Abstract
Efforts to understand molecular mechanisms of pathogenesis of the human-restricted pathogen Salmonella enterica serovar Typhi, the causative agent of typhoid fever, have been hampered by the lack of a tractable small animal model. This obstacle has been surmounted by a humanized mouse model in which genetically modified mice are engrafted with purified CD34+ stem cells from human umbilical cord blood, designated CD34+ Hu-NSG (formerly hu-SRC-SCID) mice. We have shown that these mice develop a lethal systemic infection with S. Typhi that is dependent on the presence of engrafted human hematopoietic cells. Immunological and pathological features of human typhoid are recapitulated in this model, which has been successfully employed for the identification of bacterial genetic determinants of S. Typhi virulence. Here we describe the methods used to infect CD34+ Hu-NSG mice with S. Typhi in humanized mice and to construct and analyze a transposon-directed insertion site sequencing S. Typhi library, and provide general considerations for the use of humanized mice for the study of a human-restricted pathogen.
Keywords: Humanized, Enteric fever, Typhoid, Pathogenesis, TraDIS
1. Introduction
Salmonella enterica serovar Typhi, the primary causative agent of enteric fever, is responsible for approximately 15 million infections and 200,000 deaths each year [1]. Efforts to understand the molecular mechanisms of S. Typhi virulence have relied extensively on the murine model of infection with the related serovar S. Typhimurium, a versatile pathogen capable of causing systemic infections in a variety of hosts including humans, mice, swine, chickens, and cattle [2]. In contrast, Salmonella Typhi selectively causes disease in humans, despite close genomic relatedness to S. Typhimurium [3]. Detailed comparisons have identified specific genetic differences between S. Typhi and S. Typhimurium and other non-typhoidal Salmonella serotypes [3–5]. However, the lack of a small animal model for S. Typhi has prevented the functional determination of genetic loci essential for S. Typhi virulence. Our laboratory has developed a model using mice carrying human hematopoietic cells, which are capable of sustaining a progressive systemic infection with S. Typhi. This has allowed the performance of high-throughput screening of transposon libraries to identify genetic factors important for S. Typhi virulence.
Humanized mice are constructed in immunodeficient genetic backgrounds, reducing the background presence of murine immune cells and allowing their replacement with engrafted human cells. A combination of the severe combined immunodeficiency (scid) mutation and nonobese diabetic (NOD) strain background results in the absence of murine T and B cells and reduced activity of NK cells [6–9]. Knockout of the IL-2 receptor common γ-chain impairs the murine cell response to IL-2, IL-4, IL-7, IL-9, and IL-15, leading to an absence of NK cells and superior human hematopoietic stem cell (HSC) engraftment [10, 11]. Although severely immunocompromised, NOD-scidIL2rɣnull (NSG) mice remain completely resistant to infection with S. Typhi in the absence of engrafted cells [12]. The NSG mouse is now widely used as a preferred background on which to engraft HSC and peripheral blood mononuclear cells (PBMC), as well as other normal or malignant human cell populations. Engraftment of immunodeficient mouse strains to generate CD34+Hu-NSG mice can be performed with human umbilical cord blood HSC, bone marrow, or mobilized HSC, and can also include transplantation of human fetal liver and thymus under the kidney capsule (BLT mice) [13]. Additional modifications to the NSG genetic background have been developed to improve engraftment and reduce graft-versus-host disease (GvHD) (see Jackson Laboratory website) [14].
In 2010, two laboratories reported the use of Rag2−/−IL2rɣ−/− immunodeficient mice to study S. Typhi infection. Song, et al. used Rag2−/−IL2rɣ−/− mice engrafted with human fetal liver hematopoietic stem and progenitor cells. These investigators observed dissemination of S. Typhi to the spleen and liver, with bacterial replication at those sites, but no clinical signs of infection or mortality. Analysis of cell populations in organs showed a depletion of human cells following infection, and pro-inflammatory serum cytokine levels were elevated [15]. Firoz Mian, et al. used Rag2−/−IL2rɣ−/− mice engrafted with CD34+-enriched HSC and observed evidence of meningitis and the spread of S. Typhi to liver, spleen, blood and bone marrow, but no mortality [16].
To date, the only lethal small animal model of S. Typhi infection remains the CD34+ Hu-NSG model, which uses NSG mice engrafted with CD34+ HSC derived from umbilical cord blood (Fig. 1a) [12, 17]. The presence of human hematopoietic cells in engrafted mice appears to be the key factor required to support progressive infection in vivo. In vitro studies comparing human and murine macrophages have suggested that S. Typhi is better able to replicate and survive in human macrophages [18–21]. Taken together, the in vitro and in vivo observations suggest that human macrophages are required for productive infection with S. Typhi.
Fig. 1.
