Synopsis
Acute respiratory distress syndrome (ARDS), is a syndrome of non cardiogenic pulmonary edema and hypoxia that accompanies up to 30% of deaths in pediatric intensive care units. Pediatric ARDS (PARDS) is diagnosed by the presence of hypoxia, defined by Oxygenation Index or PaO2/FiO2 ratio cutoffs, and new chest infiltrate occurring within 7 days of a known insult. The pulmonary edema in ARDS is caused by damage to the alveolar endothelial barrier in the setting of dysregulated inflammation and coagulation resulting in increased lung water and loss of aerated lung tissue. Clinical hallmarks of ARDS include hypoxemia and decreased lung compliance, increased work of breathing and impaired gas exchange. Mortality is accompanied by multiple organ failure in most cases. Although many modalities to treat PARDS have been investigated, supportive therapies and lung protective ventilator support remain the mainstay of treatment.
Keywords: Acute respiratory distress syndrome, Pediatrics, Pathophysiology, Acute Lung Injury, PARDS
Key Points
ARDS is a clinical syndrome of non-cardiogenic pulmonary edema characterized by hypoxemia, radiographic infiltrate(s), decreased functional residual capacity and decreased lung compliance.
The hallmark of pathophysiology in ARDS is the loss of the alveolar epithelial-endothelial barrier function in the setting of dysregulated inflammation and coagulation pathways complicated by concurrent loss of surfactant and impairment of lymphatic drainage.
The mainstay of management is support, including lung-protective mechanical ventilation, careful attention to fluid management, treatment of underlying condition including use of appropriate antibiotics, and general supportive care. While a number of therapeutic strategies have been tested in ARDS, use of lung protective ventilation is the only universally accepted strategy to decrease mortality. Use of neuromuscular blockade and prone positioning has been shown to lead to decreased mortality among adults with severe ARDS.
Introduction
Acute respiratory distress syndrome (ARDS) is essentially a clinical syndrome of non-cardiogenic pulmonary edema and hypoxia that contributes to significant morbidity and mortality1, 2. Initially ARDS was described as “adult” respiratory distress syndrome to differentiate it from infant respiratory distress syndrome 3. This name was later modified to acute respiratory distress syndrome in recognition of the fact that both adults and children develop ARDS 1,4. Lung development increases linearly with age and height until the adolescent growth spurt at 10 years in females and 12 years in males. Therefore, there are significant differences between adult and child ARDS pathophysiology due to remodeling, growth of the lung parenchyma and progressive maturation of immune system 5, 6.
The traditional American-European Consensus Conference (AECC) definition proposed in 1994, classified mild ARDS as acute lung injury (ALI). ALI/ARDS was defined as acute onset of severe hypoxia (ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen or P/F ratio < 300 for ALI and < 200 for ARDS) with bilateral opacities on chest x-ray in the absence of clinical evidence of left ventricular failure.7 In 2012, a panel of experts developed the Berlin definition which replaced the AECC definition and included several significant changes, specifically 1) ALI was eliminated and replaced with mild, moderate, and severe ARDS defined by a P/F ratio of 200-300, 100-200 or <100, respectively, 2) minimal ventilator settings of a positive-end expiratory pressure (PEEP) of ≥ 5 cmH20 was required, and 3) reference to the pulmonary capillary wedge pressure was removed.8 9
The current definition of pediatric acute respiratory distress syndrome (PARDS) proposed by the Pediatric Acute Lung Injury Consensus Conferences group (PALICC) followed in 2015. 10, 11 PARDS is now diagnosed by the presence of hypoxia in the context of a new lung infiltrate occurring within 7 days of a known insult. Hypoxia is defined as oxygenation index (OI, determined by the mean airway pressure divided by P/F ratio) of 4-8 (mild), 8-16 (moderate), or >16 (severe) for ventilated patients while on PEEP of ≥ 5 cm H20) or the Berlin definition P/F ratio cutoffs in non-ventilated patients.11 Although there is no age limit in PARDS, the definition excludes patients with perinatal-related lung disease. It allows use of pulse oximetry oxygen saturation to calculate the S/F ratio when PaO2 is not available. Both the use of OI (or OSI when PaO2 is not available) to define hypoxia and the requirement for radiographic evidence of any new infiltrate are major departures from the Berlin definition used in adults.
A population-based study estimated an annual incidence of ARDS of 12.8 cases per 100,000 persons in Olmsted County in Washington. Severe sepsis (with pneumonia as the infection focus) was the most common risk factor. Reported estimates for prevalence of ARDS in single center studies ranges range from 1 to 10%. 12 A multi-national point prevalence study of PARDS conducted by the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) is currently ongoing. The overall reported mortality from PARDS varies from 18 to 22%%, but it is estimated to accompany up to 30% of all pediatric intensive care unit deaths.13 Although many modalities to treat PARDS have been investigated over the last decade, supportive therapies remain the mainstay of treatment.
Causes of ARDS
ARDS is associated with many different underlying clinical conditions including pneumonia, sepsis, trauma, burns, acute pancreatitis, aspiration, toxic inhalation, transfusion, and cardiopulmonary bypass surgery 14 12,10,12,13,15 (see Table 1). Although sepsis is the most common cause of ARDS for adults, the most common underlying condition for PARDS is viral respiratory infection.
Table 1.
CAUSES of ACUTE RESPIRATORY DISTRESS SYNDROME
| DIRECT LUNG INJURY (Alveolar-Epithelial) | INDIRECT LUNG INJURY (Alveolar-Capillary) |
|---|---|
| Pneumonia | Sepsis/SIRS |
| Aspiration | Major trauma |
| Inhalation Injury | Pancreatitis |
| Drowning | Severe Burns |
| Pulmonary contusion | Massive transfusion or TRALI |
| Shock | |
| Cardiopulmonary bypass | |
| Head Injury | |
| Drug Overdose |
Physiologic Basis and Consequences of Non-Cardiogenic Pulmonary Edema
The lung’s alveolar epithelial–capillary structure provides a large surface area for efficient gas exchange and is comprised of alveolar epithelium, capillary endothelium, and basement membranes. The alveolar epithelium is coated with a thin layer of alveolar wall liquid, which is necessary for dispersion of surfactant, transfer of gases, and host defense against inhaled pathogens. Integrity of this barrier is critical for gas exchange, and separation of the aqueous and gaseous compartments (Figure 1). 16,17. Disruption of the integrity of the pulmonary endothelium and alveolar epithelium lead to accumulation of protein-rich alveolar edema fluid.1,18 Cytokines (IL1, IL8, TNFα) and lipid mediators (leukotriene B4) are attracted to alveoli and, in response to these pro-inflammatory mediators, neutrophils are recruited into the pulmonary interstitium and alveoli. Presence of protein, fibrinogen and fibrin degradation products in the alveolar fluid result in surfactant degradation (Figure 2).1,2 There is decreased functional residual capacity, increased dead space, reduced respiratory system compliance, and impaired gas exchange which leads to atelectasis and hypoxia. Loss of the epithelial barrier may also lead to sepsis in patients with bacterial pneumonia through creation of an open interface between the alveolar and circulating compartments. 1
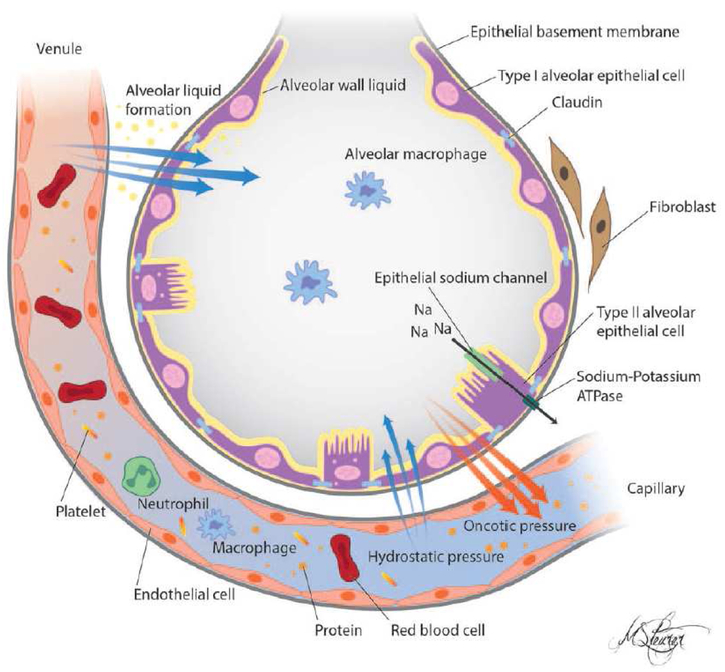
Figure 1.
