Abstract
Objective
/Background: The goal of the present study was to assess the prevalence and incidence of insomnia in the United States during the COVID-19 pandemic, and whether, among those that contracted COVID-19, insomnia predicted worse outcomes (e.g., symptoms of greater frequency, duration, or severity).
Methods
A nationwide sample of 2980 adults living in the United States were surveyed online at two points during the COVID-19 pandemic (T1 = April–June 2020; T2 = January–March 2021). Insomnia symptoms were assessed at both time points using the Insomnia Severity Index (ISI). The T2 survey also asked questions regarding COVID-19 testing and symptoms.
Results
The prevalence of insomnia (defined as ISI ≥15) was 15% at T1 and 13% at T2. The incidence rate of insomnia (i.e., new cases from T1 to T2) was 5.6%. Participants with insomnia were not more likely to contract COVID-19 relative to those participants without insomnia. Among those participants in our sample that contracted the virus during the study interval (n = 149), there were no significant group differences in COVID-19 symptom outcomes, with one exception, participants with insomnia were more likely to report a longer symptom duration (insomnia = 24.8 sick days, no insomnia = 16.1 sick days).
Conclusions
The present study suggests the prevalence of insomnia in the U.S. population remained high during the COVID-19 pandemic. The data also support that insomnia may be related to experiencing more chronic COVID-19 symptoms. These findings have more general implications for the role of sleep and insomnia on immune functioning.
Keywords: Insomnia, COVID-19, Prevalence, Symptoms
1. Introduction
The COVID-19 pandemic has had an unequivocal negative impact worldwide. To date, nearly 6.6 million COVID-related deaths have been reported as a result of the virus [1]. The pandemic has also led to greater stress related to social isolation and loneliness, unemployment, grief, and fear of contracting the disease. These factors alone would be expected to negatively impact sleep (e.g., greater stress-related acute insomnia) [[2], [3], [4], [5], [6], [7], [8]]. Beyond this, however, the stay-at-home orders, prolonged social confinement, and culture shift to increased work-from-home during the pandemic may also have led to greater instances of insomnia, possibly owing to greater sleep opportunity [9]. That is, several disease- and social-related circumstances have allowed individuals to alter their sleep schedules (e.g., changes in their time to bed or time out of bed) and subsequently increased the amount of time spent in bed or greater variability with respect to sleep timing; both of which may have deleterious short and long term effects on sleep efficiency [[10], [11], [12]]. For example, one study observed sleep patterns among a sample of individuals residing in Italy during different phases of the pandemic and found that, on average, people delayed their bedtime and risetime and increased their time in bed during the lockdowns [13].
Only a few studies, however, have directly measured whether sleep and insomnia symptoms have considerably changed since before the start of the pandemic [8,14,15]. What is known is that an appreciable segment of the population has developed insomnia symptoms during the COVID-19 pandemic. For example, Morin and colleagues (2021) reported that the prevalence of insomnia increased by over 25% relative to pre-pandemic rates, whereas data from another study suggested that the number of people reporting days with difficulty falling or staying asleep nearly doubled as compared to pre-pandemic levels [14]. Another study also found that pre-to-post pandemic changes in insomnia symptoms increased among a sample of Norwegian adults [8]. While these are the only studies to report on whether insomnia rates have increased following the pandemic, other studies [[16], [17], [18], [19]], primarily in healthcare workers, have estimated that prevalence rates for insomnia remain exceedingly high throughout the world (rates between 19 and 68% depending on insomnia criteria and sample used). A recent meta-analysis of studies that used the insomnia severity index (ISI) to assess insomnia symptoms also found that the COVID-19 pandemic is related to greater rates of subthreshold insomnia symptoms but not moderate or severe insomnia symptoms [20].
Considering the overall prevalence of insomnia has increased since the start of the COVID-19 pandemic (between 13 and 25% for moderate symptoms) [6,20,21], it is critical that studies consider if and how physical health is impacted and, as or more important, whether the increased occurrence of insomnia has altered susceptibility and/or severity to the COVID-19 virus. The primary goal of the present paper was to evaluate whether insomnia was associated with an increased risk for contracting the virus and/or worse symptom outcomes. Accordingly, the aims for the investigation were: (1) assess whether insomnia at baseline predicted who was more likely to contract COVID-19, and (2) quantify, among those that contracted COVID-19, whether insomnia led to worse outcomes (e.g., symptoms of greater frequency, duration, or severity) upon follow-up. We hypothesized that having insomnia at baseline would result in a greater likelihood of having more severe and/or chronic symptoms among those that contracted the virus. This hypothesis is based on literature that suggests that insomnia symptoms, poor sleep efficiency, and shorter habitual sleep duration are associated with a reduced immune response and lower resistance to illness [[22], [23], [24], [25], [26], [27]].
