ABSTRACT
Rabbit hemorrhagic disease virus (RHDV) typically causes a fatal disease in rabbits. In Australia, RHDV was imported to control the feral rabbit population, while it poses a severe threat to native rabbits in other countries. RHDV variants are genetically diverse and serological studies using antibodies isolated from infected rabbits or raised against RHDV virus-like particles (VLPs) have found RHDV variants antigenically distinct. In this study, we determined the X-ray crystal structure of an RHDV GI.2 (N11 strain) protruding (P) domain in complex with a diagnostic monoclonal antibody (2D9) Fab. We showed that 2D9 interacted with conserved and variable residues on top of the P domain with nanomolar affinity. To better illustrate 2D9 specificity, we determined the X-ray crystal structure of an RHDV GI.1b (Ast89 strain) that was a 2D9 non-binder. Structural analysis indicated that amino acid substitutions on the GI.1b P domain likely restricted 2D9 binding. Interestingly, a model of the GI.2 P domain-Fab complex superimposed onto a cryo-EM structure of an RHDV VLP revealed that 2D9 Fab molecules clashed with neighboring Fabs and indicated that there was a reduced antibody binding occupancy. Moreover, the RHDV GI.2 histo-blood group antigen (HBGA) co-factor binding site appeared obstructed when 2D9 was modeled on the VLP and suggested that 2D9 might also function by blocking HBGA attachment. Overall, this new data provides the first structural basis of RHDV antibody specificity and explains how amino acid variation at the binding site likely restricts 2D9 cross-reactivity.
IMPORTANCE Isolated RHDV antibodies have been used for decades to distinguish between antigenic variants, monitor temporal capsid evolution, and examine neutralizing capacities. In this study, we provided the structural basis for an RHDV GI.2 specific diagnostic antibody (2D9) binding and reveal that a small number of amino acid substitutions at the binding site could differentiate between RHDV GI.2 and GI.1b. This novel structural information provides a framework for understanding how RHDV displays a specific antigenic epitope and engages an antibody at the atomic level. Importantly, part of the 2D9 binding region was earlier reported to contain a neutralizing epitope and our structural modeling as well as recent human norovirus antibody-mediated neutralization studies, suggest that the 2D9 antibody has the potential to block HBGA attachment. These new findings should aid in characterizing antigenic variants and advance the development of novel monoclonal antibodies for diagnostics and therapeutics.
KEYWORDS: Lagovirus, X-ray crystallography, antibody binding, rabbit hemorrhagic disease virus
INTRODUCTION
Rabbit hemorrhagic disease virus (RHDV) belongs to the Lagovirus genus in the Caliciviridae family. Caliciviruses infect a broad range of animals, including humans, mice, cows, pigs, rabbits, and bats. RHDV is highly contagious and endemic in wild rabbit populations in several countries (1–6). In Australia, RHDV is used as a biocontrol measure to control the feral rabbit population (7, 8). However, molecular epidemiological and serological studies found that non-pathogenic rabbit caliciviruses (RCVs), which also co-circulate in Australia, generated antibodies in hosts that cross-protected against these RHDV biocontrol agents. Moreover, genetic analysis of emerging RHDV variants showed a modified host tropism, which likely influences antibody-mediated neutralization and reduces virulence (9, 10). In other countries, RHDV is considered a pest and an RHDV vaccine was introduced in Europe in the 1990s that provided good coverage for the strains circulating at that time (11, 12).
Lagoviruses are classified into 2 genogroups (GI and GII) based on their preferred hosts, where RHDV and RCV belong to GI infecting rabbits, and European brown hare syndrome virus (EBHSV) belongs to GII, targeting mostly hares. RHDV isolates have multiple nomenclatures (13–16) and have been termed Groups 1 to 6 (G1 to G6 [16–19]), Clades 1 to 4 (20), and Clades A to D (21). For this article, GI was divided into GI.1 to GI.4 (e.g., RHDV and RHDVa), GI.2 (e.g., RHDV2 or RHDVb), GI.3 (e.g., RCV-E1), and GI.4 (e.g., RCV-A and RCV-A1) (22). Caliciviruses are single-stranded, positive-sense RNA viruses. The Lagovirus genomic RNA is organized into 2 open reading frames (ORFs). ORF1 encodes nonstructural and structural proteins including the RNA-dependent RNA polymerase (RdRp) and the major capsid protein (VP60), whereas ORF2 encodes a minor structural protein (VP10). An additional subgenomic RNA also encodes VP60 and VP10. Genetic recombination at the RdRp and capsid junction is extensive (23, 24) and amino acid substitutions in the capsid protein have been linked to the emergence of antigenic variants (25, 26).
The RHDV capsid protein can be expressed in insect cells, and this typically results in the formation of virus-like particles (VLPs) that are morphologically and antigenically similar to the native virion, having a T = 3 icosahedral symmetry and 180 copies of VP60 (26). These VLPs have been used extensively for structural studies, host-factor binding studies, analysis of emerging variants, and reagents for serological and antigen detection assays. Structural studies have shown that the capsid protein is divided into a shell (S) and protruding (P) domain, where the S domain forms a scaffold protecting the RNA from the environment, while the P domain is more exposed and contains determinants for cell attachment, receptor/co-factor binding, and antigenic diversity. RHDV is typically detected using RT-PCR or competition ELISA using antibodies raised against RHDV VLPs. Presently, a suite of ELISAs exist to screen for exposure to wild rabbits for the various RHDV variants, and a suite of isotype ELISAs is used to infer infection dynamics (27). However, many of the antibody-based assays cross-react to various degrees, leading to difficulties in inferring the infection history of a rabbit population.
A number of studies have shown that RHDV binds histo-blood group antigens (HBGAs), which are attachment co-factors for virus infection in the host (28–30). HBGAs are polymorphic carbohydrates that are synthesized by a stepwise addition of monosaccharides to different precursor structures, aided through the action of specific glycosyltransferases. Rabbits are known to express HBGAs (A, B, and H type 2) on the duodenum surface and other areas, such as the trachea and biliary ducts, but not on hepatocytes which are the primary target cells for pathogenic RHDVs. RHDVs were found to bind to HBGAs in a strain dependent manner and with variable affinities (17). We recently discovered the HBGA binding pocket for a GI.2 RHDV variant (termed N11) and found that the HBGA binding site was located on the side of the P dimer at a dimeric interface involving residues from both monomers (28). The HBGA pockets is one of the main target regions for developing antivirals against human noroviruses and antibodies overlapping this region or interfering with the HBGA site have neutralized norovirus in cell culture (31–33).
Serological studies have characterized various strain specific, broadly reactive, and neutralizing antibodies against RHDV (2, 13, 26, 34–37). These antibodies have been used for diagnostic assays, differentiation between antigenic variants, as well as neutralization/vaccine studies. Despite antibody discovery research efforts, there is still limited structural information regarding antibody binding sites and how these antibodies might neutralize the virus (26). In this study, we determined the binding site of a GI.2 strain specific diagnostic RHDV IgG monoclonal antibody (termed 2D9) (38) using X-ray crystallography. We showed that 2D9 Fab bound to surface exposed loops on the top of the N11 P domain at a region that contained both variable and conserved amino acids among diverse RHDV and RCV variants. This structural information provides an explanation of 2D9 specificity and could aid in characterizing genetic variants.