Schematic of a healthy alveolus. The alveolar epithelium and capillary endothelium are intact. The characteristics of the pulmonary circulation and intact epithelial endothelial barrier allow for formation of the alveolar wall liquid (AWL) while maintaining the air-filled, fluid-free, status of the alveoli. The AWL facilitates gas exchange and is a medium for dispersal of surfactant and alveolar macrophages, which is essential for maintaining alveolar stability and host defenses. The intact sodium-dependent vectorial transport across type II alveolar epithelial cells regulates the removal of excess alveolar fluid.
From Sapru A, Flori H, et al. Pathobiology of acute respiratory distress syndrome.
Pediatr Crit Care Med. 2015 Jun;16(5 Suppl 1):S6–22. doi: 10.1097/PCC.0000000000000431.
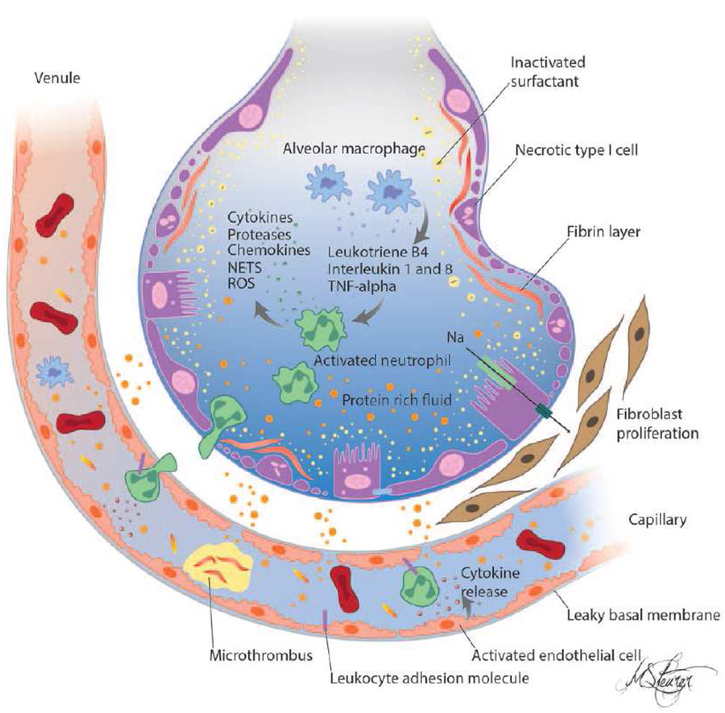
Figure 2.
Schematic of pathophysiology in acute respiratory distress syndrome. There is a loss of epithelial and endothelial barrier integrity and loss of function leading to increased permeability pulmonary edema. Solutes and large molecules such as albumin enter the alveolar space. In the presence of proinflammatory mediators and activated endothelium leukocytes traffic into the pulmonary interstitium and alveoli. There is activation of coagulation and deposition of fibrin in capillaries and alveoli with increased concentrations of fibrinogen and fibrin-degradation products in the edema fluid. Surfactant depletion and degradation result in large increases in surface tension and loss of alveolar shape and integrity. Recovery is preceded by fibroblast proliferation.
NETs = neutrophil extracellular traps, ROS = reactive oxygen species, TNF = tumor necrosis factor.
From Sapru A, Flori H, et al. Pathobiology of acute respiratory distress syndrome.
Pediatr Crit Care Med. 2015 Jun;16(5 Suppl 1):S6–22. doi: 10.1097/PCC.0000000000000431.
Alveolar Epithelial Injury and Dysfunction
Alveolar epithelium is composed of two types of cells, flat alveolar type-I cells and cuboidal alveolar type-II cells, which make up 90 percent and 10% of the alveolar surface area respectively. Type-1 cells are large thin cells and they are the primary site of gas exchange whereas type-II cells are more resistant to injury and are responsible for surfactant production, ion transport, and proliferation and differentiation to type I cells after injury.1 Type-II cells are responsible for the removal of excess alveolar fluid through sodium-dependent intracellular transport.
Direct alveolar epithelial injury (infections, inhalations, aspirations, mechanical ventilation, etc.) and indirect alveolar capillary injury (sepsis, trauma, transfusion, burns, pancreatitis, etc.) lead to breakdown of the barrier and decrease in the ability of alveolar epithelium to remove excess alveolar fluid.16,18,19 Decreased alveolar fluid clearance is associated with severity and worse clinical outcomes and increased mortality. Elevated plasma levels of surfactant protein D and the receptor for advance glycation end products (RAGE), markers of alveolar epithelial damage correlate with poor outcomes in patients with ARDS.20–23
Lung Endothelial Injury
Damage to pulmonary capillary endothelium activates inflammatory and coagulation cascades.24 There are multiple proteins along these cascades implicated in the pathogenesis of ARDS. Endothelial-specific proteins, such as von Willebrand factor and angiotensin converting enzyme activity correlate with ARDS mortality in children and adults.25–27 Thrombomodulin is a trans-membrane protein found on surface of endothelial cells that facilitates the thrombin-mediated conversion of protein C to activated protein C and has roles in coagulation, fibrinolysis, and inflammation. It is highly expressed in pulmonary alveolar capillaries 28. Elevated soluble thrombomodulin levels are associated with organ dysfunction in PARDS and with higher mortality in children with indirect lung injury.28
Transfusions and ARDS
Over recent years the incidence of antibody-mediated transfusion-related acute lung injury (TRALI) as a risk factor for ARDS has declined due to changes in blood banking practice.29 However, transfusion of RBCs is associated with an increased risk of developing ARDS in critically ill patients with sepsis.30 Cell free hemoglobin (CFH) levels are increased during sepsis and ARDS due to decreased levels of haptoglobulin, hemopexin and heme oxygenase-1 and reduced capacity to detoxify CFH 31. Increased CFH levels may lead to injury via peroxidation of lipid membranes and increased vascular permeability.32 In addition, massive transfusions can overwhelm the scavenging capacity of macrophages and lead to increased non-transferrin bound iron (NTBI) production that can promote both oxidative stress, as well as increase susceptibility to infections with siderophilic bacteria. 33
Inflammatory Dysfunction
Alveolar macrophages play a central role in orchestrating inflammation. When alveolar macrophages are activated, they recruit neutrophils and circulating macrophages to the alveoli. Neutrophils communicate between the vessel wall and platelets which results in endothelial injury and releases neutrophil extracellular traps (NETS), which may also cause damage to the lung 29,34,35.