2. Methods
2.1. Participants and procedure
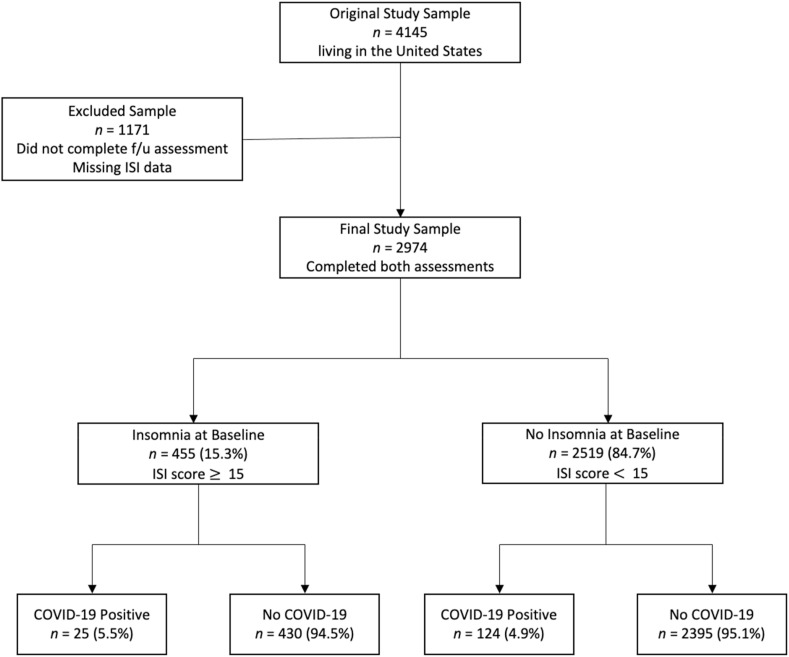
The original sample included 4145 adult participants (Mage = 45.8, SDage = 16.6; 78.7% female; 82.7% white) recruited from the United States to participate in an online study on social distancing, sleep, and mood. Study recruitment began during the first three months following the start of the COVID-19 pandemic (COVID-19 was declared a pandemic in March 2020 [28]). Convenience sampling strategies were used, such that recruitment occurred through postings on multiple social media websites (e.g., Facebook, Reddit), online newsletters, and ResesarchMatch. Participants were included in the study if they were at least 18 years old, had access to the Internet, and were able to read and write in English. The sample include participants from across the United States, including all geographic regions (% by region: 27.5 southeast, 26.7 midwest, 23.5 northeast, 12.6 west, 5.6 southwest, 4.1 rocky mountains). Eligible individuals were first invited to complete a baseline survey. This baseline survey was administered via Qualtrics XM (Provo, UT) and assessed social distancing, mood, sleep, physical activity, and basic demographic information. A follow-up survey was administered 10–12 months following the initial survey (i.e., January–March 2021). The follow-up survey consisted of similar items but also included more specific questions regarding COVID-19 testing and symptoms. Of those participants that completed the initial baseline survey, 28.1% (n = 1165) of the sample did not complete the follow-up survey and an additional 6 participants did not have complete baseline ISI data. Therefore, the final sample (i.e., those that completed surveys at both baseline and follow-up) included 2974 participants between the ages of 18 and 86 years old (Mage = 47.0, SDage = 16.7; 79.0% female; 84.1% white; please also see Fig. 1 for a description of the subject flow). The final sample, compared to those participants that were lost to follow-up (LTFU) was older (Mage in years, final sample = 47.0, LTFU = 42.8 years), had a greater percentage of participants who identified as white (%, final sample = 84.1, LTFU = 79.2), were married (%, final sample = 46.1, LTFU = 39.1), and were college-educated (%, final sample = 82.7, LTFU = 75.0). The final sample did not vary with regard to gender or employment status (i.e., unemployed, employed part-time, employed full-time). All procedures were approved by the Institutional Review Boards at the University of Arkansas and University of Pennsylvania.
Fig. 1.
Participant Flow. Covid-19 status was assessed at follow-up. “No COVID-19” group includes both those that did not test positive as well as those that were never tested.
2.2. Measures
2.2.1. Insomnia
Insomnia symptom prevalence and severity were estimated using the Insomnia Severity Index (ISI) [29,30]. The ISI is a 7-item instrument that allows for the assessment of the incidence and severity of initial, middle, and late insomnia and related daytime impairments. While the ISI can be used as a continuous measure with total scores ranging from 0 to 28 (with higher scores suggesting more severe insomnia), clinical thresholds are provided and defined as ‘no significant insomnia’ (scores 0–7); “subthreshold insomnia” (scores 8–14); “clinical insomnia, moderate severity” (scores 15–21); and “clinical insomnia, severe” (scores 22–28). For the present study, we defined insomnia as an ISI score of 15 or greater. The ISI was administered during the baseline survey and at follow-up and demonstrated good internal consistency in the current sample (baseline α = 0.89).
2.2.2. COVID-19 testing and symptoms
The follow-up survey included additional items regarding COVID-19 testing (e.g., “Have you been tested for COVID-19?” and “Have you tested positive for COVID-19?“), symptom onset (e.g., “Did you develop symptoms of COVID-19?” and “Regardless of whether you tested positive for COVID-19, since March 2020, have you experienced any of the following symptoms?“), symptom duration (“How long did your COVID-19 symptoms last?“), and overall symptom severity (“How severe were your COVID-19 symptoms?“). Symptom severity was also assessed for each individual symptom reported (e.g., “How severe was your reduced sense of taste?“). The symptom list included up to 19 different COVID-19 related symptoms (see Table 1 for a full list of symptoms). Yes/no responses were provided for questions related to testing and symptom onset. Participants responded in terms of months and days for the question related to symptom duration (this was subsequently converted to duration in days where 1 month is equal to 30 days). For symptom severity, participants responded on a 5-point Likert scale from “Not at all severe” to “Extremely severe”. In addition to an overall symptom severity score, mean symptom severity was computed based on the average severity rating of the symptoms endorsed by each participant. The survey also included questions about other clinical outcomes related to COVID-19 infection, such as whether the participant received inpatient treatment (e.g., “Were you hospitalized because of your COVID-19 symptoms?“) or respiratory assistance (e.g., “Were you provided supplemental oxygen [for example with a CPAP “mask”, oxygen cannula, oxygen “helmet”, etc.] because of your COVID-19 symptoms?” and “Were you intubated [i.e., put on a mechanical ventilator the delivered oxygen via an endotracheal tube] because of your COVID-19 symptoms?“).
