Abstract
BACKGROUND:
Abnormal glutamate and GABA (gamma-aminobutyric acid) levels have been found in the early phase of schizophrenia and may underlie cognitive deficits. However, the association between cognitive function and levels of glutamatergic metabolites and GABA has not been investigated in a large group of antipsychotic-naïve patients.
METHODS:
In total, 56 antipsychotic-naïve patients with schizophrenia or psychotic disorder and 51 healthy control subjects underwent magnetic resonance spectroscopy to measure glutamate, glutamate+glutamine (Glx), and GABA levels in dorsal anterior cingulate cortex (ACC) and glutamate and Glx levels in left thalamus. The cognitive domains of attention, working memory, and IQ were assessed.
RESULTS:
The whole group of antipsychotic-naïve patients had lower levels of GABA in dorsal ACC (p = .03), and the subgroup of patients with a schizophrenia diagnosis had higher glutamate levels in thalamus (p = .01), but Glx levels in dorsal ACC and thalamus did not differ between groups. Glx levels in dorsal ACC were positively associated with working memory (logarithmically transformed: b = −.016 [higher score indicates worse performance], p = .005) and attention (b = .056, p = .035) in both patients and healthy control subjects, although the association with attention did not survive adjustment for multiple comparisons.
CONCLUSIONS:
The findings suggest a positive association between glutamatergic metabolites and cognitive function that do not differ between patients and healthy control subjects. Moreover, our data indicate that decreased GABAergic levels in dorsal ACC are involved in schizophrenia and psychotic disorder, whereas increased glutamate levels in thalamus seem to be implicated in schizophrenia pathophysiology. The findings imply that first-episode patients with cognitive deficits may gain from glutamate-modulating compounds.
A growing body of studies suggest that glutamatergic and GABAergic (gamma-aminobutyric acidergic) neurotransmission is disturbed in first-episode patients with schizophrenia or psychosis. Glutamate and GABA levels in antipsychotic-naïve or minimally treated patients have primarily been investigated in anterior cingulate cortex (ACC) (1–11), thalamus (2,3,7,9), and dorsal striatum (12,13) using proton magnetic resonance spectroscopy (1H-MRS). These regions are part of cortico-striato-thalamo-cortical networks that are believed to be dysregulated in psychotic disorders (14,15). In the majority of the studies, higher levels of glutamine, glutamine+glutamate (Glx), glutamine/glutamate, and GABA have been found in pregenual ACC (1–6), and lower levels of glutamate (7) or Glx (11) have been found in dorsal ACC, although no differences in pregenual ACC also have been reported in some studies (5,8–10) and in a recent meta-analysis of antipsychotic-naïve/free patients (16). In dorsal striatum higher glutamate levels have been found (12,13), and in left thalamus higher glutamine and glutamate levels have been reported (2,3,7), whereas a recent study did not find alterations in glutamate levels (9). Importantly, our group recently reported that thalamic glutamate levels in twins are heritable and associated with schizophrenia spectrum disorder (17) and that higher thalamic glutamate levels and lower GABA levels in dorsal ACC of antipsychotic-naïve patients were related to subsequent poor treatment outcome (7). Moreover, two other groups have reported an association between high levels of glutamate and GABA in pregenual ACC and lack of treatment response (6,9).
Interestingly, several lines of evidence suggest that abnormal glutamatergic and GABAergic neurotransmission also may underlie cognitive deficits. For example, a reduced cortical glutamate/GABA balance is believed to cause insufficient gamma band oscillations that might impair working memory (18,19), and administration of antagonists of the NMDA receptor induces schizophrenia-like cognitive deficits in healthy control subjects (HCs) and animals in the domains attention, spatial working memory (SWM), and executive function, among others (20–24). While it is primarily cortical areas, such as dorsal ACC (25,26), that are believed to be involved in cognitive function, thalamocortical interactions might also be crucial for, for example, working memory and attention (27). Studies of mainly medicated patients have investigated the association between different cognitive domains and levels of glutamatergic metabolites and GABA. These studies report an association between prefrontal levels of glutamatergic metabolites and GABA and the cognitive domains working memory or executive functioning (28–33), attention (30,34), and intelligence (31). Importantly, the association between glutamatergic metabolites and cognitive function seems to change after treatment (28). Therefore, antipsychotic-naïve patients are needed to study the pathophysiology of cognitive deficits. To date, only 4 studies have included subgroups of antipsychotic-naïve or minimally treated patients (4,10,28,35) and reported conflicting results. In ACC, one study found a positive correlation between ACC glutamine and working memory (4), whereas others did not find correlations between glutamate or glutamine levels and working memory (28) or processing speed (10). In dorsolateral prefrontal cortex, Glx and GABA levels did not correlate with working memory and verbal learning (4,35). The studies were limited by small sample sizes (Ns = 7–26), no healthy control group (28), a long time interval (64 days) between cognitive testing and 1H-MRS (28), and the use of a 1.5T scanner (35). Thus, larger studies of antipsychotic-naïve patients are needed to clarify whether glutamatergic and GABAergic abnormalities are involved in the pathophysiology of cognitive deficits.
