Abstract
Low vertebral bone mass is a major risk factor for vertebral compression fractures. While sarcopenia has been shown to be associated with low bone mineral density (BMD), it is not known whether trunk musculature is directly associated with lumbar BMD, and whether exercise modifies this association. Using data from the Multi-Ethnic Study of Atherosclerosis (MESA), we sought to determine the association of muscle density and fat fraction of the psoas, paraspinal, and oblique muscle groups with L3 lumbar volumetric BMD, and whether these associations were modified by exercise. We obtained L3 vBMD measurements, and fat and muscle measurements (in Hounsfield units (HU)) from abdominal CT scans spanning L2-L4 intervertebral disc spaces. Muscle density was defined as the mean HU value for a muscle group area. Fat fraction was calculated as the mean HU value for the muscle group fat area/total muscle group area(sq cm). Exercise data were self-reported (MET-min/wk). We utilized multivariable linear regression to evaluate these associations, stratified by gender, and adjusting for demographics, BMI, smoking status, impaired fasting glucose, and corticosteroid and anti-resorptive medication use. Among 1923 MESA participants, mean age was 62±10, 49% were female, 40% White, 21% Black, 26% Hispanic/Latino and 13% Chinese. In fully adjusted analysis, for every 1 SD higher psoas fat fraction, there was a 3.19 SD lower L3 vBMD in men and 4.3 SD lower SD L3 vBMD in women (p<0.001). For every 1 SD higher psoas density, there was a 0.2 SD higher L3 vBMD (p<0.001) in men and 0.19 SD higher L3 vBMD (p<0.001) in women. Findings were similar for paraspinal and oblique muscles. Intentional exercise did not modify these associations. In men and women, trunk muscle density was positively associated with higher lumbar BMD, suggesting a local association. Future studies are warranted to determine the temporality of this association.
Keywords: Epidemiology, lumbar spine, bone mineral density, muscle density, muscle fat fraction
Introduction
Among US adults over the age of 65, vertebral compression fractures are associated with a two-fold higher risk of mortality.1 Vertebral compression fractures result in stooped posture, difficulty walking, and on average, two weeks of bed rest and one month of restricted activity following a fracture episode.2 A powerful predictor of risk for new vertebral compression fractures is low vertebral bone mass, which has been linked to older age, metabolic syndrome, inflammatory disease, low body mass index (BMI), poor diet, and lack of mechanical loading.3–5 Thus, uncovering predictors of vertebral bone mass is important for fracture prevention.
Mechanical loading of bone by muscle contraction increases bone mineral density (BMD), as part of the “mechanostat” hypothesis, and muscle-derived endocrine and paracrine signals additionally play an anabolic role on skeletal tissue.3–6 While this physiology is recognized, few studies have investigated the association between BMD and muscle quality at the regional level. In the Health, Aging, and Body Composition Study, Lang and colleagues showed that a one standard deviation lower density of muscle at the mid-femur was associated with a 50% higher risk of hip fracture.7 Although lower muscle density was associated with higher fracture risk at the femur, the authors did not assess the association between muscle fat fraction and femoral BMD, and whether this higher fracture risk could have been due to weakness-related gait disturbance.
As decreased mechanical loading is associated with lower muscle mass, it is not surprising that low bone mass has also been linked with low muscle mass. Miyakoshi and colleagues showed that among middle to elderly aged women with osteoporosis, 20.4% had concurrent sarcopenia, as defined by a relative skeletal muscle index.8 Trunk musculature in particular, has been shown to decline with age. Prior osteoporotic spinal compression fractures have been associated with changes in lumbar paraspinal muscle, where paraspinal cross-sectional area was less, and intramuscular fat infiltration was greater among those with spinal compression fractures compared those without.9 Further, higher muscle density has been shown to be indicative of less postural sway, suggesting a role in functional capacity;10, 11 yet muscle density declines with age, especially among women.12
Exercise has been shown to independently improve both bone mineral density and muscle area and density. Michel and colleagues showed in subjects over age 50, weight-bearing exercise was associated with an increase in lumbar bone density for exercise routines of up to 270 min/wk in women and up to 400 min/wk in men.13 Similarly for muscle, Sipila et al. showed that quadriceps muscle cross-sectional area (CSA) and lean tissue CSA were increased, while relative proportion of fat was decreased in elderly women enaging in strength training.14
Prior studies linking osteoporosis and sarcopenia have not evaluated local associations between muscle density, specifically the psoas, paraspinal, and oblique muscles at the level of the 3rd lumbar vertebra with BMD. Thus, we assessed whether these muscle groups were associated with BMD at L3; and further evaluated whether physical activity modified this association. We hypothesized that individuals with higher physical activity would have a stronger association between muscle density and lumbar BMD.
