Abstract
BACKGROUND:
Amyloid-β (Aβ) likely plays a primary role in Alzheimer’s disease pathogenesis, but longitudinal Aβ, tau, and neurodegeneration (A/T/N) measurements in the same individuals have rarely been examined to verify the temporal dynamics of these biomarkers.
METHODS:
In this study, we investigated the temporal ordering of Aβ, tau, and neurodegeneration using longitudinal biomarkers in nondemented elderly individuals. A total of 395 cognitively unimpaired individuals and 204 individuals with mild cognitive impairment (320 [53%] were female) were classified into 8 A±/T±/N± categories according to the abnormal (+)/normal (−) status of Aβ (18F-florbetapir or 18F-florbetaben) positron emission tomography (PET), 18F-flortaucipir PET, and adjusted hippocampal volume (aHCV). Follow-up Aβ PET, tau PET, and aHCV measurements at 0.6 to 4.1 years were available for 35% to 63% of the sample. Baseline Aβ, tau, and aHCV were compared between different A/T/N profiles. We investigated the associations of baseline and longitudinal Aβ, tau, and neurodegeneration in relation to one another continuously.
RESULTS:
Among T− participants, tau was higher for A+/T−/N− individuals compared with the A−/T−/N− group (p = .02). Among N− participants, neurodegeneration was worse among A+/T+/N− individuals compared with the A−/T−/N− group (p = .001). High baseline Aβ was associated (p < .001) with subsequent tau increase and high baseline tau was associated (p = .002) with subsequent aHCV decrease, whereas high tau and low aHCV at baseline were not associated with subsequent Aβ increase.
CONCLUSIONS:
These findings define a sequence of pathological events in Alzheimer’s disease that support a current model of Alzheimer’s disease pathogenesis in which Aβ appears early, followed by deposition of abnormal tau aggregates and subsequent neurodegeneration.
Amyloid-β (Aβ) plaques and neurofibrillary tangles are hallmarks of Alzheimer’s disease (AD) and appear before the presence of manifest clinical symptoms (1). Measurement of amyloid (A) and tau (T) is possible with positron emission tomography (PET) scanning and cerebrospinal fluid assays, and neurodegeneration (N) can be quantified with structural magnetic resonance imaging (MRI) or PET glucose metabolism; dichotomous ratings for each category (A±/T±/N±) may be combined to define 8 possible biomarker categories in characterizing individuals for features of AD (2). According to the dominant model of disease pathogenesis (3), Aβ aggregation plays an initiating role in disease onset, followed by the deposition of abnormal tau aggregates and subsequent neurodegeneration, which then eventuates in cognitive decline. This model generates empirically testable predictions, including the hypothesis that Aβ is the driver of subsequent downstream pathology, and the implication that the presence of abnormalities of Aβ, tau, and neurodegeneration together should be the most malignant biomarker profile. These hypotheses have been difficult to examine without longitudinal biomarker and cognitive data, but recent studies have begun to investigate them, revealing the importance of tau pathology and interactions between all 3 biomarkers in leading to cognitive decline (4,5). Characterization of individuals according to A/T/N profiles with either cerebrospinal fluid or imaging biomarkers has further demonstrated that the presence of Aβ and tau together, or all 3 abnormal biomarkers, is most strongly associated with cognitive decline in nondemented individuals (6–8).
In addition to understanding how biomarkers may interact to produce cognitive decline, longitudinal data can provide insight into the temporal dynamics of biomarker changes. For example, the current model of AD pathogenesis is built on data indicating that Aβ is associated with subsequent tau deposition (9–11) or longitudinal tau change (12). Longitudinal measurement of Aβ and neurodegeneration has also been investigated (13–16), as have relationships between tau and neurodegeneration (17), with these results generally supporting a unidirectional model with Aβ as an early event, tau an intermediate event, and neurodegeneration a late event. However, data have also suggested that neurodegeneration may arise independently of Aβ (18–21), and other studies have claimed that either cognitive decline (22) or tau aggregation (23) may precede Aβ deposition. The longitudinal model of AD is difficult to evaluate and test when only a subset of variables is analyzed. Here, we examined all variables, using a single cohort, in the proposed amyloid cascade model of AD, with a view toward understanding the directionality of each set of biomarker relationships.
In this study, we first compared Aβ, tau, neurodegeneration, and cognition dichotomously (A/T/N profiles) in nondemented adults from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database using both cross-sectional and longitudinal analyses. We subsequently investigated the relationships of continuous measures of Aβ, tau, and neurodegeneration in relation to cognition. The project aimed 1) to describe the baseline (cross-sectional) participant characteristics using the A/T/N schema and compare how abnormalities in biomarkers were related to one another; and 2) to examine the associations between continuous longitudinal Aβ, tau, and neurodegeneration in relation to each other and cognition. The ultimate end was to examine whether evidence supported a unidirectional pathway beginning with Aβ in disease pathogenesis.
METHODS AND MATERIALS
Participants
The data were obtained from the ADNI database (https://ida.loni.usc.edu). The ADNI study was approved by institutional review boards of all participating centers, and written informed consent was obtained from all participants or their authorized representatives. Cognitively unimpaired (CU) ADNI participants and ADNI participants with mild cognitive impairment (MCI) with concurrent (interval of acquisition <1 year) amyloid (18F-florbetapir [FBP] or 18F-florbetaben [FBB]) PET, 18F-flortaucipir (FTP) tau PET, structural MRI, and cognition were included in this study.
Amyloid PET Imaging
Details on FBP and FBB Aβ PET image acquisition are given elsewhere (http://adni-info.org). Baseline and follow-up FBP or FBB scans were coregistered to their corresponding baseline structural MRI scans. FreeSurfer (V5.3.0; https://surfer.nmr.mgh.harvard.edu/) was used to extract cortical tracer retention in 34 regions of interest (ROIs) as described previously (24). FBP or FBB standardized uptake value ratios (SUVRs) were calculated by referring regional florbetapir or florbetaben to that found in the whole cerebellum. A cortical summary COMPOSITE SUVR was created from a composite cortical area (comprising frontal, cingulate, parietal, and temporal regions) (24). FBP and FBB SUVRs were converted to Centiloids using the equations Centiloid = (196.9 × SUVRFBP) − 196.03 and Centiloid = (159.08 × SUVRFBB) − 151.65, respectively, as described on the ADNI website (ADNI_Centiloid_Methods-Instruction_20181113.pdf).
