Abstract
Background and Aims
Intestinal fibrosis is a common complication of inflammatory bowel diseases. Medical treatment of intestinal fibrosis is an unmet therapeutic need. CD147 overexpression can induce myofibroblast differentiation associated with extracellular matrix deposition, favouring the development of fibrosis. To understand whether CD147 may promote intestinal fibrosis, we analysed its expression and blocked its function by using its specific inhibitor AC-73 [3-{2-[([1,1’-biphenyl]-4-ylmethyl) amino]-1-hydroxyethyl} phenol] in the murine TNBS [trinitrobenzenesulfonic acid]-chronic colitis model associated with intestinal fibrosis.
Methods
TNBS chronic colitis was induced by weekly intrarectal administration of escalating doses of TNBS. Ethanol-treated and untreated mice were used as controls. Separated groups of TNBS, ethanol-treated or untreated mice received AC-73 or vehicle administered intraperitoneally from day 21 to day 49. At day 49, mice were killed, and colons collected for histological analysis, protein and RNA extraction. CD147, α-SMA and activated TGF-β1 protein levels, CD147/ERK/STAT3 signalling pathway and autophagy were assessed by Western blot, collagen and inflammatory/fibrogenic cytokines mRNA tissue content by quantitative PCR.
Results
In mice with chronic TNBS colitis, CD147 protein level increased during fibrosis development in colonic tissue, as compared to control mice. CD147 inhibition by AC-73 treatment reduced intestinal fibrosis, collagen and cytokine mRNA tissue content, without significant modulation of activated TGF-β1 protein tissue content. AC-73 inhibited CD147/ERK1/2 and STAT3 signalling pathway activation and induced autophagy.
Conclusions
CD147 is a potential new target for controlling intestinal fibrosis and its inhibitor, AC-73, might represent a potential new anti-fibrotic therapeutic option in IBD.
Keywords: Intestinal fibrosis, CD147, autophagy
1. Introduction
The inflammatory bowel diseases [IBD], including Crohn’s disease [CD] and ulcerative colitis [UC], are chronic inflammatory and multifactorial disorders with a polygenic background and multiple environmental triggers.1 Intestinal fibrosis is a common complication of IBD, particularly in patients with CD. More than one-third of CD patients develop a distinct fibrostenosing phenotype.2 Stricturing disease, both as primary or as post-surgical recurrent disease, represents the main indication for surgery in CD patients.3–5 Fibrosis is a consequence of the chronic intestinal inflammation that leads to ongoing tissue damage resulting in excessive extracellular matrix [ECM] deposition and loss of normal function.5,6 Despite the increasing ability to control inflammation, there has been little progress in preventing intestinal inflammation from progressing to fibrosis, suggesting that inflammation-independent mechanisms mediate a self-perpetuating fibrostenotic process.7,8 Indeed, recent evidence suggests that development of fibrosis and inflammation may be disconnected and that fibrosis might be reversible.9,10 ECM components are produced by different types of intestinal cells, including mesenchymal cells represented by fibroblasts, myofibroblasts and smooth muscle cells directly involved in intestinal fibrosis.6-8 Myofibroblasts are activated through multiple pathways, including proteolytic enzymes, pro-fibrogenic cytokines and growth factors such as transforming growth factor beta (TGF-β).11,12 The mucosa overlying CD strictures contains elevated levels of TGF-β1 RNA transcripts, phosphorylated intracellular TGF-β signalling proteins Smad2/3 with low levels of the inhibitor Smad7 compared with those seen in mucosa overlying non-stricture areas of the same patients.13 Furthermore, evidence suggests that the ERK1/2 pathway is an additional TGF-β signalling pathway that contributes to fibrogenesis.14 Defective autophagy has been reported to contribute to the development of fibrosis.15,16 A genetic variant of ATG16L1 (autophagy related 16-like 1) (rs2241880; leading to a T300A conversion) exhibits a strong association with the fibrostricturing phenotype in both adult and paediatric CD patients.15–17 Although the inhibition of TGF-β appears to be a logical way to control fibrogenesis, this approach may have undesirable effects because TGF-β shows highly pleiotropic actions and has immune-regulatory functions, including the induction of regulatory T cells.18 Therefore, targeting molecular pathways more directly related to the fibrogenesis and/or affecting the autophagy process might be useful in the treatment of intestinal fibrosis.
CD147, a glycosylated cell surface transmembrane protein of the immunoglobulin superfamily, is an extracellular matrix metalloproteinase [MMP] inducer [EMMPRIN] involved in the regulation of ECM remodelling in wound healing, inflammatory diseases and cancers.19,20 Expressed in numerous cell types, including epithelial cells and fibroblasts, CD147 is overexpressed on the surface of various types of tumour, such as colorectal cancer.20,21 Among its multifaceted roles and its interaction partners, CD147 has been involved in a positive ERK1/2-dependent loop of the TGF-β1/ Smad signalling pathway promoting liver fibrogenesis.22 Recently, an increased CD147 level has been proposed as a biomarker of IBD in children.23 In our experience, CD147 inhibition by using AC-73, a small molecule that specifically disrupts CD147 dimerization blocking ‘in vivo’ the action of CD147,24 is associated with increased autophagy.25 Here, to understand whether CD147 plays a key role in the development of intestinal fibrosis, we analysed its expression and blocked its function by using its inhibitor AC-73, also described as an inducer of autophagy,25 in the TNBS chronic colitis murine model of intestinal fibrosis.26
We found that CD147 protein expression increases during the development of intestinal fibrosis. AC-73 administration starting from the initial appearance of fibrosis significantly reduces intestinal fibrosis and decreases the levels of different inflammatory/fibrogenic cytokines, without significant modulation of activated TGF-β1 protein level. In line with previous studies,24,25 we found that by inhibiting CD147, AC-73 suppresses the activation/phosphorylation of STAT3 and ERK1/2 and activates autophagy, effects that contribute to the efficacy of AC-73 in reducing fibrosis in the murine TNBS chronic colitis model.
2. Materials and Methods
2.1 Mice
Male BALB/c mice [Charles River Laboratories Italy] were housed in the Istituto Superiore di Sanità [Rome, Italy] animal facility in individually ventilated cages [IVC system] containing enrichment devices. Maintenance of pathogen-free conditions was ensured by monitoring every 6 months, in accordance with the Federation of European Laboratory Animal Science Associations [FELASA] recommendations. All studies were approved by the Animal Care and Use Committee of Istituto Superiore di Sanità and the Italian Ministry of Health (no. 687/2019-PR [Prot D9997.88] #354028039#).