RESULTS
RHDV VLP reactivity against RHDV 2D9 IgG.
To confirm RHDV VLP specificity with 2D9 IgG (38), we examined the reactivity using a direct ELISA (39). RHDV VLPs were expressed in insect cells and Ast89 mainly formed native sized particles, whereas N11 assembled into both native- and smaller-sized particles (Fig. 1A), presumably T = 3 and T = 1 as recently reported (40). The monoclonal antibody 2D9 IgG was able to detect both GI.2 N11 VLPs and the corresponding P domain at comparable antibody concentrations (>4,096-fold antibody dilution), but not GI.1b Ast89 VLPs or Ast89 P domain (Fig. 1B). These indicated that 2D9 IgG was specific and likely bound on the GI.2 N11 P domain.
FIG 1.
RHDV VLPs and antibody cross-reactivity. (A) Ast89 and N11 VLPs were expressed in insect cells. Ast89 VLPs (left) were expressed in SF9 cells and showed mainly native size VLPs, whereas RHDV N11 VLPs (right) were expressed in High Five cells and showed mainly small VLPs. Scale bar represents 100 nm. (B) ELISA of Ast89 VLPs (and corresponding P domain) and N11 VLPs (and corresponding P domain) binding to 2D9 IgG (serial dilution). A cutoff limit (dash line) was set at OD490 > 0.15.
X-ray crystal structure of N11 P domain-Fab 2D9 complex.
The precise binding site of Fab 2D9 on N11 P domain was determined to 2.65 Å resolution in space group P6522 with one P domain and one Fab molecule per unit cell. Data collection and refinement statistics are provided in Table 1. The N11 P domain was reminiscent of previously published RHDV P domain structures (26, 28), whereas the Fab displayed the typical immunoglobulin fold, with 7 and 9 antiparallel β-strands in the constant and variable regions, respectively (Fig. 2A). The total interface area of N11 P domain and 2D9 Fab was 1,110 Å2 (806 Å2 for the heavy [H] chain and 304 Å2 for the kappa [κ] light chain), as calculated using the PDBe-PISA server. In this position, the complementarity-determining regions (CDRs) of the Fab directly interacted with the top side of one N11 P domain (Fig. 2A), whereas an alternative interface had less surface interface (443 Å2) and the CDRs of Fab did not interact with the N11 P domain (data not shown).
TABLE 1.
Data collection and refinement statistics for RHDV P domainsa
| Parameter | N11-Fab 2D9 complex | GI.1b P domain |
|---|---|---|
| Data collection | ||
| Space group | P6522 | P212121 |
| Cell dimensions | ||
| a, b, c (Å) | 92.29, 92.29, 493.07 | 74.59, 78.41, 100.86 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 90 |
| Resolution range (Å) | 48.81–2.65 (2.74–2.65)b | 47.64–1.89 (1.96–1.89)b |
| Rmerge | 10.67 (69.86)b | 10.12 (55.93)b |
| I/σI | 12.52 (1.93)b | 11.65 (3.21)b |
| Completeness (%) | 99.65 (98.34)b | 98.19 (96.15)b |
| Redundancy | 6.0 (6.1)b | 4.7 (4.7)b |
| CC1/2 | 99.6 (94.2)b | 99.6 (82.0)b |
| Refinement | ||
| Resolution range (Å) | 46.15–2.65 | 42.41–1.89 |
| No. of reflections | 37,730 | 47,320 |
| Rwork/Rfree | 19.40/22.41 | 16.34/19.53 |
| No. of atoms | 5,688 | 5,333 |
| Protein | 5,633 | 4,888 |
| Ligand/ion | 14 | 0 |
| Water | 41 | 445 |
| Avg B factors (Å2) | ||
| Protein | 53.61 | 19.66 |
| Ligand/ion | 69.98 | |
| Water | 40.55 | 25.59 |
| RMSD | ||
| Bond lengths (Å) | 0.004 | 0.005 |
| Bond angles (°) | 1.04 | 1.17 |
| Ramachandran favoured (%) | 96.72 | 97.75 |
| Ramachandran outliers (%) | 0 | 0 |
| MolProbity score | 1.19 | 0.83 |
Each data set was collected from single crystals, respectively.
Values in parentheses are for highest-resolution shell.
FIG 2.
The X-ray crystal structure of the N11 P domain-Fab complex. N11 P domain dimer was colored according to the subdomains, i.e., P1 (salmon) and P2 (cyan), whereas the Fab was colored according to chains, i.e., Kappa (κ) light chain (yellow) and heavy chain (green). (A) The 2D9 Fab bound to the top of the P2 subdomain interacting with one monomer of the P domain dimer. (B) A close-up view of the interacting P domain residues for chain A (GLY-306, SER-319, GLY-383, ALA-384, SER-386, ASN-387, GLN-398, LYS-402, and THR-415) and Fab residues κ chain (TRP-92) and heavy chain (THR-30, ASN-35, THR-53, GLU-57, PRO-58, TYR-99, and TYR-101). The hydrogen bond interactions included both side chain and main chain interactions. Hydrogen bonding interactions distances were between 2.4 and 3.5 Å. Water molecule-residue binding interactions were excluded from the analysis as the resolution of the complex was outside the range for precise determination.
GI.2 N11 P domain interaction with the 2D9 Fab.
The N11 P2 subdomain and 2D9 Fab interaction included 11 hydrogen bonds, 10 of which were formed between the P2 subdomain and Fab heavy chain (CDRH1, CHDH2, and CDRH3) and 1 between the P2 subdomain and Fab light chain (CDRL3) (Fig. 2B). Nine N11 P2 subdomain amino acids were involved with 2D9 Fab binding, GLY-306N11 main chain formed a hydrogen bond with 2D9 PRO-58H; SER-319N11 side chain, GLY-383N11 main chain, and GLN-398N11 side chain formed hydrogen bonds with 2D9 TYR-101H; ALA-384N11 main chain formed a hydrogen bond with 2D9 TYR-99H; SER-386N11 side chain formed a hydrogen bond with 2D9 ASN-35H; ASN-387N11 side chain formed a hydrogen bonds with 2D9 THR-30H and THR-53H; LYS-402N11 side chain formed a hydrogen bond with 2D9 GLU-57H; and THR-415N11 side chain formed a hydrogen bond with 2D9 TRP-92κ.
Superposition of the apo N11 P domain and the Fab bound N11 P domain showed that one loop located between TYR-304N11 and ASN-311N11 shifted slightly (~3.6-6.5 Å). This P domain loop movement apparently positioned the side chain of PRO-305N11 away from the Fab CDRH2 and re-positioned the main chain of GLY-306N11 to form a hydrogen bond with PRO-58H (Fig. 3). The electrostatic potential of the Fab was calculated using PyMOL, and the interacting residues on the P domain bound at mainly negatively charged pockets on the Fab (Fig. 4A and 4B), which was also observed with other human norovirus P domain and Fab complexes (31, 41). Interestingly, our structural analysis of N11-Fab complex revealed that the Fab bound in close proximity to the N11 HBGA binding pocket (Fig. 5A and 5B). In particular, residue THR-415N11 that held TRP-92κ was adjacent to SER-364N11, which firmly held the terminal N-acetylglucosamine moiety of HBGAs (Lewis Y and H types) (28).