Tau PET Imaging
Details on FTP tau PET image acquisition are given elsewhere (http://adni-info.org). Baseline and follow-up FTP scans were coregistered to the baseline MRI scan that was closest in time to the baseline FTP scan. FTP SUVRs in FreeSurfer-defined ROIs were calculated based on mean uptake over 75 to 105 minutes postinjection normalized by a mean inferior cerebellar gray matter uptake (25). FTP SUVRs in a temporal metaROI (entorhinal, amygdala, parahippocampal, fusiform, inferior temporal, and middle temporal) (26) were calculated to represent tau deposition, because this region has been commonly used to detect AD-related tau deposition in the human brain (7,27–31).
Structural MRI
Hippocampal volume (HCV) (cm3) was calculated across hemispheres from the structural MRI scans using FreeSurfer and adjusted by estimated total intracranial volume using the approach employed by Jack et al. (32): the adjusted HCV (aHCV) was calculated as the difference between the raw HCV and the expected HCV from a linear regression of raw HCV (y-axis) and total intracranial volume (x-axis) among 328 Aβ− ADNI CU participants.
Cutoffs of Biomarkers
Aβ positivity of FBP and FBB were defined as COMPOSITE FBP SUVR ≥1.11 and FBB SUVR ≥1.08 as described on the ADNI website. FTP SUVRs and aHCVs do not have clear bimodal distributions (Figures S2A and S4A). The threshold for the temporal-metaROI FTP SUVR was set as ≥1.25 according to the receiver operating characteristic curve analysis using the Youden index classifying 277 Aβ− ADNI CU participants and 176 Aβ+ ADNI MCI and AD patients as the end point (Figure S1), which was consistent with the threshold calculated in another sample (7). The threshold of abnormal aHCV was set ≤−0.82 cm3 according to a receiver operating characteristic analysis classifying 440 ADNI Aβ− CU participants and 555 Aβ+ patients with MCI and AD as the end point (Figure S3). In order to examine the effect of cutoffs selection for FTP SUVR and aHCV, we also used previously reported cutoffs for temporal-metaROI FTP SUVR (26) ≥1.23 and aHCV (32,33) ≥−0.70 cm3 defined by different samples as alternative cutoffs to define T± and N±.
Cognition
Preclinical Alzheimer’s cognitive composite (PACC) scores (34) that were concurrent with baseline Aβ PET and tau PET and up to 4-year follow-up were used to represent cognitive ability. The delayed recall portion of the Alzheimer’s Disease Assessment Scale, the delayed recall score on the logical memory IIa subtest from the Wechsler Memory Scale, the digit symbol substitution test score from the Wechsler Adult Intelligence Scale–Revised, and the Mini-Mental State Examination total score were transferred to standard z scores (using the mean values of ADNI CU participants), and these 4 cognitive z scores were summed to form the PACC score.
Statistical Analysis
Participants were classified as positive (+) or negative (−) for each biomarker, resulting in 8 A/T/N groups. A−/T−/N− participants were considered “AD-biomarker normal” and were used as the reference for statistical comparison relative to the other A/T/N groups. All other A− participants were characterized as suspected non-Alzheimer’s pathology, and all A+ groups were characterized as on the AD continuum (2). Data are presented as median (interquartile range [IQR]) or number and percentage. Different A/T/N groups were compared using a Mann-Whitney test. We assessed categorical differences using Fisher’s exact test. Baseline Aβ PET (Centiloid), tau PET (temporal-metaROI FTP SUVR), neurodegeneration (aHCV), and cognition were compared among different A/T/N groups. A false discovery rate of .05 using the Benjamini-Hochberg approach was employed for multiple comparisons correction. Linear mixed-effects (LME) (lme4 package) models investigated subsequent longitudinal changes in aHCV and the PACC score over time in different A/T/N groups based on the following independent variables: time, A/T/N group, A/T/N group × time, diagnosis, APOE-ε4 status, age, gender, and education.
In order to avoid findings dependent on dichotomous A/T/N biomarkers, LME models investigated 1) the associations between baseline FTP SUVR and aHCV as well as their interaction and subsequent change in Aβ PET over time; 2) the associations between baseline Aβ PET and aHCV as well as their interaction and subsequent change in FTP SUVR over time; and 3) the associations between baseline Aβ PET and FTP SUVR as well as their interaction and subsequent change in aHCV over time, controlling for diagnosis, APOE-ε4 status, age, and gender. Finally, we used LME models to investigate the associations between baseline Aβ PET, FTP SUVR, and aHCV as well as their interaction and subsequent change in PACC score over time, controlling for diagnosis, APOE-ε4 status, age, gender, and education. Baseline Aβ PET, FTP SUVR, and aHCV were computed as a z score (centered and scaled), and the sign of aHCV was changed to negative (−) to facilitate the comparison with Aβ and tau in these LME models with continuous Aβ, tau, and neurodegeneration variables. All the LME models included a random slope and intercept for each participant, with 2-tailed tests and p < .05 as the significance level. All statistical analyses were performed in the statistical program R (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographics
Measurements were acquired between August 12, 2015, and December 4, 2019, and the characteristics of the 599 participants included in this study can be found in Table 1. Overall, 43% of participants were AD-biomarker normal, and 39% were on the AD continuum. A total of 34% of participants had MCI, with the A+/T+/N+ group showing the largest proportion (82%). The 4 N+ groups were older than the 4 N− groups (age, median [IQR] = 79 [9] years vs. 71 [9] years; estimate = 6.56 [95% confidence interval (CI), 5.31–7.83], p < .001). The proportion of APOE-ε4 carriers was greater among the 4 A+ groups than among the 4 A− groups (49% vs. 19%; odds ratio, 4.9 [95% CI, 3.3–7.5], p < .001). Longitudinally, 121, 185, 218, and 299 participants had ≥2 Aβ PET, tau PET, structural MRI, and PACC scores, and the duration of follow-up was a median of 2.0 (IQR, 0.2; range, 0.8–4.1), 2.0 (IQR, 1.0; range, 0.6–4.0), 1.2 (IQR, 0.9; range, 0.9–4.1), and 1.2 (IQR, 1.0; range, 0.8–4.1) years, respectively.