2.2 Induction of colitis
Chronic TNBS colitis was induced in 6–7-week-old male BALB/c mice by administering escalating doses of TNBS [trinitrobenzenesulfonic acid, Sigma-Aldrich] dissolved in 45% ethanol [total injection volume,150 µL] as follows: days 0 and 7: 1.5 mg; days 14 and 21: 2 mg; days 28, 35 and 42: 2.5 mg. TNBS/ethanol or ethanol were administered via a 3.5-F catheter inserted into the rectum of lightly anaesthetized mice, as previously described.26 Control groups consisted of untreated mice and mice treated with 45% ethanol [total injection volume, 150 µL]. At day 21 mice were treated with AC-73 [3-{2-[([1,1’-biphenyl]-4-ylmethyl) amino]-1-hydroxyethyl} phenol; 20 mg/kg] or its vehicle administered intraperitoneally [i.p.] three times a week until day 49 when mice were killed and colons collected for further analysis [see Supplementary Figure 1]
2.3 AC-73 administration
AC-73 [Specs ID number AN-465/42834501] was dissolved in 10% DMSO/40% PEG 300/ 5% Tween 80 [Sigma] used as vehicle [V], according to the manufacturer’s instructions [MedChemExpress]. AC-73 [20 mg/kg] was administrated i.p. three times a week starting from day 21 [3 weeks] of TNBS treatment [see Supplementary Figure.1]. Mice were killed at day 49.
2.4 Histological examination of colonic tissue
The last distal third of the colon was immediately fixed after removal in 10% buffered formalin and stored at 4°C for at least 48 h before trimming and paraffin embedding. Two different series of transverse 3-µm sections of each colon sample were stained both with Sirius Red and Haematoxylin-Eosin staining methods and examined by an Olympus BX51 light microscope.
2.4.1 Histopathology: Morphological evaluation of colitis on H&E-stained transverse sections
Morphological evaluation of chemically induced intestinal inflammation was carried out according with the scoring system [ranging from 0 to 6] proposed in Erben et al.27
2.4.2 Fibrosis [% on total area]: automated count by ImageJ software on Sirius Red-stained sections
From each sample of both series a microphotograph of the whole section, at 4× magnification, was collected using the Image Capture 2.0 system. Computer analysis of these images was performed to calculate fibrosis using ImageJ, a free open software [http://rsbweb.nih.gov/ij/]. Fibrosis was calculated as the percentage of red tissue on total area automatically quantified by ImageJ software. In detail, for every picture % fibrosis was calculated after 1000-µm calibration and after selection in the ImageJ menu of area, limit to threshold and display label on Set Measurements , in five steps: [1] convert the image in greyscale [image>type>RGB Stack]; [2] segment [isolate] the red-stained collagen using thresholding on the green channel; [3] measuring the thresholded area1 [red area – fibrosis] in µm2; [4] segment [isolate] the total colon transverse area using thresholding on blue channel; [5] measuring the thresholded transverse total area2. For each individual transverse colon image, thresholded areas1 of fibrosis was expressed as a percentage [%] vs total colon transverse area2. For each mouse, two different images from two different transverse colon sections were evaluated as the % fibrosis expressed as the individual mean value. Group total score was expressed by averaging the individual mean values of each mouse.
2.5 Western blot analysis
Aliquots of 25 µg of total protein extract were prepared from murine colonic tissue and resolved on 10% and 12% mini-Protean TGX precast gels [Bio-Rad] for standard denaturing electrophoresis, according to the manufacturer’s instructions. Precast gels were transferred to nitrocellulose filters by using the Transblot-Turbo transfer system, according to the manufacturer’s procedures [Bio-Rad]. Membranes were saturated for 2 h in 5% milk with 0.2% Tween 20-TBS-T [10 mM Tris–HCl pH 8.0, 150 mM NaCl] solution before incubation with a primary antibody in 1% BSA/TBS-T solution overnight at 4°C. After washes in TBS-T buffer, blots were incubated with the corresponding peroxidase-conjugated secondary antibody [Amersham Biosciences] in TBS-T for 1 h. Bound antibodies were visualized by using the enhanced chemiluminescence technique [ECL] according to the manufacturer’s instructions [WesternBright ECL-spray]. Antibodies used were: CD147 monoclonal antibody (EMMPRIN/CD147 [B5] sc-46700, Santa Cruz); TGF-β1 monoclonal antibody (TGFβ1[TB21] sc-52893, Santa Cruz); α-SMA polyclonal antibody [Cell Signaling Technology, Ma, USA]; for total STAT3 protein detection, STAT3 [H-190] polyclonal antibody [sc-7179, Santa Cruz]; phospho-STAT3-S727 and phospho-ERK1/2 [p44/p42 MAPK] polyclonal antibodies [Immunological Sciences]; for total ERK1/2 protein detection, p44/42 MAPK [Erk1/2] polyclonal antibody [9102, Cell Signaling Technology]; GAPDH polyclonal antibody [Sigma-Aldrich] was used as an internal control of the loaded amounts of total proteins. Densitometric analysis was performed to assess protein expression level by using a FluorChem E system, software version 4.1.1; the intensity of each band was normalized to that of the corresponding band of GAPDH.
2.6 Autophagy detection
Western blot analysis of the autophagy-related marker LC3 and its conversion from LC3-I to LC3-II form in colonic tissues from [TNBS+AC-73]-treated mice, as compared to [TNBS+V]-treated mice and to [ETOH+V]-treated mice of controls. For detection and quantification of LC3-I and LC3-II protein expression levels, LC3B polyclonal antibody [NB600-1384, Novus Biologicals] was used.25
2.7 RNA extraction and gene expression
RNA was extracted using an RNA mini–Kit Plus [Qiagen] and RNA quality was analysed via a NanoDrop Spectrophotometer [Thermo Scientific]. cDNA was reverse transcribed from 1 µg RNA using TaqMan Reverse Transcription Reagents [Applied Biosystems, Thermo Fisher Scientific] and the reaction was performed via a MiniAmp Thermal Cycler [Applied Biosystems, Thermo Fisher Scientific]. Quantitative real-time PCR was used to determine the tissue content of connective tissue growth factor [CTGF], Col1a2 and Col3a1, TNF-α, IL-6, IL-23p19, IL-17A, IL-12p35, IFN-γ, IL-34 and IL-36α, using the following primers:
| Primer | Forward sequence 5ʹ–3ʹ | Reverse sequence 5ʹ–3ʹ |
|---|---|---|
| HPRT | CTGGTGAAAAGGACCTCTCG | TGAAGTACTCATTATAGTCAAGGGCA |
| TNF-α | TTCTGTCTACTGAACTTCGGGGTGATCGGTCC | GTATGAGATAGCAAATCGGCTGACGGTGTGGG |
| IL-6 | CTGCAAGAGACTTCCATCCAGTT | GAAGTAGGGAAGGCCGTGG |
| IFN-γ | TGGCATAGATGTGGAAGAAAAGAG | TGCAGGATTTTCATGTCACCA |
| IL-17A | CTCTCCACCGCAATGAAGAC | AGCTTTCCCTCCGCATTGA |
| IL23p19 | CAGCGGGACATATGAATCTA | CCTTGAGTCCTTGTGGGTCA |
| IL12p35 | CTGCACTGCTGAAGACATCG | CCAGGCAACTCTCGTTCTTG |
| IL-34 | TTGCTGTAAACAAAGCCCCAT | CCGAGACAAAGGGTACACATTT |
| IL-36α | CAGCATCACCTTCGCTTAGAC | AGTGTCCAGATATTGGCATGG |
| CTGF | CAGACTGGAGAAGCAGAGCC- | GCTTGGCGATTTTAGGTGTC |
| Col1a2 | TGTTGGCCCATCTGGTAAAGA | CAGGGAATCCGATGTTGCC |
| Col3a1 | TGGTTCTGGCTTCCAGACAT | GCTTTGTGCAAAGTGGAACC |
IL-13 was quantified by using commercially available primers, QuantiTect Primer Assay in combination with QuantiTect SYBR Green PCR [Qiagen].