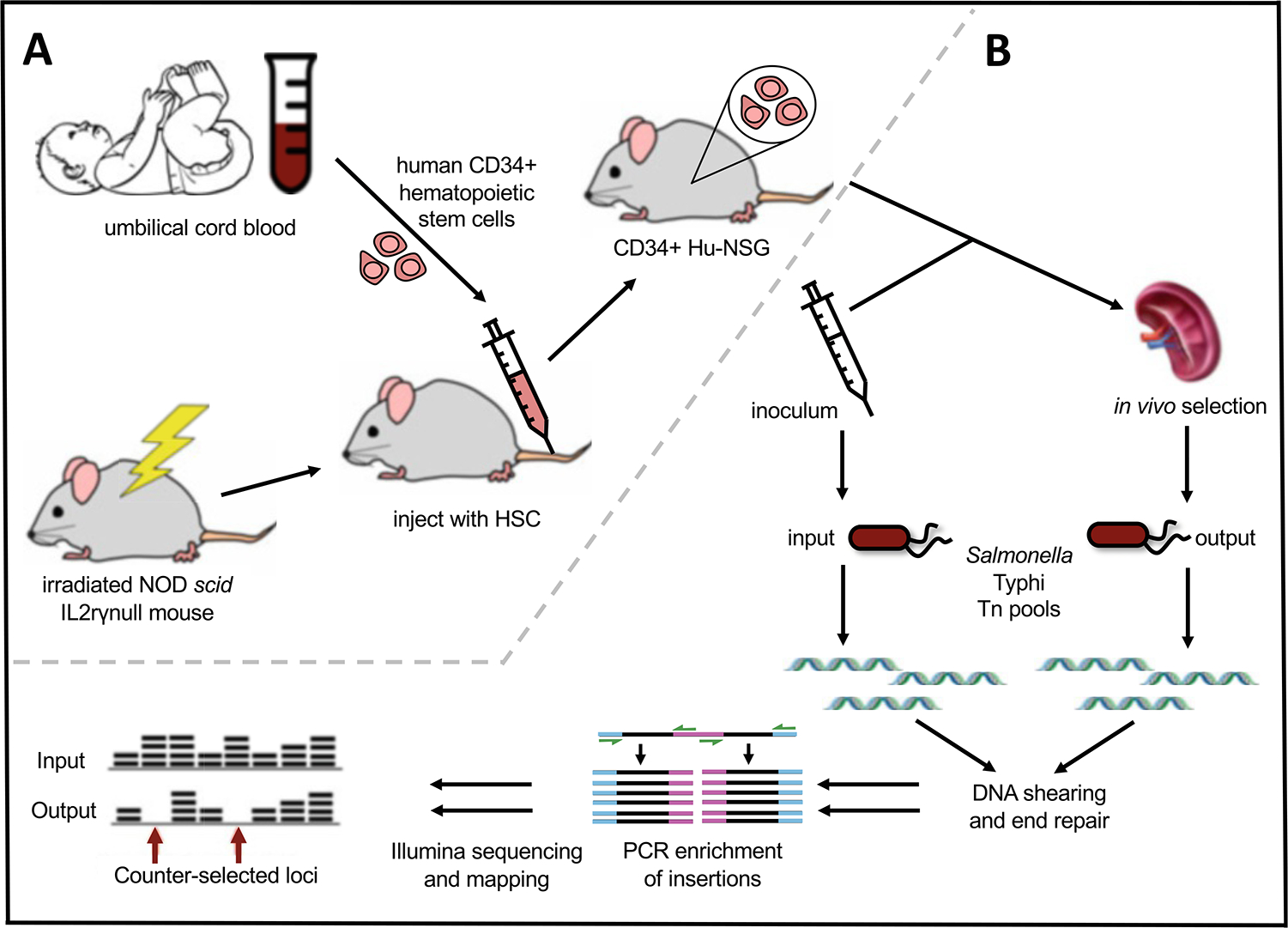
Construction of CD34+ Hu-NSG Humanized Mice and TraDIS to identify S. Typhi loci required for virulence. (a) NOD-scidIL2rɣnull mice are irradiated and injected with CD34+ human hematopoietic stem cells (HSC) derived from umbilical cord blood. Engraftment of mature human immune cells (CD45+) is confirmed in the peripheral blood 2 months after injection. (b) Humanized mice are infected with high-density S. Typhi transposon pools, and next-generation sequencing is used to compare input and output pools to identify counterselected mutants
The pathology observed in S. Typhi-infected CD34+ Hu-NSG mice resembles that of human typhoid, including hepatic Kupffer cell swelling and splenic granulomatous inflammation with multi-nucleated giant cells [12, 22, 23]. Human cytokines are elevated in serum of infected humanized mice [12]. Concentrations of human cytokines in this model may be readily measured using commercial assays.
The CD34+ Hu-NSG mouse does have important limitations. The residual presence of murine immune cells creates a chimeric immune environment that can be detrimental to the host, and CD34+ Hu-NSG mice eventually succumb to graft-versus-host disease [24]. In addition, older mice may exhibit increasing levels of T cell activation, which may be associated with reduced susceptibility to S. Typhi. Oral inoculation of S. Typhi does not result in productive systemic infection, possibly due to impaired survival of S. Typhi in the murine gut and the absence of mucosal lymphoid tissue. These mice do not appear to contain Peyer’s patches, which are the primary site of intestinal invasion following oral infection of Salmonella [17]. Gut mucosal lymphoid tissue (GALT) does not develop in CD34+ Hu-NSG mice due to the absence of the IL-2 receptor common γ-chain and whole-body irradiation required for engraftment, although newer models of NSG mice can be engrafted with human HSC in the absence of irradiation as pre-conditioning [25]. Subject-to-subject variation is observed due to variable engraftment levels and donor heterogeneity. Finally, the cost and labor required to produce CD34+ Hu-NSG mice limits experimental sample size. Despite these constraints, the CD34+ Hu-NSG model provides a unique opportunity to study the pathogenesis of S. Typhi infection.
2. Materials
2.1. Humanized Mice-Sources
When the CD34+ Hu-NSG murine model of Salmonella Typhi infection was initially reported, the only sources of these mice were the University of Massachusetts Medical School and the Jackson Laboratory [12]. Now, there are multiple companies that offer the CD34+ Hu- NOD-scidIL2rɣnull engrafted mice and NOD-scidIL2rɣnull-derivative human cell-engrafted mice. In addition, the Jackson Laboratory offers numerous NSG-mouse derivatives as engraftment backgrounds [14] (see Notes 1 and 2). Mice can now be ordered following engraftment with umbilical-cord CD34+ HSC or peripheral blood mononuclear cells from the same donor or from multiple donors. We have not investigated the role of HLA or blood groups on the growth of S. Typhi in CD34+ Hu-NSG mice, as all of our infections have been cohorts of engrafted mice from mixed donors.
2.2. Source of S. Typhi
The considerable cost of CD34+ Hu-NSG mice warrants careful consideration when choosing a strain of S. Typhi for virulence experiments. Many laboratories have a characterized strain of S. Typhi used for in vitro experimentation, such as invasion assays with epithelial cells or survival assays with phagocytic cells. For laboratories considering experimentation with humanized mice, there are several important considerations.
2.2.1. Characterized Clinical Isolates
It may be preferable to work with recent clinical S. Typhi isolates that are known to cause disease in humans. We selected the clinical isolate S. Typhi Ty2 for our studies. Laboratory-adapted strains of S. Typhi may have acquired mutations that could negatively impact infection in CD34+ Hu-NSG mice. S. Typhi Ty2 can be obtained from the American Type Culture Collection (ATCC), and the genomic sequence of this strain is available in public databases.