In this study, we initially examined whether levels of glutamatergic metabolites and GABA in dorsal ACC were lower, and levels of glutamatergic metabolites in thalamus were higher, in a large group of lifetime antipsychotic-naïve patients or only in the subgroup with a schizophrenia diagnosis. This was based on our previous findings in a subsample of the participants (7). Thereafter, we tested the primary hypotheses that Glx and GABA levels in dorsal ACC are associated with cognitive performance in tests of attention, SWM, and premorbid IQ in patients and investigated whether the associations were different from those in HCs.
METHODS AND MATERIALS
Participants
In total, 56 antipsychotic-naïve patients with schizophrenia or psychosis were recruited from January 2014 to March 2019 from psychiatric units and outpatient services in the Capital Region of Denmark, and 51 HCs matched on age, sex, and parental socioeconomic status were recruited through online advertisement. The study was conducted as part of baseline examinations in a prospective follow-up study approved by the Committee on Biomedical Research Ethics described in the Supplemental Participants and Methods. Subgroups of participants are also included in another article investigating the associations among glutamate, GABA, and treatment outcome (nPatients = 39, nHCs = 36) (7) and in two other articles including participants from separate studies to describe patterns of cortical structure and cognition (36) (nPatients = 23, nHCs = 39) and the effect of age on verbal memory functions (nPatients = 47, nHCs = 41) (37). Study procedures were fully explained before written informed consent was obtained. Inclusion criteria for patients were as follows: fulfillment of diagnostic criteria for schizophrenia, schizoaffective disorder, or nonorganic psychosis according to ICD-10 criteria assessed with the Schedules for Clinical Assessment in Neuropsychiatry (38); 18 to 45 years of age; never treated with antipsychotics or central nervous system stimulants during lifetime (confirmed by medical record); and legally competent. Participants with substance abuse during the past 3 months were excluded, but previous substance abuse and current occasional use were accepted for patients. Further exclusion criteria are reported in the Supplemental Participants and Methods. Information on alcohol, nicotine, cannabis, and drug use was obtained through self-report and confirmed with a urine test (Rapid Response; Jepsen HealthCare, Tune, Denmark). Occasional use of benzodiazepines was accepted in patients. Benzodiazepine users were excluded from the main analyses but were included in the Supplement.
Clinical and Cognitive Assessments
For patients, psychopathology was assessed by trained raters with the Positive and Negative Syndrome Scale (39), symptom severity was assessed with the Clinical Global Impression scale (40), and level of function was assessed with Global Assessment of Functioning social and occupational functioning score (41). The Personal and Social Performance Scale (42) estimated level of function in patients and HCs. Participants completed a cognitive battery from which three measures were selected a priori: SWM (outcome was SWM strategy score) and sustained visual attention tested with rapid visual information processing (RVP) (outcome was RVP A′) from the Cambridge Neuropsychological Test Automated Battery (43,44), and premorbid intelligence estimated with the Danish Adult Reading Test (DART) (45) (outcome was number of words correctly pronounced). Magnetic resonance scans and clinical and cognitive assessments were performed within 7 days.