Gene amplification was performed in duplicate at 60°C using Fast SYBR Green Master Mix in a ViiA7 real-time PCR platform system [Applied Biosystems, Thermo Fisher Scientific]. Data are expressed as log2 [relative expression] according to relative expression = 40 − Delta [∆] Ct where 40 is the maximum number of cycles and ∆Ct is the difference in number of cycles of the gene of interest with the housekeeping gene. We attributed a relative expression of 40 to samples with undetectable expression [threshold cycle ≥ 40]; next, we estimated the relative expression as described above.
2.7. Statistical analysis
Data were statistically analysed and visualized with graphs using GraphPad Prism software [v 9.0] using Student’s t test or Mann–Whitney U test as appropriate. Statistical significance was set at p < 0.05.
3. Results
3.1. CD147 protein expression increases during intestinal inflammation and fibrosis development in TNBS chronic colitis
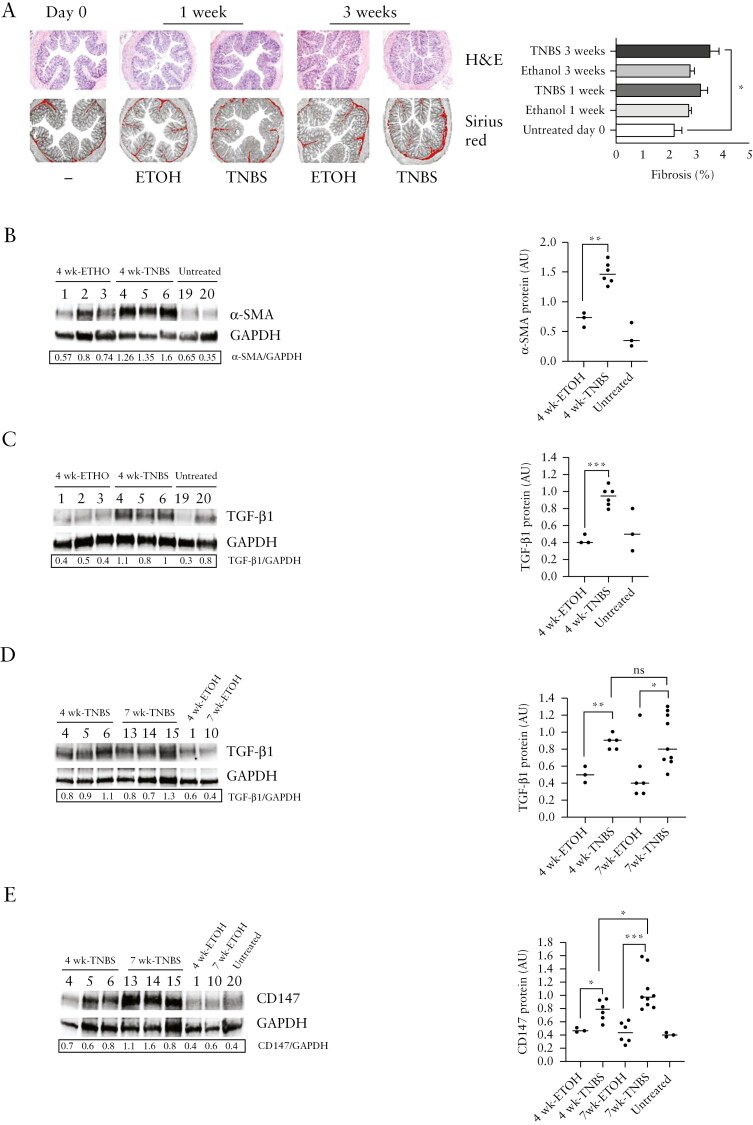
In preliminary experiments, we analysed collagen deposition and the expression of ECM proteins, including the pro-fibrotic α-smooth muscle actin [α -SMA], and transforming growth factor-β1 [TGF-β1] at different time points during the induction of TNBS chronic colitis. Ethanol [ETOH]-treated and untreated mice were evaluated as controls. We observed the microscopic appearance of fibrosis in colon sections after 3 weeks of TNBS treatment when compared with untreated mice [Figure 1A]. Therefore, additional mice were killed after 4 and 7 weeks of TNBS administration and compared with groups of ETOH-treated and untreated mice as controls. Expression of α-SMA and TGF-β1 biologically active [25 kDa] protein increased in colonic samples of TNBS-treated mice at 4 weeks, as compared to ETOH-treated and untreated control mice at 4 weeks [Figure 1B,C]. In addition, increased expression of TGF-β1 [25 kDa] biologically active protein was found in colonic samples of 4- and 7-week TNBS-treated mice, as compared to 4- and 7-week ETOH-treated control mice [Figure 1D]. However, we did not find any further significant increase of TGF-β1 [25 kDa] protein level in 7-week TNBS-treated mice when compared to 4-week TNBS-treated mice, indicating that TGF-β1 [25 kDa] expression remains high from 4 to 7 weeks of chronic TNBS treatment [Figure 1D]. Then, we found that CD147 protein expression level, higher in colonic samples of 4-week TNBS-treated mice than in 4- and 7-week ETOH-treated and untreated control mice, significantly increased in colon samples from 7-week TNBS-treated mice when compared with 4-week TNBS-treated mice [Figure 1E].