Table 1.
Demographic Characteristics of Participants
| Normal | SNAP | AD Continuum | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| A−/T−/N− | A−/T+/N− | A−/T−/N + | A−/T+/N + | A+/T−/N + | A+/T−/N− | A+/T+/N− | A+/T+/N + | |
|
| ||||||||
| Participants With Concurrent Aβ PET, Tau PET, MRI, and PACC Score at Baseline (n = 599) | ||||||||
|
| ||||||||
| A/T/N, n (%) | 259 (43) | 26 (4) | 65 (11) | 13 (2) | 36 (6) | 84 (14) | 67 (11) | 49 (8) |
|
| ||||||||
| MCI, n (%) | 57 (22) | 10 (38) | 28 (43) | 8 (62) | 13 (36) | 19 (23) | 29 (43) | 40 (81) |
|
| ||||||||
| Age, Years, Median (IQR) | 70 (8) | 74 (9) | 77 (9) | 81 (7) | 82 (10) | 72 (9) | 74 (10) | 79 (8) |
|
| ||||||||
| Education, Years, Median (IQR) | 17 (2) | 18 (4) | 18 (5) | 17 (2) | 16 (4) | 17 (3) | 16 (2) | 16 (4) |
|
| ||||||||
| Female, n (%) | 151 (58) | 15 (58) | 27 (42) | 4 (31) | 15 (42) | 40 (48) | 45 (67) | 23 (47) |
|
| ||||||||
| APOE-ε4, n (%) | 51 (20) | 4 (15) | 11 (17) | 2 (15) | 13 (36) | 41 (49) | 35 (52) | 26 (53) |
|
| ||||||||
| Participants With ≥2 Aβ PET Scans (n = 121) | ||||||||
|
| ||||||||
| A/T/N, n (%) | 54 (45) | 5 (4) | 9 (7) | 4 (3) | 3 (2) | 17 (14) | 15 (12) | 14 (12) |
|
| ||||||||
| FU Visits, Median (IQR, Range) | 2 (0, 2–3) | 2 (1, 2–3) | 2 (0, 2–3) | 2 (0, 2–2) | 2 (0, 2–2) | 2 (0, 2–3) | 2 (0, 2–2) | 2 (0, 2–3) |
|
| ||||||||
| Duration of FU, Years, Median (IQR, Range) | 2.0 (0.3, 0.8–4.1) | 2.0 (1.1, 1.1–3.7) | 2.0 (0.4, 1.1–3.7) | 2.0 (0.1, 1.8–2.1) | 2.1 (0.0, 2.0–2.1) | 1.9 (0.1, 0.8–3.7) | 2.0 (0.1, 1.5–3.0) | 2.0 (0.4, 1.0–3.3) |
|
| ||||||||
| Participants With ≥2 Tau PET Scans (n = 185) | ||||||||
|
| ||||||||
| A/T/N, n (%) | 54 (29) | 6 (3) | 11 (6) | 3 (2) | 16 (9) | 40 (22) | 34 (18) | 21 (11) |
|
| ||||||||
| FU Visits, Median (IQR, Range) | 2 (0, 2–5) | 2 (0, 2–4) | 2 (0, 2–4) | 3 (0.5, 2–3) | 2 (0, 2–3) | 2 (0, 2–4) | 2 (1, 2–4) | 2 (1, 2–4) |
|
| ||||||||
| Duration of FU, Years, Median (IQR, Range) | 1.2 (1.0, 0.6–3.8) | 1.1 (0.4, 0.8–3.1) | 1.2 (0.5, 0.7–2.9) | 2.2 (0.2, 1.9–2.3) | 1.0 (0.4, 0.6–2.1) | 1.0 (0.5, 0.7–3.3) | 1.2 (1.0, 0.8–4.0) | 1.5 (1.0, 1.0–3.1) |
|
| ||||||||
| Participants With ≥2 MRI Scans (n = 218) | ||||||||
|
| ||||||||
| A/T/N, n (%) | 74 (34) | 9 (4) | 17 (18) | 6 (3) | 15 (7) | 40 (18) | 34 (15) | 23 (11) |
|
| ||||||||
| FU Visits, Median (IQR, Range) | 2 (1,2–5) | 2 (0, 2–4) | 2 (0, 2–4) | 2 (0.75, 2–3) | 2 (0, 2–3) | 2 (0, 2–4) | 2 (0, 2–3) | 2 (1, 2–4) |
|
| ||||||||
| Duration of FU, Years, Median (IQR, Range) | 1.9 (1.1, 0.9–4.1) | 1.6 (0.9, 1.0–3.1) | 1.2 (0.9, 1.0–3.6) | 1.6 (0.9, 1.0–2.0) | 1.1 (0.2, 0.9–2.1) | 1.1 (0.6, 0.9–3.3) | 1.1 (0.5, 1.0–4.0) | 1.6 (0.9, 1.0–3.2) |
|
| ||||||||
| Participants With ≥2 PACC Scores (n = 299) | ||||||||
|
| ||||||||
| A/T/N, n (%) | 109 (36) | 12 (4) | 30 (10) | 10 (3) | 24 (8) | 47 (16) | 38 (13) | 29 (10) |
|
| ||||||||
| FU Visits, Median (IQR, Range) | 2 (1, 2–5) | 2 (0.25, 2–4) | 2 (0, 2–4) | 2 (1, 2–3) | 2 (1, 2–3) | 2 (1, 2–4) | 2 (1, 2–4) | 2 (1, 2–4) |
|
| ||||||||
| Duration of FU, Years, Median (IQR, Range) | 2.0 (1.0, 0.9–4.1) | 2.0 (1.0, 1.0–3.1) | 1.1 (1.0, 0.9–3.6) | 1.3 (0.9, 1.0–2.1) | 1.1 (1.0, 0.9–2.2) | 1.1 (1.0, 0.8–3.7) | 1.1 (1.0, 1.0–4.0) | 1.5 (0.9, 1.0–3.5) |
A/T/N, amyloid-β/tau/neurodegeneration; Aβ, amyloid-β; AD, Alzheimer’s disease; FU, follow-up; IQR, interquartile range; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; PACC, preclinical Alzheimer’s cognitive composite; PET, positron emission tomography; SNAP, suspected non-Alzheimer’s pathology.