Magnetic Resonance Imaging and Data Analysis
Magnetic resonance imaging was performed on a 3T scanner as previously described (46). Levels of Glx, glutamate, and other major metabolites were acquired with point-resolved spectroscopy (PRESS) (repetition time = 3000 ms, echo time = 30 ms, 128 averages with MOIST [Multiply Optimized Insensitive Suppression Train] water suppression) in a 2.0 × 2.0 × 2.0-cm3 voxel prescribed in dorsal ACC (Brodmann areas 24 and 32) and in a 2.0 × 1.5 × 2.0-cm3 voxel prescribed in left thalamus. Levels of GABA, Glx, and total creatine were acquired with the MEGAPRESS (Mescher–Garwood point-resolved spectroscopy) sequence (47) (echo time = 68 ms, repetition time = 2000 ms, 14-ms editing pulse applied at 1.9 and 7.5 ppm, 320 averages with MOIST water suppression) in a 3.0 × 3.0 × 3.0-cm3 voxel prescribed in dorsal ACC. Unsuppressed water reference spectra were acquired separate as inbuild sequences in the PRESS and MEGAPRESS sequences. PRESS acquisitions were analyzed with LCModel version 6.3-1J (48) and fitted in the spectral range between 0.2 and 4.0 ppm as previously described (46). Glx was the main outcome because it is generally believed that glutamate measured at 3T cannot be reliably separated from glutamine and GABA. However, glutamate levels are also reported to allow for comparison with previous MRS studies of ACC or left thalamus in antipsychotic-naïve or minimally treated patients scanned at 3T or 4T (2,3,7,9). MEGAPRESS acquisitions were analyzed with Gannet version 2.1 (49) and fitted in the spectral range between 2.79 and 3.55 ppm. The in vivo water-scaled values of metabolites reported by LCModel and Gannet were corrected for partial volume cerebrospinal fluid contamination to estimate concentration in institutional units as described in the Supplement. Details of 1H-MRS acquisitions, quality assessment, and analyses are reported in the Supplement together with motion estimates, metabolite levels scaled to total creatine (Table S5) and corrected for gray matter fraction, illustrations of and fidelity of voxel placements (Figures S1 and S2), representative spectra (Figure S3), and information about a scanner upgrade performed after inclusion of 10 patients and 4 HCs.
Statistical Analyses
Demographic and clinical variables, cognitive performance, and spectral quality of patients and HCs were compared using χ2, Fisher’s exact test, or independent t tests as appropriate. Separate multivariate linear regression models investigated whether glutamate and GABA levels in dorsal ACC (dependent variables) were different in antipsychotic-naïve patients compared with HCs (diagnosis as independent variable) with inclusion of the covariates sex and age, which may affect the metabolite levels (50,51), and smoking status that differed between patients and HCs (Table 1). Levels of other metabolites are provided in Table 2.
Table 1.
Demographic and Clinical Characteristics of Participants
| ANPs | HCs | Statistics | |
|---|---|---|---|
| n (Male/Female) | 56 (24/32) | 51 (22/29) | χ2 = 0.009, p = .98 |
| Age, Years, Mean ± SD | 22.7 ± 5.0 | 22.2 ± 4.1 | t105 = 0.54, p = .59 |
| Education, Years, Mean ± SD | 12.0 ± 2.1 | 13.9 ± 2.1 | t105 = −4.70, p < .001 |
| Parental Socioeconomic Status, High/Moderate/Low/Missing, n | 16/32/7/1 | 18/28/5/0 | χ2 = 0.57, p = .75 |
| Current Tobacco Use, Yes/No, n | 21/35 | 6/45 | χ2 = 9.37, p = .002 |
| Current Cannabis Use, Yes/No, n | 4/52 | 2/49 | Fisher’s p = .68 |
| Benzodiazepines, Daily/Occasionally/No, n | 1/5/50 | 0/0/51 | Fisher’s p = .06 |
| PSP Score, Mean ± SD | 46.3 ± 13.5 | 89.4 ± 3.4 | t101 = −21.37, p < .001 |
| GAF-F Score, Mean ± SD | 35.3 ± 6.5 | NA | |
| DUP in Weeks, Median (25th–75th Percentile) | 28 (16–130) | NA | |
| Diagnosis (ICD-10), n | |||
| Undifferentiated schizophrenia | 11 | ||
| Paranoid schizophrenia | 25 | ||
| Disorganized schizophrenia | 5 | ||
| Simplex schizophrenia | 1 | ||
| Schizoaffective, depressive type | 1 | ||
| Nonorganic psychosis | 11 | ||
| Paranoid psychosis | 2 | ||
| PANSS, Mean ± SD | |||
| Total score | 75.9 ± 14.3 | NA | |
| Positive subscore | 18.8 ± 4.4 | NA | |
| Negative subscore | 19.9 ± 5.3 | NA | |
| General subscore | 37.1 ± 7.3 | NA | |
| CGI, Mean ± SD | 4.4 ± 0.6 | NA | |
ANPs, antipsychotic-naïve patients; CGI, Clinical Global Impression scale; DUP, duration of untreated psychosis; GAF-F, Global Assessment of Functioning, social and occupational functioning score; HCs, healthy control subjects; NA, not applicable; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale.
