Abstract
Using immunohistochemical staining, we examined the presence of secretory component (SC) on epithelial cells in gastric and duodenal biopsy specimens collected from Helicobacter pylori-infected individuals and healthy controls. Gastric epithelial cells from healthy volunteers expressed low, but detectable, levels of SC. In contrast, significantly higher level of expression of SC (P < 0.001) was observed on epithelial cells in the antra of H. pylori-infected individuals. The antral SC expression correlated with staining for gamma interferon of intraepithelial and lamina propria lymphocytes (rs = 0.76 and 0.69, respectively, P < 0.001) and correlated weakly with production of tumor necrosis factor alpha (rs = 0.43, P < 0.05), but it did not correlate at all with interleukin-4 production.
Infection with the noninvasive human pathogen Helicobacter pylori gives rise to active chronic gastritis as well as to substantial production of specific immunoglobulin A (IgA) that can be detected both in serum and in gastric aspirates (21, 23, 30, 31). Furthermore, we have recently demonstrated large numbers of H. pylori-specific IgA-secreting cells in the gastric mucosae of H. pylori-infected individuals (22). In spite of the local immune response to H. pylori, the bacteria are rarely cleared and the infection is usually lifelong. Nevertheless, several animal studies demonstrated that it is possible to eradicate an already established Helicobacter infection by means of oral, therapeutic immunization (4, 9), and in some immunization studies, protection has been correlated with mucosal IgA responses to the bacterium (18, 25). On the other hand, it is possible to protect antibody-deficient mice from H. pylori infection by similar mucosal immunizations (11).
In mucosal tissues, IgA molecules are predominantly produced as dimers, which are transported in endocytotic vesicles to the apical side of epithelial cells bound to secretory component (SC), also known in its uncleaved form as the polymeric immunoglobulin receptor (3). Subsequent proteolytic cleavage of SC results in the release of secretory IgA (S-IgA). Several cytokines have been shown to upregulate SC expression in vitro, i.e., gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin-4 (IL-4) (7, 16, 26). Conflicting results regarding the presence of SC in the healthy human stomach have been published (13, 15, 17, 28, 29). An association between gastritis and increased gastric SC expression has, however, been reported (13, 29), and H. pylori infection also seems to be associated with increased expression of SC by gastric epithelial cells (10, 15). The influence of different components in the H. pylori-induced inflammation on SC expression has, however, not been established. Therefore, we have examined the expression of SC in gastric antra and corpora from H. pylori-infected subjects, both duodenal ulcer (DU) patients and asymptomatic carriers, as well as from uninfected healthy individuals. In parallel, gastric lymphocyte and granulocyte infiltration, H. pylori cell density, and local cytokine production were assessed on the individual level.
Volunteers and specimens.
The study was approved by the Human Ethical Committee of the Medical Faculty, Göteborg, Sweden, and comprised 17 subjects infected with H. pylori, of whom 9 had DU disease (mean age, 52.7 years; five males and four females) and 8 were asymptomatic H. pylori carriers (mean age, 50.9 years; seven males and one female) who had been identified among healthy blood donors by using enzyme-linked immunosorbent assay (ELISA) (12). In addition, nine healthy, uninfected subjects (mean age, 39.8 years; three males and six females) with no gastrointestinal disorders or symptoms were recruited to participate in the study. The DU patients all had chronic relapsing DU disease confirmed by endoscopy but were in clinical remission at the time of the investigation. The asymptomatic and uninfected subjects had no history of gastrointestinal disease or any other relevant illness. None of the subjects were on any medication related to gastrointestinal symptoms at the time for the study, and no premedication was used before endoscopy except for local anesthesia.
Gastric aspirates were collected at endoscopy and were immediately put on ice and adjusted to pH 6 to 8; enzymatic degradation of immunoglobulins was prevented by addition of bovine serum albumin, phenylmethylsulfonyl fluoride, and soybean trypsin inhibitor (23). The aspirates were stored at −70°C until ELISA analysis. Furthermore, biopsy specimens were collected from the duodenal, antral, and corpus regions from each subject. One specimen from each site was immediately fixed in formalin and sent for routine histology at the Department of Pathology, Göteborg University, where the presence of H. pylori and acute and chronic inflammation were assessed blindly by an experienced pathologist according to the Sydney classification system and scored from 0 to 3 (none, mild, moderate, or severe) (8). Four antral biopsy specimens were immediately snap frozen in O.C.T. compound by using liquid nitrogen and stored at −70°C until they were stained for cytokine expression. Finally, fresh biopsy specimens from the antrum were homogenized and inoculated on Skirrow blood agar plates containing 10% horse blood, which were examined for the presence of H. pylori-like colonies after 3-day incubation under microaerobic conditions at 37°C. Only individuals who were positive by both culture and histology were regarded as infected with H. pylori, and only individuals who were negative by both culture and histology were included in the uninfected group.