Materials and Methods
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-center prospective study designed to evaluate subclinical and clinical cardiovascular disease in community-dwelling individuals. MESA consists of 6,814 asymptomatic men and women aged 45–84 at baseline seen across six exams starting in April 2000 at six field centers in the United States [Columbia University, New York; Johns Hopkins University, Baltimore; Northwestern University, Chicago; UCLA, Los Angeles; University of Minnesota, Twin Cities; and Wake Forest University, Winston Salem].
The MESA cohort comprised 38% White, 28% Black, 22% Hispanic/Latino, and 12% Chinese Americans at baseline. Data collected during the clinical exams include imaging, laboratory biomarker measurement, questionnaires, sociodemographic factors, lifestyle factors, genetic information, and muscle and bone composition. Details regarding the MESA study design, recruitment and data collection methods have been previously published.15
Study Population
Of the 6,814 total MESA participants, 1,968 individuals from five field centers were randomly selected to undergo abdominal computed tomography (CT) scans for the purposes of determining the presence and extent of abdominal aortic calcification. Among this sub cohort, 1,944 participated in the MESA Abdominal Body Composition ancillary study to evaluate associations of measures of abdominal fat and muscle with cardiovascular outcomes.16 Of these 1,944 participants, 17 were excluded due to missing BMD measurements and 4 were excluded due to missing clinical information. Thus, the analytic dataset consisted of 1923 individuals. All studies were performed under institutionally approved protocols for human subject research, and all participants provided informed consent.
Measurement of Bone Mineral Density
Abdominal CT scans were performed between September 2002 and September 2005, with half occurring at visit 2 and half at visit 3. CTs were performed using electron-beam scanners with 3 mm collimation, 6 mm slice thickness, and reconstructed using 25 6-mm slices, in a 35-cm field of view, and normal kernel or with multi-detector CT.16 All scans used a standard phantom to adjust for brightness.16 Bone mineral density was quantified from CT scans of the abdomen using Image Analysis QCT 3D PLUS software program and were read at the central MESA CT reading center.16 Volumetric bone mineral density was measured in 10-mm thick slices of trabecular bone from L2–4, in the anterior one-half to one-third of the vertebral body, and excluded cortical bone. We used vBMD measurements from L3. A trained reader examined each region and excluded observations with abnormalities including fractures, osteophytes, metastatic lesions, or bone islands.16 As described by Hyder and colleagues, a random sample of 25 scans were reread on 3 occasions with 100% agreement on inclusion and exclusion criteria for the vertebrae evaluated (L2-L4). Pearson’s r was >0.98 for pairwise reads.16
Measurement of Muscle Density and Fat Fraction
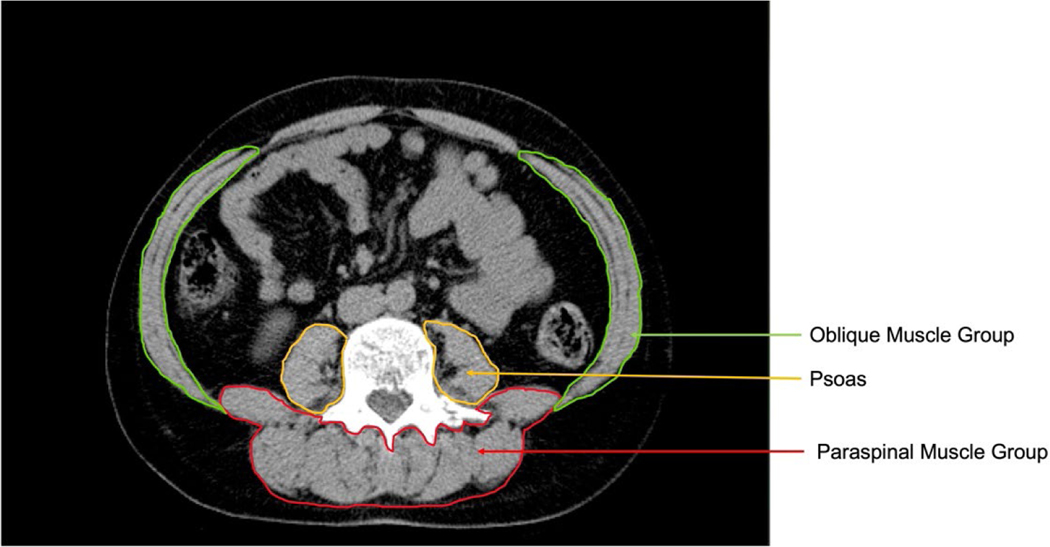
To assess abdominal muscle density and fat fraction, six transverse cross-sectional 6mm slices were analyzed: 2 slices each at L2–3, L3–4, and L4–5. In each slice, areas of interest were processed by the Medical Imaging Processing Analysis and Visualization Software (MIPAV version 4.1.2, National Institutes of Health, Bethesda, Maryland) that quantified areas of fat and lean tissue in square centimeters, as well as density in Hounsfield units (HU). For this study, fat tissue was identified as being between −190 and −30 Hounsfield units while lean tissue was identified as being between 0 and 100 Hounsfield units. The muscles measured in each slice were: bilateral psoas, rectus abdominis, obliques, quadratus lumborum, spinalis, longissimus, illiocostalis, multifidus and erector spinae. Inter- and intra-rater reliability for determining muscle groups was 0.93 and 0.98, respectively. Muscle density was calculated as the mean HU value for a muscle group area. Fat fraction was calculated as the mean HU value for the muscle group fat area / total muscle group area (in sq cm). Figure 1 presents the muscle segmentation used for analysis. The three muscle groups analyzed include the psoas, oblique muscle group [internal oblique, external oblique, and transverse abdominus], and the paraspinal muscle group [quadratus lumborum, spinalis, longissiumus, iliocostalis, erector spinae, and multifidus].