2.2.2. Presence of Vi Antigen
An important virulence characteristic of S. Typhi is production of the Vi capsular polysaccharide. Nearly all clinical isolates are Vi-antigen-producing as determined by an agglutination assay using antiserum to Vi-capsule, but this trait can be lost by serial passage. A simple slide agglutination test can be performed to confirm Vi expression (see below). The role of Vi-capsule in the biology and pathogenesis of S. Typhi infection in humans is well documented [26, 27], and a vaccine consisting of purified Vi-antigen is immunogenic and protective.
2.2.3. Multidrug Resistance Status
The antibiotic resistance profile of the S. Typhi strain to be utilized is an important consideration, as drug resistance may pose two problems. First, antibiotic resistance may complicate further genetic manipulation using common selectable markers. Second, a multiple drug-resistant (MDR) strain such as CT18 [28] complicates biosafety containment and laboratory safety. Genetic manipulation should not include antibiotic resistance markers that would prevent successful treatment in the event of a laboratory infection. When considering genetic manipulation, consult with the local Institutional Biosafety Committee or the Environmental Health and Safety offices for guidance.
2.3. Growth of S. Typhi
All solutions and media should be prepared using analytical-grade reagents.
Luria-Bertani (LB) broth and agar are available from most scientific suppliers. For consistency, it is advisable to purchase LB broth and LB agar as premade powders.
Aromix 100× is a solution of aromatic amino acids that facilitates the growth of S. Typhi in LB broth and on plates. Dissolve 40 mg mL−1 L-phenylalanine, 40 mg mL−1 L-tryptophan, 10 mg mL−1 2,3-dihydroxybenzoic acid, and 10 mg mL−1 p-amino benzoic acid in water, filter-sterilize, and store at 4 °C in a bottle wrapped in aluminum foil to protect from light; do not use if discoloration occurs. Add this mixture to LB broth and LB agar after autoclaving (see Note 3).
Dissolve antibiotic stocks in appropriate solvents and filter-sterilize when needed.
Glass culture tubes should normally be used for the cultivation of bacteria.
Vi antiserum (BD Difco, Cat# 228271).
2.4. Infection of CD34+ HU-NSG and NSG Mice
Sterile syringes with 25-gauge Luer-lock needles.
Sterile phosphate buffered saline (PBS), tissue culture grade. All solutions and chemicals used in mice must be USP grade or tissue culture grade. Exceptions require approval from the local IACUC or local animal use administrators.
2.5. Organ Harvest and Blood Collection
95% ethanol in wash bottle.
Surgical forceps and scissors, usually multiple sets. Curved scissors are advantageous.
12 × 75 mm sterile round-bottom tubes for dilutions of homogenized tissues (Falcon Cat# 352054).
Plastic round-bottom dilution tubes for homogenization of tissues.
Sterile PBS for homogenizing tissues and serial dilutions.
Homogenizer to homogenize tissues.
Wide-bore pipet tips.
Serum separation tubes (BD Microtainer Cat# 365967).
Goldenrod animal lancet 3 mm (Braintree Scientific Inc.).
2.6. Transposon Library Construction
0.45 μM nitrocellulose membrane filters.
Q Trays, 240 × 240 × 20 mm (Molecular Devices).
Dimethyl sulfoxide (DMSO).
Qiagen Blood and Tissue Kit (Qiagen Cat#69504).
Covaris microTUBE AFA Fiber Crimp-Cap 6 × 16 mm (Covaris Cat#520052).
TE buffer.
Sterile PCR tubes.
NEBNext End Repair Module (NEB Cat#E6050).
MinElute PCR Cleanup Kit (Qiagen Cat#28004,28006).
EB buffer.
- Solution of 9.5 mM dCTP and 0.5 mM ddCTP prepared as follows:
Final Stock 10 μL 20 μL 30 μL dCTP 9.5 mM 100 mM 0.95 1.9 2.85 ddCTP 0.5 mM 10 mM 0.5 1 1.5 H2O 8.55 17.1 25.65 Terminal Deoxynucleotidyl Transferase, Recombinant (Promega Cat#M1871).
Performa DTR Gel Filtration Cartridges (EdgeBio Cat#98780).
Qubit dsDNA BR Assay Kit (Invitrogen Cat#Q32850).
Qubit dsDNA HS Assay Kit (Invitrogen Cat#Q32851).
KAPA HiFi HotStart ReadyMix (Roche Cat#KK2620).
KAPA HiFi HotStart Library Amplification Kit (Roche Cat#KK2612).
SYBR Green I.
96-well round-bottom microtiter plate (Costar Cat#3795).
SPRIselect Reagent (Beckman Coulter Life Sciences Cat#B23318).
Agilent High Sensitivity DNA Kit (Agilent Cat#5067–4626).
85% ethanol.
Library Quantification Kit—Illumina/Universal (Roche Cat#KK4824).
3. Methods
3.1. Biosafety and Animal Husbandry
3.1.1. Humanized Mice
Mice containing human cells or tissues must be housed at ABSL2-level or higher containment according to the NIH Guide. Prior to ordering and working with these mice, it is important to consult with the veterinary care staff. Some suppliers of humanized mice recommend maintaining the mice on medicated water containing trimethoprim-sulfamethoxazole or Baytril (enrofloxacin). However, we have not found this to be necessary, as our mice are housed in autoclaved bedding and caging with irradiated chow and handled only in a biosafety cabinet using strict technique to minimize introduction of Pneumocystis murina and other pathogens. Additionally, the presence of antibiotics may confound infection experiments. If medicated water is used, mice should be on antibiotic-free water at least 7 days prior to infection. During the washout period, mice need to be housed in clean cages and changed every other day; mice are coprophagic and will ingest their antibiotic-containing feces, resulting in self-inoculation.