Table 2.
Neurometabolite Levels in Dorsal ACC and Left Thalamus of ANPs and HCs
| Dorsal ACC | Statisticsb | Left Thalamus | Statisticsb | |||
|---|---|---|---|---|---|---|
| ANPs, Mean ± SD, na | HCs, Mean ± SD, na | ANPs, Mean ± SD, na | HCs, Mean ± SD, na | |||
| PRESS Sequences | ||||||
| Glutamate IU | 10.65 ± 1.44, n = 48 | 10.91 ± 1.17, n = 51 | F1,98 = 1.02, p = .32 | 6.97 ± 0.89, n = 48 | 6.75 ± 0.82, n = 46 | F1,93 = 2.72, p = .10 |
| Glx IU | 14.12 ± 2.10, n = 48 | 14.30 ± 1.69, n = 51 | F1,98 = 0.19, p = .67 | 9.88 ± 1.67, n = 48 | 9.78 ± 1.35, n = 47 | F1,93 = 0.24, p = .63 |
| Glutamine IU | 3.70 ± 0.76, n = 40 | 3.65 ± 0.60, n = 40 | F1,79 = 0.76, p = .39 | – | – | – |
| NAA IU | 8.72 ± 1.01, n = 48 | 8.84 ± 0.82, n = 51 | F1,98 = 0.30, p = .58 | 7.17 ± 0.65, n = 48 | 7.30 ± 0.51, n = 47 | F1,93 = 0.20, p = .65 |
| Myo-inositol IU | 5.89 ± 0.76, n = 48 | 5.96 ± 0.67, n = 51 | F1,98 = 0.34, p = .56 | 3.51 ± 0.54, n = 48 | 3.63 ± 0.48, n = 47 | F1,93 = 0.85, p = .40 |
| Choline IU | 1.98 ± 0.30, n = 48 | 2.03 ± 0.28, n = 51 | F1,98 = 0.64, p = .43 | 1.68 ± 0.19, n = 48 | 1.71 ± 0.15, n = 47 | F1,93 = 0.26, p = .61 |
| PCr+Cr IU | 7.10 ± 0.80, n = 48 | 7.07 ± 0.64, n = 51 | F1,98 = 0.02, p = .89 | 5.59 ± 0.65, n = 48 | 5.55 ± 0.32, n = 47 | F1,93 = 0.23, p = .63 |
| Gray matter, % | 68.5 ± 7.5, n = 48 | 69.1 ± 6.0, n = 51 | F1,98 = 0.08, p = .78 | 19.3 ± 7.0, n = 48 | 18.5 ± 5.6, n = 47 | F1,93 = 1.43, p = .23 |
| White matter, % | 14.5 ± 4.4, n = 48 | 13.4 ± 3.7, n = 51 | F1,98 = 1.17, p = .28 | 79.8 ± 7.4, n = 48 | 81.3 ± 5.3, n = 47 | F1,93 = 1.92, p = .17 |
| CSF, % | 17.1 ± 7.5, n = 48 | 17.5 ± 5.9, n = 51 | F1,98 = 0.12, p = .73 | 0.9 ± 2.9, n = 48 | 0.2 ± 0.7, n = 47 | F1,93 = 0.43, p = .51 |
| MEGAPRESS Sequences | ||||||
| GABA IU | 2.24 ± 0.35, n = 37 | 2.40 ± 0.33, n = 47 | F1,83 = 5.16, p = .03 | – | – | – |
| Glx IU | 10.39 ± 1.58, n = 37 | 10.97 ± 1.21, n = 47 | F1,83 = 3.31, p = .07 | – | – | – |
| PCr+Cr IU | 14.17 ± 1.32, n = 37 | 13.92 ± 1.32, n = 47 | F1,83 = 0.04, p = .84 | – | – | – |
| Gray matter, % | 52.4 ± 5.4, n = 37 | 53.8 ± 5.3, n = 47 | F1,83 = 0.73, p = .37 | – | – | – |
| White matter, % | 31.7 ± 4.1, n = 37 | 30.8 ± 4.7, n = 47 | F1,83 = 0.13, p = .72 | – | – | – |
| CSF, % | 15.9 ± 6.7, n = 37 | 15.3 ± 5.1, n = 47 | F1,83 = 0.27, p = .61 | – | – | – |
ACC, anterior cingulate cortex; ANPs, antipsychotic-naive patients; CSF, cerebrospinal fluid; GABA, gamma-aminobutyric acid; Glx, glutamate+glutamine; HCs, healthy control subjects; IU, institutional units; MEGAPRESS, Mescher–Garwood point-resolved spectroscopy (3 × 3 × 3 cm3 voxel in dorsal ACC); NAA, N-acetyl aspartate; PCr+Cr, phosphocreatine+creatine; PRESS, point-resolved spectroscopy (2 × 2 × 2 cm3 voxel in dorsal ACC and 2 × 1.5 × 2 cm3 voxel in left thalamus).