Figure 1.
Computed tomography image of the oblique muscle group, psoas, and paraspinal muscle group at the level of the third lumbar vertebra.
Exercise
We utilized self-reported exercise data gathered from the MESA Typical Week Physical Activity Survey (TWPAS) administered at Exam 2.17 The TWPAS is adapted from the Cross-Cultural Activity Participation Study, a physical activity survey funded and validated by the National Institutes of Health Women’s Health Initiative as a tool to assess moderate activity patterns among minority women over age 40.18 The TWPAS measured total physical activity and specific categories of activity, with “Intentional Exercise” encompassing all non-work related activity. Specifically, intentional exercise was defined as walking, dancing, sports and physical conditioning, whether for exercise or pleasure. “Physical conditioning,” a subset of “Intentional Exercise,” was defined as meaningful exercise including aerobics, cycling, swimming, and using resistance machines. “Physical activity in the workplace” was also accounted for and dichotomized into moderate and vigorous activity. All data were recorded as metabolic equivalents – minutes per week (MET-min/wk), which is the ratio of metabolic rate of activity to resting metabolic rate, conventionally described as 1 kilocalorie per kilogram per hour.19
Covariates
Over a period of two administered exams, data were collected using standardized questionnaires on demographics, lifestyle factors, and medication use. Cigarette smoking was classified as never, current or former, and for our purposes dichotomized as “having ever smoked” versus “never”. Body mass index (BMI) was calculated as weight (kg) / height (m2) after participants were measured wearing light clothing and no shoes.
Blood samples were obtained after a 12-hour fast to measure glucose. Impaired glucose tolerance was classified as having a fasting glucose greater than 101 mg/dL according to the 2003 American Diabetes Association criteria.20 Oral corticosteroid use was defined as being currently prescribed corticosteroids for any reason. Use of anti-resorptive medications was defined by self-report of its current use.
Statistical Analysis
Due to marked differences across sexes in both BMD and abdominal muscle and fat, we stratified analyses a priori on gender. We evaluated baseline characteristics of the entire cohort as well as by gender using means and proportions. To analyze the associations of psoas density, paraspinal density, oblique density, and fat fraction with L3 vBMD as the outcome, we used multivariable linear regression. In each analysis, we constructed a set of multivariable models. Model 1 was unadjusted. Model 2 adjusted for age, race, BMI, smoking, impaired glucose status, use of corticosteroids, and use of anti-resorptive medications.
To determine whether intentional exercise modified the association between muscle density, fat fraction, and L3 vBMD, we included an interaction term for exercise. Specifically, for intentional exercise, “high” versus “low” exercisers were dichotomized based on median exercise values for men and women. For both conditioning and work, we dichotomized these variables as ‘any amount of physical activity’ versus ‘none at all’ as the median values equaled zero for conditioning and work. We conducted analyses using SPSS Statistics (version 24), and p-values < .05 determined statistical significance.
Because intentional exercise did not significantly modify the association between muscle density, fat fraction, and L3 vBMD, we adjusted for it in Model 3 in our fully adjusted analysis of the main effects.
Results
Characteristics of the cohort are summarized in Table 1. Of the 1923 participants in this analysis, the mean age was 62 ± 9.7, 49% were women, 40% were White, 13% were Chinese, 21% were Black, and 26% were Hispanic/Latino. Approximately half (49%) of participants, 60% of women and 39% of men, had never smoked. Additionally, 33% of participants, 35% of men and 27% of women, had impaired fasting glucose. Average BMI was 28.2 ± 5.2 kg/m2 for all participants, with women at 28.4 ± 5.2 kg/m2 and men at 27.9 ± 4.4 kg/m2. One percent of participants, 0.8% of men and 1.5% of women, reported current oral corticosteroid use; and 13% of all participants, .8% of men and 24.7% of women reported current use of anti-resorptive medication. On average, men were engaged in 900 met-min/wk total intentional exercise, and women were engaged in 660 met-min/wk. Less than half of all respondents reported engaging in any conditioning. Less than half of all women in the cohort reported any significant physical activity at work, while the median value of physical activity at work for men was 3038 met-min/wk. Table 1 also lists the median values for L3 vBMD as well as density and fat fraction of psoas, paraspinals, and oblique. Average L3 vBMD for men was 120 ± 39 mg/cm3 and for women was 111 ± 40 mg/cm3.