Figure 1.
CD147 protein expression increases with fibrosis development in TNBS chronic colitis. [A] left: H&E and Sirius red collagen staining of representative colon cross-sections [magnification, 4×] of mice at time 0 and after TNBS or ethanol [ETOH] intrarectal administration for 1 and 3 weeks; right: % fibrosis. Data are mean values ± SE from five untreated mice on day 0, six ethanol-treated and TNBS-treated mice at 1 week, five ethanol-treated and six TNBS-treated mice at 3 weeks. *p<0.05 by Student’s t test. [B] Western blot analysis of α-SMA protein [42 kDa] and [C] activated TGF-β1 [25 kDa] protein expression levels in colonic tissue from 4-week TNBS-treated mice, as compared to 4-week ETOH treated mice and untreated control mice. One representative western blot out of three is shown; GAPDH is an internal control of total protein extracts. [D] Western blot analysis of activated TGF-β1 [25 kDa] protein expression level in colonic tissue from mice after 4 weeks [4wk-TNBS] and 7 weeks [7wk-TNBS] of intrarectal TNBS administration, as compared to mice receiving ethanol [4wk-ETOH, 7wk-ETOH]. [E] Western blot analysis of CD147 protein [50 kDa] expression level in colonic tissue from mice after 7 weeks of intrarectal TNBS administration [7wk-TNBS], as compared to colonic tissue from mice after 4 weeks of intrarectal TNBS administration [4wk-TNBS] and from control mice receiving ethanol alone [4wk-ETOH, 7wk-ETOH] or untreated. [B–E] Left: one representative western blotting with densitometric analysis of protein expression levels compared with GAPDH levels, out of three is shown; GAPDH is an internal control of total protein extracts; right: densitometric data [AU, arbitrary units] is represented as individual plots from 4-week TNBS [n = 6] and 7-week TNBS [n = 9] treated mice, 4-week ETOH [n = 3] and 7-week ETOH [n = 6] treated mice, and untreated mice [n = 3]. Lines indicate median values. ns [not significant]; *p<0.05; **p<0.01; ***p<0.001 from a Mann–Whitney test.
Altogether, the increased expression of CD147 associated with the increased expression of α-SMA related to collagen deposition and TGF-β1 production in TNBS-treated mice when compared with control mice, suggests a potential role of CD147 in the development of fibrosis in TNBS chronic colitis.
3.2. AC-73 treatment reduces intestinal fibrosis without significant modulation of activated TGF-β1 level
To investigate the potential role of CD147 in the development of the fibrotic process, we inhibited its function by using AC-73. Different groups of mice were treated as described in the Methods and Supplementary Figure 1. AC-73 was administered i.p. three times a week starting from day 21. Mice were killed at day 49 [after 7 weeks of TNBS/ethanol intrarectal administration], and colons were collected for histological analysis, and RNA and protein extraction.
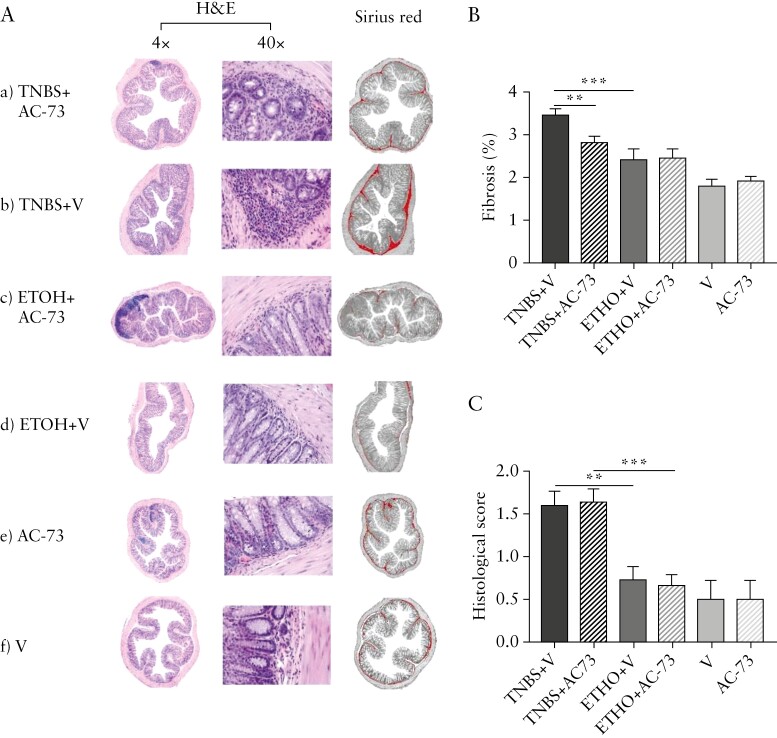
As shown in Figure 2, we detected a significant increase of collagen staining in 7-week TNBS+Vehicle [TNBS+V]-treated mice colonic sections, as compared to ethanol+vehicle [ETOH+V]-treated mice [Figure 2Ab,d,B]. Colonic sections of TNBS+AC-73-treated mice [TNBS+AC-73, Figure 2Aa,B] show significantly less collagen staining when compared with TNBS+V treated mice [Figure 2Ab,B] and a collagen staining comparable to control mice, i.e. [i] ETOH+V [Figure 2Ad,B] and ETOH+AC-73-treated mice [Figure 2Ac,B], and [ii] mice treated with vehicle V [Figure 2Af,B] or with AC-73 [Figure 2Ae,B] only. Assessment of colonic inflammation revealed, in the context of a mild inflammation, a significantly increased histological score in TNBS-treated vs ethanol-treated and untreated mice irrespective of treatment with AC-73 but no difference in TNBS-treated mice with AC-73 compared with TNBS-treated mice with vehicle [Figure 2C].The collagen staining on histology sections was mirrored by connective tissue growth factor, Type I and III collagen mRNA colonic tissue content: PCR analysis showed a significant reduction of CTGF, Collagen type I alpha 2 chain [Col1a2] and Collagen type III alpha 1 chain [Col3a1] mRNA expression in colonic tissue from TNBS+AC-73-treated mice, as compared to Col1a2 and Col3a1 mRNAs overexpressed in TNBS-treated mice [TNBS+V, Figure 3A], while a quite similar level of Col1a2 and Col3a1 mRNA expression was found in colonic samples of TNBS+AC-73-treated mice when compared to mice from control groups [ETOH+V, ETOH+AC-73, V and AC-73 mice] [ Figure 3A]. We also analysed α-SMA protein expression and observed a significant increase in TNBS+V-treated mice, as compared to ETOH+V-treated control mice and a significant decrease in colon samples from TNBS+AC-73-treated mice, as compared to colon samples from TNBS+V-treated mice [Figure 3B]. Together, the data show that AC-73 treatment significantly reduces intestinal fibrosis in TNBS-induced chronic colitis.