FIG 3.
Close-up of loop between TYR-304N11 and ASN-311N11. The apo N11 P domain (light gray) and N11-Fab complex (dark gray) were superimposed. The P domain loop between TYR-304 and ASN-311 was colored accordingly, P domains of N11 apo (cyan) and N11-Fab (red). The hydrogen bond (dashed line) between N11 P domain GLY-306N11 and 2D9 PRO-58H is shown. This P domain loop (aa 304–311) shift may have aided 2D9 engagement.
FIG 4.
The binding site on the 2D9 Fab was coordinated by negative charge regions on the Fab. (A) N11 P domain was colored as in Fig. 2A. The 2D9 Fab surface was colored according to contact potential, where red was negative and blue was positive. The scale was from -66 to 66 kT/e using protein contact potential in PyMol software. (B) A close-up view of the contact potential on the Fab, showing the P domain side chain residues on chain A that interacted with the Fab. N11 P domain side chains bound near the negative charge (red) regions on the Fab.
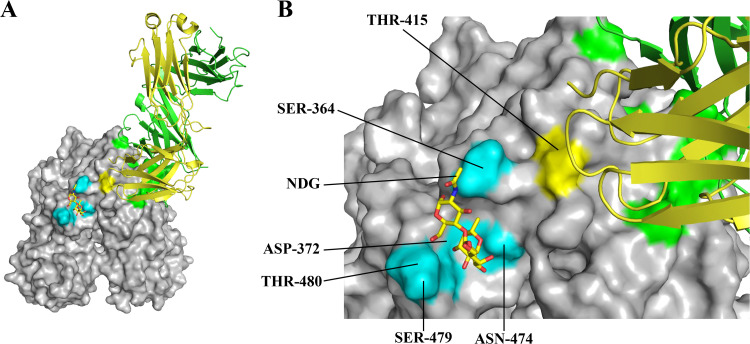
FIG 5.
HBGA and 2D9 binding site. (A) N11 P domain HBGA complex structure (4X1X) was superpositioned on the N11 P domain 2D9 complex structure. The P domain (gray) was shown as a surface representation and the HBGA as sticks (Lewis Y, yellow). P domain residues binding HBGAs (cyan), heavy chain (green), and kappa chain (yellow) where highlighted. (B) Close-up view showing THR-415N11 that held TRP-92κ was adjacent to residue SER-364N11, which held the terminal N-acetylglucosamine (NDG) moiety of HBGAs (Lewis Y and H types) (28).
X-ray crystal structure of GI.1b Ast89 P domain.
In order to determine the structural basis of 2D9 IgG non-reactivity with RHDV GI.1b Ast89, we determined the structure of the unbound Ast89 P domain using X-ray crystallography. The Ast89 P domain was refined to a resolution of 1.89 Å in space group P212121 with one P domain dimer in the asymmetric unit. Data collection and refinement statistics for Ast89 P domain are provided in Table 1. The electron density was well defined for most of the Ast89 P dimer, having an overall B-value of 19.66 Å2. The Ast89 P domain was subdivided into P1 and P2 subdomains as proposed for RHDVa (26) (Fig. 6A). The Ast89 P1 subdomain contained an α-helix and several β-sheets, whereas the P2 subdomain contained six antiparallel β-sheets that formed a barrel-like structure. Three extended loops were located on the Ast89 P2 subdomain between residues 304–312, 343-350, and 382–390.
FIG 6.
The X-ray crystal structure of Ast89 P domain. (A) The Ast89 P domain dimer was colored according to the subdomains, i.e., P1 (smudge) and P2 (pink). The P2 subdomain extended loops protruded out from the side of the P domain (aa 304–312, 343-350, and 382–390). (B) Superposition of Ast89 and N11 P dimers. Ast89 and N11 VP60 had an amino acid identity of 84% and the P dimer Ast89: N11 P domain had an RMSD of 0.28–0.32 Å. (C) Amino acid variations between Ast89 and N11 P dimers. Amino acid changes (red) were highlighted on the Ast89 P dimer (side and top views), where the changes were numbered according to a change from N11 to Ast89. The N11 P domain residues that bound the 2D9 binding are colored accordingly, conserved amino acids (green) and substitutions (lightgold). Two HBGAs (yellow sticks: PDB 4X1X) were modeled into the structure and residues having a direct hydrogen bond with HBGAs were colored (cyan). (D) Close-up of N11 P domain residues (cyan) interacting with 2D9 Fab and superpositioned Ast89 equilivalent residues (pink: Ast89 numbering).
Superposition of apo Ast89 and N11 (4X1W) P domains showed that their overall structures were similar (Fig. 6B), where the root mean square deviation (RMSD) for Cα atoms was calculated to be 0.28–0.32 Å (Ast89 chains A and B). However, amino acid variation at the 2D9 binding pocket (Fig. 6C) and several equivalent Ast89 P domain residues (GLN-398 and PRO-415) that would interact with 2D9 were positioned away from 2D9 (Fig. 6D) and likely accounted for the lack of Ast89 binding to 2D9 (Fig. 1B).
Specificity of 2D9 IgG.
The amino acid sequence identity between Ast89 and N11 VP60 was 84%. An amino acid sequence alignment of representative RHDV sequences showed that 4 of 9 P domain residues interacting with 2D9 Fab were highly conserved (GLY-306, ASN-387, GLN-398, and LYS-402) and 2 residues were semi-conserved (GLY-383 and ALA-384) (Fig. 7). In the case of Ast89, six of 9 Fab binding residues were conserved and 3 were substituted (SER-319N11 to ASN-319Ast89; SER-386N11 to GLY-386Ast89; and THR-415N11 to PRO-415Ast89). These substitutions likely inhibited Ast89 from binding 2D9 IgG (38), although other residues surrounding the Fab binding pocket and/or contiguous to binding residues might have also contributed to the lack of 2D9 binding (Fig. 6C).
FIG 7.
Amino acid alignment of RHDV VP60 sequences. Nineteen RHDV capsid sequences were aligned to the consensus N11 capsid sequence (partial VP60 shown). The N11 P domain residues that interacted (hydrogen bonds) with the 2D9 Fab were highlighted accordingly, heavy chain (green) and kappa chain (yellow). The residues having direct hydrogen bonds with HBGAs were highlighted (cyan). The asterisk represents conserved amino acids.
Binding properties of N11 P domain and 2D9 IgG.
ITC was used to analyze the thermodynamics of N11 P domain and 2D9 IgG. Three independent experiments were performed, and a representative of binding is shown (Fig. 8). The binding reaction was exothermic and was fitted into a one-site binding model (stoichiometry value, 0.86) with a binding affinity of Kd = 10 nM. These results revealed that N11 P domain had a strong affinity toward 2D9 IgG.