3.1.2. Salmonella enterica
S. Typhi is considered a risk group 2 (RG2) pathogen in most countries, and all in vitro and in vivo work must be performed with BSL2 and ABSL2 containment. Consultation with local biosafety or Environmental Health and Safety officers is important prior to beginning work with these agents. All standard BSL2 containment practices and procedures must be followed. Any aerosol-generating activities should be performed in a certified Class II biosafety cabinet. For best practices, growth of liquid cultures should be performed in a separate incubator. There should be a protocol in place for cleaning an accidental spill of an RG2 agent. Additional guidance can be found at http://www.cdc.gov and https://www.cdc.gov/labs/BMBL.html.
3.1.3. Laboratory Animal Husbandry Staff
Laboratory staff should be offered vaccination against S. Typhi. There are presently two widely available vaccines: the oral live attenuated Ty21a vaccine and an injectable Vi-antigen preparation. Consult medical professionals and local Occupational Health specialists about receiving these vaccines.
3.2. Vi Agglutination Assay
Before working with S. Typhi, the presence of Vi must be confirmed by agglutination assay. Use a proper negative control such as S. Typhimurium, an E. coli K12 strain, or a Vi mutant strain of S. Typhi (vexA or tviA mutation).
Add 10 μL of overnight culture of S. Typhi to glass slide.
Add 10 μL of Vi antiserum and mix.
Allow to sit at room temperature for 10–15 min. A positive agglutination will appear as a firm and granular clumping of the culture.
3.3. Determination of S. Typhi Inoculum for CD34+ Hu-NSG Infection
As the costs of CD34+ Hu-NSG mice are significant, taking the time to accurately and reproducibly prepare the infection inoculum is crucial (see Note 4). We have not attempted to inoculate humanized mice with more than 2 × 105 CFU of wild-type S. Typhi Ty2 (see Note 5). Dosing of other strains and other mouse suppliers should be determined empirically. The purpose of the following procedure is to accurately determine the number of viable bacteria grown in culture. We routinely perform a practice preparation three times on three separate days. All cultures are initiated using freshly prepared LB broth with 1× aromix. All cultures are started from freezer stocks and grown for 18 h (overnight) at 37 °C on a platform shaker.
Day 0
-
1
Start cultures of S. Typhi strains by inoculating 5 mL LB in a glass culture tube directly from freezer stock and incubate at 37 °C with shaking at 250 rpm for 18 h (overnight).
Day 1
-
2
Measure OD600 on a spectrophotometer, and adjust cultures to OD600 = 1.0 in sterile PBS as a means of normalizing all cultures.
-
3
Make appropriate serial dilutions in sterile PBS and plate on LB agar. Incubate overnight at 37 °C.
Days 2–3
-
4
Determine the colony forming units (CFU) of viable bacteria per mL of the culture adjusted to OD600 = 1.0. Use this calculation to determine the volume of OD600 = 1.0 that will be used for infection. Dilute bacteria in sterile PBS so that the injection volume per mouse is 0.5 mL. Although we typically use this volume, smaller volumes can be used.
-
5
Repeat steps 1–4 at least three times on three separate days. Although this might sound excessive, the cost of the mice warrants extra precautions to ensure reproducibility.
3.3.1. Determination of S. Typhi Inoculum for Competitive Infections
Competitive infections are used to compare the virulence of two strains, typically mutant and wild-type [29]. Strains to be compared must have distinctive antibiotic resistance profiles to allow individual enumeration. It is not recommended to exceed 2 × 105 CFU per mouse, or 1 × 105 CFU of each strain.
-
6
Complete steps 1–4 for each strain three times to determine the number of viable bacteria per mL at OD600 = 1.0.
-
7
Mix two strains in a 1:1 ratio (by number of viable bacteria), and plate serial dilutions on LB agar plates. Incubate overnight at 37 °C.
-
8
On the following day, pick 100 colonies and patch onto selective and nonselective LB agar plates to determine the actual ratio of the two strains. The Competitive Index (CI) is determined by dividing the output ratio (CFU mutant/CFU wild type) by the input ratio (CFU mutant/CFU wild type).
-
9
Adjust volumes if needed and repeat steps 7 and 8 three times on three separate days.
3.4. Infection of CD34+ Hu-NSG Mice or NSG Mice
Grow S. Typhi cultures exactly as done for the inoculum determination.
Dilute and plate the inoculum onto LB agar to determine the actual dose. Incubate plates overnight at 37 °C and count the following day.
Infect the mice by intraperitoneal injection (see Notes 6 and 7). There is no reason to save the inoculum for additional viability determination, as S. Typhi is viable in PBS for many hours.
Mice should be monitored according to the stipulations on the IACUC protocol. We monitor mice twice daily and more frequently if they appear ill (see Note 8). Consult with veterinary and animal care staff for protocols regarding after-hours health checks. Mice that are developing systemic infection will show the following symptoms: reduced body temperature, hunched body position, reduced nesting behavior, slow and unsteady movement, and frequently, a loose yellow rectal discharge. Mice that exhibit these characteristics are becoming moribund and will need to be euthanized, as they will not recover (see Notes 9 and 10). Development of symptoms can occur within 24–48 h of inoculation. The NSG nonengrafted mice should not develop any of these symptoms, even following administration of high doses of wild type S. Typhi, and should be included as controls.
3.5. Blood Collection
Humanized mice can be periodically bled to assay murine and human cytokine levels. The frequency and method of bleeding, and volume of blood removed, is dictated by the institution’s IACUC protocol. We use the submandibular puncture method to collect blood postinfection.
Use Goldenrod 3 mm lancet to pierce the mandibular vein.
Collect blood in BD Microtainer Serum Separator Tubes (SST), and allow to sit for approximately 5 min.
Centrifuge at 5000 rpm (2300 × g) for 5 min at room temperature using a microcentrifuge.
Remove serum to a clean microfuge tube and freeze at −80° until ready to analyze.
3.6. Organ Homogenization for CFU Determination
Tissue homogenization and plating of serial dilutions of the homogenate are required to determine the bacterial burden following infection (see Note 11). There are multiple methods to homogenize tissues. Our laboratory uses reusable homogenizers that can be cleaned and sterilized with gas or hydrogen peroxide methods that are used in a hospital central supply facility, to avoid autoclaving. Metal homogenizers can be used, but a method to clean and decontaminate between samples will have to be developed. Our laboratory uses the following homogenizers and handheld power unit: Fisher Brand 150 (#15–340-167), Omni homogenizers (#15–340-104), and an adapter for the homogenizer Fisher Brand (#15–340-11). All homogenization must be carried out in a Class II Biosafety Cabinet due to the generation of infectious aerosols.