SC expression.
Formalin-fixed mucosal biopsy specimens were paraffin embedded and subsequently stained for SC by the immunoperoxidase technique according to the manufacturer’s instructions, following trypsin digestion with 1 mg of Trypsin (Boehringer Mannheim) per ml at 37°C for 30 min. Endogenous peroxidase activity was blocked with 1% H2O2 and 0.02% NaN3 followed by incubation with 1% goat serum. Thereafter the sections were incubated with a preparation of polyclonal horseradish peroxidase (HRP)-labelled goat antibody to human SC (Nordic Immunological Laboratories, Tilburg, Holland) at 1 μg/ml at room temperature for 1 h. The sections were then incubated with diaminobenzidine substrate (Vector Laboratories, Inc., Burlingame, Calif.) for 15 min, rinsed in distilled water, counterstained with hematoxylin, dehydrated, and mounted with Mountex (Histolab, Göteborg, Sweden). The intensity of SC staining was scored on an arbitrary scale from 0 (negative) to 5 (very intense) and was evaluated blindly by two independent investigators, whose estimates correlated well (Pearson correlation coefficient [r] = 0.87).
A polyclonal HRP-labelled goat antibody raised to mouse IgG (Jackson Immuno Research Laboratories, Inc., West Grove, Pa.) diluted to 1 μg/ml was used as a negative control, and sections stained with this antibody were always completely negative. In addition, the specificity of the SC-reactive antibody preparation was examined by absorption with 25 μg of S-IgA purified from human colostrum (Sigma, St. Louis, Mo.) per ml at 4°C for 3 h. This treatment completely abolished SC staining of both duodenal and gastric specimens, while a parallel incubation with human serum IgA did not affect the staining intensity.
The ability of a single biopsy to represent the SC staining of the different mucosal compartments was determined before the start of the study. From each of three volunteers (two H. pylori-infected individuals and one uninfected individual), three specimens were collected from each of the duodenum, antrum, and corpus and stained as described above. With one exception, all three specimens collected from the same site in one individual were judged to have the same staining intensity when evaluated blindly. Only the duodenal specimens from one H. pylori-infected individual had different staining intensities, ranging from 2 to 4.
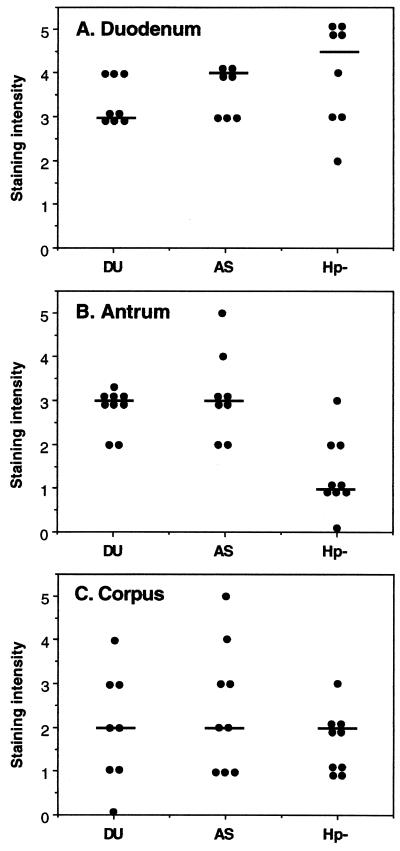
When the expression of SC in duodenal and gastric (antrum and corpus mucosa) biopsy specimens was evaluated, the duodenal epithelial cells were usually found to have a higher intensity of SC staining than the gastric epithelial cells, either from the antrum or the corpus (Fig. 1), from the same individual, and H. pylori infection did not seem to affect duodenal SC expression (Fig. 1A). The SC staining of antral sections was always more intense on epithelial cells in the neck region of the gastric glands than on the epitheliums at the surface or deeper in the glands (Fig. 2A). The same staining pattern, although not as pronounced, was seen also in corpus tissue, and has also been observed in previous studies of gastric inflammation (13, 29). Therefore, the staining intensity reported for gastric specimens is the value obtained in the neck region. In healthy individuals, the level of gastric expression of SC was much lower than the level seen in the duodenum (Fig. 1B and C). Nevertheless, SC was detected on epithelial cells in the antrum for all but one of the healthy volunteers (Fig. 1B), indicating that translocation of locally produced IgA and IgM across the gastric epithelium can occur in healthy individuals. When analyzing biopsy specimens from H. pylori-infected individuals, we found that the infection is associated with an increased expression of SC in the antrum region of the stomach compared to the expression in uninfected individuals (P < 0.001, Wilcoxon rank sum test; Fig. 1B), but increased expression was not observed in the corpus (P > 0.05; Fig. 1C). On the other hand, there was no difference in epithelial SC expression between asymptomatic H. pylori carriers and DU patients, suggesting that SC expression and IgA translocation are probably not important factors in determining the outcome of an H. pylori infection.