FIG 8.
Binding properties of N11 P domain and 2D9 IgG. The N11 P domain was titrated into 2D9 IgG in the presence of PBS. ITC titration curve (upper figure) and binding isotherm (lower figure) showing the calculated heat of injection against the molar ratio. The thermodynamic properties were Kd = 10.80 nM ± 8.89e−9 nM; dH = −12.9 kJ/mol ± 0.55 kJ/mol; and −TdS = −32.6 kJ/mol.
Structural basis of 2D9 antibody engagement.
To gain a better insight into 2D9 possible attachment to RHDV virions, the P domain-Fab complex was superimposed onto an RHDV cryo-EM VLP structure (Fig. 9A and 9B) (26). In this model, the Fab clashed with neighboring Fabs, when Fab molecules were positioned on a neighboring P domain dimer or when both antibody binding sites were occupied on one P domain dimer. These steric clashes likely further intensified for a complete IgG molecule. Although not directly overlapping the HBGA pocket on the N11 P dimer, the HBGA binding site was much less exposed to the outside when 2D9 Fab bound (Fig. 9B).
FIG 9.
Model of 2D9 antibody binding to RHDV particle. (A) N11-Fab complex and N11-HBGA complex (PDB ID: 4X1X) were superpositioned onto the cryo-EM structure of RHDV VLPs (EMD-5410), where the heavy chains (green), kappa chains (yellow), and HBGAs (cyan) are shown bound to the P dimers. Fab where colored as Fig. 1. The Fabs (transparent) from neighboring P dimers clashed, suggesting that not all P dimers bind 2D9 simultaneously at the two possible binding sites per dimer. (B) A close-up view showing the Fabs likely block the HBGA binding site and likely prevent the virus from attachment to host cells. (C) Superposition of norovirus P domain-Fab complexes and RHDV P domain-2D9 Fab onto a norovirus GII.4 P dimer. Two antibodies bind per dimer (one shown). Antibodies are colored accordingly, 10E9 (brown: PDB ID 6EWB), A1431 (bright pink: 6N8D), noro-320 (pink: 7JIE), and 512 (blue: 5KW9).
DISCUSSION
Molecular epidemiological studies have shown that RHDV is one of the fastest evolving viruses, and as such RHDV variants are antigenically diverse (42–44). The evolution of RHDV is shaped through processes such as natural selection, immune selection, genetic mutation, and recombination. In viruses such as caliciviruses and influenza viruses, this genetic diversification enables key changes in the viral capsid allowing them to escape herd immunity. For RHDV, strain specific and cross-reactive antibodies have been described (2, 13, 34–37, 45–47). However, structural information regarding RHDV antibodies as well as temporal capsid amino acid structural modifications among variants is an unexplored area.
Earlier studies indicated that exposed loops on the RHDV P dimers contributed to antigenic variation as the S domain is typically more conserved (45, 48, 49). To describe the precise binding site of the 2D9 diagnostic antibody (38) we determined the X-ray crystal structure of a GI.2 RHDV (N11 strain) P domain-Fab complex. We also determined the X-ray crystal structure of a GI.1b RHDV (Ast89 strain) P domain to determine the molecular basis of 2D9 specific recognition. We showed that 2D9 Fab bound to the top of the N11 P2 subdomain with a mostly conserved set of binding residues (contributed by both main and side chains). A small number of amino acid substitutions (3 of 9) at the equivalent antibody binding site on the Ast89 P2 subdomain likely inhibited 2D9 from binding. This variation likely distinguishes antigenic variants and because it is an external and environment-exposed loop and not a secondary structure like alpha helix or beta-sheet, mutations likely accumulate without impacting capsid functions. In comparison to human norovirus structural studies, several antibodies also bound to the top of the norovirus P2 subdomain (31, 33, 50) (Fig. 9C). These norovirus antibodies were typically strain specific, as this region is also comparatively more variable than the P1 subdomain. Additional studies with other RHDV strains using the rapid detection system with 2D9 IgG and sequence analysis of RHDV capsids could aid in determining the specificity.
In order to describe the 2D9 binding interaction in the context of the entire particle, the N11 P domain-Fab complex was fitted into the cryo-EM structure of the RHDV VLP. When the occupancy was fitted with one Fab per P domain dimer on the particle, no apparent clashes with neighboring Fabs were observed, but when the occupancy was fitted with 2 Fabs per P domain dimer clashes with neighboring Fabs were clearly observed (Fig. 9A and 9B). These clashes likely reduced the antibody occupancy on the particles, which was similarly observed with several human norovirus bound antibodies (Fig. 9C) (31, 33, 50). Likewise, an earlier low resolution RHDV antibody cryo-EM study indicated that a neutralizing antibody (termed E3) that bound to the top of the RHDV P domain had an apparent occupancy of 50%, due to steric hindrance between neighboring antibodies (51). Interestingly, recent studies have discovered that norovirus P domain dimers on the particle could undergo significant movement in respect to the S domain, whereby the P domain dimers could be lowered or raised off the S domain as well as rotate around (i.e., looking directly down) the S domain (41, 52–56). The P domain is connected to the S domain via a flexible hinge region, which apparently can allow these P domain movements. Further studies of RHDV VLP bound IgG might help explain this occupancy and possible P domain movement upon antibody binding.
Interestingly, several human norovirus antibody-mediated neutralization studies have shown that norovirus-specific IgG and IgA antibodies could block the HBGA pocket on the capsid and this steric hindrance appeared as a possible neutralization mechanism (Fig. 9C) (31, 33, 50), which leads us to speculate that 2D9 might also have a similar mode of action. Moreover, part of the 2D9 binding region was reported to contain a neutralizing epitope for GI.1a (aa 304–314) (see Fig. 3) and antibodies raised against this region provided protection in rabbits (26). These results suggest that 2D9 IgG might provide host protection from RHDV infection, as the antibody engagement partially also blocked the HBGA binding site. Overall, these findings are expected to assist with the further characterization of other diagnostic antibodies and possibly the development of new RHDV therapeutics that can target the capsid and block the HBGA pocket.
MATERIALS AND METHODS
Protein expression and purification of RHDV P domains.
The RHDV GI.1b (strain: Ast89) and RHDV GI.2 (strain: N11) P domains (GenBank accession numbers: Z49271 and KM878681, respectively) were codon optimized for Escherichia coli expression and cloned into a modified pMal-c2X expression vector (28, 57). Briefly, the pMal-c2X P domains were transformed into BL21(DE3) cells and grown in LB medium at 37°C until the optical density reached OD600 = 0.6, after which the temperature was reduced to 16°C for Ast89 or 20°C for N11 and induced with 0.7 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 18 h. Cells were harvested by centrifugation at 6,500 rpm for 15 min at 4°C and disrupted by sonication on ice. Following the centrifugation, the clarified supernatant containing the His-tagged MBP-P domain fusion protein was purified over a Ni-NTA agarose column and digested with HRV-3C protease (Novagen) overnight at 4°C. Cleaved P domains were separated from the His-tagged MBP on Ni column (Qiagen) and then dialyzed in gel filtration buffer (GFB: 50 mM Tris-HCl [pH 7.6] and 250 mM NaCl) overnight at 4°C. The P domains were purified by size exclusion chromatography using a Superdex-200 column (GE Healthcare), concentrated to 3 mg/mL (Ast89) or 8 mg/mL (N11), and stored in GFB at 4°C.