Baseline Aβ, Tau, Neurodegeneration, and Cognition of Different A/T/N Groups
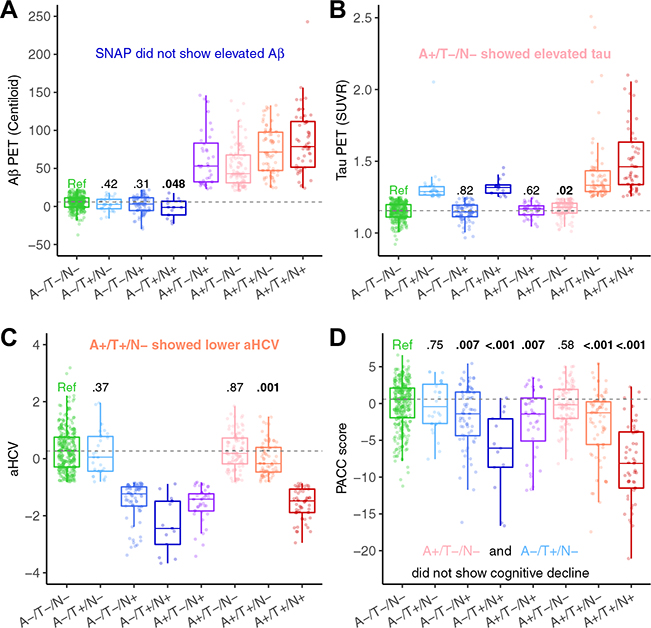
Cross-sectional continuous biomarker levels are shown in Figure 1, and these were elevated according to the classification schemes. Compared with the reference (A−/T−/N−), among T− participants (Figure 1B and Figure S5B), the A+/T−/N− individuals had a higher FTP SUVR (estimate = 0.02 [95% CI, 0.004 to 0.03], p= .02), and the A+/T−/N+ individuals had weakly but not significantly higher FTP SUVR; however, among A− participants, the A−/T+ individuals did not show higher Aβ PET, and A−/T+/N+ individuals even had significantly lower Aβ PET, perhaps owing to atrophy (Figure 1A and Figure S5A). Among N− participants, A+/T+/N− individuals had lower aHCV (estimate = −0.301 [95% CI, −0.487 to −0.119], p= .001) (Figure 1C and Figure S5C), whereas A−/T−/N+ individuals did not show either higher Aβ or tau (Figure 1A, B and Figure S5A, B), consistent with Aβ and tau lowering hippocampal volume even in N− individuals, but there was no effect of neurodegeneration on Aβ or tau in A−/T− individuals. PACC scores were lower than the reference in all groups except the A−/T+/N− and A+/T−/N− groups (p ≤ .007). The A+/T+/N+ group had lower (p < .001) PACC scores than other groups, except for the A−/T+/N+ group (Figure 1D). The details of comparisons of baseline Aβ, tau, aHCV, and PACC between different A/T/N profiles and the reference group can be found in Tables S1 and S2. The results were substantially the same using the alternative cutoffs for FTP SUVR and aHCV to define T± and N± (Figure S6).
Figure 1.
Baseline amyloid-β (Aβ) (A), tau (T), neurodegeneration (N), and cognition of different A/T/N groups. Comparison of baseline (A) Aβ positron emission tomography (PET), (B) tau PET, (C) adjusted hippocampal volume (aHCV), and (D) preclinical Alzheimer’s cognitive composite (PACC) of suspected non-Alzheimer’s pathology (SNAP) and Alzheimer’s disease continuum groups with the reference group (Ref) (A−/T−/N−). The boxplot whiskers extend to the lowest and highest data points within 1.5 times the interquartile range, from the lower and upper quartiles. The dots represent individual points of each A/T/N group. Gray dashed lines represent the median values of the reference. Values at the top of the bar indicate the p values of the comparisons with the reference. SUVR, standardized uptake value ratio.
Longitudinal Changes of Neurodegeneration and Cognition Over Time of Different A/T/N Groups
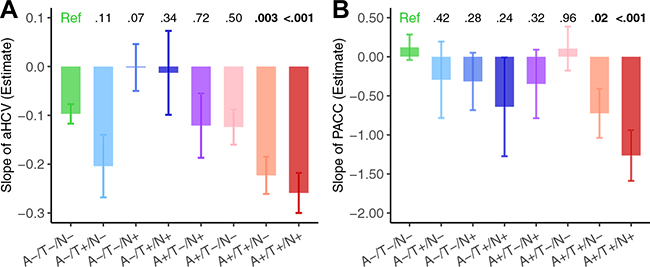
Results of LME models showed that both the A+/T+/N+ and A+/T+/N− groups showed greater decline than the reference group in aHCV (n = 218; A+/T+/N− vs. A−/T−/N−: estimate = −0.127 [95% CI, −0.211 to −0.043], SE = 0.043, p = .003; A1T+/N+ vs. A−/T−/N−: estimate = −0.162 [95% CI, −0.252 to −0.072], SE = 0.046, p < .001) (Figure 2A) and PACC (n = 299; A+/T+/N− vs. A−/T−/N−: estimate = −0.85 [95% CI, −1.54 to −0.15], SE = 0.36, p = .02; A1T+/N+ vs. A−/T−/N−: estimate = −1.39 [95% CI, −2.10 to −0.67], SE = 0.36, p < .001) (Figure 2B). The details of comparisons of longitudinal changes of aHCV and PACC between different A/T/N profiles and the reference group can be found in Table S3. The results were substantially the same using the alternative cutoffs for FTP SUVR and aHCV to define T± and N± (Figure S8).
Figure 2.