Inflammation mediated by the innate immune system in response to infectious agents or tissue damage is triggered by the presence of pathogen-associated molecular patterns and endogenous danger (or damage) associated molecular patterns.34,36–38 These patterns are recognized by pattern recognition receptors such as Toll-like receptors and cytosolic nucleotide-binding oligomerization domain like receptors (NLRs), which leads to increased expression of inflammatory cytokines and chemokines such as IL-1B, TNFα,IL-6, IL-8.37,38 A number of human studies have demonstrated evidence for dysregulation of inflammatory pathways in ARDS and their association with outcome.39-41 A recent multicenter study of inflammatory pathways in pediatric ARDS identified a strong relationship between mortality and elevated plasma levels of both pro-inflammatory (IL-6, IL-8, IL-18, MIP-1β, TNF-α) and anti-inflammatory cytokines (IL-1RA, IL-10, and TNF-R2). These cytokines were associated with ARDS illness severity, including the P/F ratio, OI, pediatric risk of mortality score (PRISM-3), ICU morbidity, and biochemical evidence of endothelial injury, including elevated plasma angiopoietin 2 and soluble thrombomodulin. The addition of inflammatory cytokines to the OI improved risk-stratification in a heterogeneous ARDS population.42
Surfactant Dysfunction
One of the hallmarks of ARDS is a reduction surfactant expression and surfactant dysfunction. Surfactant is secreted into the alveolar space by type-II cells and its main function is to lower surface tension at the air-liquid interface of the alveoli. Surfactant contains 4 major proteins; surfactant protein B and C are responsible for lowering the surface tension, while surfactant protein A and D are important in innate and adaptive immune responses against pathogens. Surfactant proteins A, B, and D levels are low in the bronchoalveolar lavage fluid of adults with ARDS. Loss of surfactant leads to the decreased compliance and alveolar instability observed in ARDS, and which leads to atelectasis. Further, increased serum levels of these proteins in children and adults are associated with alveolar epithelial cell injury.43–46
Coagulation and Fibrinolysis Dysfunction
Inflammation and coagulation are critical host responses to infection and injury; however, in ARDS, there is a significant imbalance between coagulation and fibrinolysis. This favors fibrin formation. Microthrombi and pulmonary vascular injury occur early in ARDS. Stimuli such as hypoxia, cytokines and chemokines, and inflammatory mediators lead to activation of the endothelium. 27,47 Endothelial cells respond by shifting from their normal anti-thrombotic and anti-inflammatory phenotype to an “activated” state of endothelial “dysfunction”,48 characterized by pro-thrombotic and pro-adhesive properties. Key events in this transformation are the expression of adhesion molecules, tissue factor, 49 and von Willebrand factor.26 Furthermore, it appears that alveolar epithelium can up-regulate tissue factor in response to inflammatory stimuli.50 The pro-coagulant environment with ARDS is also due in part to the elevated plasminogen activator inhibitor-1 (PAI-1), and elevated PAI-1 levels are associated with increased mortality in adults and children with ARDS. 51
Platelets can also contribute to lung injury in ARDS. Platelets can directly interact with neutrophils and monocytes, and are themselves a source of proinflammatory cytokines. There is evidence that platelets can also alter the barrier function of alveolar capillaries, regulate pulmonary vascular permeability, and influence pulmonary vascular reactivity.52
Ventilator Induced Lung Injury in ARDS
Current treatment regimens for ARDS include oxygen therapy and mechanical ventilation.53 Unfortunately, side effects of these treatments include exacerbation of lung injury by barotrauma, volutrauma, and propagation of lung inflammation. These changes are clinically recognized as ventilator-induced lung injury.54 Over the past two decades, except for the introduction of low-tidal volume ventilation,55 few if any new therapeutic approaches have shown improvements in patient survival.56,57 Recently it has been proposed that stretch-activated ion channels, such as 2-pore-domain potassium channels, may play an important role in the development and propagation of low-tidal volume ventilation58 by regulating inflammatory mediator secretion, 59,60 epithelial cell detachment and cytoskeletal remodeling,61 and alveolar-capillary barrier function.57
Resolution of ARDS
Although ARDS causes extensive damage to lung tissue, it can fully resolve. However, restoring the balance of proinflammatory and anti-inflammatory responses is essential to prevent lung fibrosis. Repair of the epithelium is a complex process that appears to involve epithelial cell spreading, migration, proliferation and differentiation. Scar tissue is formed during healing to preserve alveolar integrity and prevent further alveolar edema. This fibrotic tissue is removed by matrix metalloproteinases, a family of enzymes that digest extracellular fibers.62 Matrix metalloproteinases are up-regulated during the repair process and appear to be involved in facilitating migration in the remodeling.63 The migration and proliferation of epithelial progenitor cells is also regulated by transforming growth factor alpha (TGFα), fibroblast growth factor, hepatocyte growth factor, and keratinocyte growth factor. Interestingly, there is also evidence of a multipotent mesenchymal stem cells that may be involved in repair of lung alveoli.64. There is an ongoing Phase II clinical trial to test the role of mesenchymal stem cells in ARDS in adults.
Mortality in ARDS
The overall mortality associated with PARDS is decreasing over time. The estimated mortality varies from 18 to 35%. Studies that include both invasively and noninvasively ventilated patients report 18-22% mortality whereas among studies limited to invasively ventilated patients mortality tends to be somewhat higher in the range of 26-35%.15,65 While the pooled mortality from several studies is about 30%, it is clear that children with certain comorbidities and immunodeficiency have worse outcomes.65 The majority of deaths in ARDS are attributable to sepsis or multi-organ dysfunction rather than primary respiratory causes. Multiple organ system dysfunction is the single most important independent clinical risk factor for mortality in children with only a minority of deaths attributable solely to lung failure and refractory hypoxemia. 1,65 Interestingly a combination of OI level and history of cancer/stem cell transplant predicts mortality as well as complex models incorporating measures of overall severity of illness and severity of lung injury.66
Management of ARDS
Management of PARDS consists mainly of addressing gas exchange and work of breathing, providing nutrition, and providing general ICU supportive care, with the goal of treating the underlying cause of PARDS and minimizing iatrogenic harm. Most treatment recommendations in PARDS are based on extrapolated adult studies, small studies in pediatric populations, and the clinical expertise of bedside physicians. To promote optimization and consistency of care for children with PARDS, the Pediatric Acute Lung Injury Consensus Conference (PALICC) published treatment recommendations in 2015.10 Despite advances in management treatment modalities, the clinical benefits and risks of these innovations remain mixed.10 In the following, we review current management, citing relevant adult and pediatric studies along with the published PALICC recommendations. Since ARDS is characterized by impaired gas exchange, support of oxygenation and ventilation with supplemental oxygen, noninvasive ventilation, and invasive mechanical ventilation are the cornerstone of PARDS therapy.