FIG. 1.
Expression of SC on epithelial cells in duodenal and gastric mucosae. The expression of SC on duodenal (A), antral (B), and corpus (C) epithelial cells was determined by using immunohistochemistry, and the staining intensity was graded from 0 to 5 (negative to very intense). Tissues from DU patients (DU), asymptomatic H. pylori carriers (AS), and H. pylori-negative healthy volunteers (Hp−) were examined. Each dot represents the individual value for one volunteer, and the horizontal bars denote the medians for individual groups. The staining intensity for H. pylori-infected individuals (DU and AS) was significantly different from that for uninfected individuals (P < 0.001) for the antrum but not (P > 0.05) for the duodenum and corpus.
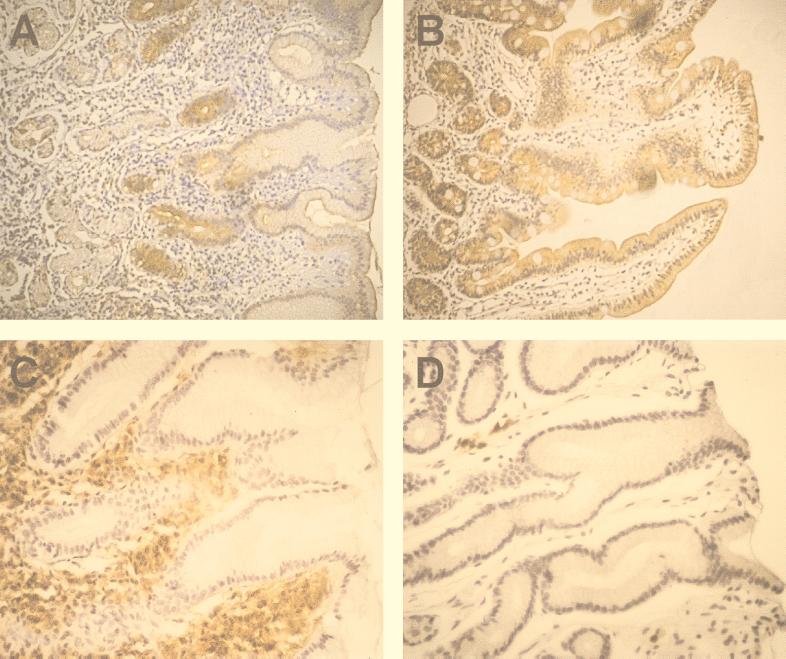
FIG. 2.
Immunohistochemical staining of SC and IgA in gastrointestinal tissues. (A and B), staining of SC in sections of the antrum (A) and duodenum (B) from an H. pylori-infected individual. (C and D), staining of IgA in sections of the antra from an H. pylori-infected individual (C) and an uninfected healthy individual (D). Original magnification, ×200.
IgA production.
Formalin-fixed antral biopsy specimens were stained for IgA, using an HRP-conjugated goat antibody to human IgA (Southern Biotechnology Inc., Birmingham, Ala.), according to the protocols described for SC. S-IgA content in gastric juice was determined by ELISA as previously described (23), using commercially available S-IgA (Sigma) as a standard. The immunohistochemical staining of IgA-positive cells demonstrated that H. pylori infection is accompanied by a large increase in the frequencies of IgA-positive cells in the antrum (Fig. 2C and D, Table 1). The actual frequencies of IgA-positive cells were difficult to determine, due to a strong staining of the entire lamina propria in specimens from some infected individuals, probably caused by extracellular IgA. The high frequencies of IgA-positive cells in H. pylori-infected volunteers are in accordance with previous studies by our group documenting substantially increased frequencies of IgA-secreting cells in the gastric mucosa of H. pylori-infected individuals (22), as well as studies by others showing increased amounts of IgA in gastric tissue homogenates from H. pylori-infected subjects (21, 31). Increased frequencies of IgA-positive cells have also been shown in earlier studies of inflamed gastric tissue (13, 29). Furthermore, total IgA production and H. pylori-specific IgA production in the gastric mucosa are very similar in asymptomatic carriers and DU patients (22), again arguing that local S-IgA production in response to natural infection does not seem to influence the outcome of H. pylori infection. It is surprising that the combined increase in gastric IgA production and SC expression did not result in increased levels of S-IgA in gastric aspirates of H. pylori-infected subjects compared to the levels in uninfected volunteers. This is probably caused by the relatively small number of volunteers in the study and by the fact that IgA concentrations were determined only at a single time point. In addition, reflux of duodenal contents to the stomach might conceal the differences in gastric S-IgA output between H. pylori-infected and uninfected subjects.