Longitudinal changes of neurodegeneration and cognition over time of different amyloid-β (A)/tau (T)/neurodegeneration (N) groups. Comparisons of slopes of (A) adjusted hippocampal volume (aHCV) decreases in 218 participants with longitudinal magnetic resonance imaging data and (B) preclinical Alzheimer’s cognitive composite (PACC) cognitive score decline in 299 participants with longitudinal cognitive data. Error bars reflect the standard error of estimated slope in linear mixed-effects model analyses. Values at the top of the bar indicate the p values of the comparisons with the reference (Ref).
Aβ, Tau, and Neurodegeneration in Relation to Each Other
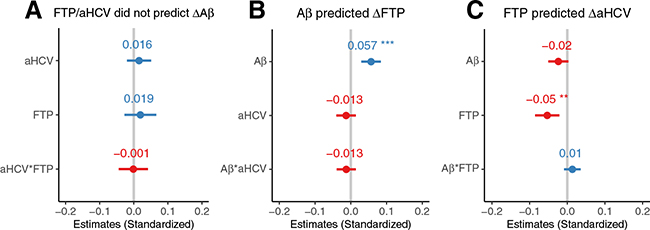
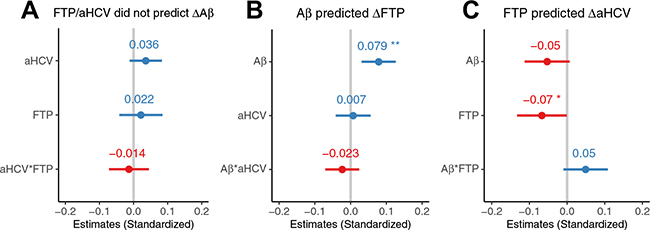
In 121 participants with longitudinal Aβ PET, neither high baseline FTP SUVR nor low aHCV (Figure 3A) was associated with subsequent Aβ PET increases. However, in 185 participants with longitudinal tau PET, high baseline Aβ PET (but not low aHCV) was associated (estimate = 0.059 [95% CI, 0.031 to 0.087], p < .001) with subsequent tau PET increase (Figure 3B). In addition, subsequent longitudinal aHCV decreases of 218 participants with longitudinal aHCV data were significantly associated with high baseline tau PET (estimate = −0.053 [95% CI, −0.086 to −0.021], p= .002) and were marginally associated with high Aβ PET (estimate = −0.024 [95% CI, −0.051 to 0.003], p = .07), and there was no significant interaction between Aβ PET and tau PET (estimate = 0.014 [95% CI, −0.009 to 0.036], p = .23) (Figure 3C). In order to avoid the different longitudinal sample sizes of Aβ PET, tau PET, and MRI scans affecting the conclusions, we also did the same analyses based on 76 participants with longitudinal Aβ PET, tau PET, and MRI scans all available at follow-up. The results were substantially the same (Figure 4 and Supplement).
Figure 3.
Amyloid-β (Aβ) (A), tau (T), and neurodegeneration (N) in relation to each other. (A) Baseline high tau positron emission tomography (PET) and low adjusted hippocampal volume (aHCV) were not associated with subsequent Aβ PET increase (ΔAb) in 121 participants with longitudinal Aβ PET data. (B) Baseline high Aβ PET but not low aHCV was associated with subsequent tau PET increase (Δ18F-flortaucipir [ΔFTP]) in 185 participants with longitudinal tau PET data. (C) Baseline high tau PET but not high Aβ PET was associated with subsequent aHCV decrease (ΔaHCV) in 218 participants with longitudinal magnetic resonance imaging data. The error bars indicate 95% confidence interval of the estimated β coefficient. aHCV*FTP, Aβ*aHCV, and Aβ*FTP indicate the interaction between aHCV and FTP, Aβ and aHCV, and Aβ and FTP, respectively. **p < .01; ***p < .001.
Figure 4.
Amyloid-β (Aβ) (A), tau (T), and neurodegeneration (N) in relation to each other in 76 participants with longitudinal Aβ positron emission tomography (PET), tau PET, and adjusted hippocampal volume (aHCV) data all available. (A) Baseline high tau PET and low aHCV were not associated with subsequent Aβ PET increase (ΔAb). (B) Baseline high Aβ PET but not low aHCV was associated with subsequent tau PET (Δ18F-flortaucipir [ΔFTP]). (C) Baseline high tau PET but not high Aβ PET was associated with subsequent aHCV decrease (ΔaHCV). The error bars indicate 95% confidence interval of the estimated β coefficient. HCV*FTP, Aβ*aHCV, and Aβ*FTP indicate the interaction between aHCV and FTP, Aβ and aHCV, and Aβ and FTP, respectively. *p < .05; **p < .01.
Aβ, Tau, and Neurodegeneration in Relation to Cognition
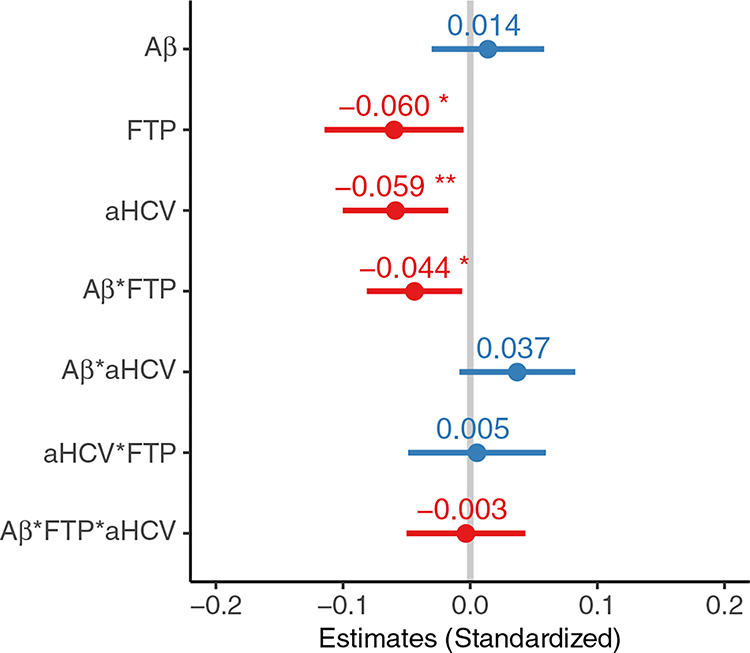
Results of LME models showed that high baseline tau PET (estimate = −0.060 [95% CI, −0.115 to −0.005], p = .04) and low aHCV (estimate = −0.059 [95% CI, −0.100 to −0.017], p= .003) (but not high Aβ PET) were associated with subsequent longitudinal PACC score decline over a median of 2.0 (IQR, 1.0) years of follow-up in 299 participants with longitudinal cognitive data (Figure 5). In addition, there was an interaction between baseline high Aβ PET and tau PET in relation to subsequent PACC score decline (estimate = −0.044 [95% CI, −0.081 to −0.006], p = .02), but no other significant interactions (Aβ × aHCV: estimate = 0.037 [95% CI, −0.009 to 0.083], p = .11) were detected.