High Flow Nasal Cannula and Noninvasive Ventilation
In adults with ARDS, some studies have demonstrated worse mortality in patients treated with these non-invasive approaches.67–69 These poorer outcomes may be due to delay in initiation of lung-protective ventilation and tend to be more pronounced in patients with severe disease. Conversely, there may be benefit to limiting exposure of patients to invasive mechanical ventilation and its associated complications.14,70 A recent study in adult patients with acute hypoxemic respiratory failure (defined as a P/F ratio of ≤ 300) found those randomized to receive high flow nasal cannula oxygen had more ventilator-free days at day 28 and a lower 90-day mortality than those randomized to facemask or noninvasive ventilation.71 A prospective randomized control trial (RCT) comparing noninvasive ventilation and standard oxygen therapy in children with respiratory failure after extubation showed no difference between groups.72 Finally, in a propensity score-matched cohort study evaluating outcomes in children admitted to the ICU, those receiving noninvasive ventilation as first-line therapy had reduced mortality, length of ventilation, and length of ICU stay.73 Though the exact role of high flow nasal cannula and noninvasive ventilation in PARDS remains unknown, these studies suggest that in appropriately selected patient populations these approaches may be valuable. Ongoing assessment of patients and an early change to invasive mechanical ventilation among nonresponders are key to successful use of noninvasive ventilation.74
Invasive Mechanical Ventilation
Invasive mechanical ventilation strategies balance support of gas exchange with toxicity from volutrauma, barotrauma, and free-radical injury.55,74 In their landmark publication on lung-protective ventilation, the ARDS Network found a decrease in mortality with lower tidal volume ventilation (6 ml/kg of predicted body weight) compared to conventional ventilation volumes at that time (12 ml/kg of predicted body weight).55 Two subsequent meta-analyses in adult ARDS further supported that low-tidal volume ventilation reduced in-hospital mortality.75,76 Current recommendations in adults include lung-protective ventilation with tidal volumes of less than 6ml/kg of estimated ideal body weight, limitation of plateau pressures < 30 cm H2O, and use of adequate PEEP.55,75,76 Given the strength of the adult ARDS data, standard PARDS therapy involves lung-protective ventilation as well. PALICC recommends tidal volumes of 3-6 mL/kg predicted body weight for patients with poor compliance and closer to physiologic range (5-8 mL/kg predicted body weight) for patients with preserved compliance.10 In addition, they suggest an inspiratory plateau pressure of < 28 cm H2O (29-32 cm H2O in those with increased chest wall elastance).10 Permissive hypercapnia (pH of 7.15-7.30) may be used if necessary to limit further injury to the lung caused by potentially injurious ventilator pressure.74,77 A goal-directed strategy to minimize further lung injury also includes adjusting the supplemental oxygen to maintain saturations 92-97% for mild PARDS and 88-92% in those with PEEP of 10 cm H2O.74,77
Additional invasive ventilation considerations include how best to treat hypoxia with supplemental oxygen and PEEP. The goal of applied PEEP in PARDS is to maximize alveolar recruitment, provide optimal FRC, and prevent recruitment/decruitment cycles. When originally investigated by the ARDS Network, there was no difference in outcome between low PEEP strategies (range of 5 to 24 cmH2O) and high PEEP strategies (range of 12 to 24 cmH2O), so many centers favor a low PEEP strategy given the concern for barotrauma, pneumothoraces, and hemodynamic compromise that can be associated with high PEEP.78 However, subsequent studies in adult ARDS suggest a significantly reduced mortality with a high PEEP strategy in severe ARDS.79–81 Open lung ventilation is another emerging technique that combines low tidal-volume ventilation and setting a PEEP at least 2 cm H2O above the lower inflection point of the patient’s pressure-volume curve. Small studies suggest a reduced mortality with this strategy.82,83 Neither high PEEP or open lung ventilation has been evaluated in PARDS. PALICC suggests PEEP should be 10-15 cm H2O with levels above 15 cm H2O used in severe PARDS with careful attention to inspiratory plateau pressures.10
High Frequency Oscillatory Ventilation
Despite its widespread clinical use, studies investigating outcomes in high-frequency oscillatory ventilation (HFOV) in ARDS have not shown any benefit in lung-protective ventilation. The OSCAR trial found no difference in mortality in those randomized to HFOV compared to conventional ventilation.84 A similar Canadian study was stopped due to futility and suggested a mortality increase in HFOV patient. 85 While there are no RCTs of HFOV dedicated to PARDS, two recent secondary analyses have shown tendency towards harm among children with PARDS managed by early HFOV.86,87 Most recently, a propensity score matched analysis evaluating sedation in children found early (within 48 hours of intubation) initiation of HFOV to be associated with longer duration of mechanical ventilation, but no mortality difference when compared to late initiation or conventional ventilation.87
Inhaled Nitric Oxide
Use of inhaled nitric oxide (iNO) in ARDS became popular when Rossaint et al showed that it improved oxygenation.88 Subsequent studies, including three systematic reviews with primarily adult patients, found no significant effect of iNO on mortality or number of ventilator-free days.89–91 Further, in some adults iNO may increase renal impairment, suggesting the benefits may not outweigh the risks in certain adult populations.91,92 Three RCTs have demonstrated an oxygenation benefit with iNO in PARDS,93–95 but this benefit is not sustained and does not lead to improved overall outcomes. The most notable was a multicenter trial which showed iNO improved oxygenation at 12 and 24 hours, but not at 72 hours after initiation.94 In 2014, Bronicki et al published an RCT that showed iNO not only improved oxygenation at 12 hours, but also reduced duration of mechanical ventilation and increased ECMO-free survival.96 It should be noted that ECMO-free survival was not a primary endpoint, which leaves the true survival benefit of iNO still unresolved.96 While PALICC does not recommend routine use of iNO in PARDS, they do recommend use in severe cases or in cases complicated by pulmonary hypertension or severe right heart strain.10
Surfactant
Because there is surfactant deficiency and dysfunction in ARDS, exogenous surfactant could potentially be beneficial, particularly in early stages of lung injury.97 However, the literature on the use of surfactant has not been conclusive. Two studies of recombinant surfactant protein in ARDS yielded improvement in oxygenation, but no effect on survival.98,99 A post-hoc analysis of this study suggested patients with severe ARDS from pneumonia or aspiration had an improvement in 28-day survival with surfactant administration;99 however, a follow-up study in this patient population was halted for futility and demonstrated no significant benefit in mortality, oxygenation, or ventilator-free days.100
Several studies have shown surfactant improves lung function, oxygenation and gas exchange.101–103 The most notable was a multicenter RCT conducted by PALISI which demonstrated an overall mortality benefit after early surfactant administration.100 Of note, this was the first trial for which a post-hoc analysis suggested benefit in direct lung injury. However, one of the major limitations of this trial was a greater proportion of immunocompromised patients in the control group leading to a higher than expected mortality.101 Another recent international, multicenter RCT on the use of lucinactant in infants with early PARDS demonstrated no significant reduction in ventilator-free days or mortality.104 Further, a 2013 study focused on PARDS secondary to direct lung injury was halted for futility, finding no significant difference in oxygenation, ventilator-free days, or 90-day mortality with surfactant administration.105 PALICC does not recommend the routine use of surfactant in PARDS.10
Fluid Management
In addition to disrupting gas exchange, PARDS leads to fluid overload and to a proinflammatory response by the endothelium due to increasing intravascular pressure. Interstitial edema is a frequent complication.106,107 In the FACTT trial published in 2006, an even fluid balance over the first seven days of mechanical ventilation improved lung function, shortened duration of mechanical ventilation, and shortened duration of ICU stay.108 There was no significant difference in 60-day mortality and no increase in nonpulmonary-organ failures between conservative and liberal fluid management.108 More recent adult ARDS literature suggests that early treatment with hemofiltration not only reduces overall lung water and improves cardiac function but also may reduce cytokine levels and systemic inflammation.109
Of the four studies to investigate fluid balance in PARDS, three have demonstrated an association between fluid balance and mortality. Specifically: 1) Flori, et al demonstrated an association between cumulative fluid balance and pediatric ICU mortality:110 2) Wilson et al showed an association between cumulative fluid balance and mortality within the first week of illnes;111 and 3) Hu et al found a daily fluid balance of ≤ 10 ml/kg/day was associated with lower mortality in patients with acute hypoxemic respiratory failure.112 Lower cumulative fluid balance was also found to increase ventilator-free days.110,111,113 Current PALICC consensus suggests that, after initial fluid resuscitation, goal-directed fluid management should be used in order to maintain adequate intravascular volume while aiming to prevent a positive fluid balance.10
Nutrition
In general, critically ill children have reduced mortality when enteral nutrition is started within 48 hours of their admission.114 Further, emerging research suggests a role for gut dysfunction in pathogenesis of ARDS.114 The EDEN study, which attempted to address the optimal amount of enteral nutrition in ARDS found that, compared to full enteral feeding, a strategy of initial trophic enteral feeding for up to 6 days did not improve ventilator free days, 60-day mortality, or infectious complications, but was associated with less gastrointestinal intolerance.115 Trials investigating omega-3 fatty acid-enriched lipid emulsion found them to be clinically safe and to modulate eicosanoid values, which may have important immunomodulatory implications.116,117 Overall, the area of optimal nutrition is a burgeoning field within ARDS research. PALICC stresses the importance of nutrition, preferably enteral, in maintaining growth, meeting metabolic needs, and facilitating recovery.10
Prevention of Inflammation
Inflammation plays a key role in the pathophysiology of PARDS both in the lungs and systemically. Corticosteroids are used in 20-60% of patients with PARDS, though evaluating this use is confounded by indications including peri-extubation airway management, shock, and hypercytokinemia.118–120 Despite the prevalence of corticosteroid use, a definitive role for these drugs in PARDS has not been established. In two adult ARDS meta-analyses, there is a suggestion of reduced mortality and increased ventilator-free days when steroids are initiated at onset ARDS.121,122 In the pediatric population, data consists of small studies with wide variability in timing, duration, dose, and type of corticosteroids used.120 In a recent RCT of low-dose methylprednisolone infusion in PARDS, there was no difference in length of mechanical ventilation, duration of ICU stay, duration of hospitalization, or mortality between intervention and control groups.123 However, a single-center prospective observational study found corticosteroid exposure for more than 24 hours and cumulative corticosteroid dose were both independently associated with fewer ventilator-free days at 28 days and a shorter overall duration of ventilation.118 Due to this inconsistency, the recent PALICC recommendations state that corticosteroids cannot be recommended as part of routine therapy.10,124 That said, systemic steroids may benefit ARDS patients with certain co-morbidities including bronchopulmonary dysplasia, reactive airway disease, pneumonia in general, and pneumocystis pneumonia. Much more study of these indications is warranted.