TABLE 1.
IgA in the stomachs of H. pylori-infected subjects (both DU patients and asymptomatic carriers) and uninfected healthy subjects
| Subjects | Frequency of IgA-positive cells [mean (range)a, P valueb] | Concn (μg/ml) of S-IgA in gastric juice [mean (range), P valueb] |
|---|---|---|
| H. pylori-infected | 2.9 (1–5) | 16 (0.27–162) |
| Uninfected | 1 (1–1), <0.001 | 5.9 (0.27–26), NS |
Frequency of IgA-positive cells graded on an arbitrary scale from 0 to 5.
H. pylori-infected subjects compared to uninfected individuals, evaluated by Wilcoxon rank sum test. NS, nonsignificant.
Gastric inflammation.
The grade of acute and chronic inflammation and presence of H. pylori in gastric biopsy specimens were evaluated according to the Sydney system (8) and scored from 0 to 3 (none, mild, moderate, or severe). The inflammation scores were significantly higher in the antrum than in the corpus for the H. pylori-infected individuals (P < 0.01, Wilcoxon signed rank test). The mean scores for chronic gastritis were 1.8 (range, 1 to 3) for the antrum and 1.2 (range, 0 to 2) for the corpus, and the mean scores for active gastritis were 1.5 (range, 0 to 3) for the antrum and 0.8 (range, 0 to 3) for the corpus. All but one of the uninfected subjects (who had grade 1 lymphocytic gastritis in the corpus) had normal mucosa without inflammation. The Helicobacter-like organism (HLO) density scores for both the antrum and the corpus varied between 0 and 3 for the specimens from infected subjects. The mean score for the antrum (2.5) was higher than that for the corpus (1.8), but the difference was not statistically significant. Expression of SC did not seem to be directly related to the density of H. pylori or inflammatory cells in either the antrum or the corpus. Thus, when the correlation of the SC score with each of HLO density and lymphocyte and granulocyte scores was evaluated, granulocyte infiltration and SC expression were found to correlate weakly for the corpus (rs = 0.54, P < 0.05) but no other analysis revealed any significant correlation. Furthermore, the observation that the inflammation in the corpus of the H. pylori-infected volunteers was not accompanied by an increase in SC expression compared to the expression in uninfected individuals suggests that the presence of inflammatory cells per se is not a crucial factor influencing the expression of SC. In accordance, it has been shown that SC expression on dysplastic epithelial cells is actually inversely related to the degree of inflammation in ulcerative colitis patients (27).
Antral cytokine production and SC expression.
Several in vitro studies have shown that SC expression by intestinal epithelial cell lines is upregulated by IFN-γ, IL-4, and TNF-α, either alone or in combinations (7, 16, 26). Since increased levels of IFN-γ and TNF-α are hallmarks of H. pylori-associated gastritis (5, 6, 14, 24), we examined if local cytokine production might influence SC expression.