Table 1.
Full list of COVID-19 related symptoms.
| COVID-19 Related Symptoms | # (%) Participants Endorsed |
|---|---|
| Fever | 285 (9.6) |
| Dry Cough | 394 (13.2) |
| Body Aches | 565 (19.0) |
| Muscle Pain | 508 (17.1) |
| Chills | 291 (9.8) |
| Shaking | 76 (2.6) |
| Fatigue | 884 (29.7) |
| Runny Nose | 796 (26.8) |
| Stuffy Nose | 672 (22.6) |
| Sore Throat | 578 (19.4) |
| Shortness of Breath | 261 (8.8) |
| Difficulty Breathing | 105 (3.5) |
| Vomiting | 82 (2.8) |
| Diarrhea | 448 (15.1) |
| Headaches | 893 (30.0) |
| Reduced Smell | 147 (4.9) |
| Odd Smell | 60 (2.0) |
| Reduced Taste | 147 (4.9) |
| Odd Taste | 76 (2.6) |
2.3. Statistical analyses
All analyses were completed in SPSS (version 26.0). Insomnia status was coded as 0 [no insomnia] and 1 [insomnia]. COVID-19 testing and positivity rates were also coded as binary variables (no/yes). COVID-19 symptom duration and severity were treated as continuous variables and entered as dependent variables in separate models. Insomnia prevalence and incidence rates were computed first. Here, the proportion of subjects who met criteria for ISI-based insomnia at baseline and follow-up were calculated. Next, a series of chi-square tests were used to estimate whether insomnia status at baseline was related to COVID-19 testing and positivity rates assessed at follow-up. Finally, a series of one-way ANOVAs were used to compute whether insomnia at baseline predicted COVID-19 outcomes among those participants who contracted COVID-19 during the study period (as assessed at follow-up). Analyses that adjusted for covariates (e.g., age, gender, race, marital status, educational history, employment status and history of chronic illnesses) were also computed.
3. Results
3.1. Prevalence and incidence of insomnia at baseline and follow-up
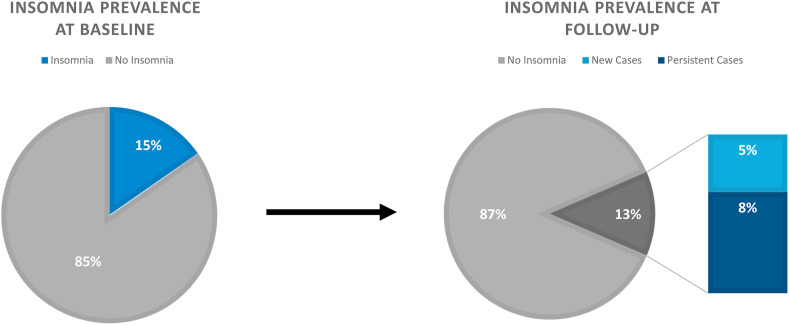
According to the data collected at baseline, the sample mean score on the ISI was 8.2 (SD = 5.9). The prevalence of ISI-defined insomnia (ISI total score ≥15 [threshold for clinical insomnia, moderate severity]) was 15.3% (n = 455). The sample mean at follow-up was 7.4 (SD = 5.8). The overall prevalence of insomnia at follow-up was 13.0% (n = 386). Of those that endorsed clinical levels of insomnia at baseline, 44.0% (n = 200) no longer met criteria for clinical insomnia at follow-up. In contrast, the incidence of insomnia (i.e., percent of new cases at follow-up) was 5.6% (n = 140). Please see Fig. 2 for more information regarding the prevalence and incidence of insomnia during the COVID-19 pandemic.
Fig. 2.
Insomnia prevalence reported as the percent of cases with an ISI ≥15 at baseline and follow-up. Incidence rate is reflected at the number of new cases during the intervening period.
3.2. Demographic differences by insomnia status [baseline]
Table 2 summarizes means, standard deviations and percentiles for all demographic variables and whether there were any group differences at baseline. According to these data, the insomnia group (as determined by baseline ISI scores) was slightly younger, more likely to identify as a woman, had a lower percentage of people who were married, educated, and employed, and less likely to report having a history of at least one chronic illness (e.g., cardiovascular disease, diabetes). While the omnibus test was not statistically significant, the insomnia group was also more likely to identify as Black or Latinx.
Table 2.
