Bmal1 and Clock (Fig. 8 A and B). To further confirm whether circadian clock regulates cell senescence through MAPK pathways, the pathway inhibitors PD98059 (20 µM) and SB203580 (20 µM) were used. Bmal1 knockdown cells or Clock knockdown cells were pretreated with PD98059 and SB203580 for 1 h. As shown in Fig. 9, the MAPK inhibitors reversed the up-regulated cellular senescence expression after knocking down Bmal1 and Clock. The result indicates that MAPK signalling pathways were involved in the anti-aging functions of circadian clock.
Fig. 8.
Knockdown of Bmal1 or Clock modulates cigarette smoke-induced cell senescence: role of intracellular signalling pathways
Fig. 9.
MAPK inhibitors reversed the up-regulation senescence in Bmal1 or Clock knockdown cells
Discussion
COPD patients exhibit daily rhythms with increased symptoms associated with sleep disruption [33]. Due to the daily nature of COPD exacerbation and its influence on sleep quality, we suggested that disruption of the circadian rhythm may be a vital role in the pathogenesis of COPD. Several pathways by which circadian clock genes control life cycle regulation and organ aging. In this study, we investigated two core clock genes, BMAL1 and CLOCK, in regulating cellular senescence in COPD. We are the first time showing that level of Clock-Bmal1 were reduced in the serum of COPD patients, and CSE caused the inhibition of expression of Bmal1 and Clock coincided with increased expression of cell senescence in human bronchial epithelial cells, which is through the MAPK pathways. The data might uncovered new insights about the accelerated aging of lung the in the development of COPD .
CS exposure changed clock genes expression of the lung in both rats and mice [34, 35]. The expression of p16 and p21 were increased in Emphysematous lungs, which are markers of cell senescence [36]. In our study, We demonstrated that CSE significantly reduced the expression of Bmal1 and Clock. We also found that cell senescence was induced by CSE in Beas-2B cells. This notion is basically the same as that of a previous study, acrolein treatment caused deletion of Clock and Bmal1 in HUVECs [37]. Recently, other studies have shown that disruption of the circadian molecular clock has a significant effect on lung function and lung pathophysiology [38–40]. A recent study also finds link between biological clock and aging [41]. Therefore, we hypothesized that circadian clock might accelerated aging process in COPD.
Disturbance in circadian rhythms are involved in the pathological processes of many diseases, such as inflammatory and metabolic diseases [42]. It has been found sleep disturbances and night-time symptoms are common in COPD patients, suggesting disrupted circadian rhythms in COPD patients [43]. Oishi and colleagues are the first found Clock gene expression in the lungs [44] and Gibbs and colleagues [38] reported molecular clock function in bronchial epithelial cells. We are the first time found that CSE treatment disrupted rhythms of Clock and Bmal1 in bronchial epithelial cells. In addition, we also found the relation between circadian rhythms and cell senescence. The expression of p16, p21 and pH2AX increased significantly in Clock or Bmal1 knockdown cells. However, over-expression of Bmal1 or Clock decreased the expression of p16, p21 and pH2AX significantly. These results are in line with a previous study that Bmal1−/− mice display early signs of aging, possibly via cellular senescence [25]. In the present study, we have found that circadian clock dysfunction contributes to accelerated cellular senescence in bronchial epithelial cells. Therefore, our results showed that circadian clock may accelerated lung cellular senescence and premature aging, the pivotal pathogenesis of COPD.
MAPK pathways play a vital role in the development of COPD. In our study, we demonstrated that circadian clock regulated cellular senescence through the MAPK pathway. In a previous study, Cellular senescence can promote the transformation of benign skin papilloma to cancer by enhancing p38 MAPK and ERK MAPK signaling pathways [45]. Goldsmith et al. found that circadian clock genes were regulated by MAPK pathways in mammalian SCN [46]. Therefore, we speculated that MAPK pathway is likely to be involved in circadian clock regulating CSE-induced cell senescence. In our study, CSE significantly activated p38 MAPK and ERK MAPK pathway, whereas knockdown of Bmal1 or Clock further upregulated the effect of CSE on the MAPK pathways in Beas-2B cells. We further used the p38 and ERK inhibitor (SB203580 and PD98059) to confirm the above results, which reduced the increased cell senescence after knocking down Bmal1 or Clock. Our findings implied that circadian clock regulated cellular senescence in response to CSE.
In our study, DEX increased the protein level of Bmal1 and Clock in Beas-2B cells. Accumulating evidence has confirmed that dexamethasone could induced the expression of clock genes in bronchial epithelial cells, peripheral blood mononuclear cells, lymphocytes, and fibroblasts [47]. These findings suggest that molecular clock genes is mechanistically associated with the anti-inflammatory effects of glucocorticoids. Therefore, designing compounds that normalize the expression of circadian clock genes in the lung may be a new approach to treating COPD in the future. In addition, Molecules that enhance circadian clock genes have been developed as an effective alternative to steroid hormones in the treatment of COPD. However, it remains to be seen whether these chronopharmacological drugs can be used to effectively treat cellular senescence in COPD.