Protein expression and purification of RHDV VLPs.
Ast89 and N11 capsid genes were cloned into a baculovirus expression system as previously described (58). Ast89 VLPs were expressed in Sf9 cells, while N11 seed virus (purified from Sf9 cells) was used to infect High Five insect cells and produce VLPs. The VLPs present in the supernatant were pelletized by ultracentrifugation at 30,000 rpm (Bechman 45Ti) for 2 h at 4°C and resuspended in PBS (pH 7.4). VLPs were purified using CsCl gradient ultracentrifugation at 35,000 rpm (SW41Ti) for 18 h at 4°C and resuspended in PBS (pH 7.4). The integrity of the VLPs was confirmed by negative-stain electron microscopy (EM). VLPs were diluted in water, applied on carbon coated EM grids and stained with 1% uranyl acetate. The grids were examined on a Zeiss 910 electron microscope (Zeiss) at 50,000-fold magnification.
Preparation of 2D9 Fab fragment.
The 2D9 IgG was developed at the University of Oviedo and Certest produced 65 mg of IgG for ELISA and Fab preparation as previously described (41). The IgG was reduced in 100 mM DTT (pH 7.6) for 1 h at 37°C. Subsequently, DTT was removed by dialyzing the reduced IgG in GFB supplemented with 20 mM HEPES (pH 7.7) for 1 h at 4°C. The IgG was alkylated in the same buffer supplemented with 2 mM iodoacetamide for 48 h at 4°C. To remove the iodoacetamide, dialysis was performed again in GFB-HEPES for 1 h at 4°C. The IgG was oxidized and desalted in a digestion buffer containing 5 mM EDTA in 0.1 M citrate buffer (pH 6) supplemented with 4.39 mg/mL cysteine using a Zebra spin desalting column. The Fab was cleaved from the Fc using Ficin for 4 h at 37°C. The Fab was separated from the Fc on a Protein A column. The Fab was dialyzed in GFB and further purified by size exclusion chromatography using a Superdex-200 column, concentrated to 5 mg/mL, and then stored in GFB at 4°C.
Antibody cross-reactivity.
The cross-reactivity of RHDV VLPs and P domains were analyzed with 2D9 (38) monoclonal antibody using a direct ELISA as previously described (39). Briefly, 96-well microtiter plates were coated with 100 μL/well of Ast89 or N11 VLPs (10 μg/mL) or P domains (10 μg/mL) for 1 h at 37°C. Plates were washed three times with PBS containing 0.1% Tween 20 (PBS-T) and then blocked with PBS containing 5% skim milk (PBS-SM) for 1 h at 25°C. Antibodies were 2-fold serially diluted (from a starting dilution of 1:200) in PBS-T containing 0.5% skim milk (PBS-T-SM). Plates were washed three times with PBS-T and then 100 μL/well of serially diluted antibody was added to triplicate wells for 1 h at 37°C. After washing with PBS-T, 100 μL/well of secondary antibody (anti-mouse-HRP-conjugated antibody, Sigma) was added and incubated for 1 h at 37°C. After washing the plate with PBS-T, 100 μL o-phenylenediamine and H2O2 in phosphate citrate buffer was added to each well for 30 min at 25°C. The reaction was stopped after 30 min with 3 M HCl and the absorbance measured at 490 nm. A cutoff limit was set at OD490 > 0.15, which was ~3 times the value of the negative control (PBS).
Preparation and co-crystallization of Ast89 P domain and N11 P domain-Fab complex for X-ray crystallography.
The Ast89 P domain was crystallized using the hanging drop method. Briefly, a 1:1 ratio of Ast89 P domain and mother solution containing 0.1 M citric acid (pH 4) and 30% PEG6000 were mixed and incubated at 18°C. The N11 P domain and 2D9 Fab were mixed 1:1.4 for 1 h at 25°C. The P domain-Fab complex was purified by size exclusion chromatography and concentrated to 8 mg/mL. Crystals of the N11 P domain-Fab complex were grown by the hanging drop vapor diffusion method, mixing the protein and reservoir solution containing 0.05 M cadmium sulfate, 1 M sodium acetate (pH 7), and 0.1 M HEPES (pH 7.5) in a 1:1 ratio at 18°C. Prior to flash freezing, crystals were transferred to a cryoprotectant containing mother liquor and 30% ethylene glycol.
X-ray crystallography data collection, structure solution, and refinement.
X-ray diffraction data were collected at the European Synchrotron Radiation Facility (ESRF), France at beamlines ID23-1 and BM30A (currently called BM07) and processed with XDS (59). Structures were solved by molecular replacement using previously published RHDV GI.2 P domain (PDB ID: 4X1W) and Fab fragment structures (PDB ID: 3V7A) as search models in PHASER (60). The N-terminal amino acid sequences for 2D9 Fab heavy and kappa chains were determined at Absolute Antibody. Structures were refined in multiple rounds of manual model building in COOT (61) and further refined with PHENIX (62). Structures were validated with Procheck (63) and Molprobity (64). Fab P domain interactions were analyzed using PyMOL (version 1.2r3pre) and Accelrys Discovery Studio (Version 4.1), with hydrogen bonding interactions distances between 2.4 and 3.5 Å. Alternative binding interfaces derived from the crystal packing were analyzed using an online server PDBePISA. Figures and protein contact potentials were generated using PyMOL and Chimera (version 1.10.2).
Isothermal titration calorimetry (ITC) measurements.
ITC experiments were performed using an ITC-200 (GE Healthcare). Samples were dialyzed in PBS (pH 7.4) and filtered (0.45 μM) before experiments. Titrations were performed at 25°C by injecting consecutive (2.5 μL) aliquots of 149 μM N11 P domain into 18 μM 2D9 IgG in 150 s intervals. Injections were performed until saturation was achieved. To correct for the heat of dilution from titrants, control experiments were performed by titrating 2D9 IgG into PBS. Data were fitted using a one-site binding model (MicroCal PEAQ-ITC software).
Sequence analysis.
Amino acid sequences of the complete capsid gene of 19 RHDV sequences representing different variants (22) were aligned in Clustal X, GI.1a (AB300693, AF258618, and MF598301 [K5]), GI.1b (Z49271 [Ast89], AJ319594, and KJ683903), G1.1c (AF231353, EU003579, and KF594473 [Czech]), GI.1d (EF363035 and FR823354), GI.2 (KM878681 [N11], and KT280060), GI.3 (AJ006019 and LT708128), GI.4a (EU871528), GI.4b (GU368898), GI.4c (GU368889), and GI.4d (LT708121).
Data availability.