Participant Demographic Information by Insomnia Status. P-values were determined using t-tests for continuous variables and chi-square analyses for categorical variables.
| Total Sample |
Insomnia |
No Insomnia |
p-value | |
|---|---|---|---|---|
| (n = 2974) | (n = 455) | (n = 2519) | ||
| Age, mean (SD) | 47.0 (16.7) | 45.0 (15.2) | 47.4 (17.0) | <0.01 |
| Gender, n (%) | <0.01 | |||
| Female | 2354 (79.0) | 380 (83.5) | 1970 (78.2) | |
| Male | 567 (19.0) | 61 (13.4) | 504 (20.0) | |
| Transgender Female | 4 (0.1) | 2 (0.4) | 2 (0.1) | |
| Transgender Male | 11 (0.4) | 3 (0.7) | 8 (0.3) | |
| Gender Variant/Non-Conforming | 29 (1.0) | 5 (1.1) | 24 (1.0) | |
| Other | 13 (0.4) | 3 (0.7) | 10 (0.4) | |
| Race/Ethnicity, n (%) | 0.06 | |||
| American Indian, Native American, or Alaska Native | 12 (0.4) | 3 (0.7) | 9 (0.4) | |
| Asian or Asian American | 121 (4.1) | 15 (3.3) | 105 (4.2) | |
| Black, African American, or African | 133 (4.5) | 29 (6.4) | 104 (4.1) | |
| Latino or Latina | 87 (2.9) | 16 (3.5) | 71 (2.8) | |
| Middle Eastern or Arab | 10 (0.3) | 1 (0.2) | 9 (0.4) | |
| Native Hawaiian or Other Pacific Islander | 4 (0.1) | 2 (0.4) | 2 (0.1) | |
| White or Caucasian | 2507 (84.1) | 369 (81.1) | 2133 (84.7) | |
| Multi-racial | 82 (2.8) | 18 (4.0) | 64 (2.5) | |
| Other | 24 (0.8) | 2 (0.4) | 22 (0.9) | |
| Marital Status, n (%) | <0.001 | |||
| Married | 1371 (46.0) | 161 (35.4) | 1206 (47.9) | |
| Widowed | 113 (3.8) | 15 (3.3) | 98 (3.9) | |
| Divorced | 411 (13.8) | 80 (17.6) | 331 (13.1) | |
| Separated | 35 (1.2) | 12 (2.6) | 23 (0.9) | |
| Never Married | 1046 (35.1) | 185 (40.7) | 859 (34.1) | |
| Educational History, n (%) | <0.001 | |||
| less than high school | 9 (0.3) | 3 (0.7) | 6 (0.2) | |
| high school graduate | 131 (4.4) | 30 (6.6) | 101 (4.0) | |
| some college | 374 (12.6) | 81 (17.8) | 293 (11.6) | |
| 2-year degree | 213 (7.1) | 39 (8.6) | 174 (6.9) | |
| 4-year degree | 1068 (35.8) | 149 (32.7) | 918 (36.4) | |
| professional degree | 934 (31.3) | 119 (26.2) | 812 (32.2) | |
| Doctorate | 239 (8.0) | 29 (6.4) | 208 (8.3) | |
| Employment, n (%) | 0.02 | |||
| Unemployed | 1091 (36.6) | 195 (42.9) | 895 (35.5) | |
| employed 1–20 h | 286 (9.6) | 40 (8.8) | 246 (9.8) | |
| employed 20–30 h | 196 (6.6) | 32 (7.0) | 163 (6.5) | |
| employed full time (40+ hours) | 1392 (46.7) | 186 (40.9) | 1202 (47.7) | |
| Chronic Illness, n (%) | <0.001 | |||
| No | 1316 (46.2) | 137 (32.1) | 1176 (48.7) | |
| Yes | 1530 (53.8) | 290 (67.9) | 1237 (51.3) |
3.3. Baseline insomnia and COVID-19 testing rates
Overall, 58.2% of the sample reported getting tested for COVID-19 (assessed at follow-up). Participants with insomnia at baseline (62.8% tested) were more likely to get tested for COVID-19 compared to participants without insomnia (57.4% tested), χ2 = 4.60, p = 0.03. Among those that reported on their test results (n = 1706), 149 participants tested positive for the virus (5% of the overall study sample). The positive test rate did not vary by group, insomnia [baseline] = 8.8% vs no insomnia 8.7%, χ2 = 0.002, p > 0.20 (Table 3 ). In terms of number of positive cases per total group, 25 participants (5.5%) in the insomnia group tested positive for COVID-19, whereas 124 participants (4.9%) in the control group tested positive (Fig. 1). Alternatively, mean baseline ISI scores did not differ by group (COVID positive = 8.9, no COVID = 8.6).
Table 3.
COVID-19 testing and self-reported symptom rates and percentages by group.
| Insomnia |
No Insomnia |
χ [2] | |
|---|---|---|---|
| (n = 455) | (n = 2519) | ||
| # participants tested (% of full sample) | 285 (62.8) | 1442 (57.4) | 4.60∗ |
| # participants tested positive (% of tested sample) | 25 (8.8) | 124 (8.7) | 0.002 |
| # participants with COVID-19 symptoms (% untested sample) | 19 (11.4) | 79 (7.4) | 3.13 |
| # participants report “yes” to having COVID (% untested sample) | 15 (8.9) | 39 (3.6) | 9.70∗∗ |
∗p < 0.05, ∗∗p < 0.01.
3.4. Insomnia and COVID-19 self-reported symptom rates
Among those who did not receive any COVID-19 tests during the study period (n = 1235), 11.4% of participants with insomnia at baseline (n = 167) reported experiencing COVID-19 symptoms during the past six months, compared to 7.4% of participants without insomnia (n = 1068), χ2 = 3.13, p = 0.08. When asked if they believe they contracted COVID-19 during the past six months, 8.9% of participants with insomnia at baseline (n = 15) said “yes” compared to only 3.6% of participants without insomnia (n = 39), χ2 = 9.70, p < 0.01 (see also Table 3).