The present study has several weakness. Firstly, this study was conducted only in one cell line, whereas, we should use primary cells from patients and controls ideally. Secondly, the exact mechanism by which CSE alters circadian clock levels is unclear, and further studies are needed to clarify the mechanism of CSE-induced deletion of Bmal1 and Clock, and whether genetic modifications of Bmal1 or Clock can subtle aging processes in COPD models.
In summary, we revealed that Clock-Bmal1 may be a vital factor to accelerate cellular senescence of the lung leading to COPD. The study provides data for circadian clock level in the plasma of COPD patients, and further searches the potential role of circadian clock in the development of COPD in vitro studies, which might shed a light on a novel mechanism linked to the accelerated lung aging in the COPD formation.
B.CSE decreased the protein levels of Bmal1 and Clock in Beas-2B cells at concentrations of CSE (0.25– 1%) for 24 h. Relative densities (% of control) are shown of Bmal1 and Clock in Beas-2B cells at concentrations of CSE (0.25– 1%) for 24 h. B. 0.5% CSE decreased the protein levels of Bmal1 and Clock in Beas-2B cells at times of CSE treatment (24–72 h). Relative densities (% of control) are shown of Bmal1 and Clock in Beas-2B cells at times of CSE (24–72 h). Values are expressed as means ± SD of 3 replications. *p < 0.05, **p < 0.01 vs. control group. Full-length gels are presented in Supplementary Fig. 1.
A.CSE increased the protein levels of pH2AX in Beas-2B cells at concentrations of CSE (0.25– 1%) for 24 h. Relative densities (% of control) are shown of pH2AX in Beas-2B cells at concentrations of CSE (0.25–1%) for 24 h. B. Cultured cells were exposed to CSE (0.25%, 0.5% and 1%) for 24 h, The mRNA expression of p16 was examined by real time PCR. C. Cultured cells were exposed to CSE (0.25%, 0.5% and 1%) for 24 h, The mRNA expression of p21 was examined by real time PCR. D. Cultured cells were exposed to CSE (0.25%, 0.5% and 1%) for 24 h, Senescence-associated β-galactosidase (SA-β-gal) activity was assessed. Values are expressed as means ± SD of 3 replications. *p < 0.05, ***p < 0.001 vs. control group. Full-length gels are presented in Supplementary Fig. 2.
A.Beas-2B cells were incubated with CSE (0.5%) at 6 h intervals between 6 and 24 h time points. The protein levels of Bmal1 and Clock were examined by Western blotting. Relative densities (% of control) are shown of Bmal1 and Clock in Beas-2B cells. B-G. The mRNA expression of clock genes Bmal1, Clock, Cry1, Cry2, Per1 as well as Per2 with GAPDH as an internal reference. Values are expressed as means ± SD of 3 replications. *p < 0.05, **p < 0.01 vs. control group. Full-length gels are presented in Supplementary Fig. 3.
A.Beas-2B cells were transfected with Bmal1 and Clock siRNAs separately. The mRNA expression of Bmal1 and Clock were detected by PCR. B. Beas-2B cells were transfected with Bmal1 and Clock siRNAs separately. The protein expression of Bmal1 and Clock were detected by Western blotting. C. Relative densities (% of control) are shown of Bmal1 and Clock in Beas-2B cells. D. p16 and p21 mRNA expression after knockdown of Bmal1 or Clock. E,F. pH2AX protein expression after knockdown of Bmal1 or Clock. Values are expressed as means ± SD of 3 replications. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group. Full-length gels are presented in Supplementary Fig. 4.
A.Beas-2B cells overexpressing Bmal1 or Clock were established. The protein expression of Bmal1 and Clock were detected by Western blotting. Relative densities (% of control) are shown of Bmal1 and Clock in Beas-2B cells. B. Beas-2B cells overexpressing Bmal1 or Clock were established. The mRNA expression of Bmal1 and Clock were detected by PCR. C. p16 and p21 expression after overexpression of Bmal1 or Clock. D. pH2AX protein expression after overexpression of Bmal1 or Clock. Values are expressed as means ± SD of 3 replications. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group. Full-length gels are presented in Supplementary Fig. 5.
Cells were pretreated with dexamethasone (Dex) (10, 100, and 1000 pM)for 1 h prior to incubation with 0.5% CSE for 24 h. The protein expressions of Bmal1 and Clock were detected by Western blotting. Relative densities (% of control) are shown of Bmal1 and Clock in Beas-2B cells. Values are expressed as means ± SD of 3 replications. *p < 0.05 vs. control group. Full-length gels are presented in Supplementary Fig. 6.