Figure 5.
Amyloid-β (Aβ) (A), tau (T), and neurodegeneration (N) in relation to cognition. Estimates of association with longitudinal preclinical Alzheimer’s cognitive composite changes of Aβ positron emission tomography (PET), 18F-flortaucipir (FTP) standardized uptake value ratio, and adjusted hippocampal volume (aHCV), as well as their interactions in a linear mixed-effects model in 299 participants with longitudinal cognitive data. The error bars indicate 95% confidence interval of the estimated β coefficient. Aβ*FTP, Aβ*aHCV, aHCV*FTP, and Aβ*FTP*aHCV indicate the interaction between Aβ and FTP; Aβ and aHCV; aHCV and FTP; and Aβ, and FTP and aHCV, respectively. *p < .05; **p < .01.
DISCUSSION
This study provides cross-sectional and longitudinal evidence for the proposed sequence of pathological biomarker changes in AD that occur in one direction only, beginning with Aβ deposition, through tau deposition, neurodegeneration, and cognitive decline. We first used cross-sectional data to examine variability within the negative/normal ranges of Aβ, tau, and neurodegeneration biomarkers in order to identify the earliest signs of abnormality in A/T/N groups. This showed that tau in the negative range was elevated among A+/T−/N− individuals, presumably indicating very early signs of tau elevation following Aβ positivity among individuals. Conversely, Aβ was not elevated in the Aβ− range among A−/T+ individuals, suggesting that tau positivity does not lead to early Aβ changes. Compared with the A−/T−/N− group, we also found that hippocampal volumes were smaller among A+/T+/N− individuals, while there was no evidence for higher Aβ or tau among the A−/T−/N+ individuals. These cross-sectional data imply that elevated Aβ affects tau at an early stage and that Aβ and tau together are especially malignant with regard to hippocampal volume, but there is no evidence that elevated tau affects Aβ or that neurodegeneration affects either Aβ or tau. The longitudinal biomarker data extend these findings by indicating that high baseline Aβ was associated with subsequent tau accumulation, whereas baseline high tau was not associated with subsequent increase in Aβ. High baseline tau was associated with subsequent loss in hippocampal volume, and the most pronounced hippocampal atrophy was observed in A+/T+/N± individuals. In contrast, there is no evidence that either high baseline tau or low hippocampal volume is associated with subsequent Aβ increases, nor is there evidence that low baseline hippocampal volume was associated with subsequent increases in tau.
While these results support a unidirectional model beginning with Aβ, they do not prove that Aβ is the cause of AD. For example, it is possible that another more fundamental and undiscovered process results in Aβ deposition, or that Aβ is an epiphenomenon that reports on an entirely different causal event resulting in tau deposition. However, while these results do not prove causation, they argue strongly against a model in which tau deposition, neurodegeneration, or cognitive decline is consistently the initiator.
Other data would seem to contradict these results but are more consistent with the likelihood that there is more than one pathway to each individual biomarker abnormality. For example, it is well recognized from both pathological data and biomarker results that tau accumulation can occur in the absence of or can precede Aβ, particularly in normal older people (23,35). While this has been attributed to non-AD processes such as primary age-related tauopathy (36), it is nevertheless possible that primary age-related tauopathy represents a substrate for the development of AD. However, there is no evidence from our data that this tau deposition leads to the deposition of Aβ, as has been proposed by others (12). In fact, our data echo previous findings that brain tau is rarely substantially elevated in the absence of abnormal Aβ (28,37,38), and that even in Aβ− individuals, higher Aβ and faster Aβ deposition predict later brain tau (9–11). Similarly, observations that cognitive decline may occur prior to Aβ deposition (22) are consistent with non-AD processes leading to cognitive decline, and with the observation that subthreshold, but elevated, Aβ levels predict cognitive decline (11,39). Our results do not support the idea that cognitive decline is an initiating process itself.
A subset of individuals labeled as suspected non-Alzheimer’s pathology (2,32) have neurodegeneration without significant Aβ. The A−/N+ suspected non-Alzheimer’s pathology individuals (13% of the whole cohort) in our data showed cross-sectional cognitive decline, as they have in other studies (33,40,41). However, both the cross-sectional and longitudinal analyses provided no evidence that neurodegeneration is associated with Aβ accumulation (13,14,33), nor is there evidence that neurodegeneration may lead to tau deposition. By contrast, high levels of tau but not of Aβ were associated with faster subsequent hippocampal volume loss, implying that the late-involved tau deposition is more related to subsequent AD-related neurodegeneration than to Aβ accumulation on the AD continuum (17,42).
It appears likely that Aβ and tau together have stronger effects on both neurodegeneration (15,43) and cognition (38,44–46) than either biomarker alone. We found that the A+/T+/N− and A+/T+/N+ groups showed the fastest rates of hippocampal volume loss, but continuous Aβ PET and tau PET did not show a synergistic effect for subsequent hippocampal volume loss, indicating that late-involved abnormal tau is closer to subsequent AD-related neurodegeneration than abnormal Aβ. There is an emerging consensus in the literature that the A+/T+/N+ group shows the fastest cognitive decline among nondemented individuals (6–8). In line with previous studies, we found that cross-sectional cognitive performance was generally lower in most groups than in the reference group, and was lowest in those abnormal on all 3 biomarkers; longitudinally, only the A+/T+ groups showed evidence of cognitive decline, and the A+/T+/N+ group showed the most rapid decline. In addition, we found that continuous baseline high tau and low hippocampal volume but not high Aβ were independently associated with subsequent cognitive decline, although we found that continuous baseline high Aβ and tau were synergistically associated with subsequent cognitive decline. These findings imply that the late-involved tau and neurodegeneration are closer to cognitive decline than Aβ, while Aβ deposition may have synergistic effects upon tau-related cognitive decline on the AD continuum (46).