Table 1:
Population characteristics, including L3 bone mineral density, muscle density, fat fraction, and physical activity of 1923 MESA participants in the MESA Body Composition Ancillary Study
| All | Men | Women | |
|---|---|---|---|
| N (%) | 1923 | 973 (50.6) | 950 (49.4) |
| Age, years(SD) | 62.07 (9.72) | 63 (10) | 64 (9.5) |
| Ethnicity, n(%) | |||
| White | 769 (39.9) | 409 (42) | 360 (37.9) |
| Chinese | 256 (13.3) | 135 (13.9) | 121 (12.7) |
| Black | 399 (20.7) | 179 (18.4) | 220 (23.2) |
| Hispanic/Latino | 499 (25.9) | 250 (25.7) | 249 (26.2) |
| Never smoked, n(%) | 947 (49.2) | 376 (38.7) | 571 (60.2) |
| Impaired Fasting Glucose, n(%) | 635 (33) | 360 (34.9) | 280 (26.8) |
| BMI, kg/m2 (SD) | 28.2 (5.2) | 27.9 (4.4) | 28.4 (5.8) |
| Oral steroid use, n(%) | 22 (1.1) | 8 (0.8) | 14 (1.5) |
| Anti-resorptive medication use, n(%) | 243 (12.6) | 8 (0.8) | 235 (24.7) |
| Mean (SD) L3 vBMD, muscle density, and fat fraction of psoas, paraspinal, and oblique muscles groups | |||
|
| |||
| vBMD L3, mg/cm3 | 116 (40) | 120 (39) | 111 (40) |
| Total Psoas Area, sq cm | 16.4 (5.7) | 20 (5.5) | 13 (3.3) |
| Psoas Fat Fraction % | 0.07 (0.05) | 0.07 (0.05) | 0.08 (0.06) |
| Psoas Density, HU | 39 (11.3) | 41 (10.7) | 36.7 (11.6) |
| Total Paraspinal Area, sq cm | 63.1 (14.6) | 72.1 (12.7) | 53.6 (9.8) |
| Paraspinal Fat Fraction % | 0.11 (0.07) | 0.09 (0.06) | 0.13 (0.07) |
| Paraspinal Density, HU | 29.4 (14.7) | 34 (13.4) | 24.6 (14.5) |
| Total Oblique Area, sq cm | 37.7 (15.5) | 44.2 (16.1) | 31.6 (12.2) |
| Oblique Fat Fraction % | 0.11 (0.07) | 0.1 (0.06) | 0.13 (0.08) |
| Oblique Density, HU | 22.4 (13.1) | 26.4 (11.4) | 18.8 (13.6) |
| Median, 25th, and 75th percentile of self-reported exercise and work | |||
|
| |||
| Total Intentional Exercise (MET-min/week) | 742 [96, 1834] | 900 [165, 2100] | 660 [0, 1601] |
| Moderate Conditioning | 0 [0, 330] | 0 [0, 495] | 0 [0,330] |
| Vigorous Conditioning | 0 [0,0] | 0 [0, 0] | 0 [0, 0] |
| Total Conditioning | 0 [0, 495] | 0 [0, 660] | 0 [0, 483.75] |
| Moderate Work | 0 [0,900] | 0 [0,1800] | 0 [0, 450] |
| Vigorous Work | 0 [0,0] | 0 [0,0] | 0 [0,0] |
| Total Work * | 450 [0,4950] | 3038 [0, 5850] | 0 [0,4207.5] |
IFG = Impaired fasting glucose. For mean values, SD is presented in parentheses. For median values, 25th and 75th quartiles are presented in brackets.
Light work was not calculated
Table 2 presents the parameter estimates for the association between paraspinal, oblique, and psoas density with L3 vBMD in unadjusted, covariate adjusted, and adjusted for total intentional exercise analyses. When evaluating associations between muscle density and vBMD, we observed higher muscle density was associated with higher L3 vBMD. To better understand the magnitude of effect, we present here the standard deviation (SD) change in L3 vBMD in response to a 1 SD change in muscle density. In fully adjusted analysis, for every 1 SD higher psoas density, there was a 0.20 SD higher L3 vBMD (p<.001) in men and a 0.19 SD higher L3 vBMD (p<.001) in women. For every 1 SD higher paraspinal density, there was a 0.14 SD (p<.001) higher L3 vBMD in men, and 0.18 SD (p<.001) higher L3 vBMD in women. For every 1 SD higher oblique density, there was a 0.09 SD (p=.003) higher L3 vBMD in men and 0.12 SD higher L3 vBMD in women.