3.5. Predicting COVID-19 symptom severity and course
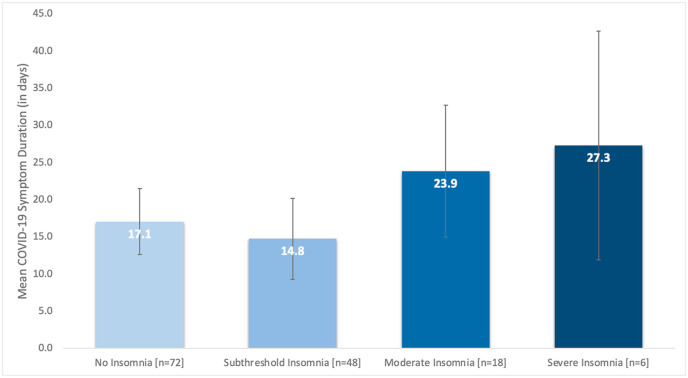
Only those participants who tested positive for COVID-19 during the study period (n = 149) were included in these analyses. No significant group differences were observed on any of the COVID-19 outcome measures (e.g., % participants endorsing symptoms, symptoms severity, symptom count) between those with and without insomnia at baseline, except for differences in symptom duration (see Table 4 for all parameter estimates and effect sizes). Specifically, participants with insomnia at baseline, on average, reported experiencing symptoms for more days than participants without insomnia, mean symptom duration in days, insomnia = 24.8 days, no insomnia = 16.1 days, F(1,142) = 4.2, p = 0.04, Hedge's g = 0.45. The parameter estimates for these analyses were comparable when controlling for age, gender, race, marital status, educational history, and employment status (unadjusted B = 8.61; adjusted B = 8.06). In contrast, there was a meaningful association between insomnia status, chronic health status, and mean COVID symptom duration. Participants who reported a positive history of chronic disease (i.e., endorsed at least one chronic health condition, such as cardiovascular disease, diabetes, COPD, etc.) and insomnia reported a greater mean symptom duration compared to participants without insomnia or a history of any chronic conditions; mean symptom duration in days, insomnia and chronic illness, 29.9 days vs. insomnia and no chronic illness, 12.3 days, Hedge's g = 0.71; mean symptom duration in days, insomnia and chronic illness, 29.9 days vs. no insomnia and chronic illness, 18.6 days, Hedge's g = 0.55. A secondary analysis of this data was also conducted where mean symptom duration was compared across the different ISI clinical thresholds. Consistent with our primary analysis, participants with the most severe insomnia symptoms at baseline reported longer COVID-19 symptoms (mean symptom duration in days, no insomnia [n = 72] = 17.1, subthreshold insomnia [n = 48] = 14.8, clinical insomnia, moderate [n = 18] = 23.9, clinical insomnia, severe [n = 6] = 27.3), however these differences were not statistically significant; F(3,140) = 1.55, p = 0.21) (Fig. 3 ). Finally, only six participants reported being hospitalized for COVID-19 (or 4% of COVID-positive sample). Additional analyses on these data were not completed given the small sample size.
Table 4.
Means, 95% confidence intervals, and percentages for COVID-19 symptom data among those participants who tested positive for COVID-19. Statistical tests of significance were determined using chi-square (for categorical variables) and t-tests (for continuous variables). Effect sizes (Hedge's g) were also provided for all continuous outcome variables.
| Insomnia |
No Insomnia |
p-value | g | |
|---|---|---|---|---|
| (n = 25) | (n = 124) | |||
| # participants that endorsed symptoms, n (%) | 22 (88.0) | 114 (92.0) | n.s. | – |
| Symptom count, mean (95% CI) | 7.40 (5.85–8.95) | 7.02 (6.32–7.71) | n.s. | 0.10 |
| Overall symptom severity rating, mean (95% CI) | 1.32 (0.94–1.70) | 1.02 (0.85–1.20) | n.s. | 0.31 |
| Mean symptom severity rating, mean (95% CI) | 1.86 (1.53–2.19) | 1.54 (1.40–1.69) | 0.08 | 0.39 |
| Symptom duration in days, mean (95% CI) | 24.8 (17.09–32.41) | 16.1 (12.72–19.57) | 0.04 | 0.45 |
Note: overall symptom severity ratings were based on a single-item response to “How severe were your COVID-19 symptoms?“, whereas mean symptom severity was computed based on the average severity rating of the symptoms endorsed by each participant.
Fig. 3.
Mean COVID-19 symptom duration by insomnia symptom severity.
4. Discussion
The COVID-19 pandemic has had a substantial impact on society. Until recently, one aspect that has often been overlooked is the effect it has had on sleep health (for a review on sleep dysfunction and COVID-19 please see Bhat & Chokroverty, 2022) [31]. For example, data from the present study support that, in general, the prevalence of clinical insomnia (13–15%) has increased since the start of the pandemic (compared to 6–10% for pre-pandemic chronic insomnia) [32]. These data are consistent with other research that has also shown greater insomnia prevalence rates during the post-pandemic period, though some studies have shown even larger increases in moderate-to-severe insomnia symptoms (e.g., 16–25% prevalence) [6,15,20].