A.Cells were treated with Bmal1-directed siRNA3 for 48 h before cells were exposed to 0.5% CSE for a further 1 h compared with siControl cells. The effects of siRNA3 knockdown of Bmal1 on 0.5% CSE-induced MEK/ERK (p-ERK) and p38 MAPK (p-p38) activation was determined after 1 h. B. Cells were treated with Clock-directed siRNA1 for 48 h before cells were exposed to 0.5% CSE for a further 1 h compared with siControl cells. The effects of siRNA1 knockdown of Clock on 0.5% CSE-induced MEK/ERK (p-ERK) and p38 MAPK (p-p38) activation was determined after 1 h. Values are expressed as means ± SD of 3 replications. *p < 0.05 and ***p < 0.001 vs. control group. #p < 0.05 and ##p < 0.001 vs. siControl group. Full-length gels are presented in Supplementary Fig. 7.
(A) Bmal1 knockdown Cells were pre-treated with MEK/ERK (PD98059, 20 µM) inhibitor, pH2AX protein expression, p16 and p21 mRNA expression were assessed. (B) Bmal1 knockdown Cells were pre-treated with p38 MAPK (SB203580, 20 µM) inhibitor, pH2AX protein expression, p16 and p21 mRNA expression were assessed. (C) Clock knockdown Cells were pre-treated with MEK/ERK (PD98059, 20 µM) inhibitor for 1 h, pH2AX protein expression, p16 and p21 mRNA expression were assessed. (D) Clock knockdown Cells were pre-treated with p38 MAPK (SB203580, 20 µM) inhibitor for 1 h, pH2AX protein expression, p16 and p21 mRNA expression were assessed. Values are expressed as means ± SD of 3 replications. ***p < 0.001 vs. control group. ###p < 0.001 vs. siRNA group. Full-length gels are presented in Supplementary Fig. 8.
Supplementary Information
Acknowledgements
The authors would like to thank Jie Yang and Zhiwei Lu for their technical advice and assistance.
Authors’ contributions
L.L and M.S designed the study and wrote the main manuscript text, M.Z and Y.Z contributed to methodology, C.L and Y.C contributed to the interpretation of the manuscript. All authors read and approved the final manuscript.
Funding
The design of the study and collection, analysis, and interpretation of data were supported by Key projects of Wannan Medical College (WK2021ZF11) and Anhui Provincial Key projects of Natural Science Foundation for Colleges and Universities (KJ2021A0834).
Availability of data and materials
All supporting data are included within the main article and are available by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
All patients wrote the informed consents. The study protocol was approved by the Medical Ethical Committee of the First Affiliated Hospital of Wannan Medical College. The clinical data of patients was maintained with confidentiality and in compliance with the Declaration of Helsinki. All experiments were performed under the guidelines. The authors declare that there are no competing interests associated with the manuscript.
Consent for publication
Not applicable.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.May SM, Li JT. Burden of chronic obstructive pulmonary disease: Healthcare costs and beyond. Allergy Asthma Proc. 2015;36:4–10. doi: 10.2500/aap.2015.36.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir Soc. 2014;44:1055–68. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilleminault L, Rolland Y, Didier A. Characteristics of non-pharmacological interventions in the elderlywith COPD. Smoking cessation, pulmonary rehabilitation, nutritional management and patient education. Rev Mal Respir. 2018;35:626–41. doi: 10.1016/j.rmr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–13. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fragoso CAV, Gill TM. Defining chronic obstructive pulmonary disease in an aging population. J Am Geriatr Soc. 2010;58:2224. doi: 10.1111/j.1532-5415.2010.03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dall. G, Semilla CWest, Storm MV. (2013) An aging population and growing disease burden will require alarge and specialized health care workforce by 2025. Health Aff. 32,2013–2020. [DOI] [PubMed]
- 7.Raherison G. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–21. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 8.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 9.Mohawk, Green T. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrecht Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–60. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Dibner, Schibler A. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 12.Lamia, Storch, Weitz. P(2008) hysiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. U. S. A. 105, 15172–15177. [DOI] [PMC free article] [PubMed]
- 13.Gabryelska S, Turkiewicz B. (2020) Relationship between HIF-1 and Circadian Clock Proteins in Obstructive Sleep Apnea Patients—Preliminary Study. J. Clin. Med. 2020;9:1599. doi: 10.3390/jcm9051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borsboom, et al. Diurnal variation in lung function in subgroups from two dutch populations: consequences for longitudinal analysis. Am J Respir Crit Care Med. 1999;159:1163–71. doi: 10.1164/ajrccm.159.4.9703106. [DOI] [PubMed] [Google Scholar]
- 15.Casale P. Cosinor analysis of circadian peak expiratory flow variability in normal subjects, passive smokers, heavy smokers, patients with chronic obstructive pulmonary disease and patients with interstitial lung disease. Respiration; Int Rev Thorac Dis. 1997;64:251–6. doi: 10.1159/000196682. [DOI] [PubMed] [Google Scholar]