Label and preweigh all 12 × 75 mm sterile collection tubes; the homogenizer fits these tubes. Label LB agar plates.
Note the ear notch pattern identification for each mouse or other identifying marks on the mouse. This information will be needed to correlate data to engraftment level for the particular animal.
After euthanasia, surface decontaminate the mouse with 95% ethanol. Flood the surface to remove debris.
Aseptically remove tissues from the mouse, and add to labeled and preweighed tubes. Make sure to keep the dissection instruments in beakers of 95% ethanol.
Weigh each tube with the organ to determine the CFU per gram of tissue.
Add 1 mL sterile PBS to tissue and homogenize (see Note 12).
Serially dilute homogenized tissue by tenfold dilutions. For the first one or two dilutions, use wide-bore pipette tips to facilitate pipetting.
Plate 100 μL of one dilution per LB agar plates in a range of dilutions to so that 30–300 colonies per plate can be counted accurately. Depending on the strain being used, antibiotics may be included in the agar plates.
Incubate plates overnight at 37 °C. Tissue homogenates can be stored on ice at 4 °C and rediluted and replated the following day, if colony counts are not possible (see Note 13). If there is contamination with other bacteria, replate the homogenates onto xylose lysine deoxycholate (XLD) agar, which is selective for Salmonella.
Count the number of colonies and calculate bacterial burden per organ.
3.7. Organ Preservation
A small section of each organ may be saved for histology by preservation in 4% neutral buffered formalin. Remove a section for histology prior to weighing the remainder of organ for CFU determination.
3.8. Genetic Manipulation of Salmonella enterica
Standard methods for the genetic manipulation of Gram-negative bacteria can be utilized for Salmonella Typhi. Lambda red-mediated site-specific recombination is a useful tool for disrupting chromosomal genes in this serotype [30]. Transformation is commonly performed by electroporation. Unfortunately, there are no generalized transducing phages for Salmonella Typhi, and each mutation must be made separately. We recommend the use of low- to medium-copy number plasmid vectors for expression and complementation studies. Useful plasmids include pSC101-based plasmids, including the pWSK series [31], pACYC184 series (p15A-based) [32], or the RK2-RP4-based plasmids (pRB3) [33]. Plasmids that are stable in the absence of selection should be used where possible for animal infections. ColE1-based replicons (e.g., pUC, pSK) are unstable in Salmonella and are not recommended.
3.9. Transposon Library Construction, Infection, and Analysis
Construction of a transposon mutant library in S. Typhi is possible using a Tn5-based transposon, and such a library has been screened in humanized mice using transposon-directed insertion site sequencing (TraDIS) to identify genes required for virulence (Fig. 1b) [34, 35] (see Note 14).
3.9.1. S. Typhi Transposon Library Construction
Transposon mutagenesis of S. Typhi is performed by conjugal mating of pLG100 containing transposable element T22 (ISlacZ-Tn2/FRT with selectable kanamycin marker) [36]. All matings and recoveries should be performed in a Class II Biosafety Cabinet.
Grow donor strain pLG100/Rho3 in LB broth with carbenicillin and 2,6-diaminopimelic acid (DAP) to OD600 ~ 1.0.
Mix donor strain with recipient strain S. Typhi grown in LB broth with 1× aromix to OD600 ~ 1.0 at a ratio of 0.1:1.
Spot onto a sterile nitrocellulose membrane filter seeded on an LB agar plate, and incubate at 37 °C for 1 h.
Perform eleven independent matings (steps 1–3).
Add filters to 1 mL LB broth with aromix and vortex, then pool together.
Plate pooled matings onto ten QTrays containing LB agar with aromix and kanamycin (no DAP); incubate at 37 °C for ~18 h (overnight).
The following day, harvest each plate with 15 mL of LB broth, then pool the samples.
Add DMSO to 10%, and freeze in 1 mL aliquots at −80 °C.
Thaw one library aliquot and plate serial dilutions on LB agar to determine the viable titer of the transposon library.
For library infection of humanized mice, use a freshly thawed library aliquot and calculated viability to prepare the inoculum. Infect humanized mice as described in Subheading 3.4.
3.9.2. Recovery and Archiving of “Input” and “Output” Samples
Add 0.5 mL of the thawed library aliquot used for the infection inoculum to a 125 mL Erlenmeyer flask containing 25 mL LB broth + 1× aromix + antibiotic selection.
Incubate at 37 °C for 18 h with shaking at 200 rpm.
Add DMSO to 10% and store aliquots at −80 °C (this constitutes the “Input” samples).
When mice have reached the appropriate infection time point, harvest organs as described in Subheading 3.6. After organ homogenization in sterile PBS, a portion of each organ can be plated for CFU determination.
Place the remainder of the homogenized organ in a 125 mL Erlenmeyer flask containing 25 mL LB broth + 1X aromix + antibiotic selection.
Incubate at 37 °C for 18 h with shaking at 200 rpm.
Filter outgrowths through a 70 μM tissue strainer, add DMSO to 10%, and store aliquots at −80 °C (this constitutes the “Output” samples).
3.9.3. Isolation of DNA for TraDIS
Prepare total DNA from both thawed “Input” and “Output” samples using the Qiagen Blood and Tissue Kit.
Finish DNA isolation with an additional ethanol precipitation at the end to concentrate DNA and remove impurities.
Resuspend DNA in 100 μL TE and quantify. A minimum of 1.5 μg of high-quality DNA is required for the TraDIS library construction.
3.9.4. DNA Shearing
For each sample, add 1–1.5 μg total DNA to a Covaris tube, filling the tube to 130 μL total volume with additional TE buffer.
Shear to a fragment size of ~300 bp using a Covaris LE220 Ultrasonicator (Covaris, Woburn, MA) with rack PN500282, duty factor 30%, (W) 450 and 200 cycles 200 for 60 s.