In contrast to most prior research, the current study evaluated the prevalence and incidence of clinical insomnia during different phases of the pandemic. That is, insomnia symptoms were assessed at the start of the pandemic (April–June 2020) and then again during the first COVID-19 surge (January–March 2021). Findings from the current study suggest that the prevalence of insomnia (based on ISI-defined clinical thresholds) what highest during the initial months of the pandemic, but that the overall rates remained high in the general U.S. population (13–15%). Like other studies that have assessed insomnia symptoms longitudinally [33,34], overall mean insomnia scores were relatively consistent across different phases of the pandemic. In this study, five-to-six percent of insomnia cases at follow-up were new cases (i.e., incidence rate), and nearly half of participants with insomnia at baseline recovered by the follow-up assessment. These data are consistent with a prior study by Wang and colleagues (2022) that observed different trajectories of insomnia symptoms during the pandemic (e.g., chronic dysfunction vs. recovery), and in general, research suggesting that many, if not most, people with acute insomnia recover within a year's time [35]. While it's unclear why the proportion of people with insomnia decreased, it may be the case that, for many, the pandemic offered greater flexibility in setting their sleep/wake schedules (and presumably match their sleep timing with their preferred circadian rhythm). This may have led to greater average sleep durations [11] and subsequently fewer concerns or complaints about their sleep. That said, at least one other study found that this flexibility in changing one's sleep timing (as assessed by the relative increase/decrease in pandemic-related social jet lag) led to greater insomnia symptoms [12].
The effect that these persistent sleep problems have had on our physical health, and in particular, our ability to fight off the COVID-19 infection itself are unknown (e.g., does having insomnia increases one's risk for contracting COVID-19, more severe symptoms, and/or long-COVID?). According to the current study findings, while participants with insomnia at baseline were more likely to get tested for COVID-19, the rates of infection were comparable. This is an interesting discordance, and potentially speaks to the behavioral patterns of patients with insomnia during the pandemic. It may be the case, for example, that several of the biopsychosocial factors that are related to insomnia (e.g., general test anxiety, social isolation, heightened anxiety and/or depression symptoms during the initial wave of the pandemic, etc.) may mediate or moderate the likelihood that someone would have opted to get tested [for COVID] regardless of whether they were actually infected.
The primary hypothesis that insomnia at baseline would be associated with a greater likelihood of having more severe and/or chronic COVID-19 symptoms among infected participants was partially supported. Specifically, among those participants that tested positive, there were no differences in COVID-19 symptom count or severity between those with and without insomnia at baseline. Those with insomnia at baseline were however more likely to experience a more chronic course of COVID-19 (8.5 additional days sick on average; a small-to-medium effect size), especially among those that had a history of at least one chronic health condition (large effect). Put differently, having insomnia at baseline did not impact the number of COVID-19 symptoms that they reported experiencing and/or the severity of those symptoms, however, it did impact the duration of symptoms. Participants with insomnia reported taking longer to recover from COVID-19 relative to those participants without insomnia. This is not surprising considering that patients with insomnia exhibit heightened sympathetic and β-adrenergic activation [36,37], which can increase production of pro-inflammatory cytokines [38]. Studies have also shown that insomnia is associated with elevated plasma- and serum-based markers of inflammation, such as interluekin-6 (IL-6), C-reactive protein (CRP), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) [[39], [40], [41]]. Data from a meta-analysis supported these initial findings that insomnia symptoms were associated with higher levels of IL-6 and CRP [42]. This is also consistent with other research suggesting that short sleep duration increases susceptibility to respiratory infections [26,27]. Put differently, these data support the notion that our immune system produces an inflammatory response to the insomnia itself (or the shortened sleep associated with insomnia). Overtime, increased cytokine levels may make the immune system less efficient at handling a real immunological threat (such as the COVID-19 virus). For example, multiple studies have now demonstrated that sleep disturbance is associated with producing fewer antibodies in response to the influenza vaccine [22,43]. Put together, this literature may explain why those participants with insomnia that contracted COVID-19 did not necessarily experience more severe symptoms but did take longer to recover from the virus.
The present findings are promising and have implications beyond the COVID-19 pandemic, including the need for (1) sleep assessment both during the acute onset of COVID-19 symptoms and during recovery and (2) further evaluation on the relationship between insomnia, COVID-19, and the potential for an immunological response. Notably, the study has a number of important strengths. Insomnia symptoms were assessed prospectively via a validated instrument. The overall study sample was large and surveyed people from different geographic regions of the United States. The study was also reasonably comprehensive in its assessment of COVID-19 related-outcomes (i.e., positivity rate, symptom count, severity, chronicity, etc.). That said, the study has a number of important limitations that should be highlighted. First, the primary analyses were limited to a sub-sample of 149 people (or 5% of the sample that completed the follow-up survey). While a strength of this study is that subjects were surveyed during the initial phases of the COVID-19 pandemic, the drawback is that few people in our study contracted the virus during the assessment interval. This may be because the majority of subjects were surveyed during the height of the stay-at-home orders which ultimately delayed the spread of the virus, thus decreasing the likelihood that subjects would be diagnosed with COVID-19. Ultimately, the study is likely underpowered, limiting the ability to detect differences between groups. The small sample size also limited our ability to evaluate the effects of other variables. For example, people with poor health or pre-existing chronic illnesses are more likely to experience insomnia symptoms and take longer to recover from COVID-19. That said, in the present sample, a lower proportion of participants with insomnia reported a history of chronic illness relative to those participants without insomnia. Either way, additional efforts should be made to comprehensively assess the impact of initial health status on the link between sleep and COVID-19 outcomes. Secondly, the COVID-19 symptom and testing data were based on self-report, thus there was no objective way of knowing whether if those who endorsed testing positive for COVID-19 actually had the virus. Furthermore, it may be the case that individuals with more severe COVID-19 and/or longer inflammatory responses experience greater sleep disturbance as a consequence of the infection. The direction of this relationship remains to be elucidated. Future studies should consider a study design that uses more frequent sampling in order to temporally map out these events, which will allow fora more specific evaluation of insomnia's prospective relationship with immune functioning (i.e., does insomnia precede and predict more severe infection?). Finally, while relatively large, the overall sample was not representative of the general population living in the United States and generalizations to the population should be made with appropriate caution. Despite these limitations, these data are still an important contribution to the literature but should be appropriately contextualized.