Table 2.
Association between psoas, paraspinal, and oblique muscle density (HU) and L3 vBMD (mg/cm3) in 950 women and 973 men in the Multi-Ethnic Study of Atherosclerosis
| Psoas | ||||
|---|---|---|---|---|
|
|
||||
| Men | Women | |||
|
| ||||
| β (SD) | P value | β (SD) | P value | |
|
|
||||
| Unadjusted | 1.10 (0.11) | <0.001 | 1.23 (0.11) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use adjusted | 0.78 (0.11) | <0.001 | 0.67 (0.10) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, BP use, intentional exercise adjusted * | 0.75 (0.11) | <0.001 | 0.67 (0.10) | <0.001 |
|
| ||||
| Paraspinal | ||||
|
|
||||
| Men | Women | |||
|
| ||||
| β (SD) | P value | β (SD) | P value | |
|
|
||||
| Unadjusted | 0.86 (0.09) | <0.001 | 1.03 (0.09) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use adjusted | 0.45 (0.10) | <0.001 | 0.59 (0.09) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, BP use, intentional exercise adjusted * | 0.43 (0.10) | <0.001 | 0.52 (0.09) | <0.001 |
|
| ||||
| Oblique | ||||
|
|
||||
| Men | Women | |||
|
| ||||
| β (SD) | P value | β (SD) | P value | |
|
|
||||
| Unadjusted | 0.74 (0.12) | <.0001 | 0.75 (0.10) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use adjusted | 0.34 (0.11) | <0.001 | 0.33 (0.09) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, BP use intentional exercise adjusted * | 0.33 (0.11) | 0.003 | 0.36 (0.09) | <0.001 |
SD= standard deviation. BMI = Body mass index (kg/m2). IFG=Impaired fasting glucose. CCS = Corticosteroid. Analysis for muscle density was for increments of 1 HU.
Following evaluation of intentional exercise as an effect modifier on the association between muscle density, fat fraction, and L3 vBMD, we found no significant interaction. Therefore, we considered it a confounder and adjusted for it in Model 3.
As shown in Table 3, the association between fat fraction and L3 vBMD was similar for all 3 muscle groups where higher fat fraction was significantly associated with lower L3 vBMD in fully adjusted analysis. To better understand the magnitude of effect, we present here the standard deviation (SD) change in L3 vBMD in response to a 1 SD change in muscle fat fraction. In particular, for every 1 SD higher fat fraction of psoas, there was a 3.19 lower SD of L3 vBMD in men (p<0.001), and 4.3 lower SD of L3 vBMD in women (p<0.001). For every 1 SD higher fat fraction in the paraspinal muscle group, there was a 1.4 SD and 3.9 SD lower L3 vBMD in men and women, respectively (p=0.012 and p<0.001). For the oblique muscle group, every 1 SD higher fat fraction was associated with a 1.6 SD lower L3 vBMD in men (p=0.034) and 2.2 SD lower L3 vBMD in women (p=0.001)
Table 3:
Association between psoas, paraspinal, and oblique muscle group fat fraction and L3 vBMD (mg/cm3) in 950 women and 973 men in the Multi-Ethnic Study of Atherosclerosis
| Psoas | ||||
|---|---|---|---|---|
|
|
||||
| Men | Women | |||
|
| ||||
| β (SD) | P value | β (SD) | P value | |
|
|
||||
| Unadjusted | −220.0 (26.5) | <0.001 | −188.5 (21.7) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use adjusted | −154.2 (26.3) | <0.001 | −104.3 (20.3) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use, intentional exercise adjusted * | −147.3 (26.3) | <0.001 | −104.2 (20.3) | <0.001 |
|
| ||||
| Paraspinal | ||||
|
|
||||
| Men | Women | |||
|
| ||||
| β (SD) | P value | β (SD) | P value | |
|
|
||||
| Unadjusted | −138.0 (19.9) | <0.001 | −168.0 (16.3) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use adjusted | −53.0 (21.2) | 0.011 | −86.7 (16.6) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use, intentional exercise adjusted * | −50.3 (21.2) | 0.012 | −86.0 (16.4) | <0.001 |
|
| ||||
| Oblique | ||||
|
|
||||
| Men | Women | |||
|
| ||||
| β (SD) | P value | β (SD) | P value | |
|
|
||||
| Unadjusted | −80.9 (24.6) | 0.001 | −76.8 (16.9) | <0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use adjusted | −52.2 (22.5) | 0.022 | −47.12 (15.3) | 0.001 |
| Age, race, BMI, smoking, IFG, CCS use, anti-resorptive use, intentional exercise adjusted * | −51.8 (22.5) | 0.034 | −48.2 (15.3) | 0.001 |
SD= standard deviation. BMI = Body mass index (kg/m2). IFG=Impaired fasting glucose. CCS = Corticosteroid. Analysis for fat fraction was in increments of 10%.