3.9.5. End Repair
- For each sample, prepare the following end-repair reaction. Keep all reagents on ice.
Sheared DNA 130 μL 10× end-repair buffer 15.5 μL End-repair enzyme mix 7.5 μL Sterile water (adjust as needed) 2 μL Total reaction volume 155 μL Incubate at 20 °C for 30 min in a thermocycler, dividing each reaction into two tubes of 77.5 μL each.
Purify DNA using MinElute PCR Cleanup Kit. Elute from each column with 2 × 10 μL EB buffer, for a total elution volume of 20 μL.
Optional: quantify DNA by fluorometry.
3.9.6. C-Tailing
-
For each sample, prepare the following C-Tailing Reaction. Keep all reagents on ice.
End-repaired DNA 18.6 μL Fresh solution of 9.5 mM dCTP and 0.5 mM ddCTP 2.8 μL 5× TdT reaction buffer 5.6 μL Terminal transferase enzyme 1.0 μL Total reaction volume 28 μL As a negative control, include a sample with no TdT enzyme.
Incubate at 37 °C for 60 min, then at 75 °C for 20 min.
Purify the DNA using Performa DTR gel filtration cartridges. Centrifuge the cartridges for 2 min at 800 × g, then store at −20 °C.
Optional: assay 2 μL of each sample by Qubit dsDNA BR Assay Kit.
3.9.7. PCR1
-
For each sample, prepare the following PCR reaction. Keep all reagents on ice.
EDGE-purified C-tailed DNA, ~150 ng 7.4 μL 2× KAPA HiFI HotStart ReadyMix 25 μL 10 μM primer olj376 (Ultramer) 3 μL 10 μM primer T22-87_Left 1 μL 100× SYBR green 0.25 μL PCR-grade sterile water 13.35 μL Total reaction volume 50 μL As a negative control, include the end-repaired and C-tailed sample with no TdT enzyme (there should be no amplification).
Run the PCR in a thermocycler under the following conditions: 95 °C for 2 min, 24× (98 °C for 30 s, 64 °C for 30 s, 72 °C for 1.5 min, read), 72 °C for 2 min, then hold at 10 °C. The inflection point should be approximately 24 cycles with 150 ng of sample.
3.9.8. PCR2
-
For each sample, prepare the following PCR reaction. Keep all reagents on ice.
PCR1 product 1.2 μL 2× KAPA HiFI HotStart ReadyMix 25 μL 10 μM primer T22_PAIR_AmpF_Left 3 μL 10 μM primer TdT_Index_XX 3 μL 100× SYBR green 0.25 μL PCR-grade sterile water 17.55 μL Total reaction volume 50 μL See Table 1 for primer sequences for T22 insertion mutant pool specific for left end.
-
Run the PCR in a thermocycler under the following conditions: 95 °C for 2 min, 25–30×* (98 °C for 30 s, 64 °C for 30 s, 72 °C for 1.5 min, read), 72 °C for 2 min, then hold at 10 °C.
*Run PCR2 for 25–30 cycles to determine the inflection point of each sample.
3.9.9. PCR for Sequencing
Repeat run of PCR2, stopping each reaction near the inflection point determined in the initial PCR2 run.
3.9.10. SPRI Size Selection
Use SPRI beads for 0.8–0.61 size selection and a range of 230–660 base-pairs (this method selects first the right side of the bp range, then the left).
Set up the 96-well microtiter plate: Add samples and the appropriate amount of SPRI beads (95 μL sample volume * 0.61× = 58 μL SPRI beads). Mix ten times and incubate for 1 min at room temperature.
Place on an Agencourt SPRIPlate 96R ring super magnet plate (Beckman Coulter) for 2 min.
Remove 145 μL supernatant to a new well while on the magnet, then remove from magnet.
Add appropriate amount of beads to the supernatant (145 μL sample volume * (0.8–0.61×) = 27.6 μL SPRI beads). Mix ten times and incubate 1 min at room temperature.
Place on the magnet for 2 min.
Discard the supernatant on the magnet, then add 180 μL fresh 85% ethanol and incubate for 30 s on the magnet at room temperature.
Discard ethanol and air-dry for 2 min on the magnet at room temperature.
Transfer supernatant to a new microfuge tube. Store at −20 °C.
3.9.11. Quantification
Perform quantification using an Agilent Bioanalyzer 2100 (1 μL) (size ~250–600 bp, average size ~400 bp, ~15 nM).
- Use the qPCR KAPA Library Quantification Kit for Illumina platforms.
- Run both 1:5000 and 1:10,000 dilutions in triplicate with the Illumina primers included in the kit.
- Run a 1:5000 dilution in triplicate with custom primers “T22_custom_1stRead_SEQ_Left” and “P7c’“ to determine the amount of transposon-specific products in the library.
- Use the Data Analysis Template from KAPA Biosystems to determine library quantitation.
- For multiplexing, use genomics facility’s specifications for concentration as determined by Agilent and proper dilution for Qubit dsDNA HS Assay.
- Pool to a final concentration of 2 nM of each library.
- Assay 3 μL of pooled library by Qubit dsDNA HS Assay Kit.
- Optional: perform qPCR KAPA Library Quantification (use dilutions at 1:500 and 1:1000 for Illumina primers and 1:500 for Tn-specific primers).
3.9.12. Next-Generation Sequencing
- Submit samples to genomics facility.
- ~30 μL 2 nM pooled library.
- ~20 μL 100 μM HPLC-purified custom primer.
- Platform: HiSeq Rapid Run (65 °C) (2 lanes if multiplexing 24 libraries).
- 50 SR.
- 15 pM final library concentration, with a 6% spike-in of a 12.5 pM hiX library: redenature by heating to 95 °C for 5 min, then plunge into ice bath.
4. Notes
NSG mice, whether nonengrafted or engrafted, are similarly sensitive to S. Typhimurium as C57Bl/6 or BALB/c mice.
Refer to the Jackson Laboratory website for information on NSG mouse backgrounds and engraftment options: https://www.jax.org/jax-mice-and-services/in-vivo-pharmacology/humanized-mice.