Inflammatory pathways provide an ongoing target for novel therapies in the treatment of PARDS. Possible alternatives to systemic corticosteroids currently being investigated include: 1) inhaled corticosteroids which improve dynamic lung compliance, improve oxygenation, and decrease local inflammation;125,126 2) angiotensin II, which induces NF-kB gene expression and blocks the renin-angiotensin axis127, 3) peroxisome proliferator receptor agonists which negatively control proinflammatory gene expression;128 and 4) hypoxia-inducible factor-1a (HIF1a), which inhibits the proteasome and induces anti-inflammatory effects.129,130
Prone Positioning
Prone positioning improves oxygenation in ARDS, likely through a combination of improved lung ventilation-perfusion matching, recruitment of lower-lobe atelectasis, reduced ventilator-induced lung injury due to maintenance of open lung units, improved secretion clearance, and improved right ventricular dysfunction.54,131 The Supine-Prone Study Group was among the first to demonstrate improved oxygenation in adult ARDS patients.114,132 However, improvement in oxygenation in this study and several others that followed did not translate into a mortality benefit for the general ARDS population.133–137
In the pediatric population, a RCT investigating prone position in PARDS was halted early for futility.138 Like previous adult studies, despite improved oxygenation this investigation demonstrated no significant benefit of prone positioning on ventilator-free days or 28-day mortality.138 However, the mortality rate in the control group was unusually low at 8% suggesting the true utility of prone positioning in PARDS may still be unknown.138 Guerin et al found a 28-day and 90-day mortality benefit in patients with moderated to severe ARDS who were placed prone more than 16 hours per day.139 The difference between this outcome and that of previous studies is likely due to targeting children with more severe ARDS, longer time periods spent prone, and improved expertise in the prone position procedure. Lack of expertise in prone positioning, the potential for complications, and uncertainty as to which population most benefits from this therapy has led to variability in the adoption of prone positioning for PARDS.54,133 PALICC currently recommends consideration of prone positioning in severe cases of PARDS.10
Sedation and Neuromuscular Blockade
Deep sedation and neuromuscular blockade (NMB) likely benefits ARDS patients by limiting: 1) lung injury arising from ventilator-patient asynchrony; 2) expiratory muscle function which can cause collapse and derecruitment; and 3) the potential release of inflammatory cytokines.54 A multicenter RCT in adults with moderate to severe ARDS (specifically patients with a P/F ratio <150) found lower adjusted 90-day in-hospital mortality and increased ventilator-free days in patients with NMB and deep sedation compared to deep sedation alone.140 Currently, a multicenter study is being conducted by the NHLBI-funded PETAL network to assess the benefits and risks of NMB. There have been no studies investigating the effects of sedation and NMB in PARDS. PALICC recommends the use of NMB at the minimum effective dose in patients who are unable to achieve effective mechanical ventilation with sedation alone.10 Further, they recommend close monitoring, titration, and consideration of a daily NMB holiday in patients in which full chemical paralysis is used.10
Extracorporeal life support
Extracorporeal life support (ECLS) is likely beneficial in PARDS because it limits the volutrauma, barotrauma, and oxygen toxicity associated with mechanical ventilation. 141,142 The CESAR trial showing a survival benefit for adult patients with respiratory failure randomized to receive care in ECLS centers. ECLS is now an important therapeutic option for adult ARDS.143
The proper timing of initiation ECLS in respiratory failure is not defined and must balance the potential benefit of lung rest against morbidities associated with ECLS. Several observational adult ARDS studies have found that improved outcome is associated with a shorter duration of mechanical ventilation prior to initiation of ECLS.144–147 Current adult ARDS recommendations suggest that patients mechanically ventilated for more than 7 days may be less likely to benefit from ECLS for their respiratory failure.148 Zabrocki et al evaluated this question in the PARDS population.149 They found that patients mechanically ventilated for ≤14 days prior to ECLS initiation had similar survivals (56-61%), while those ventilated >14 days dropped their survival to 38%. 149 Nance et al reported a statistically significant survival decrease of 2.9% for each pre-ECLS ventilator day.150 Therefore, the most current ELSO guidelines for pediatrics suggest that implementing ECLS is most appropriate within the first 7 days of mechanical ventilation at high levels of support.149
Many other variables are important in applying ECLS in the PARDS population, including the method of support (venovenous in patients with respiratory failure and preserved cardiac function or venoarterial for patients with cardiopulmonary failure), type of cannula, location of cannulation, and approach to anticoagulation.151,152 Although venovenous ECLS can replace lung function, this is correlated with the efficiency of ECLS, which is affected by the maximum achievable flow and the extent of recirculation. Therefore, some ventilator support might be required to augment ventilation and/or oxygenation.151 However, in some patient populations, extubation while on ECLS is an emerging management strategy.76,153 Finally, new data suggests that ECLS for carbon dioxide removal may be a beneficial adjuvant for patients without life-threatening hypoxia.152 ECLS is an important and growing management strategy in PARDS. PALICC recommends consideration of ECLS in severe PARDS where the cause is likely to be reversible or the child is suitable for consideration of lung transplantation. 154
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
Disclose any relationship with a commercial company that has a direct financial interest in subject matter or materials discussed in article or with a company making a competing product.