5. Conclusion
While several studies now support that greater sleep problems have had an adverse impact on people's mental health during the pandemic, it's unclear whether these increased rates of insomnia have adversely affected physical health, including an individual's ability to respond to the SARS-CoV-2 virus. The present data provide some of the first evidence that insomnia may prolong an individual's recovery from COVID-19. While these findings have clear implications for role of sleep during the ongoing pandemic (and mitigating the effects of the virus), they also highlight the importance of continuing to investigate the impact of insomnia on immune functioning. These results, for example, further support what sleep scientists have been advocating for several decades: insomnia is not only bad for your sleep, it's bad for your health [[44], [45], [46]]. Specifically, persistent bad sleep may augment adaptive immunity and compromise the immune system, and as these data suggest, it may not be that the immune system becomes weakened but rather less efficient (resulting in longer illness duration). The clinical implications are clear, it is important to treat insomnia now more than ever. Especially now that there is a threat of pandemic viruses (i.e., we are more vulnerable to future pandemics), but more generally, treating insomnia may reduce the overall burden of disease by increasing resiliency to disease and infection.
Financial disclosure
None.
Non-financial disclosure
None.
Data availabilty statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgments
This work was supported by the National Institutes of Health: K23HL141581 (PI: Vargas), K24AG055602 (PI: Perlis).
References
- 1.World Health Organization . World Heal Organ; 2021. WHO coronavirus disease (COVID-19) dashboard with vaccination data | WHO coronavirus (COVID-19) dashboard with vaccination data; pp. 1–5.https://covid19.who.int/ [Google Scholar]
- 2.Drake C.L., Richardson G., Roehrs T., Scofield H., Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. doi: 10.1093/sleep/27.2.285. http://www.ncbi.nlm.nih.gov/pubmed/15124724 [DOI] [PubMed] [Google Scholar]
- 3.Ellis J., Gehrman P., Espie C., Riemann D., Perlis M.L. Acute insomnia: current conceptualizations and future directions. Sleep Med Rev. 2012;16(1):5–14. doi: 10.1016/j.smrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.O’regan D., Jackson M.L., Young A.H., Rosenzweig I. Understanding the impact of the covid-19 pandemic, lockdowns and social isolation on sleep quality. Nat Sci Sleep. 2021;13:2053–2064. doi: 10.2147/NSS.S266240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilcher J.J., Dorsey L.L., Galloway S.M., Erikson D.N. Social isolation and sleep: manifestation during COVID-19 quarantines. Front Psychol. 2022;12(January:1–7. doi: 10.3389/fpsyg.2021.810763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzierzewski J.M., Dautovich N.D., Ravyts S.G., Perez E., Soto P., Donovan E.K. Insomnia symptoms during the COVID-19 pandemic: an examination of biopsychosocial moderators. Sleep Med. 2022;91:175–178. doi: 10.1016/j.sleep.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheshmehzangi A., Chen H., Su Z., Zou T., Xiang Y.T., Dawodu A. How does the COVID-19 fuel insomnia? Brain, Behav Immun - Heal. 2022;21 doi: 10.1016/j.bbih.2022.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ø Halsøy, Johnson S.U., Hoffart A., Ebrahimi O.V. Insomnia symptoms in the general population during the COVID-19 pandemic. Front Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.762799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilcher J.J., Dorsey L.L., Galloway S.M., Erikson D.N. Social isolation and sleep: manifestation during COVID-19 quarantines. Front Psychol. 2022;12(January:1–7. doi: 10.3389/fpsyg.2021.810763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rome O., Sinai L., Sevitt R., et al. Owls and larks do not exist: COVID-19 quarantine sleep habits. Sleep Med. 2021;77:177–183. doi: 10.1016/j.sleep.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins R., Affouf M., Weaver M.D., et al. Estimated sleep duration before and during the COVID-19 pandemic in major metropolitan areas on different continents: observational study of smartphone app data. J Med Internet Res. 2021;23(2) doi: 10.2196/20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandão L.E.M., Martikainen T., Merikanto I., et al. Social jetlag changes during the COVID-19 pandemic as a predictor of insomnia - a multi-national survey study. Nat Sci Sleep. 2021;13:1711–1722. doi: 10.2147/NSS.S327365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte F., Cellini N., De Rosa O., et al. Dissociated profiles of sleep timing and sleep quality changes across the first and second wave of the COVID-19 pandemic. J Psychiatr Res. 2021;143(August):222–229. doi: 10.1016/j.jpsychires.2021.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisler G.C., Twenge J.M. Sleep characteristics of U.S. adults before and during the COVID-19 pandemic. Soc Sci Med. 2021:276. doi: 10.1016/j.socscimed.2021.113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin C.M., Vézina-Im L.-A., Ivers H., et al. Prevalent, incident, and persistent insomnia in a population-based cohort tested before (2018) and during the first-wave of COVID-19 pandemic (2020) Sleep. October 2021 doi: 10.1093/sleep/zsab258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokou-Kpolou C.K., Megalakaki O., Laimou D., Kousouri M. Insomnia during COVID-19 pandemic and lockdown: prevalence, severity, and associated risk factors in French population. Psychiatr Res. 2020;290 doi: 10.1016/j.psychres.2020.113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappa S., Ntella V., Giannakas T., Giannakoulis V.G., Papoutsi E., Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulah D.M., Musa D.H. Insomnia and stress of physicians during COVID-19 outbreak. Sleep Med X. 2020;2 doi: 10.1016/j.sleepx.