Following evaluation of intentional exercise as an effect modifier on the association between muscle density, fat fraction, and L3 vBMD, we found no significant interaction. Therefore, we considered it a confounder and adjusted for it in Model 3.
We examined the effect of total intentional exercise on the association between muscle density and muscle fat fraction with L3 vBMD for men and women. Men and women were dichotomized on their median MET-min/wk values, 900 and 660, respectively, and evaluated for all muscle groups. There was no significant interaction between intentional exercise and muscle density on L3 vBMD; and no significant interaction between intentional exercise and fat fraction on L3 vBMD for both men and women. Thus, in our analysis of the main effects as shown in Table 2 and Table 3, we considered intentional exercise a covariate, and adjusted for it in Model 3.
We also examined the effect of moderate, vigorous, and total activity spent during both conditioning and work on the association between muscle density and fat fraction with L3 vBMD. In this analysis, participants were stratified based on their median reported value of moderate, vigorous, and total activity. In fully adjusted analysis, there was no significant interaction between activity and muscle density on L3 vBMD or between activity and fat fraction and L3 vBMD except for oblique density and fat fraction in women. For the latter, in the high exercise group, for every 1 SD higher oblique density there was a 0.2 SD higher L3 vBMD (p<0.001) compared to a 0.13 SD higher L3 vBMD in the low exercise group (p=0.002) (pinteraction=0.05). For every 1 SD higher oblique fat fraction in the high exercise group, there was 1.7 SD L3 vBMD compared to a 1.05 SD lower L3 vBMD in the lower exercise group (pinteraction = 0.05). This interaction was also present in women who participated in high versus low conditioning for oblique density (pinteraction = 0.005) and fat fraction (pinteraction<0.001). Specifically, in the high conditioning group, a 1 SD higher oblique density was associated with a 0.24 SD higher L3 vBMD (p<0.001), whereas it was associated with a 0.12 SD higher L3 vBMD in the low conditioning group (p=0.001). For every 1 SD higher oblique fat fraction in the high conditioning group, there was a 2.3 SD lower L3 vBMD (p=<0.001) compared to a 0.86 SD lower L3 vBMD in the low conditioning group (p=0.02).
To assess whether L3 vBMD and muscle group density and fat fraction differed for high vs. low exercisers, we dichotomized men and women based on the median MET-min/wk value for intentional exercise. There was no significant difference in mean L3 vBMD between high and low volume exercisers for both women and men. Psoas density was significantly higher in female high volume exercisers (p=0.04); and psoas and paraspinal fat fraction were significantly higher in the low volume exercisers, for both women (p=0.01, p=0.01) and men (p=0.003, p=0.02). (Table 4)
Table 4:
Psoas, paraspinal, and oblique muscle density, fat fraction, and L3 vBMD in high and low volume exercisers, stratified by gender, in the Multi-Ethnic Study of Atherosclerosis
| Male high volume exercisers | Male low volume exercisers | P-value | Female high volume exercisers | Female low volume exercisers | P-value | |
|---|---|---|---|---|---|---|
|
|
||||||
| N (%) | 480 (50.2) | 475 (49.7) | 448 (49.3) | 461 (50.1) | ||
| vBMD L3, mg/cm3 | 123.3 (40.7) | 118.5 (37) | 0.119 | 112.1 (39.8) | 111.1 (39.5) | 0.471 |
| Total Psoas Area, sq cm | 20.1 (5.5) | 19.5 (5.4) | 0.96 | 13.1(3.3) | 12.9 (3.3) | 0.96 |
| Psoas Fat Fraction, % | 0.06 (.04) | 0.07 (.05) | 0.003 | 0.08 (0.05) | 0.09 (0.07) | 0.01 |
| Psoas Density, HU | 42.03 (9.8) | 40.02 (11.3) | 0.08 | 37.6 (10.2) | 35.8 (12.6) | 0.04 |
| Total Paraspinal Area, sq cm | 72.2 (12.8) | 72.04 (12.6) | 0.59 | 30.9 (12.3) | 32.5 (12.2) | 0.70 |
| Paraspinal Fat Fraction, % | 0.08 (.06) | 0.09 (.07) | 0.02 | 0.12 (0.07) | 0.13 (0.08) | 0.01 |
| Paraspinal Density, HU | 34.8 (12.7) | 33.3 (14) | 0.157 | 20.2 (13.1) | 17.5 (13.8) | 0.266 |
| Total Oblique Area, sq cm | 44.13 (15.3) | 44.3 (16.8) | 0.073 | 53.6 (9.9) | 53.9 (9.8) | 0.41 |
| Oblique Fat Fraction, % | 0.09 (0.05) | 0.1 (0.05) | 0.639 | 0.12 (0.07) | 0.13 (0.08) | 0.09 |
| Oblique Density, HU | 27.1 (11.5) | 25.6 (11.2) | 0.64 | 26.04 (13.7) | 23.4 (15.1) | 0.18 |
Discussion
In this multi-ethnic community-living population, muscle density and fat fraction of the psoas, paraspinal, and oblique muscle groups were associated with L3 volumetric bone mineral density. Specifically, in fully adjusted analysis, higher psoas, paraspinal, and oblique muscle density was significantly associated with higher volumetric bone mineral density at L3 in men and women, while higher fat fraction in these muscles was associated with lower L3 vBMD in both women and men. These associations were not modified by intentional exercise, conditioning, or work, except in the oblique muscles for women, where the interaction was quantitative. Our findings are the first to demonstrate that muscle density and fat fraction of the psoas, paraspinal, and oblique muscle groups are associated with L3 BMD and warrant further investigation into potentially a direct relationship between these muscle groups and the spine.