The Authors have nothing to disclose
References:
- 1.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med 2000;342(18):1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Sapru A, Flori H, Quasney MW, Dahmer MK. Pathobiology of Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2015;16(5):S6–S22. doi: 10.1097/PCC.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL LB. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 4.Katz R Adult respiratory distress syndrome in children. Clin Chest Med 1987;8(4):635–639. [PubMed] [Google Scholar]
- 5.Sherrill DL, Camilli a, Lebowitz MD. On the temporal relationships between lung function and somatic growth. Am Rev Respir Dis 1989;140(3):638–644. doi: 10.1164/ajrccm/140.3.638. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med 1994;20(3):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 8.Kress JP, Accp I, Care C, Brd M. Acute Respiratory Distress Syndrome. JAMA. 2012;307(23):2526–2533. doi: 10.1378/ccmbr.20th.279. [DOI] [PubMed] [Google Scholar]
- 9.The ARDS Definition Task Force, Ranieri V, Rubenfeld G, et al. Acute Respiratory Distress Syndrome. Jama. 2012;307(23):1. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Jouvet P, Thomas NJ, Willson DF, et al. Pediatric Acute Respiratory Distress Syndrome: Consensus Recommendations From the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit CARE Med 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2015;16(5):S23–S40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124(1):87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 13.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med 2015;43(5):937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 14.Cheifetz IM. Year in Review 2015: Pediatric ARDS. Respir Care. 2016;61(7):980–985. doi: 10.4187/respcare.05017. [DOI] [PubMed] [Google Scholar]
- 15.Flori H, Glidden D, Rutherford G, Matthay M. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005;171(9):995–991001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 16.Lai-Fook SJ. Perivascular interstitial fluid pressure measured by micropipettes in isolated dog lung. J Appl Physiol 1982;52(1):9–15. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya J, Nakahara K, Staub N. Effect of edema on pulmonary blood flow in the isolated perfused dog lung lobe. J Appl Physiol 1980;48(3):444–449. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya J Hydraulic conductivity of lung venules determined by split-drop technique. J Appl Physiol 1988;64(6):2562–2567. http://www.ncbi.nlm.nih.gov/pubmed/3403440. [DOI] [PubMed] [Google Scholar]
- 20.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008;63(12):1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003;58(11):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo WA, Knight PR, Raghavendran K. The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med 2012;38(10):1588–1598. doi: 10.1007/s00134-012-2624-y. [DOI] [PubMed] [Google Scholar]
- 23.Determann RM, Royakkers AA, Haitsma JJ, et al. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med 2010;10:6. doi:1471-2466-10-6 [pii]\r10.1186/1471–2466-10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wort SJ, Evans TW. The role of the endothelium in modulating vascular control in sepsis and related conditions. Br Med Bull 1999;55(1):30–48. [DOI] [PubMed] [Google Scholar]
- 25.Orfanos SE, Armaganidis A, Glynos C, et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation. 2000;102(16):2011–2018. [DOI] [PubMed] [Google Scholar]
- 26.Sabharwal AK, Bajaj SP, Ameri A, et al. Tissue factor pathway inhibitor and von Willebrand factor antigen levels in adult respiratory distress syndrome and in a primate model of sepsis. Am J Respir Crit Care Med 1995;151(3 Pt 1):758–767. doi: 10.1164/ajrccm/151.3_Pt_1.758. [DOI] [PubMed] [Google Scholar]
- 27.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med 2004;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 28.Orwoll BE, Spicer AC, Zinter MS, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): a prospective observational cohort study. Crit Care. 2015;19:435. doi: 10.1186/s13054-015-1145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: Incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janz DR, Zhao Z, Koyama T, et al. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care. 2013;3(1):33. doi: 10.1186/2110-5820-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janz DR, Ware LB. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J intensive care. 2015;3:20. doi: 10.1186/s40560-015-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeulen Windsant IC, de Wit NCJ, Sertorio JTC, et al. Blood transfusions increase circulating plasma free hemoglobin levels and plasma nitric oxide consumption: a prospective observational pilot study. Crit Care. 2012;16(3):R95. doi: 10.1186/cc11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arezes J, Jung G, Gabayan V, et al. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe 2015;17(1):47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 2014;306(8):L709–25. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 2012;122(7):2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovach MA, Standiford TJ. Toll like receptors in diseases of the lung. Int Immunopharmacol 2011;11(10):1399–1406. doi: 10.1016/j.intimp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolle LB, Standiford TJ. Danger-associated molecular patterns (DAMPs) in acute lung injury. J Pathol 2013;229(2):145–156. doi: 10.1002/path.4124. [DOI] [PubMed] [Google Scholar]
- 38.Xiang M, Fan J. Pattern recognition receptor-dependent mechanisms of acute lung injury. Mol Med 2010;16(1–2):69–82. doi: 10.2119/molmed.2009.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164(10 Pt 1):1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 40.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005;288(3):L426–31. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 41.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33(1):1–2. [DOI] [PubMed] [Google Scholar]
- 42.Zinter MS, Orwoll BE, Spicer AC, et al. Incorporating Inflammation into Mortality Risk in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med February 2017. doi: 10.1097/CCM.0000000000002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory TJ, Longmore WJ, Moxley MA, et al. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 1991;88(6):1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunther A, Schmidt R, Feustel A, et al. Surfactant subtype conversion is related to loss of surfactant apoprotein B and surface activity in large surfactant aggregates. Experimental and clinical studies. Am J Respir Crit Care Med 1999;159(1):244–251. doi: 10.1164/ajrccm.159.1.9612005. [DOI] [PubMed] [Google Scholar]
- 45.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 1999;160(6):1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 46.Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 2003;31(1):20–27. doi: 10.1097/01.CCM.0000045028.46623.C2. [DOI] [PubMed] [Google Scholar]
- 47.Flori HR, Ware LB, Milet M, Matthay MA. Early elevation of plasma von Willebrand factor antigen in pediatric acute lung injury is associated with an increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med 2007;8(2):96–101. doi: 10.1097/01.PCC.0000257097.42640.6F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 2006;291(3):H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 49.Scarpati EM, Sadler JE. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem 1989;264(34):20705–20713. [PubMed] [Google Scholar]
- 50.Bastarache JA, Wang L, Geiser T, et al. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax 2007;62(7):608–616. doi: 10.1136/thx.2006.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sapru A, Curley MAQ, Brady S, Matthay MA, Flori H. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med 2010;36(1):157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dushianthan A, Grocott MPW, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J 2011;87(1031):612–622. doi: 10.1136/pgmj.2011.118398. [DOI] [PubMed] [Google Scholar]
- 54.Baron RM, Levy BD. Recent advances in understanding and treating ARDS. F1000Research. 2016;5. doi: 10.12688/f1000research.7646.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 56.Needham CJ, Brindley PG. Best evidence in critical care medicine: The role of neuromuscular blocking drugs in early severe acute respiratory distress syndrome. Can J Anaesth 2012;59(1):105–108. doi: 10.1007/s12630-011-9615-2. [DOI] [PubMed] [Google Scholar]