Each n denotes the number of analyzed spectra (detailed information is provided in the Supplemental Results).
Results are corrected for age, sex, and smoking status.
The primary hypotheses that levels of Glx and GABA in dorsal ACC were associated with cognitive performance was examined separately for DART, RVP A′, and SWM strategy scores as dependent variables in the following multivariate linear regression model: cognitive score = b0 + b1 × metabolite + b2 × metabolite2 + b3 × diagnosis + metabolite × diagnosis + metabolite2 × diagnosis, with the significance level adjusted for 6 comparisons (3 cognitive tests associated with Glx and GABA) (p < .0083). The metabolite2 (included to test a U-shaped association), metabolite × diagnosis (testing whether the association differed between patients and HCs), and metabolite2 × diagnosis interactions all were insignificant and therefore removed from the main analyses. SWM strategy scores were logarithmically transformed due to non-normality.
Pearson’s correlation was used to explore associations between Glx and GABA levels as well as correlations among metabolites, psychopathology, and level of function.
Statistical analyses were performed using SAS version 7.1 (SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
Table 1 summarizes participant characteristics. In total, 43 patients had a schizophrenia diagnosis and 13 were diagnosed with nonorganic or paranoid psychosis. The patients had significantly less education and a lower level of function, and more were smokers and used benzodiazepines. There were no differences in sex, age, parental educational status, or current cannabis use.
Levels of Glx, Glutamate, and GABA in Antipsychotic-Naïve Patients and Healthy Control Subjects
Spectral Quality.
For the PRESS data, 99 dorsal ACC spectra (48 patients and 51 HCs) and 95 thalamus spectra (48 patients and 47 HCs) were included. For the MEGAPRESS data, 84 spectra (37 patients and 47 HCs) were included. Details of excluded data are provided in the Supplemental Participants and Methods. Spectral quality measures were good for both glutamate and Glx as reported in Tables S1 and S2. Mean metabolite levels, the fraction of cerebrospinal fluid and gray and white matter, and statistics after adjustment for sex, age, and smoking status are shown in Table 2 for all participants and in Table S3 for the subgroup with a schizophrenia diagnosis. A significant main effect of sex was found for Glx levels in dorsal ACC as described below, but no other significant main effects of sex, age, and smoking status were found for metabolite levels in the two regions.
Dorsal ACC: Glx.
The levels of Glx did not differ between antipsychotic-naïve patients and HCs (main effect of diagnosis insignificant (F1,98 = 0.21, p = .64). Adjustment for covariates did not change the results (Table 2) but revealed a significant main effect of sex due to higher levels in male individuals (p = .001). In addition, there was no difference when analyzing only patients with a schizophrenia diagnosis (p = .67; after including covariates: p = .48).
Dorsal ACC: Glutamate.
For glutamate levels, there were no significant group differences (p = .30) and adjustment for covariates (Table 2), and analyzing only patients with a schizophrenia diagnosis did not alter the results (p values = .93–.96).
In addition, adjustment for gray matter fraction or excluding participants scanned before the upgrade did not alter the Glx and glutamate findings (Supplemental Results).
Dorsal ACC: GABA.