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voitsidis P., Gliatas I., Bairachtari V., et al. Insomnia during the COVID-19 pandemic in a Greek population. Psychiatr Res. 2020;289 doi: 10.1016/j.psychres.2020.113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlRasheed M.M., Fekih-Romdhane F., Jahrami H., et al. The prevalence and severity of insomnia symptoms during COVID-19: a global systematic review and individual participant data meta-analysis. Sleep Med. 2022;100:7–23. doi: 10.1016/j.sleep.2022.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.al Mamun F., Gozal D., Hosen I., Misti J.M., Mamun M.A. Predictive factors of insomnia during the COVID-19 pandemic in Bangladesh: a GIS-based nationwide distribution. Sleep Med. 2022;91:219–225. doi: 10.1016/j.sleep.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor D.J., Kelly K., Kohut M.L., Song K.S. Is insomnia a risk factor for decreased influenza vaccine response? Behav Sleep Med. 2017;15(4):270–287. doi: 10.1080/15402002.2015.1126596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S., Doyle W.J., Alper C.M., Janicki-Deverts D., Turner R.B. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169(1):62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besedovsky L., Lange T., Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin M.R. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- 26.Prather A.A., Janicki-Deverts D., Hall M.H., Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015;38(9):1353–1359. doi: 10.5665/sleep.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prather A.A., Janicki-Deverts D., Adler N.E., Hall M., Cohen S. Sleep habits and susceptibility to upper respiratory illness: the moderating role of subjective socioeconomic status. Ann Behav Med. 2017;51(1):137–146. doi: 10.1007/s12160-016-9835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention CDC museum COVID-19 timeline | david J. Sencer CDC museum | CDC. CDC. 2022. https://www.cdc.gov/museum/timeline/covid19.html
- 29.Morin C.M. Guilford Press; New York: 1993. Insomnia: psychological assessment and management. [DOI] [Google Scholar]
- 30.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 31.Bhat S., Chokroverty S. Sleep disorders and COVID-19. Sleep Med. 2022;91:253–261. doi: 10.1016/j.sleep.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NIH. NIH . In: State-of-the- science conference statement on manifestations and management of chronic insomnia in adults. Bethesda, editor. 2005. MD) [Google Scholar]
- 33.Ø Halsøy, Johnson S.U., Hoffart A., Ebrahimi O.V. Insomnia symptoms in the general population during the COVID-19 pandemic. Front Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.762799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner GG, Cludius B, Sckopke P, Stefan A, Schönbrodt F, Zygar-Hoffmann C. The predictive power of insomnia symptoms on other aspects of mental health during the COVID-19 pandemic: a longitudinal study. J Sleep Res. 2022:e13641. doi:10.1111/jsr.13641. [DOI] [PMC free article] [PubMed]
- 35.Perlis M.L., Vargas I., Ellis J.G., et al. The natural history of Insomnia: the incidence of acute insomnia and subsequent progression to chronic insomnia or recovery in good sleeper subjects. Sleep. 2020;43(6):1–8. doi: 10.1093/sleep/zsz299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonnet M.H., Arand D.L. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):97–108. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 37.De Zambotti M., Covassin N., De Min Tona G., Sarlo M., Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20(2):318–325. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- 38.Goebel M.U., Mills P.J., Irwin M.R., Ziegler M.G. Interleukin-6 and tumor necrosis factor-α production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med. 2000;62(4):591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Mills P.J., Von Känel R., Norman D., Natarajan L., Ziegler M.G., Dimsdale J.E. Inflammation and sleep in healthy individuals. Sleep. 2007;30(6):729–735. doi: 10.1093/sleep/30.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liukkonen T., Räsänen P., Ruokonen A., et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 birth cohort study. Psychosom Med. 2007;69(8):756–761. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 41.Ghilotti F., Bellocco R., Trolle Lagerros Y., et al. Relationship between sleep characteristics and markers of inflammation in Swedish women from the general population. J Sleep Res. 2021;30(2) doi: 10.1111/jsr.13093. [DOI] [PubMed] [Google Scholar]
- 42.Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatr. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prather A.A., Pressman S.D., Miller G.E., Cohen S. Temporal links between self-reported sleep and antibody responses to the influenza vaccine. Int J Behav Med. 2021;28(1):151–158. doi: 10.1007/s12529-020-09879-4. [DOI] [PubMed] [Google Scholar]
- 44.Taylor D.J.D.J., Lichstein K.L.K.L., Durrence H.H. Insomnia as a health risk factor. Behav Sleep Med. 2003;1(4):227–247. doi: 10.1207/S15402010BSM0104. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Mendoza J., Vgontzas A.N. Insomnia and its impact on physical and mental health. Curr Psychiatr Rep. 2013;15(12) doi: 10.1007/S11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyle S.D., Morgan K., Espie C.A. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69–82. doi: 10.1016/j.smrv.2009.07.004. [DOI] [PubMed] [Google Scholar]