- 57.Schwingshackl A, Teng B, Makena P, et al. Deficiency of the two-pore-domain potassium channel TREK-1 promotes hyperoxia-induced lung injury. Crit Care Med 2014;42(11):e692–701. doi: 10.1097/CCM.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwingshackl A, Meduri GU. Rationale for Prolonged Glucocorticoid Use in Pediatric ARDS: What the Adults Can Teach Us. Front Pediatr 2016;4:58. doi: 10.3389/fped.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwingshackl A, Teng B, Ghosh M, Waters CM. Regulation of Monocyte Chemotactic Protein-1 secretion by the Two-Pore-Domain Potassium (K2P) channel TREK-1 in human alveolar epithelial cells. Am J Transl Res 2013;5(5):530–542. [PMC free article] [PubMed] [Google Scholar]
- 60.Schwingshackl A, Teng B, Ghosh M, et al. Regulation of interleukin-6 secretion by the two-pore-domain potassium channel Trek-1 in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2013;304(4):L276–86. doi: 10.1152/ajplung.00299.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roan E, Waters CM, Teng B, Ghosh M, Schwingshackl A. The 2-pore domain potassium channel TREK-1 regulates stretch-induced detachment of alveolar epithelial cells. PLoS One. 2014;9(2):e89429. doi: 10.1371/journal.pone.0089429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davey A, McAuley DF, O’Kane CM. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur Respir J 2011;38(4):959–970. doi: 10.1183/09031936.00032111. [DOI] [PubMed] [Google Scholar]
- 63.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010;298(6):L715–31. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. N Engl J Med 2011;364(19):1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Flori H, Dahmer MK, Sapru A, Quasney MW, Pediatric Acute Lung Injury Consensus Conference Group. Comorbidities and assessment of severity of pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5 Suppl 1):S41–50. doi: 10.1097/PCC.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 66.Spicer AC, Calfee CS, Zinter MS, et al. A Simple and Robust Bedside Model for Mortality Risk in Pediatric Patients With Acute Respiratory Distress Syndrome. Pediatr Crit Care Med 2016:1. doi: 10.1097/PCC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Messika J, Ben Ahmed K, Gaudry S, et al. Use of High-Flow Nasal Cannula Oxygen Therapy in Subjects With ARDS: A 1-Year Observational Study. Respir Care. 2015;60(2):162–169. doi: 10.4187/respcare.03423. [DOI] [PubMed] [Google Scholar]
- 68.Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007;35(1):18–25. doi: 10.1097/01.CCM.0000251821.44259.F3. [DOI] [PubMed] [Google Scholar]
- 69.Bellani G, Laffey JG, Pham T, et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med 2017;195(1):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 70.Chiumello D, Brioni M. Severe hypoxemia: which strategy to choose. Crit Care. 2016;20(1):132. doi: 10.1186/s13054-016-1304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 72.Fioretto JR, Ribeiro CF, Carpi MF, et al. Comparison between noninvasive mechanical ventilation and standard oxygen therapy in children up to 3 years old with respiratory failure after extubation: a pilot prospective randomized clinical study. Pediatr Crit Care Med 2015;16(2):124–130. doi: 10.1097/PCC.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 73.Morris J V, Ramnarayan P, Parslow RC, Fleming SJ. Outcomes for Children Receiving Noninvasive Ventilation as the First-Line Mode of Mechanical Ventilation at Intensive Care Admission: A Propensity Score-Matched Cohort Study. Crit Care Med March 2017. doi: 10.1097/CCM.0000000000002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheifetz IM. Pediatric Acute Respiratory Distress Syndrome. Respir Care. 2011;56(10):1589–1599. doi: 10.4187/respcare.01515. [DOI] [PubMed] [Google Scholar]
- 75.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 2009;151(8):566–576. [DOI] [PubMed] [Google Scholar]
- 76.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane database Syst Rev 2013;(2):CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdelsalam M, Cheifetz IM. Goal-directed therapy for severely hypoxic patients with acute respiratory distress syndrome: permissive hypoxemia. Respir Care. 2010;55(11):1483–1490. [PubMed] [Google Scholar]
- 78.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 79.Mercat A, Richard J-CM, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 80.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 81.Santa Cruz R, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane database Syst Rev 2013;(6):CD009098. doi: 10.1002/14651858.CD009098.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 83.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34(5):1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 84.Young D, Lamb SE, Shah S, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med 2013;368(9):806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 85.Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 86.Gupta P, Green JW, Tang X, et al. Comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. JAMA Pediatr 2014;168(3):243–249. doi: 10.1001/jamapediatrics.2013.4463. [DOI] [PubMed] [Google Scholar]
- 87.Bateman ST, Borasino S, Asaro LA, et al. Early High-Frequency Oscillatory Ventilation in Pediatric Acute Respiratory Failure. A Propensity Score Analysis. Am J Respir Crit Care Med 2016;193(5):495–503. doi: 10.1164/rccm.201507-1381OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 1993;328(6):399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 89.Adhikari NKJ, Burns KEA, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adhikari NKJ, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med 2014;42(2):404–412. doi: 10.1097/CCM.0b013e3182a27909. [DOI] [PubMed] [Google Scholar]
- 91.Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis. Anesth Analg 2011;112(6):1411–1421. doi: 10.1213/ANE.0b013e31820bd185. [DOI] [PubMed] [Google Scholar]
- 92.Hunt JL, Bronicki RA, Anas N. Role of Inhaled Nitric Oxide in the Management of Severe Acute Respiratory Distress Syndrome. Front Pediatr 2016;4:74. doi: 10.3389/fped.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dobyns EL, Cornfield DN, Anas NG, et al. Multicenter randomized controlled trial of the effects of inhaled nitric oxide therapy on gas exchange in children with acute hypoxemic respiratory failure. J Pediatr 1999;134(4):406–412. [DOI] [PubMed] [Google Scholar]
- 94.Day RW, Allen EM, Witte MK. A randomized, controlled study of the 1-hour and 24-hour effects of inhaled nitric oxide therapy in children with acute hypoxemic respiratory failure. Chest. 1997;112(5):1324–1331. [DOI] [PubMed] [Google Scholar]
- 95.Ibrahim T, El-Mohamady H. Inhaled Nitric Oxide and Prone Position: How Far They Can Improve Oxygenation in Pediatric Patients with Acute Respiratory Distress Syndrome? J Med Sci 2007;7(3):390–395. [Google Scholar]
- 96.Bronicki RA, Fortenberry J, Schreiber M, Checchia PA, Anas NG. Multicenter randomized controlled trial of inhaled nitric oxide for pediatric acute respiratory distress syndrome. J Pediatr 2015;166(2):365–9.e1. doi: 10.1016/j.jpeds.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt R, Markart P, Ruppert C, et al. Time-dependent changes in pulmonary surfactant function and composition in acute respiratory distress syndrome due to pneumonia or aspiration. Respir Res 2007;8:55. doi: 10.1186/1465-9921-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spragg RG, Lewis JF, Walmrath H-D, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med 2004;351(9):884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 99.Taut FJH, Rippin G, Schenk P, et al. A Search for subgroups of patients with ARDS who may benefit from surfactant replacement therapy: a pooled analysis of five studies with recombinant surfactant protein-C surfactant (Venticute). Chest. 2008;134(4):724–732. doi: 10.1378/chest.08-0362. [DOI] [PubMed] [Google Scholar]
- 100.Spragg RG, Taut FJH, Lewis JF, et al. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med 2011;183(8):1055–1061. doi: 10.1164/rccm.201009-1424OC. [DOI] [PubMed] [Google Scholar]
- 101.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293(4):470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 102.Hermon MM, Golej J, Burda G, et al. Surfactant therapy in infants and children: three years experience in a pediatric intensive care unit. Shock. 2002;17(4):247–251. [DOI] [PubMed] [Google Scholar]
- 103.Yapicioglu H, Yildizdas D, Bayram I, Sertdemir Y, Yilmaz HL. The use of surfactant in children with acute respiratory distress syndrome: efficacy in terms of oxygenation, ventilation and mortality. Pulm Pharmacol Ther 2003;16(6):327–333. doi: 10.1016/S1094-5539(03)00088-9. [DOI] [PubMed] [Google Scholar]
- 104.Thomas NJ, Guardia CG, Moya FR, et al. A pilot, randomized, controlled clinical trial of lucinactant, a peptide-containing synthetic surfactant, in infants with acute hypoxemic respiratory failure. Pediatr Crit Care Med 2012;13(6):646–653. doi: 10.1097/PCC.0b013e3182517bec. [DOI] [PubMed] [Google Scholar]
- 105.Willson DF, Thomas NJ, Tamburro R, et al. Pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 2013;14(7):657–665. doi: 10.1097/PCC.0b013e3182917b68. [DOI] [PubMed] [Google Scholar]