Atomic coordinates and structure factors were deposited into the Protein Data Bank (PDB) as apo Ast89 P domain (PDB ID: 8CYL) and N11 P domain-Fab complex (PDB ID: 8CZ5).
ACKNOWLEDGMENTS
We thank Dr Penny Rudd (Institute for Glycomics) and Dr Patrice Guillon (Institute for Glycomics) for their critical review of the manuscript. We are grateful to the staff at the European Synchrotron Radiation Facility (ESRF) and the European Molecular Biology Laboratory (EMBL-Grenoble) for assistance and support in using beamlines ID23-1 and BM30A.
We acknowledge the protein crystallization platform within the excellence cluster CellNetworks of the University of Heidelberg for initial crystal screening and DKFZ for the EM facility. G.S.H. was funded by the CHS Foundation and the Deutsche Forschungsgemeinschaft (DFG) (FOR2327). F.P. was supported by grants AGL2013-48550-C2-1-R from the Spanish Ministerio de Economía y Competitividad, cofinanced by FEDER and GRUPIN14-099 from Principado de Asturias (Spain). K.P.D. is supported by grant number MCI-21-PID2020-120349RB-100 from the Spanish Ministerio de Economía y Competitividad.
Contributor Information
Grant S. Hansman, Email: g.hansman@griffith.edu.au.
Colin R. Parrish, Cornell University
REFERENCES
- 1.Camacho-Sillero L, Caballero-Gómez J, Gómez-Guillamón F, Martínez-Padilla A, Agüero M, Miguel ES, Zorrilla I, Rayas E, Talavera V, García-Bocanegra I. 2019. Monitoring of the novel rabbit haemorrhagic disease virus type 2 (GI.2) epidemic in European wild rabbits (Oryctolagus cuniculus) in southern Spain, 2013–2017. Vet Microbiol 237:108361. 10.1016/j.vetmic.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Fordham DA, Cooke BD, Cox T, Mutze G, Strive T. 2014. Distribution and prevalence of the Australian non-pathogenic rabbit calicivirus is correlated with rainfall and temperature. PLoS One 9:e113976. 10.1371/journal.pone.0113976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller A, Freitas J, Silva E, Le Gall-Reculé G, Zwingelstein F, Abrantes J, Esteves PJ, Alves PC, van der Loo W, Kolodziejek J, Nowotny N, Thompson G. 2009. Evolution of rabbit haemorrhagic disease virus (RHDV) in the European rabbit (Oryctolagus cuniculus) from the Iberian Peninsula. Vet Microbiol 135:368–373. 10.1016/j.vetmic.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 4.White PJ, Trout RC, Moss SR, Desai A, Armesto M, Forrester NL, Gould EA, Hudson PJ. 2004. Epidemiology of rabbit haemorrhagic disease virus in the United Kingdom: evidence for seasonal transmission by both virulent and avirulent modes of infection. Epidemiol Infect 132:555–567. 10.1017/s0950268804002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke BD. 2002. Rabbit haemorrhagic disease: field epidemiology and the management of wild rabbit populations. Rev Sci Tech 21:347–358. 10.20506/rst.21.2.1337. [DOI] [PubMed] [Google Scholar]
- 6.Schwensow NI, Cooke B, Kovaliski J, Sinclair R, Peacock D, Fickel J, Sommer S. 2014. Rabbit haemorrhagic disease: virus persistence and adaptation in Australia. Evol Appl 7:1056–1067. 10.1111/eva.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke BD, Fenner F. 2002. Rabbit haemorrhagic disease and the biological control of wild rabbits, in. Wildl Res 29:689–706. 10.1071/WR02010. [DOI] [Google Scholar]
- 8.Cox TE, Ramsey DSL, Sawyers E, Campbell S, Matthews J, Elsworth P. 2019. The impact of RHDV-K5 on rabbit populations in Australia: an evaluation of citizen science surveys to monitor rabbit abundance. Sci Rep 9:15229. 10.1038/s41598-019-51847-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke B, McPhee S, Robinson AJ, Capucci L. 2002. Rabbit haemorrhagic disease: Does a pre-existing RHDV-like virus reduce the effectiveness of RHD as a biological control in Australia? Wildl Res 29:673–682. 10.1071/WR00092. [DOI] [Google Scholar]
- 10.Jahnke M, Holmes EC, Kerr PJ, Wright JD, Strive T. 2010. Evolution and phylogeography of the nonpathogenic calicivirus RCV-A1 in wild rabbits in Australia. J Virol 84:12397–12404. 10.1128/JVI.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arguello Villares JL. 1991. Viral haemorrhagic disease of rabbits: vaccination and immune response. Rev Sci Tech 10:459–480. 10.20506/rst.10.2.554. [DOI] [PubMed] [Google Scholar]
- 12.Abrantes J, van der Loo W, Le Pendu J, Esteves PJ. 2012. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet Res 43:12. 10.1186/1297-9716-43-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capucci L, Fallacara F, Grazioli S, Lavazza A, Pacciarini ML, Brocchi E. 1998. A further step in the evolution of rabbit hemorrhagic disease virus: the appearance of the first consistent antigenic variant. Virus Res 58:115–126. 10.1016/s0168-1702(98)00106-3. [DOI] [PubMed] [Google Scholar]
- 14.Dalton KP, Nicieza I, Balseiro A, Muguerza MA, Rosell JM, Casais R, Alvarez AL, Parra F. 2012. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis 18:2009–2012. 10.3201/eid1812.120341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puggioni G, Cavadini P, Maestrale C, Scivoli R, Botti G, Ligios C, Le Gall-Recule G, Lavazza A, Capucci L. 2013. The new French 2010 rabbit hemorrhagic disease virus causes an RHD-like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet Res 44:96. 10.1186/1297-9716-44-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Gall-Recule G, Lavazza A, Marchandeau S, Bertagnoli S, Zwingelstein F, Cavadini P, Martinelli N, Lombardi G, Guerin JL, Lemaitre E, Decors A, Boucher S, Le Normand B, Capucci L. 2013. Emergence of a new lagovirus related to rabbit haemorrhagic disease virus. Vet Res 44:81. 10.1186/1297-9716-44-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nystrom K, Le Gall-Recule G, Grassi P, Abrantes J, Ruvoen-Clouet N, Le Moullac-Vaidye B, Lopes AM, Esteves PJ, Strive T, Marchandeau S, Dell A, Haslam SM, Le Pendu J. 2011. Histo-blood group antigens act as attachment factors of rabbit hemorrhagic disease virus infection in a virus strain-dependent manner. PLoS Pathog 7:e1002188. 10.1371/journal.ppat.1002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gall G, Arnauld C, Boilletot E, Morisse JP, Rasschaert D. 1998. Molecular epidemiology of rabbit haemorrhagic disease virus outbreaks in France during 1988 to 1995. J Gen Virol 79:11–16. 10.1099/0022-1317-79-1-11. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall-Reculé G, Zwingelstein F, Laurent S, de Boisséson C, Portejoie Y, Rasschaert D. 2003. Phylogenetic analysis of rabbit haemorrhagic disease virus in France between 1993 and 2000, and the characterisation of RHDV antigenic variants. Arch Virol 148:65–81. 10.1007/s00705-002-0908-1. [DOI] [PubMed] [Google Scholar]