It is possible to grow S. Typhi in other rich media. However, we have not characterized growth and virulence of S. Typhi in other media besides LB.
Practicing growth of the Salmonella inoculum culture is essential to ensure the exact dose, and three separate growth experiments are recommended.
The dose of S. Typhi may have to be determined empirically depending on the source of humanized mice and source of donor used. We do not recommend exceeding 2 × 105 total wild-type S. Typhi per CD34+ Hu-NSG mouse.
Humanized mice do not support oral infection with S. Typhi, as the poor survival of S. Typhi in the murine gut and the absence of normal GALT, including Peyer’s patches, does not allow Salmonella to invade the intestinal epithelium. Thus, intraperitoneal or intravenous infection is recommended.
Mice should be infected within 5 days of receipt. Engraftment eventually leads to GvHD, causing thick and tough skin, and a lack of grooming. This can be accompanied by anemia, evidenced by pale footpads. Prompt infection will help to avoid the confounding effects of GvHD.
It is advised to infect mice earlier in the day so that they can be monitored in the hours immediately following infection. Mice should be monitored at regular intervals for signs of infection, including weight loss, dehydration (skin tenting), hunched appearance, slow movement, and lack of nesting behavior. Mice sacrificed when moribund will achieve the best results in terms of organ analysis, while mice that succumb to infection prior to organ harvest will likely yield unreliable results.
Be prepared with endpoint materials for euthanasia of mice at different times. Mice may exhibit different sensitivity to S. Typhi due to variation in engraftment and donor background.
CD34+ Hu-NSG mice infected with 4 × 105 S. Typhi transposon library experienced acute infections, showing signs of infection within 24–36 h. Infected humanized mice had >4 × 105 CFU in livers and spleens at these timepoints, indicating growth of S. Typhi in vivo. Some mice never showed signs of infection, and subsequent analysis of organism burden showed <4 × 105 CFU. Lack of infection in some mice may reflect inconsistency in engraftment levels and donor variability. Output library analysis from mice without progressive infections showed indiscriminate counterselection of genes, and analysis of these genes did not pass the false discovery rate calculations [34]. For this reason, we generally assay library outputs at 24 h postinfection.
Organs in which S. Typhi can be found include the liver, spleen and gall bladder, as well as blood. Liver and spleen are commonly used for determination of organism burden. Infected spleens tend to be smaller than in uninfected mice, while infected livers become fragile. Both may show visible infection lesions, but absence of these lesions does not necessarily indicate lack of infection.
We have successfully reused tissue homogenizers for over 5 years using gas sterilization rather than autoclaving, which will eventually cause them to break down.
It is important to save tissue homogenates overnight, packed on ice, in case replating is needed.
Sufficient density of transposon insertion within the genome is important to ensure that all genes contain multiple insertions. Essential genes will not be represented in the library, and inactivation of genes that confer a severe growth defect will be poorly represented. We opted for 50× coverage of the S. Typhi genome when constructing our library, using conjugation of a Tn5-derivative transposon [34]. Because S. Typhi has ~4000 genes, we aimed for the conjugation to result in 200,000 individual colonies to achieve 50× coverage. CD34+ HU-NSG mice were infected with 400,000 bacteria for full library coverage. The use of a sufficiently high-complexity library and infecting mice with enough bacteria to encompass the full library helps to compensate for bottleneck effects [35].
Table 1.
Primer Sequences
| Primer name | Sequence 5’- 3’ |
|---|---|
| T22—87 Left (pLG100) | ATCCCCCTAGGGCGCGCCGAAGT |
| T20-87_Left (pLG66a or pJK804) | GGATCCCTAGGGCGCGCCGAAGT |
| olj376 | GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTGGGGGGGGGGGGGGGG |
| T22_PAIR_AmpF_LEFT | AATGATACGGCGACCACCGAGATCTACACTAGAGAATAGGAACTTCGGAATAGGAACTTCTTAGATGTGTATAAGAG |
| TdT_Index_01_ATCACG | CAAGCAGAAGACGGCATACGAGAT CGTGAT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_02_CGATGT | CAAGCAGAAGACGGCATACGAGAT ACATCG GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_03_TTAGGC | CAAGCAGAAGACGGCATACGAGAT GCCTAA GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_04_TGACCA | CAAGCAGAAGACGGCATACGAGAT TGGTCA GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_05_ACAGTG | CAAGCAGAAGACGGCATACGAGAT CACTGT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_06_GCCAAT | CAAGCAGAAGACGGCATACGAGAT ATTGGC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_07_CAGATC | CAAGCAGAAGACGGCATACGAGAT GATCTG GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_08_ACTTGA | CAAGCAGAAGACGGCATACGAGAT TCAAGT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_09_GATCAG | CAAGCAGAAGACGGCATACGAGAT CTGATC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_10_TAGCTT | CAAGCAGAAGACGGCATACGAGAT AAGCTA GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_11_GGCTAC | CAAGCAGAAGACGGCATACGAGAT GTAGCC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_12_CTTGTA | CAAGCAGAAGACGGCATACGAGAT TACAAG GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_13_AGTCAA | CAAGCAGAAGACGGCATACGAGAT TTGACT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_14_AGTTCC | CAAGCAGAAGACGGCATACGAGAT GGAACT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_15_ATGTCA | CAAGCAGAAGACGGCATACGAGAT TGACAT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_16_CCGTCC | CAAGCAGAAGACGGCATACGAGAT GGACGG GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_18_GTCCGC | CAAGCAGAAGACGGCATACGAGAT GCGGAC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_19_GTGAAA | CAAGCAGAAGACGGCATACGAGAT TTTCAC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_20_GTGGCC | CAAGCAGAAGACGGCATACGAGAT GGCCAC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_21_GTTTCG | CAAGCAGAAGACGGCATACGAGAT CGAAAC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_22_CGTACG | CAAGCAGAAGACGGCATACGAGAT CGTACG GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_23_GAGTGG | CAAGCAGAAGACGGCATACGAGAT CCACTC GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_25_ACTGAT | CAAGCAGAAGACGGCATACGAGAT ATCAGT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| TdT_Index_27_ATTCCT | CAAGCAGAAGACGGCATACGAGAT AGGAAT GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT |
| T22_custom_1stRead_SEQ_Left | CCGAGATCTACACTAGAGAATAGGAACTTCGGAATAGGAACTTCTTAGATGTGTATAAGAG |
| P7c’ | CAAGCAGAAGACGGCATACGAGAT |
Note: When designing/choosing a set of index primers for multiplexing, red-channel and green-channel diversity requirements must be considered for the barcode bases (see Illumina documentation)
All primers were ordered from IDT as ultramers, except T22_custom_1stRead_SEQ_Left, which was HPLC purified
Acknowledgments
This work was supported by NIH grants AI112640 (F.C.F.), AI132963 (M.A.B. and L.D.S.), OD018259 (L.D.S.), and CA034196 (L.D.S.).