Cryopreserved antral biopsy specimens were analyzed for the presence of cells containing IFN-γ, TNF-α, or IL-4 according to the protocol of Andersson et al. (1). Briefly, thin (8-μm) cryosections were fixed in 4% paraformaldehyde and permeabilized with 0.1% saponin (Sigma) before staining with cytokine-specific monoclonal antibodies (MAbs) (for IFN-γ clone DIK 1, Chromogenix, Mölndal, Sweden; for TNF-α, clone mAb-1, Pharmingen, San Diego, Calif.; for IL-4 clone 8F12, ImmunoKontakt, Bioggio, Switzerland), followed by the stepwise addition of a biotinylated goat antibody to mouse IgG1 (Caltag Laboratories, South San Francisco, Calif.), HRP-labelled avidin biotin complex (Vector), and diaminobenzidine substrate. The specificity of the assay was ascertained by parallel incubation with a control mouse IgG1 MAb. Cytokine expression detected by immunohistochemistry by to this procedure has previously been shown to correlate closely to in situ hybridization with cytokine mRNA (20). IFN-γ-, TNF-α-, and IL-4-containing cells were seen in the antral mucosae of most of the H. pylori-infected individuals. The individual variation in cytokine expression was large, but as a whole, H. pylori-infected individuals had a significantly greater expression of IFN-γ and TNF-α than healthy ones, whereas staining for IL-4 was similar for H. pylori-infected and healthy individuals (Table 2). These results have in part been reported previously (19), but analyses have now been extended. The correlation between SC expression and IFN-γ, TNF-α, or IL-4 staining of lamina propria and intraepithelial lymphocytes was evaluated by using Spearman’s rank correlation. SC expression correlated well with the frequencies of IFN-γ-positive lymphocytes, both intraepithelial cells and lamina propria cells (rs = 0.76 and 0.69, respectively, P < 0.001). The expression of TNF-α by lamina propria lymphocytes also correlated weakly with SC expression (rs = 0.43, P < 0.05). In contrast, IL-4 expression did not at all correlate with SC expression (rs = 0.10). It is also interesting that in our study, as well as in previous studies of gastric inflammation (13, 29), the most intense staining for SC in the stomach is found in the neck region of the gastric glands, which is actually the area where we detected the highest frequencies of IFN-γ-containing cells. These observations are the first in vivo correlates to the established capacity of IFN-γ to enhance SC expression in transformed epithelial cell lines. In analogy with the findings for the stomach, several intestinal inflammatory diseases, such as celiac disease and Crohn’s disease, which are strongly associated with increased IFN-γ production, also result in increased SC expression by enterocytes (2).
TABLE 2.
Frequencies of cytokine-expressing lymphocytes in the antra of H. pylori-infected subjects (both DU patients and asymptomatic carriers) and uninfected healthy subjects
| Subjects | Cytokine-expressing cells/mm2 of gastric mucosa, median (range), P valuea
|
|||||
|---|---|---|---|---|---|---|
| IFN-γ
|
TNF-α
|
IL-4
|
||||
| Intraepithelial lymphocytes | Lamina propria lymphocytes | Intraepithelial lymphocytes | Lamina propria lymphocytes | Intraepithelial lymphocytes | Lamina propria lymphocytes | |
| H. pylori infected | 0.8 (0–4.1) | 2.6 (0–26) | 0 (0–1.3) | 0.5 (0–7.1) | 0 (0–1.0) | 1.2 (0–27) |
| Uninfected | 0 (0–0.2), <0.001 | 0 (0–1.7), <0.01 | 0, NS | 0, <0.001 | 0 (0–0.6), NS | 1.3 (0–3.8), NS |
H. pylori-infected subjects compared to uninfected individuals, evaluated by Wilcoxon rank sum test. NS, nonsignificant.
In conclusion, our findings confirm that gastric epithelial cells have the potential to translocate locally produced IgA across the epithelium by SC-mediated transcytosis. We also show that this capacity is greatly enhanced following infection with H. pylori, possibly due to gastric cytokine production.
Acknowledgments
We are grateful to Ulf Andersson, Huddinge Hospital, Stockholm, for valuable help with establishing the cytokine staining technique. We thank all volunteers who participated in this study and the staff at the Gastroenterology unit at Sahlgrenska University Hospital.
This study was supported by a grant from Astra Research Center, Boston.