- 106.Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med 2016;42(5):699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ingelse SA, Wosten-van Asperen RM, Lemson J, Daams JG, Bem RA, van Woensel JB. Pediatric Acute Respiratory Distress Syndrome: Fluid Management in the PICU. Front Pediatr 2016;4:21. doi: 10.3389/fped.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 109.Kuzovlev A, Tishkov E, Bukaev O. Effect of continuous high-volume hemofiltration on patients with acute respiratory distress syndrome. Crit care. 2013;17:S160–S161. doi: 10.1186/cc12369. [DOI] [Google Scholar]
- 110.Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Willson DF, Thomas NJ, Tamburro R, et al. The relationship of fluid administration to outcome in the pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 2013;14(7):666–672. doi: 10.1097/PCC.0b013e3182917cb5. [DOI] [PubMed] [Google Scholar]
- 112.Hu X, Qian S, Xu F, et al. Incidence, management and mortality of acute hypoxemic respiratory failure and acute respiratory distress syndrome from a prospective study of Chinese paediatric intensive care network. Acta Paediatr 2010;99(5):715–721. doi: 10.1111/j.1651-2227.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- 113.Valentine SL, Sapru A, Higgerson RA, et al. Fluid balance in critically ill children with acute lung injury. Crit Care Med 2012;40(10):2883–2889. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilson B, Typpo K. Nutrition: A Primary Therapy in Pediatric Acute Respiratory Distress Syndrome. Front Pediatr 2016;4:108. doi: 10.3389/fped.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rice TW, Wheeler AP, Thompson BT, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects on hemodynamics and gas exchange of omega-3 fatty acid-enriched lipid emulsion in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Lipids Health Dis 2008;7:39. doi: 10.1186/1476-511X-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects of an omega-3 fatty acid-enriched lipid emulsion on eicosanoid synthesis in acute respiratory distress syndrome (ARDS): A prospective, randomized, double-blind, parallel group study. Nutr Metab (Lond). 2011;8(1):22. doi: 10.1186/1743-7075-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yehya N, Servaes S, Thomas NJ, Nadkarni VM, Srinivasan V. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med 2015;41(9):1658–1666. doi: 10.1007/s00134-015-3953-4. [DOI] [PubMed] [Google Scholar]
- 119.Wong JJ-M, Loh TF, Testoni D, Yeo JG, Mok YH, Lee JH. Epidemiology of pediatric acute respiratory distress syndrome in singapore: risk factors and predictive respiratory indices for mortality. Front Pediatr 2014;2:78. doi: 10.3389/fped.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lim JKB, Lee JH, Cheifetz IM. Special considerations for the management of pediatric acute respiratory distress syndrome. Expert Rev Respir Med 2016;10(10):1133–1145. doi: 10.1080/17476348.2016.1219656. [DOI] [PubMed] [Google Scholar]
- 121.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang BMP, Craig JC, Eslick GD, Seppelt I, McLean AS. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 2009;37(5):1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 123.Drago BB, Kimura D, Rovnaghi CR, et al. Double-blind, placebo-controlled pilot randomized trial of methylprednisolone infusion in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2015;16(3):e74–81. doi: 10.1097/PCC.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 124.Tamburro RF, Kneyber MCJ. Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5 Suppl 1):S61–72. doi: 10.1097/PCC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 125.Walther S, Jansson I, Berg S, Lennquist S. Pulmonary granulocyte accumulation is reduced by nebulized corticosteroid in septic pigs. Acta Anaesthesiol Scand 1992;36(7):651–655. [DOI] [PubMed] [Google Scholar]
- 126.Festic E, Ortiz-Diaz E, Lee A, et al. Prehospital use of inhaled steroids and incidence of acute lung injury among patients at risk. J Crit Care. 2013;28(6):985–991. doi: 10.1016/j.jcrc.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao S, Feng D, Wu Q, Li K, Wang L. Losartan attenuates ventilator-induced lung injury. J Surg Res 2008;145(1):25–32. doi: 10.1016/j.jss.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 128.Paola R Di, Cuzzocrea S Peroxisome proliferator-activated receptors and acute lung injury. PPAR Res 2007;2007:63745. doi: 10.1155/2007/63745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Orlicky S, Tang X, Neduva V, et al. An allosteric inhibitor of substrate recognition by the SCF(Cdc4) ubiquitin ligase. Nat Biotechnol 2010;28(7):733–737. doi: 10.1038/nbt.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eckle T, Brodsky K, Bonney M, et al. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoSBiol 2013;11(9):e1001665. doi: 10.1371/journal.pbio.1001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soo Hoo GW. In prone ventilation, one good turn deserves another. N Engl J Med 2013;368(23):2227–2228. doi: 10.1056/NEJMe1304349. [DOI] [PubMed] [Google Scholar]
- 132.Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 133.Mok YH, Lee JH, Rehder KJ, Turner DA. Adjunctive treatments in pediatric acute respiratory distress syndrome. Expert Rev Respir Med 2014;8(6):703–716. doi: 10.1586/17476348.2014.948854. [DOI] [PubMed] [Google Scholar]
- 134.Gattinoni L, Vagginelli F, Carlesso E, et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 2003;31(12):2727–2733. doi: 10.1097/01.CCM.0000098032.34052.F9. [DOI] [PubMed] [Google Scholar]
- 135.Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 136.Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 137.Sud S, Sud M, Friedrich JO, Adhikari NKJ. Effect of mechanical ventilation in the prone position on clinical outcomes in patients with acute hypoxemic respiratory failure: a systematic review and meta-analysis. CMAJ. 2008;178(9):1153–1161. doi: 10.1503/cmaj.071802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Curley MAQ, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guerin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 140.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 141.Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 142.Lewandowski K Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care. 2000;4(3):156–168. doi: 10.1186/cc689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet (London, England). 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 144.Pranikoff T, Hirschl RB, Steimle CN, Anderson HL 3rd, Bartlett RH. Mortality is directly related to the duration of mechanical ventilation before the initiation of extracorporeal life support for severe respiratory failure. Crit Care Med 1997;25(1):28–32. [DOI] [PubMed] [Google Scholar]
- 145.Beiderlinden M, Eikermann M, Boes T, Breitfeld C, Peters J. Treatment of severe acute respiratory distress syndrome: role of extracorporeal gas exchange. Intensive Care Med 2006;32(10):1627–1631. doi: 10.1007/s00134-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 146.Mols G, Loop T, Geiger K, Farthmann E, Benzing A. Extracorporeal membrane oxygenation: a ten-year experience. Am J Surg 2000;180(2):144–154. [DOI] [PubMed] [Google Scholar]
- 147.Lewandowski K, Rossaint R, Pappert D, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med 1997;23(8):819–835. [DOI] [PubMed] [Google Scholar]
- 148.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365(20):1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 149.Zabrocki LA, Brogan T V, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med 2011;39(2):364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 150.Nance ML, Nadkarni VM, Hedrick HL, Cullen JA, Wiebe DJ. Effect of preextracorporeal membrane oxygenation ventilation days and age on extracorporeal membrane oxygenation survival in critically ill children. J Pediatr Surg 2009;44(8):1606–1610. doi: 10.1016/j.jpedsurg.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 151.Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator-induced lung injury. BMC Med 2013;11:85. doi: 10.1186/1741-7015-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morelli A, Del Sorbo L, Pesenti A, Ranieri VM, Fan E. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med 2017;43(4):519–530. doi: 10.1007/s00134-016-4673-0. [DOI] [PubMed] [Google Scholar]
- 153.Marhong JD, Munshi L, Detsky M, Telesnicki T, Fan E. Mechanical ventilation during extracorporeal life support (ECLS): a systematic review. Intensive Care Med 2015;41(6):994–1003. doi: 10.1007/s00134-015-3716-2. [DOI] [PubMed] [Google Scholar]
- 154.Dalton HJ, Macrae DJ. Extracorporeal support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16(5 Suppl 1):S111–7. doi: 10.1097/PCC.0000000000000439. [DOI] [PubMed] [Google Scholar]