References
- 1.Duff N, Steele AD, Garrett D (2020) Global action for local impact: the 11th international conference on typhoid and other invasive Salmonelloses. Clin Infect Dis 71:S59–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos RL, Zhang S, Tsolis RM et al. (2001) Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect 3:1335–1344 [DOI] [PubMed] [Google Scholar]
- 3.Johnson R, Ravenhall M, Pickard D et al. (2018) Comparison of Salmonella enterica serovars Typhi and Typhimurium reveals typhoidal serovar-specific responses to bile. Infect Immun 86:e00490–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabbagh SC, Lepage C, McClelland M et al. (2012) Selection of Salmonella enterica serovar Typhi genes involved during interaction with human macrophages by screening of a transposon mutant library. PLoS One 7:e36643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gal-Mor O, Boyle EC, Grassl GA (2014) Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosma MJ, Carroll AM (1991) The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol 9:323–350 [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P, Iacomini J, Johnson RS et al. (2018) RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68(5):869–877. https://pubmed.ncbi.nlm.nih.gov/1547488/ [DOI] [PubMed] [Google Scholar]
- 8.Shinkai Y, Rathburn G, Lam KP et al. (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68(5):855–867. https://pubmed.ncbi.nlm.nih.gov/1547487/ [DOI] [PubMed] [Google Scholar]
- 9.van der Loo JC, Hanenberg H, Cooper RJ et al. (1998) Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse as a model system to study the engraftment and mobilization of human peripheral blood stem cells. Blood 92:2556–2570 [PubMed] [Google Scholar]
- 10.Ito M, Hiramatsu H, Kobayashi K et al. (2002) NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182 [DOI] [PubMed] [Google Scholar]
- 11.Brehm MA, Cuthbert A, Yang C et al. (2010) Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol 135:84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby SJ, Brehm MA, Greiner DL et al. (2010) Humanized nonobese diabetic-scid IL2rγnull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci U S A 107:15589–15594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong KSM, Her Z, Chen Q (2018) Humanized mice as unique tools for human-specific studies. Arch Immunol Ther Exp 66:245–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shultz LD, Keck J, Burzenski L et al. (2019) Humanized mouse models of immunological diseases and precision medicine. Mamm Genome 30:123–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J, Willinger T, Rongvaux A et al. (2010) A mouse model for the human pathogen Salmonella Typhi. Cell Host Microbe 8:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firoz Mian M, Pek EA, Chenoweth MJ et al. (2011) Humanized mice are susceptible to Salmonella Typhi infection. Cell Mol Immunol 8:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson T, Greiner DL, Shultz LD (2008) Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. Chapter 15:Unit 15.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vladoianu IR, Chang HR, Pechère JC (1990) Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb Pathog 8:83–90 [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi Y, Arai T (1995) Salmonella typhi does not inhibit phagosome-lysosome fusion in human monocyte-derived macrophages. FEMS Immunol Med Microbiol 12:55–61 [DOI] [PubMed] [Google Scholar]
- 20.Schwan WR, Huang XZ, Hu L et al. (2000) Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun 68:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascopella L, Raupach B, Ghori N et al. (1995) Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect Immun 63:4329–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallory FA (1898) Histological study of typhoid fever. J Exp Med 3(6):611–638. https://rupress.org/jem/article/3/6/611/7544/A-HISTOLOGICAL-STUDY-OF-TYPHOID-FEVER [PMC free article] [PubMed] [Google Scholar]
- 23.Bharadwaj S, Anim JT, Ebrahim F et al. (2009) Granulomatous inflammatory response in a case of typhoid fever. Med Princ Pract 18:239–241 [DOI] [PubMed] [Google Scholar]
- 24.Shultz LD, Brehm MA, Garcia JV et al. (2012) Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 12:786–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh BE, Brown ME, Duffin BM et al. (2015) Nonirradiated NOD,B6.SCID Il2rγ−/− KitW41/W41 (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Rep 4:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins JD, Robbins JB (1984) Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis 150:436–449 [DOI] [PubMed] [Google Scholar]
- 27.Looney RJ, Steigbigel RT (1986) Role of the Vi antigen of Salmonella typhi in resistance to host defense in vitro. J Lab Clin Med 108:506–516 [PubMed] [Google Scholar]
- 28.Parkhill J, Dougan G, James KD et al. (2001) Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852 [DOI] [PubMed] [Google Scholar]
- 29.Richardson AR, Payne EC, Younger N et al. (2011) Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar Typhimurium. Cell Host Microbe 10:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang RF, Kushner SR (1991) Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195–199 [PubMed] [Google Scholar]
- 32.Chang AC, Cohen SN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134:1141–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berggren RE, Wunderlich A, Ziegler E et al. (1995) HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol 10:489–495 [PubMed] [Google Scholar]
- 34.Karlinsey JE, Stepien TA, Mayho M et al. (2019) Genome-wide analysis of Salmonella enterica serovar Typhi in humanized mice reveals key virulence features. Cell Host Microbe 26:426–434.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cain AK, Barquist L, Goodman AL et al. (2020) A decade of advances in transposon-insertion sequencing. Nat Rev Genet 21:526–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher L, Turner C, Ramage E et al. (2007) Creating recombination-activated genes and sequence-defined mutant libraries using transposons. Meth Enzymol 421:126–140 [DOI] [PubMed] [Google Scholar]