REFERENCES
- 1.Andersson J, Abrams J, Björk L, Funa K, Litton M, Ågren K, Andersson U. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994;83:16–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg P, Halstensen T S, Hiutfeldt H S, Krajci P, Kvale D, Scott H, Thrane P S. Epithelial expression of HLA, secretory component (poly-Ig-receptor), and adhesion molecules in the human alimentary tract. Ann N Y Acad Sci. 1992;664:157–179. doi: 10.1111/j.1749-6632.1992.tb39758.x. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 4.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Krahenbuhl J-P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree J E, Shallcross T M, Heatley R V, Wyatt J I. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473–1477. doi: 10.1136/gut.32.12.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 7.Denning G M. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases. J Immunol. 1996;156:4807–4814. [PubMed] [Google Scholar]
- 8.Dixon M F, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The upgraded Sydney system. International Workshop on the Histopathology of Gastritis. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Doidge P, Gust I, Lee A, Buck F, Hazell S, Manne U. Therapeutic immunization against Helicobacter infection. Lancet. 1994;343:914–915. doi: 10.1016/s0140-6736(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 10.El Kaissouni L, Bene M C, Faure G C. Activation of epithelial cells in gastritis. Digestion. 1998;59:53–59. doi: 10.1159/000007467. [DOI] [PubMed] [Google Scholar]
- 11.Ermak T H, Giannasca P J, Nichols R, Myers G A, Nedrud J, Weltzin R, Lee C K, Kleanthous H, Monath T P. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamlet A K, Erlandsson K I M, Olbe L, Svennerholm A-M, Backman V E M, Pettersson A B. A simple, rapid and highly reliable capsule-based 14C urea breath test for diagnosis of H. pylori infection. Scand J Gastroenterol. 1995;30:1058–1063. doi: 10.3109/00365529509101607. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson P. Immunoperoxidase study of the secretory immunoglobulin system and lysozyme in normal and diseased gastric mucosa. Gut. 1982;23:578–588. doi: 10.1136/gut.23.7.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karttunen R, Karttunen T, Ekre H-P, MacDonald T T. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–345. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazi J I, Sinniah R, Jaffrey N A, Alam S M, Zaman V, Zuberi S J, Kazi A M. Cellular and humoral immune responses in Campylobacter pylori-associated chronic gastritis. J Pathol. 1989;159:231–237. doi: 10.1002/path.1711590310. [DOI] [PubMed] [Google Scholar]
- 16.Kvale D, Brandtzaeg P, Lovhaug D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor alpha and gamma interferon. Scand J Immunol. 1988;28:351–357. doi: 10.1111/j.1365-3083.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai A Fat R F, McClelland D B, van Furth R. In vitro synthesis of immunoglobulins, secretory component, complement and lysozyme by human gastrointestinal tissues. I. Normal tissues. Clin Exp Immunol. 1976;23:9–19. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 19.Lindholm C, Quiding-Järbrink M, Lönroth H, Hamlet A, Svennerholm A-M. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litton M J, Sander B, Murphy E, O’Garra A, Abrams J S. Early expression of cytokines in lymph nodes after treatment in vivo with staphylococcus enterotoxin B. J Immunol Methods. 1994;175:47–58. doi: 10.1016/0022-1759(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 21.Luzza F, Imeneo M, Maletta M, Monteleone G, Doldo P, Biancone L, Pallone F. Isotypic analysis of specific antibody response in serum, saliva, gastric and rectal homogenates of Helicobacter pylori-infected patients. FEMS Immunol Med Microbiol. 1995;10:285–288. doi: 10.1111/j.1574-695X.1995.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 22.Mattsson A, Quiding-Järbrink M, Lönroth H, Hamlet A, Ahlstedt I, Svennerholm A-M. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect Immun. 1998;66:2705–2712. doi: 10.1128/iai.66.6.2705-2712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattsson A, Tinnert A, Hamlet A, Lönroth H, Bölin I, Svennerholm A-M. Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin Diagn Lab Immunol. 1998;5:288–293. doi: 10.1128/cdli.5.3.288-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss S F, Legon S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappo J, Thomas W R, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips J O, Everson M P, Moldoveanu Z, Lue C, Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by epithelial cells. J Immunol. 1990;145:1740–1744. [PubMed] [Google Scholar]
- 27.Rognum T O, Elgjo K, Fausa O, Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in ulcerative colitis with dysplasia. Gut. 1982;23:123–133. doi: 10.1136/gut.23.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsumi Y, Nagura H, Watanabe K. Immune aspects of intestinal metaplasia of the stomach: an immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1984;403:345–359. doi: 10.1007/BF00737285. [DOI] [PubMed] [Google Scholar]
- 29.Valnes K, Brandtzaeg P, Elgjo K, Stave R. Specific and non-specific humoral defence factors in the epithelium of normal and inflamed gastric mucosa. Gastroenterology. 1984;86:402–412. [PubMed] [Google Scholar]
- 30.Veenendaal R A, Götz J M, Schroijen V, Kurban F, Bernards A T, Veselic M, Pena A S, Lamers C B H W. Diagnosis of Helicobacter pylori infection by specific gastric mucosal IgA and IgG pylori antibodies. J Clin Pathol. 1995;48:990–993. doi: 10.1136/jcp.48.11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt J I, Rathbone B J. Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl. 1988;142:44–49. [PubMed] [Google Scholar]