- 16.Petty Circadian variations in chronic asthma and chronic obstructive pulmonary disease. Am J Med. 1988;85:21–3. doi: 10.1016/0002-9343(88)90237-9. [DOI] [PubMed] [Google Scholar]
- 17.Tsai, Brenner C. Circadian-rhythm differences among emergency department patients with chronic obstructive pulmonary disease exacerbation. Chronobiol Int. 2007;24:699–713. doi: 10.1080/07420520701535753. [DOI] [PubMed] [Google Scholar]
- 18.Thomas, Petro, Konietzko N. The circadian rhythm of ciliary beat frequency of human nasal cilia in probands with healthy lungs and in patients with chronic obstructive lung disease. Includes adrenergic stimulation by terbutaline. Pneumologie. 1993;47:526–30. [PubMed] [Google Scholar]
- 19.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135(1):173–80. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 20.Mercado N, Ito, Barnes. (2015)Accelerated ageing of the lung in COPD: new concepts. Thorax. 70: 5: 482-489. [DOI] [PubMed]
- 21.Lopez Otin. Blasco, Partridge, Serrano and Kroemer. (2013) the hallmarks of aging. Cell 153: 6: 1194–1217. [DOI] [PMC free article] [PubMed]
- 22.Dimri, Lee, Basile, Acosta, Scott R, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–45. doi: 10.1016/S0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- 24.Hood A. The aging clock: circadian rhythms and later life. J Clin Investig. 2017;127:437–46. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in bmal1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–73. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park Z. Mirek, na, Raduwan, Anthony, Belden. (2019) BMAL1 associates with chromosome ends to control rhythms in TERRA and telomeric heterochromatin. PLoS ONE. 14, e0223803. [DOI] [PMC free article] [PubMed]
- 27.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132–52. doi: 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF- B activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–9. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 29.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF- B and post-translational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–26. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 31.White RR, Vijg J. Do DNA double-strand breaks drive aging? Mol Cell. 2016;63:729–38. doi: 10.1016/j.molcel.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–7. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 33.Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respiratory care. 2010;55:1333–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, et al. TNF-alpha suppresses the expression of clock genes by interfering with e-box-mediated transcription. Proc Natl Acad Sci U S A. 2007;104:12843–8. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, et al. The circadian clock period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006;74:4750–6. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuder RM, Kern JA, Miller YE. (2012) Senescence in Chronic Obstructive Pulmonary Disease. Proc. Am. Thorac. Soc. 9, 62–63. [DOI] [PMC free article] [PubMed]
- 37.Wu X, Chen L, Zeb F, Li C, Jiang P, Chen A, et al. Clock-Bmal1 mediates MMP9 induction in acrolein-promoted atherosclerosis associated with gut microbiota regulation. Environ Pollut Sep. 2019;252(Pt B):1455–63. doi: 10.1016/j.envpol.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon AS Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology. 2009;150:268–76. doi: 10.1210/en.2008-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadden H, Soldin SJ, Massaro D. (1985) Circadian disruption alters mouse lung clock gene expression and lung mechanics. J Appl Physiol. 2012:385–392. [DOI] [PMC free article] [PubMed]
- 40.Sukumaran S, Jusko WJ, Dubois DC, Almon RR. Light–dark oscillations in the lung transcriptome: implications for lung homeostasis, repair, metabolism, disease, and drug action. J Appl Physiol. 2011;110:1732–47. doi: 10.1152/japplphysiol.00079.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondratov RV. A role of the circadian system and circadian proteins in aging. Ageing Res Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Med Chem. 2011;3(5):623e638. doi: 10.4155/fmc.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between bmal1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- 45.Alimirah, Pulido, Valdovinos, Alptekin C, et al. Cellular Senescence promotes skin carcinogenesis through p38MAPK and p44/42MAPK signaling. Cancer Res. 2020;80:3606–19. doi: 10.1158/0008-5472.CAN-20-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldsmith B-P. Diverse roles for MAPK signaling in circadian clocks. Adv Genet. 2013;84:1e39. doi: 10.1016/B978-0-12-407703-4.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burioka N, Takata M, Okano Y, Ohdo S, Fukuoka Y, Miyata M. Dexamethasone influences human clock gene expression in bronchial epithelium and peripheral blood mononuclear cells in vitro. Chronobiol Int. 2005;22:585–90. doi: 10.1081/CBI-200062416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data are included within the main article and are available by contacting the corresponding author.