The levels of GABA were lower in the antipsychotic-naïve patients compared with HCs (main effect of diagnosis: F1,83 = 4.79, p = .03) (Figure 1A) also when adjusting for sex, age, and smoking status (Table 2), but not when restricting the analysis to include only patients with a schizophrenia diagnosis (p = .28; after including covariates: p = .31). Similar results were obtained when adjusting for gray matter fraction, and a similar trend was seen when including only subjects scanned after the upgrade (Supplemental Results).
Figure 1.
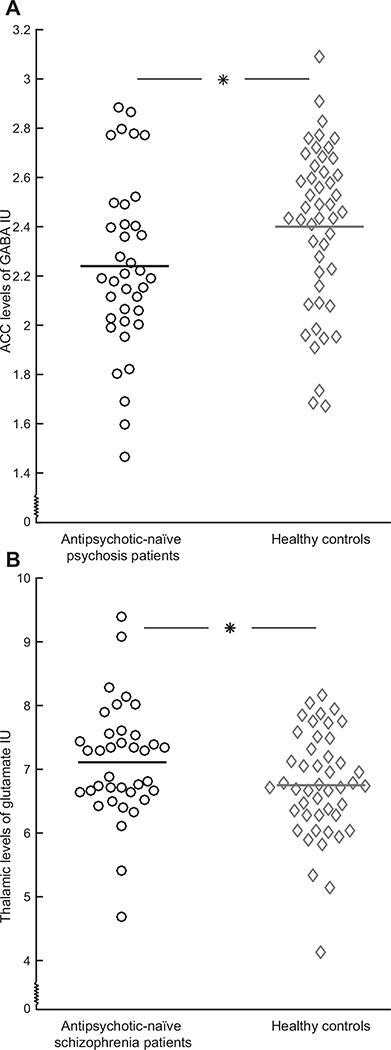
Glutamate and GABA (gamma-aminobutyric acid) levels in antipsychotic-naïve patients (circles) and healthy control subjects (diamonds). (A) Levels of GABA in dorsal anterior cingulate cortex (ACC) in patients with schizophrenia or psychosis (n = 37) are significantly lower compared with healthy control subjects (n = 47). (B) Levels of glutamate in left thalamus in the subgroup of patients with a schizophrenia diagnosis (n = 37) are significantly higher compared with healthy control subjects (n = 46). Patients with a psychosis diagnosis (n = 12) were removed from the analyses of left thalamus. Horizontal bars represent mean levels. *p < .05. IU, institutional units.
Left Thalamus: Glx.
The levels of Glx did not differ between antipsychotic-naïve patients and HCs (main effect of diagnosis insignificant: F1,94 = 0.24, p = .63). Similar results were found when adjusting for covariates (Table 2), restricting analyses to only patients with a schizophrenia diagnosis (p = .29; after including covariates: p = .20), or adjusting for gray matter fraction and excluding participants scanned before the upgrade (Supplemental Results).
Left Thalamus: Glutamate.
There was a trend for higher levels of glutamate in left thalamus of all antipsychotic-naïve patients compared with HCs after adjustment for the covariates (p = .10) (Table 2). The increase was borderline significant when restricting the analysis to include only patients with a schizophrenia diagnosis (p = .05) and significant after adjusting for covariates (F1,82 = 6.21, p = .01) (Figure 1B). Similar results were found after exclusion of participants scanned before the upgrade, and results were at trend level after adjustment for gray matter fraction (Supplemental Results).
Association Between Glx and GABA Levels in Dorsal ACC and Cognitive Performance
Cognitive testing was completed in all HCs and all but 2 antipsychotic-naïve patients, although some patients did not complete the entire cognitive battery. The number of participants completing each test, mean scores for cognitive tests, and statistics for main effects of Glx, GABA, and diagnosis on cognition are provided in Table 3 and are briefly described below.
Table 3.