Figure 2.
AC-73 administration significantly reduces intestinal fibrosis in mice with TNBS chronic colitis. [A] H&E staining and ImageJ analysis on Sirius red collagen staining [magnification 4×] of representative colon cross-sections of mice: [a] receiving intrarectal [i.r.] TNBS and AC-73 intraperitoneally [i.p.] [TNBS+AC-73]; [b] receiving i.r. TNBS and vehicle i.p. [TNBS+V]; [c] receiving ethanol i.r. and AC-73 i.p. [ETOH+AC-73]; [d] receiving ethanol i.r. and vehicle i.p. [ETOH+V]; [e] receiving AC-73 i.p. only [AC-73].]; [f] receiving Vehicle i.p. only [V]. H&E staining: [a] and [b]: some inflammatory cells infiltrating the sub-mucosa; [c] and [d]: few inflammatory cells infiltrating the sub-mucosa; [e] and [f]: rare inflammatory cells infiltrating the sub-mucosa [magnification, 4× and 40×]. [B] % fibrosis. Fibrosis was assessed as in the Methods. Data are mean values ± SE from at least: ten TNBS-treated mice per group, five ethanol-treated mice per group and three mice per group receiving vehicle or AC-73 only. [C] Histological scores. Mean values ± SE from at least: ten TNBS-treated mice per group, five ethanol-treated mice per group and three mice per group receiving vehicle or AC-73 only. **p<0.01, ***p<0.001 from Student’s t-test [B] or a Mann–Whitney test [C].
Figure 3.
AC-73 administration decreases intestinal fibrosis without any significant modulation of CD147 and TGF-β1 protein expression levels in mice with TNBS chronic colitis. [A] Connective tissue growth factor [CTGF], collagen type I alpha 2 chain [Col1a2] and collagen type III alpha 1 chain [Col3a1] mRNA content by qPCR in colonic tissue from TNBS+V: mice receiving intrarectal [i.r.] TNBS and vehicle intraperitoneally [i.p.]; TNBS+AC-73: mice receiving TNBS i.r. and AC-73 i.p.; ETOH+V: mice receiving ethanol i.r. and vehicle i.p.; ETOH+AC-73: mice receiving ethanol i.r. and AC-73 i.p.; V: mice receiving vehicle i.p. only; AC-73: mice receiving AC-73 i.p. only. Data are represented as individual plots from at least: ten TNBS-treated mice per group, five ethanol-treated mice per group and three mice per group receiving vehicle or AC-73 only. Lines indicate median values. *p<0.05, ***p<0.001, from Mann-Whitney test. [B–D] Western blot analysis of [B] α-SMA protein, [C] CD147 protein and [D] TGF-β1 active protein levels. [B–D] Left: one representative western blot out of three is shown, with respective densitometry analysis of protein expression levels compared with GAPDH levels; GAPDH is an internal control of total protein extracts; right: densitometry analysis [A.U. = arbitrary units]: data are represented as individual plots from at least: ten TNBS-treated mice per group, five ethanol-treated mice per group and three mice per group receiving vehicle or AC-73 only. Mice group as in [A]. Lines indicate median values. ns, not significant, *p<0.05, **p<0.01, from a Mann–Whitney test.
CD147 protein level was significantly increased in colonic samples of 7-week TNBS+V fibrotic mice, as compared to 7-week ETOH+V or ETOH+AC-73 treated control mice [Figure 3C]. AC-73 treatment had no significant effect on CD147 protein level in colon samples from TNBS+AC-73 mice, as compared to TNBS+V mice [Figure 3C]. Notably AC-73 treatment had no significant effect on activated TGF-β1 [25 kDa] protein expression level in TNBS+AC-73-treated mice, as compared to TNBS+V-treated mice [Figure 3D], indicating that blockade of CD147 does not affect the overall tissue content of activated TGF-β1 [Figure 3D].
3.3. AC-73 treatment is associated with reduction of cytokine mRNA tissue content in mice with TNBS chronic colitis
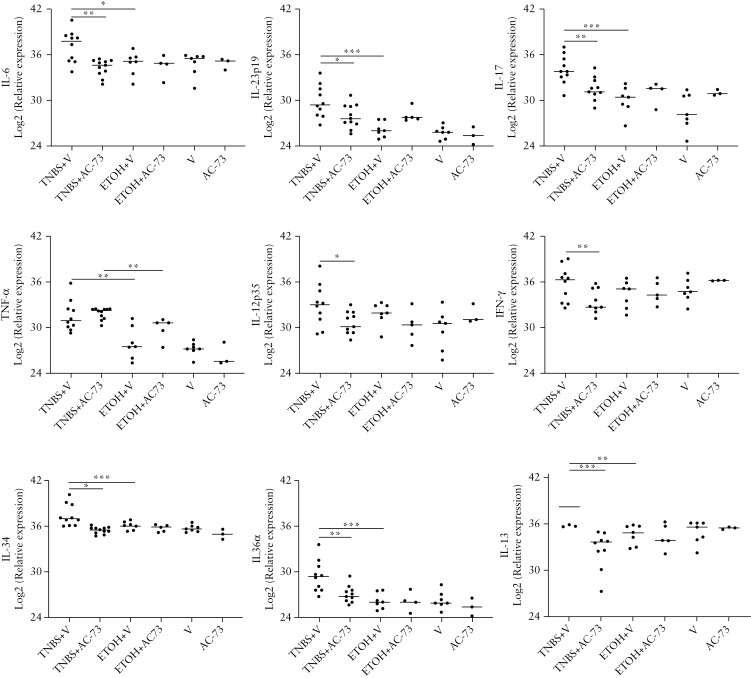
We analysed the mRNA cytokine content of colonic samples from TNBS+V-treated mice, as compared to TNBS+AC-73-treated mice and control mice [Figure 4].
Figure 4.
Effect of AC-73 administration on cytokine mRNA tissue content Cytokine mRNA content by qPCR in colonic tissue from TNBS+V: mice receiving i.r. TNBS and vehicle i.p.; TNBS+AC-73: mice receiving i.r. TNBS and AC-73 i.p.; ETOH+V: mice receiving ethanol i.r. and vehicle i.p.; ETOH+AC-73: mice receiving ethanol i.r. and AC-73 i.p.; V: mice receiving vehicle i.p. only; AC-73: mice receiving AC-73 i.p. only. Data are represented as individual plots from at least: ten TNBS-treated mice per group, five ethanol-treated mice per group and three mice per group receiving vehicle or AC-73 only. Lines indicate median values. *p<0.05, **p<0.01, ***p<0.001 from a Mann–Whitney test.