A key strength of this study is that we analyzed both the cross-sectional and longitudinal associations between amyloid, tau, and neurodegeneration in relation to their prediction of subsequent cognitive decline in one large cohort, which is important to understand the temporal sequences of AD key pathologies. However, it is important to note several limitations in our study. All A/T/N biomarkers contain important continuous information for assessing progression of the disease. However, abnormal amyloid, tau, and neurodegeneration were defined to classify individuals into different A/T/N profiles in order to study how their abnormalities contribute to the progression of AD. Some A/T/N groups had relatively small sample sizes owing to the classification of 8 A/T/N profiles. We did not observe significant tau elevations in the A+/T−/N+ group, suggesting that the tau increase in A+/T− individuals may be subtle and early. Further study may be needed to explore these results in this group. We cannot compare longitudinal changes of Aβ and tau in different A/T/N profiles because of limited longitudinal data. The number of visits and duration of follow-up of longitudinal Aβ PET, tau PET, and structural MRI data were limited and differed from one another; thus, those findings may need to be validated by further longitudinal data with more visits and longer follow-up in the future. For example, while we did not find associations between baseline Aβ and cognition, studies that include at least 4 years of observation have been able to detect this (47). As the academic community becomes increasingly concerned about the overuse and misinterpretation of significance testing and p values (48), our results do not exclude important biological significance for results with p values between .05 and .1.
Together, these findings support the current model of AD pathogenesis in which a unidirectional progression of AD biomarkers begins with Aβ elevation, followed by subsequent tau deposition, neurodegeneration, and cognitive decline. These results leave unanswered the ultimate question as to AD causation, as well as the roles of other pathological processes in leading to cognitive impairment. For example, cerebrovascular disease is an important determinant of cerebral atrophy measurements reflected in neurodegeneration biomarkers (49) and may also be associated with tau or Aβ deposition (50). Alzheimer’s clinical syndrome in older people is well recognized to be a multifactorial process, so the data presented here do not explain the full spectrum of the disorder. However, among the core pathological processes that define the neurobiology of the disease, data consistently demonstrate patterns of biomarker association and causal effects on cognition that support a unidirectional model that begins with Aβ.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | N/A | |||
| Bacterial or Viral Strain | N/A | |||
| Biological Sample | N/A | |||
| Cell Line | N/A | |||
| Chemical Compound or Drug | N/A | |||
| Commercial Assay Or Kit | N/A | |||
| Deposited Data; Public Database | Alzheimer’s Disease Neuroimaging Initiative (ADNI) database | ADNI database (ida.loni.usc.edu) | RRID:SCR_003007 | |
| Genetic Reagent | N/A | |||
| Organism/Strain | N/A | |||
| Peptide, Recombinant Protein | N/A | |||
| Recombinant DNA | N/A | |||
| Sequence-Based Reagent | N/A | |||
| Software; Algorithm | R v3.6.2 | The R Foundation for Statistical Computing | R package: lme4, RRID:SCR_015654 | |
| Transfected Construct | N/A | |||
| Other | N/A |
ACKNOWLEDGMENTS
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant No. U01 AG024904) and the Department of Defense ADNI (Grant No. W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC.; Johnson & Johnson Pharmaceutical Research and Development LLC.; Lumosity; Lundbeck; Merck and Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found online (http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf).
Footnotes
DISCLOSURES
SML has served as a consultant to Cortexyme and NeuroVision. WJJ has served as a consultant to Genentech, Novartis, Curasen, and Grifols and owns an equity interest in Optoceutics. The other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2020.06.029.
Contributor Information
Tengfei Guo, Helen Wills Neuroscience Institute, University of California, Berkeley; Molecular Biophysics and Integrated Bioimaging, Lawrence Berkeley National Laboratory, Berkeley, California.
Deniz Korman, Helen Wills Neuroscience Institute, University of California, Berkeley.
Suzanne L. Baker, Molecular Biophysics and Integrated Bioimaging, Lawrence Berkeley National Laboratory, Berkeley, California
Susan M. Landau, Helen Wills Neuroscience Institute, University of California, Berkeley Molecular Biophysics and Integrated Bioimaging, Lawrence Berkeley National Laboratory, Berkeley, California.
William J. Jagust, Helen Wills Neuroscience Institute, University of California, Berkeley Molecular Biophysics and Integrated Bioimaging, Lawrence Berkeley National Laboratory, Berkeley, California.