The Effect of Diagnosis and Levels of Glx and GABA in Dorsal ACC on Cognition
| Cognitive Domain (Outcome Measure) | Cognitive Tests, n | Mean Test Score, ± SD or (25th–75th Percentile) | Main Effects | Main Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glx | Diagnosis | GABA | Diagnosis | |||||||||
| ANPs | HCs | ANPs | HCs | F | p | F | p | F | p | F | p | |
| Sustained Visual Attention (RVP A′) | 38 | 51 | 0.89 ± 0.05 | 0.94 ± 0.04 | 4.59 | .035 | 26.88 | <.001 | 0.03 | .86 | 17.64 | <.001 |
| Spatial Working Memorya,b (SWM Strategy) | 45 | 51 | 27.6(22.0–32.0) | 23.0 (19.0–30.0) | 8.21 | .005 | 2.56 | .11 | 0.02 | .88 | 1.18 | .28 |
| Premorbid IQ (DART) | 44 | 51 | 17.1 ± 6.4 | 21.5 ± 5.3 | 2.30 | .13 | 12.61 | .0006 | 0.07 | .79 | 18.85 | <.001 |
ANPs, antipsychotic-naive patients; DART, Danish Adult Reading Test; GABA, gamma-aminobutyric acid; Glx, glutamate+glutamine; HCs, healthy control subjects; RVP A′, rapid visual information processing A; SWM, spatial working memory.
Data were logarithmically transformed prior to statistical analyses due to non-normality.
High score indicates worse performance. Significance level was set to p < .008 (adjusted for six comparisons).
Glx Levels and Cognitive Performance: Attention.
There was a positive association between Glx levels and RVP A′ score in the combined group of antipsychotic-naïve patients and HCs (p = .035) (Table 3 and Figure S4), although it was not significant when adjusting for multiple comparisons. The antipsychotic-naïve patients had significantly lower RVP A′ scores than the HCs (p < .001) (Table 3).
Glx Levels and Cognitive Performance: Spatial Working Memory.
There was a negative association between Glx levels and the logarithmically transformed SWM strategy score (higher score indicates worse performance) in the combined group of antipsychotic-naïve patients and HCs (p = .005) (Table 3 and Figure S5) also after correcting for multiple comparisons. The logarithmically transformed SWM strategy score was not significantly higher in patients (Table 3).
Glx Levels and Cognitive Performance: Premorbid Intelligence.
There was no significant association between Glx levels and DART score (Table 3). Similar results were obtained when including glutamate levels in the model instead of Glx (Table S4).
GABA Levels and Cognitive Performance: Attention, Spatial Working Memory, and Premorbid Intelligence.
The main effects of GABA on all cognitive tests were insignificant (Table 3). Patients had significantly lower RVP A′ and DART scores compared with HCs but did not differ in logarithmically transformed SWM strategy scores (Table 3).
Association Between Glx Levels in Left Thalamus and Cognitive Performance
In explorative analyses, there were no significant associations between levels of Glx in thalamus and tests of cognitive performance (main effects: attention: p = .61; working memory: p = .77; premorbid intelligence: p = .69).
Associations Between Glx and GABA Levels as Well as Between Metabolites and Clinical Measures
Glx and GABA levels in dorsal ACC were positively associated in all participants (nPatients = 37, nHCs = 47, R = .28, p = .01) and were at trend level when analyzing HCs and patients separately (both ps = .06). Glx levels in thalamus were not associated with levels of Glx (p = .10) or GABA (p = .07) in dorsal ACC.
In the patients, there were no significant correlations between glutamate and GABA levels and Positive and Negative Syndrome Scale total scores or subscores, Global Assessment of Functioning social and occupational functioning scores, or Personal and Social Performance Scale scores.
DISCUSSION
This study investigated the association between Glx and GABA levels and cognitive function in a large group of lifetime antipsychotic-naïve patients with schizophrenia or psychosis. Our main findings are that Glx levels in dorsal ACC of both patients and HCs are associated with SWM and at trend level with attention. Moreover, we found lower levels of GABA in a larger dorsal ACC voxel in patients with psychosis or schizophrenia and found higher glutamate levels in thalamus of patients with schizophrenia.
The positive association between Glx levels in dorsal ACC and performance on cognitive tests suggests that glutamatergic neurotransmission or metabolism is involved in the cognitive domains of attention and SWM. We found a positive association between Glx levels and cognitive function and no difference in Glx levels between the patients and HCs. This is in line with the existing literature of mainly medicated patients, where studies tend to find that whenever levels of glutamate, Glx, or glutamine/glutamate in the patient group are lower or similar compared with HCs, there is a positive association with cognitive performance (29,35,52–54). In contrast, a negative correlation is found when levels are higher in patients (30,55–57). Commonly, these studies suggest that there is an optimal range of glutamatergic metabolite levels for cognitive function, although the studies are very heterogeneous with regard to age span, medication status, cognitive tests included, anatomical area studied, and field strength of the scanner. An optimal range of activation for cognitive function is also found for cortical D1 and D2/3 receptors (58,59). However, we were not able to show an inverted U-curved association in our study, probably owing to small variation of Glx levels.