These findings are consistent with those shown by Nichols and colleagues, who found that regional lean tissue mass was correlated with BMD, specifically in the thigh.21 Similarly, Blain and others showed an association between quadriceps strength and femoral neck BMD but not lumbar BMD.22 Although these studies correlate mass and strength to BMD, our findings are the first to show a site-specific association between regional trunk muscle density and lumbar BMD in a community-living population.
These findings also build on previous literature regarding muscle fat content and BMD. Sollmann and colleagues examined 39 postmenopausal women using MRI and showed a correlation between proton density fat fraction of erector spinae muscles and that of lumbar vertebral bodies.23 A similar study by Farr analyzed 444 young females using peripheral quantitated computed tomography and showed that calf skeletal muscle fat content was inversely correlated with tibial weight-bearing bone strength.24 Further, Kim and colleagues demonstrated that women with osteoporotic spinal compression fractures had higher fatty infiltration of the paraspinal muscles compared to women without osteoporotic spinal compression fractures.9 Our findings mirror these associations between muscle fat content and bone density, and expand them to different trunk muscles in a larger multi-ethnic cohort of men and women while accounting for key contributors to bone and muscle density.
We found that physical activity overall did not modify the association between trunk muscle density and vBMD or fat fraction and vBMD at the level of the 3rd lumbar vertebra. Although numerous studies confirm the positive impact weight-bearing exercise can have on bone density, and how strength-training can improve muscle quality, no studies directly address whether exercise modifies the relationship between muscle density, muscle fat fraction, and BMD. Our findings suggest that the way muscle and bone interact with one another, through mechanical and molecular signaling, may not differ by low versus high intentional exercise, conditioning, or work. Further, intentional exercise did not significantly attenuate the association between muscle density, muscle fat fraction, and vBMD when evaluated as a covariate. This finding could suggest that either this association between trunk muscle and lumbar bone density is robust or that the effect of exercise must be specific as our category of ‘intentional exercise’ was quite broad and included various forms of aerobic activity versus targeted lumbar/abdominal strengthening exercise.
A proposed mechanism for the association between psoas, paraspinal, and oblique muscle density and lumbar bone mineral density, outside of mechanotransduction, is the increased production of osteogenic myokines by denser muscle. Hamrick, et. Al showed that two osteogenic factors, IGF-1 and FGF-2 are secreted by muscle in vitro, and furthermore, receptors for these factors are present on bone periosteum.25 It is our hypothesis that muscle less infiltrated by fat is more efficient at secreting osteogenic factors than less dense muscle. Further work to elucidate this theory would involve in-vivo cytology, and measurement of osteogenic myokines in rodent models comparing regional muscle and bone density, highlighting possible opportunities for pharmaceutical intervention.
Our study had several limitations. Because it was a cross-sectional evaluation, we were unable to establish temporal associations of trunk muscle density and fat fraction with lumbar bone density. Thus, we are unable to determine whether poor muscle density leads to poor bone density or vice versa. In addition, we did not have measures of muscle density and muscle fat fraction in other muscle groups, e.g., quadriceps, or measures of bone mineral density at other anatomic sites. Thus, we can not confirm that the association of psoas, paraspinal, and oblique muscle groups with L3 vBMD was a ‘local’ versus ‘global’ association. Our exercise data are broad and self-reported, and could reflect inaccurate measurements of physical activity. This potential for non-differential misclassification could bias our results toward the null. Given these limitations, strengths of our study include a large and diverse population that allow us to evaluate these associations between muscle density and fat fraction and bone density in men and women separately as muscle and fat were measured using gold standard computed tomography and automated segmenting software.