As previously reported,28 we found that TNBS chronic colitis was associated at a late time-point [day 49] with increased IL-6, TNF-α, IL-23p19, IL-17 and IL-13 mRNA tissue content while IL-12p35 and IFN-γ tissue contents were not increased when compared with controls [Figure 4]. Administration of AC-73 in mice with TNBS chronic colitis was associated with a significant reduction of IL-6 mRNA, whereas TNF-α mRNA tissue content was not influenced by AC-73 treatment. IL-23p19, IL-17 and IL-13 mRNA tissue contents were also significantly reduced when compared to TNBS+V-treated mice, showing values comparable to controls [Figure 4]. This reduction was also observed in the IL-12p35 and IFN-γ mRNA tissue content of TNBS+AC-73-treated mice, as compared to TNBS+V-treated mice [Figure 4]. A significant reduction in TNBS+AC-73-treated mice, as compared to TNBS+V-treated mice, was also found for IL-34 and IL-36 mRNA tissue content [Figure 4], two cytokines that were shown to have the ability of directly stimulate collagen synthesis by isolated intestinal fibroblasts in addition to their ability to sustain inflammatory pathways in the gut.29-32
Together, our data show that the observed reduction of intestinal fibrosis after AC-73 administration in mice with TNBS chronic colitis is associated with decreased mRNA tissue content of different colonic inflammatory/pro-fibrotic cytokines comparable to those observed in control mice.
3.4. AC-73 inhibits the activation of ERK1/2 and STAT3 signalling pathways and induces autophagy in TNBS chronic colitis
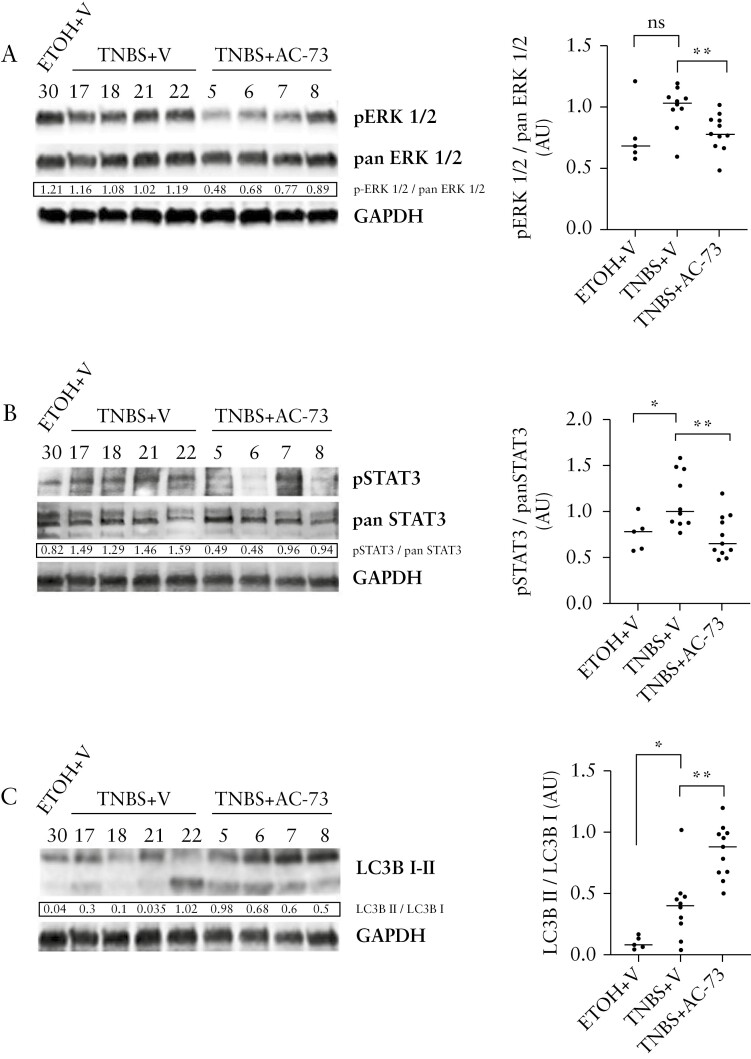
Considering that AC-73 suppresses the activation of the ERK1/2 and STAT3 signalling pathways in hepatocellular carcinoma and in acute myeloid leukaemia [AML]24,25 and induces autophagy in AML,25 we analysed the effects of AC-73 treatment on the CD147 signalling pathway in TNBS chronic colitis.
Phosphorylation of ERK1/2 and STAT3 [pERK1/2 and pSTAT3[S727]] in colonic samples of TNBS+V mice [Figures 5A,B] indicates that both ERK and STAT3 signalling pathways are activated in TNBS-treated mice. Notably, AC-73 treatment inhibits ERK1/2 and STAT3 phosphorylation, as shown by the decrease of pERK1/2 and pSTAT3 protein expression level, without affecting the total protein level of ERK1/2 [pan ERK1/2] and STAT3 [pan STAT3], in colonic samples from TNBS+AC-73-treated mice [ Figures 5A,B].
Figure 5.
AC-73 inhibits activation of the CD147/ERK1/2 and STAT3 signalling pathways and induces autophagy in mice with chronic TNBS colitis. [A, B] Western blot analysis of [A] ERK1/2 total [pan ERK1/2] and phosphorylated ERK1/2 [pERK1/2] protein expression levels, [B] STAT3 total [pan STAT3] and phosphorylated STAT3 [pSTAT3] protein expression levels. [A, B] Left: densitometric data are presented as the ratio of activated ERK to total ERK [pERK1/2/pan ERK1/2] and the ratio of activated STAT3 to total STAT3 [p-STAT3/pan STAT3]. [C] Western blot analysis of autophagy-related protein LC3 and its conversion from LC3-I to LC3-II in TNBS+AC-73, TNBS+V and ETOH+V mice; one representative western blot out of three is shown with densitometric data of the LC3B-II to LC3B-I ratio; GAPDH is an internal control of total protein extracts; right: densitometric data [A.U. = arbitrary units] represented as individual plots from at least: ten TNBS-treated mice per group and five ethanol-treated mice. Lines indicate median values. ns [not significant], *p<0.05, **p<0.01 from a Mann–Whitney test.