- 20.Kerr PJ, Kitchen A, Holmes EC. 2009. Origin and phylodynamics of rabbit hemorrhagic disease virus. J Virol 83:12129–12138. 10.1128/JVI.01523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnear M, Linde CC. 2010. Capsid gene divergence in rabbit hemorrhagic disease virus. J Gen Virol 91:174–181. 10.1099/vir.0.014076-0. [DOI] [PubMed] [Google Scholar]
- 22.Le Pendu J, Abrantes J, Bertagnoli S, Guitton JS, Le Gall-Reculé G, Lopes AM, Marchandeau S, Alda F, Almeida T, Célio AP, Bárcena J, Burmakina G, Blanco E, Calvete C, Cavadini P, Cooke B, Dalton K, Delibes Mateos M, Deptula W, Eden JS, Wang F, Ferreira CC, Ferreira P, Foronda P, Gonçalves D, Gavier-Widén D, Hall R, Hukowska-Szematowicz B, Kerr P, Kovaliski J, Lavazza A, Mahar J, Malogolovkin A, Marques RM, Marques S, Martin-Alonso A, Monterroso P, Moreno S, Mutze G, Neimanis A, Niedzwiedzka-Rystwej P, Peacock D, Parra F, Rocchi M, Rouco C, Ruvoën-Clouet N, Silva E, Silvério D, Strive T, Thompson G, et al. 2017. Proposal for a unified classification system and nomenclature of lagoviruses. J Gen Virol 98:1658–1666. 10.1099/jgv.0.000840. [DOI] [PubMed] [Google Scholar]
- 23.Buehler M, Jesse ST, Kueck H, Lange B, Koenig P, Jo WK, Osterhaus A, Beineke A. 2020. Lagovirus europeus GI.2 (rabbit hemorrhagic disease virus 2) infection in captive mountain hares (Lepus timidus) in Germany. BMC Vet Res 16:166. 10.1186/s12917-020-02386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester NL, Moss SR, Turner SL, Schirrmeier H, Gould EA. 2008. Recombination in rabbit haemorrhagic disease virus: possible impact on evolution and epidemiology. Virology 376:390–396. 10.1016/j.virol.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Hall RN, Capucci L, Matthaei M, Esposito S, Kerr PJ, Frese M, Strive T. 2017. An in vivo system for directed experimental evolution of rabbit haemorrhagic disease virus. PLoS One 12:e0173727. 10.1371/journal.pone.0173727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Xu F, Liu J, Gao B, Liu Y, Zhai Y, Ma J, Zhang K, Baker TS, Schulten K, Zheng D, Pang H, Sun F. 2013. Atomic model of rabbit hemorrhagic disease virus by cryo-electron microscopy and crystallography. PLoS Pathog 9:e1003132. 10.1371/journal.ppat.1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strive T, Piper M, Huang N, Mourant R, Kovaliski J, Capucci L, Cox TE, Smith I. 2020. Retrospective serological analysis reveals presence of the emerging lagovirus RHDV2 in Australia in wild rabbits at least five months prior to its first detection. Transbound Emerg Dis 67:822–833. 10.1111/tbed.13403. [DOI] [PubMed] [Google Scholar]
- 28.Leuthold MM, Dalton KP, Hansman GS. 2015. Structural analysis of a rabbit hemorrhagic disease virus binding to histo-blood group antigens. J Virol 89:2378–2387. 10.1128/JVI.02832-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Pendu J, Nystrom K, Ruvoen-Clouet N. 2014. Host-pathogen co-evolution and glycan interactions. Curr Opin Virol 7:88–94. 10.1016/j.coviro.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Rademacher C, Krishna NR, Palcic M, Parra F, Peters T. 2008. NMR experiments reveal the molecular basis of receptor recognition by a calicivirus. J Am Chem Soc 130:3669–3675. 10.1021/ja710854r. [DOI] [PubMed] [Google Scholar]
- 31.Koromyslova AD, Morozov VA, Hefele L, Hansman GS. 2019. Human norovirus neutralized by a monoclonal antibody targeting the histo-blood group antigen pocket. J Virol 93:e02174-18. 10.1128/JVI.02174-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindesmith LC, McDaniel JR, Changela A, Verardi R, Kerr SA, Costantini V, Brewer-Jensen PD, Mallory ML, Voss WN, Boutz DR, Blazeck JJ, Ippolito GC, Vinje J, Kwong PD, Georgiou G, Baric RS. 2019. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity 50:1530–1541. 10.1016/j.immuni.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarado G, Salmen W, Ettayebi K, Hu L, Sankaran B, Estes MK, Venkataram Prasad BV, Crowe JE, Jr., 2021. Broadly cross-reactive human antibodies that inhibit genogroup I and II noroviruses. Nat Commun 12:4320. 10.1038/s41467-021-24649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzner A, Niedbalski W. 2016. Serological survey for RHD antibodies in rabbits from two types of rabbit breeding farms. Pol J Vet Sci 19:597–607. 10.1515/pjvs-2016-0075. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Kerr PJ, Wright JD, Strive T. 2012. Serological assays to discriminate rabbit haemorrhagic disease virus from Australian non-pathogenic rabbit calicivirus. Vet Microbiol 157:345–354. 10.1016/j.vetmic.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Nagesha HS, McColl KA, Collins BJ, Morrissy CJ, Wang LF, Westbury HA. 2000. The presence of cross-reactive antibodies to rabbit haemorrhagic disease virus in Australian wild rabbits prior to the escape of virus from quarantine. Arch Virol 145:749–757. 10.1007/s007050050668. [DOI] [PubMed] [Google Scholar]
- 37.Capucci L, Nardin A, Lavazza A. 1997. Seroconversion in an industrial unit of rabbits infected with a non-pathogenic rabbit haemorrhagic disease-like virus. Vet Rec 140:647–650. 10.1136/vr.140.25.647. [DOI] [PubMed] [Google Scholar]
- 38.Dalton KP, Podadera A, Granda V, Nicieza I, Del Llano D, González R, de Los Toyos JR, García Ocaña M, Vázquez F, Martín Alonso JM, Prieto JM, Parra F, Casais R. 2018. ELISA for detection of variant rabbit haemorrhagic disease virus RHDV2 antigen in liver extracts. J Virol Methods 251:38–42. 10.1016/j.jviromet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Hansman GS, Natori K, Shirato-Horikoshi H, Ogawa S, Oka T, Katayama K, Tanaka T, Miyoshi T, Sakae K, Kobayashi S, Shinohara M, Uchida K, Sakurai N, Shinozaki K, Okada M, Seto Y, Kamata K, Nagata N, Tanaka K, Miyamura T, Takeda N. 2006. Genetic and antigenic diversity among noroviruses. J Gen Virol 87:909–919. 10.1099/vir.0.81532-0. [DOI] [PubMed] [Google Scholar]