REFERENCES
- 1.Jagust W (2018): Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci 19:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. (2018): NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. (2013): Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ (2018): Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91:e859–e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knopman DS, Lundt ES, Therneau TM, Vemuri P, Lowe VJ, Kantarci K, et al. (2019): Entorhinal cortex tau, amyloid-β, cortical thickness and memory performance in non-demented subjects. Brain 142:1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soldan A, Pettigrew C, Fagan AM, Schindler SE, Moghekar A, Fowler C, et al. (2019): ATN profiles among cognitively normal individuals and longitudinal cognitive outcomes. Neurology 92:e1567–e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR Jr, Wiste HJ, Therneau TM, Weigand SD, Knopman DS, Mielke MM, et al. (2019): Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA 321:2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J-T, Li J-Q, Suckling J, Feng L, Pan A, Wang Y-J, et al. (2019): Frequency and longitudinal clinical outcomes of Alzheimer’s AT(N) biomarker profiles: A longitudinal study. Alzheimers Dement 15:1208–1217. [DOI] [PubMed] [Google Scholar]
- 9.Leal SL, Lockhart SN, Maass A, Bell RK, Jagust WJ (2018): Subthreshold amyloid predicts tau deposition in aging. J Neurosci 38:4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosun D, Landau S, Aisen PS, Petersen RC, Mintun M, Jagust W, Weiner MW (2017): Association between tau deposition and antecedent amyloid-β accumulation rates in normal and early symptomatic individuals. Brain 140:1499–1512. [DOI] [PubMed] [Google Scholar]
- 11.Guo T, Landau SM, Jagust WJ (2020): Detecting earlier stages of amyloid deposition using PET in cognitively normal elderly adults. Neurology 94:e1512–e1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. (2019): Association of amyloid and tau with cognition in preclinical Alzheimer disease. JAMA Neurol 76:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR Jr, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, et al. (2014): Rates of β-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology 82:1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, Benzinger TLS (2016): Longitudinal β-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non-Alzheimer disease pathophysiology. JAMA Neurol 73:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, et al. (2011): Amyloid-β associated volume loss occurs only in the presence of phospho-tau. Ann Neurol 70:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman DS, Jack CR Jr, Lundt ES, Wiste HJ, Weigand SD, Vemuri P, et al. (2015): Role of β-amyloidosis and neurodegeneration in subsequent imaging changes in mild cognitive impairment. JAMA Neurol 72:1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J, et al. (2020): Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med 12:eaau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, et al. (2013): Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81:1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. (2014): Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: A cross-sectional study. Lancet Neurol 13:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, Landau SM, et al. (2013): Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol 70:1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knopman DS, Jack CR Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, et al. (2013): Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Ann Neurol 73:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas KR, Bangen KJ, Weigand AJ, Edmonds EC, Wong CG, Cooper S, et al. (2020): Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology 94:e397–e406.s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weigand AJ, Bangen KJ, Thomas KR, Delano-Wood L, Gilbert PE, Brickman AM, Bondi MW (2020): Is tau in the absence of amyloid on the Alzheimer’s continuum? A study of discordant PET positivity. Brain Commun 2:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau SM, Fero A, Baker SL, Koeppe R, Mintun M, Chen K, et al. (2015): Measurement of longitudinal-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J Nucl Med 56:567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. (2017): Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage 157:448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. (2017): Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR Jr, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, et al. (2018): Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 141:1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR Jr, Wiste HJ, Botha H, Weigand SD, Therneau TM, Knopman DS, et al. (2019): The bivariate distribution of amyloid-β and tau: Relationship with established neurocognitive clinical syndromes. Brain 142:3230–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J-C, Han S-H, Yi D, Byun MS, Lee JH, Jang S, et al. (2019): Plasma tau/amyloid-β1-42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 142:771–786. [DOI] [PubMed] [Google Scholar]
- 30.Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, et al. (2019): White matter hyperintensities: Relationship to amyloid and tau burden. Brain 142:2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Botha H, Mantyh WG, Graff-Radford J, Machulda MM, Przybelski SA, Wiste HJ, et al. (2018): Tau-negative amnestic dementia masquerading as Alzheimer disease dementia. Neurology 90:e940–e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. (2012): An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilgel M, An Y, Helphrey J, Elkins W, Gomez G, Wong DF, et al. (2018): Effects of amyloid pathology and neurodegeneration on cognitive change in cognitively normal adults. Brain 141:2475–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. (2014): The preclinical Alzheimer cognitive composite: Measuring amyloid-related decline. JAMA Neurol 71:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price JL, Morris JC (1999): Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45:358–368. [DOI] [PubMed] [Google Scholar]
- 36.Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. (2014): Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pontecorvo MJ, Devous MD, Navitsky M, Lu M, Salloway S, Schaerf FW, et al. (2017): Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain 140:748–763e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Betthauser TJ, Koscik RL, Jonaitis EM, Allison SL, Cody KA, Erickson CM, et al. (2020): Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 143:320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landau SM, Horng A, Jagust WJ (2018): Memory decline accompanies subthreshold amyloid accumulation. Neurology 90:e1452–e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machulda MM, Hagen CE, Wiste HJ, Mielke MM, Knopman DS, Roberts RO, et al. (2017): Practice effects and longitudinal cognitive change in clinically normal older adults differ by Alzheimer imaging biomarker status. Clin Neuropsychol 31:99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Tudorascu DL, Lopez OL, Cohen AD, Mathis CA, Aizenstein HJ, et al. (2018): Amyloid β deposition and suspected non-Alzheimer pathophysiology and cognitive decline patterns for 12 years in oldest old participants without dementia. JAMA Neurol 75:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon BA, McCullough A, Mishra S, Blazey TM, Su Y, Christensen J, et al. (2018): Cross-sectional and longitudinal atrophy is preferentially associated with tau rather than amyloid β positron emission tomography pathology. Alzheimers Dement (Amst) 10:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascoal TA, Mathotaarachchi S, Mohades S, Benedet AL, Chung C-O, Shin M, et al. (2017): Amyloid-β and hyperphosphorylated tau synergy drives metabolic decline in preclinical Alzheimer’s disease. Mol Psychiatry 22:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pascoal TA, Mathotaarachchi S, Shin M, Benedet AL, Mohades S, Wang S, et al. (2017): Synergistic interaction between amyloid and tau predicts the progression to dementia. Alzheimers Dement 13:644–653. [DOI] [PubMed] [Google Scholar]
- 45.Hanseeuw BJ, Betensky RA, Schultz AP, Papp KV, Mormino EC, Sepulcre J, et al. (2017): Fluorodeoxyglucose metabolism associated with tau-amyloid interaction predicts memory decline. Ann Neurol 81:583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, et al. (2019): The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol 85:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donohue MC, Sperling RA, Petersen R, Sun C-K, Weiner MW, Aisen PS (2017): Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 317:2305–2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington D, D’Agostino RB, Gatsonis C, Hogan JW, Hunter DJ, Normand SLT, et al. (2019): New guidelines for statistical reporting in the journal. N Engl J Med 381:285–286. [DOI] [PubMed] [Google Scholar]
- 49.Brickman AM, Tosto G, Gutierrez J, Andrews H, Gu Y, Narkhede A, et al. (2018): An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology 91:E1402–E1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC (2013): Risk factors for β-amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol 70:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.