To assess the effect of AC-73 on the level of the autophagic indicators LC3-BI and LC3-BII in TNBS+AC-73-treated mice, as compared to TNBS+V-treated mice, we performed western blot analysis [Figure 5C]. We found an increase of both LC3-BI and LC3-BII, the cleaved and active forms of LC3-B, protein levels in colon samples from TNBS+AC-73-treated mice, as compared to fibrotic TNBS+V-treated mice and control ETOH+V-treated mice [Figure 5C]. The significant increase in the LC3-BII/LC3-BI ratio in TNBS+AC-73-treated mice indicates an induction of autophagy by AC-73 when compared to TNBS+V-treated mice [Figure 5C].
Together, our data indicate that AC-73 inhibits the activation of both ERK and STAT3 signalling pathways and activates autophagy in TNBS chronic colitis.
4. Discussion
In the present study, we demonstrate the involvement of CD147 in the intestinal fibrosis process and the ability of AC-73 [a small molecule able to inhibit CD147 signalling and to induce autophagy] administration to inhibit the fibrogenic process in TNBS chronic colitis. CD147 expression has been recently reported to be increased in biopsies of paediatric IBD patients both in intestinal epithelial cells and mononuclear cells infiltrating the mucosa.23 We observed a progressive increase of CD147 protein expression during the development of intestinal fibrosis, suggesting that CD147 may have an important role in fibrogenesis. Recent observations point to a definite role of CD147 expression and signalling in a ERK1/2-dependent TGF-β1–CD147 positive feedback loop in hepatic stellate cells promoting liver fibrosis.22 Specifically, TGF-β1 increases CD147 expression which, in turn, via ERK1/2 signalling and downstream Sp1, upregulates α-SMA, collagen I and TGF-β1 synthesis. A similar mechanism may be operating during TNBS chronic colitis as we observed a concomitant increase in TGF-β1, CD147 and α-SMA expression during the development of fibrosis. Moreover, we observed that, in line with previous studies,25 administration of AC-73 is associated with inhibition of tissue ERK1/2 phosphorylation, suggesting that the anti-fibrogenic effect of AC-73 administration may, in part, be due to the inhibition of the above-described positive feedback loop. Of interest, ERK1/2 cell signalling has been demonstrated to be active in TGF-β-induced CTGF and collagen I production by fibroblasts isolated from strictured and macroscopically normal serosa of patients with CD.14 Furthermore, increased phosphorylation of STAT3[S727] seen in mice with TNBS colitis is inhibited by AC-73 administration. This effect might contribute to the reduction of fibrosis, as recently observed in TNBS colitis mice treated with a selective STAT3 inhibitor.33 In addition to the effect of AC-73 administration on tissue fibrosis and collagen I and III mRNA tissue content, we also observed decreased mRNA tissue content of different inflammatory/fibrogenic cytokines, such as IL-6, and IL-23, IL-17, IL-13, IL-36 and IL-34 that, overexpressed in TNBS-treated mice, are comparable to controls in mice with TNBS colitis treated with AC-73. These inflammatory/fibrogenic cytokines are able, in the context of chronic inflammation, to activate intestinal mesenchymal cells leading to fibrosis and/or promoting epithelial–mesenchymal transition.34,35 In particular, IL-6 produced by activated mesenchymal cells may induce STAT3[S727] phosphorylation and nuclear translocation to regulate TGF-β1 and collagen expression, as previously described in muscle of stricturing CD.33 Although AC-73-mediated inhibition of ERK1/2 and STAT3 activation might be responsible for the reduction of cytokine mRNA tissue content,36 the ability of AC-73 administration to increase autophagy, as previously demonstrated 25 and confirmed in the present study, might indeed contribute to the inhibition of fibrosis observed in the present study. In addition to impaired autophagy observed in epithelial cells of the damaged mucosa of IBD patients37 and the reported ability of autophagy stimulators to reduce intestinal inflammation and improve acute murine colitis,38 recent evidence suggests a role of autophagy in modulating the fibrosis process. In a murine model of intestinal fibrosis, it has been demonstrated that autophagy inhibition aggravates, while autophagy stimulation prevents, fibrosis.39 In addition to the ‘in vitro’ observation that fibroblast autophagy, and its pharmacological regulation, affects collagen degradation, the tissue inflammatory response that accompanied fibrosis was significantly affected by autophagy modulation. In particular, in mice treated with the autophagy inhibitor, an increased expression of pro-inflammatory mediators and pro-fibrogenic40 CD16+-M2 macrophage infiltration was observed, while stimulation of autophagy with rapamycin increased the expression in mice of anti-inflammatory mediators and infiltration of macrophages with a regulatory/anti-inflammatory profile.39 Thus, autophagy, macrophages and related cytokine modulation seem to play a relevant role in the fibrogenic process. This view is reinforced by the observations made in the murine model of TNBS chronic colitis. In this model, it has been demonstrated that induction of autophagy via mTOR inhibition in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis.41
Together, our data demonstrate that CD147 expression and signalling are relevant for the development of fibrosis in chronic TNBS colitis and the early use of an inhibitor of CD147 signalling reduces fibrosis by modulating multiple processes involving inhibition of the ERK1/2 and STAT3 pathways, and autophagy induction. In conclusion, CD147 represents a new potential target to reduce fibrosis in IBDs and its inhibitor, AC-73, might represent a possible anti-fibrotic drug in the management of fibrostenosing CD.
Supplementary Material
Acknowledgements
The authors acknowledge the staff of the Center for animal experimentation and well-being, Istituto Superiore di Sanità, for the care and surveillance of mice.
Contributor Information
Alessia Butera, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Maria Teresa Quaranta, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Luca Crippa, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Isabella Spinello, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Ernestina Saulle, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Nazzareno Di Carlo, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Doriana Campanile, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Monica Boirivant, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Catherine Labbaye, Istituto Superiore di Sanità, National Center for Drug Research and Evaluation, Rome, Italy.
Funding
This work was supported by the National Center for Drug Research and Evaluation, Istituto Superiore di Sanità intramural funding assigned to M.B. and C.L.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Study conception and design: M.B. and C.L. Study supervision: M.B. and C.L. Acquisition of data: A.B., MT.Q., I.S., E.S., N.DC. and D.C. Histology and histological score: L.C. Analysis and interpretation of data: A.B., MT.Q., L.C., I.S., E.S., M.B. and C.L. Drafting of manuscript: M.B. and C.L. All authors approved the final version submitted.
Data Availability Statements
Data are available on request.