- 40.Dalton KP, Alvarado C, Reytor E, Del Carmen Nunez M, Podadera A, Martinez-Alonso D, Alonso JMM, Nicieza I, Gomez-Sebastian S, Dalton RM, Parra F, Escribano JM. 2021. Chimeric VLPs bearing VP60 from two serotypes of rabbit haemorrhagic disease virus are protective against both viruses. Vaccines (Basel) 9. 10.3390/vaccines9091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansman GS, Taylor DW, McLellan JS, Smith TJ, Georgiev I, Tame JR, Park SY, Yamazaki M, Gondaira F, Miki M, Katayama K, Murata K, Kwong PD. 2012. Structural basis for broad detection of genogroup II noroviruses by a monoclonal antibody that binds to a site occluded in the viral particle. J Virol 86:3635–3646. 10.1128/JVI.06868-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovaliski J, Sinclair R, Mutze G, Peacock D, Strive T, Abrantes J, Esteves PJ, Holmes EC. 2014. Molecular epidemiology of rabbit haemorrhagic disease virus in Australia: when one became many. Mol Ecol 23:408–420. 10.1111/mec.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eden JS, Read AJ, Duckworth JA, Strive T, Holmes EC. 2015. Resolving the origin of rabbit hemorrhagic disease virus: insights from an investigation of the viral stocks released in Australia. J Virol 89:12217–12220. 10.1128/jvi.01937-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahar JE, Nicholson L, Eden JS, Duchêne S, Kerr PJ, Duckworth J, Ward VK, Holmes EC, Strive T. 2016. Benign rabbit caliciviruses exhibit evolutionary dynamics similar to those of their virulent relatives. J Virol 90:9317–9329. 10.1128/jvi.01212-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capucci L, Frigoli G, Ronshold L, Lavazza A, Brocchi E, Rossi C. 1995. Antigenicity of the rabbit hemorrhagic disease virus studied by its reactivity with monoclonal antibodies. Virus Res 37:221–238. 10.1016/0168-1702(95)00033-m. [DOI] [PubMed] [Google Scholar]
- 46.Laurent S, Vautherot JF, Le Gall G, Madelaine MF, Rasschaert D. 1997. Structural, antigenic and immunogenic relationships between European brown hare syndrome virus and rabbit haemorrhagic disease virus. J Gen Virol 78:2803–2811. 10.1099/0022-1317-78-11-2803. [DOI] [PubMed] [Google Scholar]
- 47.Marchandeau S, Le Gall-Reculé G, Bertagnoli S, Aubineau J, Botti G, Lavazza A. 2005. Serological evidence for a non-protective RHDV-like virus. Vet Res 36:53–62. 10.1051/vetres:2004049. [DOI] [PubMed] [Google Scholar]
- 48.Rodak L, Granatova M, Valicek L, Smid B, Vesely T, Nevorankova Z. 1990. Monoclonal antibodies to rabbit haemorrhagic disease virus and their use in the diagnosis of infection. J Gen Virol 71:2593–2598. 10.1099/0022-1317-71-11-2593. [DOI] [PubMed] [Google Scholar]
- 49.Schirrmeier H, Reimann I, Köllner B, Granzow H. 1999. Pathogenic, antigenic and molecular properties of rabbit haemorrhagic disease virus (RHDV) isolated from vaccinated rabbits: detection and characterization of antigenic variants. Arch Virol 144:719–735. 10.1007/s007050050538. [DOI] [PubMed] [Google Scholar]
- 50.Shanker S, Czako R, Sapparapu G, Alvarado G, Viskovska M, Sankaran B, Atmar RL, Crowe JE, Jr, Estes MK, Prasad BV. 2016. Structural basis for norovirus neutralization by an HBGA blocking human IgA antibody. Proc Natl Acad Sci USA 113:E5830–E5837. 10.1073/pnas.1609990113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thouvenin E, Laurent S, Madelaine MF, Rasschaert D, Vautherot JF, Hewat EA. 1997. Bivalent binding of a neutralizing antibody to a calicivirus involves the torsional flexibility of the antibody hinge. J Mol Biol 270:238–246. 10.1006/jmbi.1997.1095. [DOI] [PubMed] [Google Scholar]
- 52.Devant JM, Hansman GS. 2021. Structural heterogeneity of a human norovirus vaccine candidate. Virology 553:23–34. 10.1016/j.virol.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Koromyslova AD, Devant JM, Kilic T, Sabin CD, Malak V, Hansman GS. 2020. Nanobody-mediated neutralization reveals an achilles heel for norovirus. J Virol 94:e00660-20. 10.1128/JVI.00660-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu L, Salmen W, Chen R, Zhou Y, Neill F, Crowe JE, Jr, Atmar RL, Estes MK, Prasad BVV. 2022. Atomic structure of the predominant GII.4 human norovirus capsid reveals novel stability and plasticity. Nat Commun 13:1241. 10.1038/s41467-022-28757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song C, Takai-Todaka R, Miki M, Haga K, Fujimoto A, Ishiyama R, Oikawa K, Yokoyama M, Miyazaki N, Iwasaki K, Murakami K, Katayama K, Murata K. 2020. Dynamic rotation of the protruding domain enhances the infectivity of norovirus. PLoS Pathog 16:e1008619. 10.1371/journal.ppat.1008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katpally U, Voss NR, Cavazza T, Taube S, Rubin JR, Young VL, Stuckey J, Ward VK, Virgin H, Wobus CE, Smith TJ. 2010. High-resolution cryo-electron microscopy structures of murine norovirus 1 and rabbit hemorrhagic disease virus reveal marked flexibility in the receptor binding domains. J Virol 84:5836–5841. 10.1128/JVI.00314-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansman GS, Biertumpfel C, Georgiev I, McLellan JS, Chen L, Zhou T, Katayama K, Kwong PD. 2011. Crystal structures of GII.10 and GII.12 norovirus protruding domains in complex with histo-blood group antigens reveal details for a potential site of vulnerability. J Virol 85:6687–6701. 10.1128/JVI.00246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hansman GS, Natori K, Oka T, Ogawa S, Tanaka K, Nagata N, Ushijima H, Takeda N, Katayama K. 2005. Cross-reactivity among sapovirus recombinant capsid proteins. Arch Virol 150:21–36. 10.1007/s00705-004-0406-8. [DOI] [PubMed] [Google Scholar]
- 59.Kabsch W. 1993. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr 26:795–800. 10.1107/S0021889893005588. [DOI] [Google Scholar]
- 60.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. 2007. Phaser crystallographic software. J Appl Crystallogr 40:658–674. 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emsley P, Lohkamp B, Scott WG, Cowtan K. 2010. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris AL, MacArthur MW, Hutchinson EG, Thornton JM. 1992. Stereochemical quality of protein structure coordinates. Proteins 12:345–364. 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- 64.Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66:12–21. 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Atomic coordinates and structure factors were deposited into the Protein Data Bank (PDB) as apo Ast89 P domain (PDB ID: 8CYL) and N11 P domain-Fab complex (PDB ID: 8CZ5).