In conclusion, we have shown that muscle density of the psoas, paraspinal, and oblique muscle groups was positively associated with lumbar spine BMD, and that psoas, paraspinal, and oblique muscle fat fraction was inversely associated with lumbar spine BMD in a multi-ethnic community living population, regardless of activity or exercise level. Future studies are warranted to confirm a direct association; and determine mechanisms and temporality of muscle quality and bone density to prevent this potentially synergistic degenerative process.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Funding
This research was supported by awards K01 HL122394 and R03 HL146875, by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute; by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS); by award R01 AG065876 from the National Institute of Aging; and by the Miami-Marquette Research Challenge Grant from the Foundation for Physical Therapy.
Footnotes
Financial Disclosures
The authors have no financial support from commercial sources or conflicts of interest to disclose.
References
- 1.Lau E, Ong K, Kurtz S, Schmier J and Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90:1479–86. [DOI] [PubMed] [Google Scholar]
- 2.Darba J, Kaskens L, Perez-Alvarez N, Palacios S, Neyro JL and Rejas J. Disability-adjusted-life-years losses in postmenopausal women with osteoporosis: a burden of illness study. BMC Public Health. 2015;15:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272–7. [DOI] [PubMed] [Google Scholar]
- 4.Kawao N and Kaji H. Interactions between muscle tissues and bone metabolism. J Cell Biochem. 2015;116:687–95. [DOI] [PubMed] [Google Scholar]
- 5.Tella SH and Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomfield SA. Disuse osteopenia. Curr Osteoporos Rep. 2010;8:91–7. [DOI] [PubMed] [Google Scholar]
- 7.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB and Health ABCS. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyakoshi N, Hongo M, Mizutani Y and Shimada Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. J Bone Miner Metab. 2013;31:556–61. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Chae SU, Kim GD and Cha MS. Changes of paraspinal muscles in postmenopausal osteoporotic spinal compression fractures: magnetic resonance imaging study. J Bone Metab. 2013;20:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson DE, Quinn E, Parker E, Allaire BT, Muir JW, Rubin CT, Magaziner J, Hannan MT, Bouxsein ML and Kiel DP. Associations of Computed Tomography-Based Trunk Muscle Size and Density With Balance and Falls in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA and Tylavsky FA. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–7. [DOI] [PubMed] [Google Scholar]
- 12.Johannesdottir F, Allaire B, Anderson DE, Samelson EJ, Kiel DP and Bouxsein ML. Population-based study of age- and sex-related differences in muscle density and size in thoracic and lumbar spine: the Framingham study. Osteoporos Int. 2018;29:1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel BA, Bloch DA and Fries JF. Weight-bearing exercise, overexercise, and lumbar bone density over age 50 years. Arch Intern Med. 1989;149:2325–9. [PubMed] [Google Scholar]
- 14.Sipila S and Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol (1985). 1995;78:334–40. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 16.Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ and Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkbey EB, Jorgensen NW, Johnson WC, Bertoni AG, Polak JF, Diez Roux AV, Tracy RP, Lima JA and Bluemke DA. Physical activity and physiological cardiac remodelling in a community setting: the Multi-Ethnic Study of Atherosclerosis (MESA). Heart. 2010;96:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitt MC, DuBose KD, Ainsworth BE and Tudor-Locke C. Walking patterns in a sample of African American, Native American, and Caucasian women: the cross-cultural activity participation study. Health Educ Behav. 2004;31:45S–56S. [DOI] [PubMed] [Google Scholar]
- 19.Byrne NM, Hills AP, Hunter GR, Weinsier RL and Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol (1985). 2005;99:1112–9. [DOI] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P, Expert Committee on the D and Classification of Diabetes M. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. [DOI] [PubMed] [Google Scholar]
- 21.Nichols DL, Sanborn CF, Bonnick SL, Gench B and DiMarco N. Relationship of regional body composition to bone mineral density in college females. Med Sci Sports Exerc. 1995;27:178–82. [PubMed] [Google Scholar]
- 22.Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F and Jeandel C. Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology. 2001;47:207–12. [DOI] [PubMed] [Google Scholar]
- 23.Sollmann N, Dieckmeyer M, Schlaeger S, Rohrmeier A, Syvaeri J, Diefenbach MN, Weidlich D, Ruschke S, Klupp E, Franz D, Rummeny EJ, Zimmer C, Kirschke JS, Karampinos DC and Baum T. Associations Between Lumbar Vertebral Bone Marrow and Paraspinal Muscle Fat Compositions-An Investigation by Chemical Shift Encoding-Based Water-Fat MRI. Front Endocrinol (Lausanne). 2018;9:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, Laudermilk M, Lohman TG and Going SB. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26:2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamrick MW, McNeil PL and Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10:64–70. [PMC free article] [PubMed] [Google Scholar]