References
- 1. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med 2020; 383:2652–64. [DOI] [PubMed] [Google Scholar]
- 2. Rieder F, Latella G, Magro F, et al. European Crohn’s and colitis organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn’s Disease. J Crohns Colitis 2016;10:873–85. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein CN, LoftusNg EVSC, Lakatos PL, Moum B.. Epidemiology and natural history task force of the international organization for the study of inflammatory bowel disease (IOIBD). Hospitalisations and surgery in Crohn’s disease. Gut 2012;61:622–9. [DOI] [PubMed] [Google Scholar]
- 4. Olaison G, Smedh K, Sjödahl R.. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut 1992;33:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pallone F, Boirivant M, Stazi MA, Cosintino R, Prantera C, Torsoli A.. Analysis of clinical course of postoperative recurrence in Crohn’s disease of distal ileum. Dig Dis Sci 1992;37:215–9. [DOI] [PubMed] [Google Scholar]
- 6. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Latella G, Rogler G, Bamias G, et al. Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis 2014;8:1147–65. [DOI] [PubMed] [Google Scholar]
- 8. Herrera J, Henke CA, Bitterman PB.. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 2018;128:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hünerwadel A, Fagagnini S, Rogler G, et al. Severity of local inflammation does not impact development of fibrosis in mouse models of intestinal fibrosis. Sci Rep 2018;8:15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Latella G, Rieder F.. Intestinal fibrosis: ready to be reversed. Curr Opin Gastroenterol 2017;33:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pariente B, Hu S, Bettenworth D, et al. Treatments for Crohn’s disease-associated bowel damage: a systematic review. Clin Gastroenterol Hepatol 2019;17:847–56. [DOI] [PubMed] [Google Scholar]
- 12. Biernacka A, Dobaczewski M, Frangogiannis NG.. TGF-β signaling in fibrosis. Growth factors 2011;29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Sabatino A, Jackson CL, Pickard KM, et al. Transforming growth factor beta signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut 2009;58:777–89. [DOI] [PubMed] [Google Scholar]
- 14. Mulsow JJW, Watson RWG, Fitzpatrick JM, O’Connell PR.. Transforming growth factor-beta promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Ann Surg 2005;242:880–7, discussion 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zorzi F, Calabrese E, Monteleone G.. Pathogenic aspects and therapeutic avenues of intestinal fibrosis in Crohn’s disease. Clin Sci [Lond] 2015;129:1107–13. [DOI] [PubMed] [Google Scholar]
- 16. Shao BZ, Yao Y, Zhai JS, Zhu JH, Li JP, Wu K.. The role of autophagy in inflammatory bowel disease. Front Physiol 2021;12:621132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strisciuglio C, Auricchio R, Martinelli M, et al. Autophagy genes variants and paediatric Crohn’s disease phenotype: a single-centre experience. Dig Liver Dis 2014;46:512–7. [DOI] [PubMed] [Google Scholar]
- 18. Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A.. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev 2014;25:45–55. [DOI] [PubMed] [Google Scholar]
- 19. Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem 2016;59:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Landras A, Reger de Moura C, Jouenne F, Lebbe C, Menashi S, Mourah S.. CD147 is a promising target of tumor progression and a prognostic biomarker. Cancers [Basel] 2019;11:1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu T, Zhou M, Peng L, et al.. Upregulation of CD147 promotes cell invasion, epithelial-to-mesenchymal transition and activates MAPK/ERK signaling pathway in colorectal cancer. Int J Clin Exp Pathol 2014;7:7432–41. [PMC free article] [PubMed] [Google Scholar]
- 22. Li H-Y, Ju D, Zhang D-W, et al.. Activation of TGF-β1–CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci Rep 2015;5:16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Ye J, Liu R, et al.. Clinical significance of CD147 in children with inflammatory bowel disease. Biomed Res Int 2020;2020:7647181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu ZG, Wang L, Cui HY, et al.. A novel small-molecule compound targeting CD147 inhibits the motility and invasion of hepatocellular carcinoma cells. Oncotarget 2016;7:9429–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spinello I, Saulle E, Quaranta MT, et al.. The small-molecule compound AC-73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica 2019;104:973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wirtz S, Popp V, Kindermann M, et al.. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 2017;12:1295–309. [DOI] [PubMed] [Google Scholar]
- 27. Erben U, Loddenkemper C, Doerfel K, et al.. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 2014;7:4557–76. [PMC free article] [PubMed] [Google Scholar]
- 28. Fichtner-Feigl S, Fuss IJ, Young CA, et al.. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol 2007;178:5859–70. [DOI] [PubMed] [Google Scholar]
- 29. Franzè E, Dinallo V, Laudisi F, et al.. Interleukin-34 stimulates gut fibroblasts to produce collagen synthesis. J Crohns Colitis 2020;14:1436–45. [DOI] [PubMed] [Google Scholar]
- 30. Franzè E, Monteleone I, Cupi ML, et al.. Interleukin-34 sustains inflammatory pathways in the gut. Clin Sci [Lond] 2015;129:271–80. [DOI] [PubMed] [Google Scholar]
- 31. Scheibe K, Kersten C, Schmied A, et al.. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 2019;156:1082–1097.e11. [DOI] [PubMed] [Google Scholar]
- 32. Elias M, Zhao S, Le HT, et al.. IL-36 in chronic inflammation and fibrosis – bridging the gap? J Clin Invest 2021;131:e144336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Iness A, Yoon J, et al.. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscle of stricturing Crohn’s disease. J Immunol 2015;194:3422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li C, Kuemmerle JF.. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm Bowel Dis 2014;20:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang HJ, Zhang YN, Zhou H, Guan L, Li Y, Sun MJ.. IL-17A promotes initiation and development of intestinal fibrosis through EMT. Dig Dis Sci 2018;63:2898–909. [DOI] [PubMed] [Google Scholar]
- 36. Newton K, Dixit VM.. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol 2012;4:a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ortiz-Masiá D, Cosín-Roger J, Calatayud S, et al.. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal Immunol 2014;7:929–38. [DOI] [PubMed] [Google Scholar]
- 38. Macias-Ceja DC, Cosín-Roger J, Ortiz-Masiá D, et al.. Stimulation of autophagy prevents intestinal mucosal inflammation and ameliorates murine colitis. Br J Pharmacol 2017;174:2501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cosin-Roger J, Canet F, Macias-Ceja DC, et al.. Autophagy stimulation as a potential strategy against intestinal fibrosis. Cells 2019;8:10781078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salvador P, Macías-Ceja DC, Gisbert-Ferrándiz L, et al.. CD16+ macrophages mediate fibrosis in inflammatory bowel disease. J Crohns Colitis 2018;12:589–99. [DOI] [PubMed] [Google Scholar]
- 41. Mathur R, Alam MM, Zhao XF, et al.. Induction of autophagy in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol 2019;12:612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.