Patients performed significantly worse than HCs in the test of attention, and a similar but nonsignificant trend was seen for SWM. However, the association between Glx levels in dorsal ACC and cognitive function did not differ between patients and HCs. Therefore, factors other than Glx levels measured in rest may cause cognitive deficits in patients. First, a recent functional 1H-MRS study found that glutamate levels in dorsal ACC of HCs increased during a cognitive task, but in medicated patients this increase was delayed (60). Thus, the differences in cognitive function between patients and HCs may be caused by deficits in the dynamic increase of glutamatergic metabolites during a cognitive task rather than differences in resting levels. This might reflect deficient glutamatergic neurotransmission or dysfunction of metabolic processes during cognitive processing (61). In that case, our finding of a positive association might reflect a partial compensating effect of higher resting Glx levels in patients on a dysfunctional upregulation during cognitive performance. Second, neurotransmitter disturbances other than glutamatergic ones are believed to be implicated in cognitive deficits. Prefrontal dopaminergic function has also been related to optimal performance of attention and working memory (59,62,63), and dopamine receptors in the cortex are regulated by glutamate, among other neurotransmitters (64,65). The difference in cognitive function therefore may be due to insufficient glutamatergic modulation of the dopaminergic receptors in patients, which would not be reflected by 1H-MRS measures. Third, glutamatergic and GABAergic dysfunction in areas other than dorsal ACC may be related to cognitive deficits. Dorsolateral prefrontal cortex also plays a key role in cognitive function (66), although the majority of MRS studies of dorsolateral prefrontal cortex did not find significant associations between GABA, glutamate, or Glx levels and cognitive performance (4,29,35,54,56).
GABA levels measured in a larger dorsal ACC voxel were not significantly correlated with cognitive performance. This is congruent with one study that did not find an association between GABA levels in medial prefrontal cortex and working memory in minimally treated patients. However, it contrasts with three studies of ACC or medial prefrontal cortex in medicated patients within a broad age range, where positive associations between GABA levels and tests of attention (34), working memory (33), and intelligence (31) were reported. We speculate whether these differences can be explained by age and medication status. GABA levels decline with age (33) and may be reduced by antipsychotic treatment (6), although this is not consistently reported (7). It is likely that the association between GABA levels and cognition changes with age and treatment, as is also indicated for glutamate (28).
In addition, we did not find an association between thalamic Glx levels and cognitive performance. Proper thalamic function has been associated with working memory and attention in preclinical studies (67–70), and especially the mediodorsal nuclei are believed to be related to cognitive deficits (27,71). Only 2 in vivo studies of first-episode patients exist, and these report a negative association between glutamine levels and working memory (28) but no significant association between glutamate levels in thalamus and cognition (28,54). However, a recent study of subjects at ultrahigh risk for psychosis found a positive association between thalamic glutamate and attention (72). Further studies are needed to clarify the possible association between glutamatergic metabolites in thalamus and cognitive performance.
In the whole group of antipsychotic-naïve patients with psychosis or schizophrenia, we found decreased GABA levels in dorsal ACC corresponding with a previous study of minimally treated patients (11) and our previous finding in a subsample of the patient group (7). In contrast, studies of the pregenual ACC or mPFC have mainly found increased GABA levels (1–6), which probably reflects the different receptor distribution of AMPA, NMDA, GABAA, and GABAB receptors in these two subregions of ACC (73). In thalamus, we found increased glutamate levels but not increased Glx levels in the 37 patients with a schizophrenia diagnosis, but not in the entire sample of patients also including those with a psychosis diagnosis (N = 48). Thus, increased glutamate levels seem to be associated with more severe psychopathology, which is in accordance with our previous findings that baseline thalamic glutamate can predict poor treatment outcome in initially antipsychotic-naïve patients (7). However, there was no group difference in Glx levels, which might reflect that the difference in glutamatergic metabolites is small. Interestingly, approximately 80% of the thalamic voxel consisted of white matter (Table 2), suggesting that the glutamatergic abnormalities might be related to dysfunction in thalamic connections.
Metabolites estimated with 1H-MRS are commonly reported using either water or total creatine as a reference, and currently there is not consensus about one method being superior. We previously reported decreased glutamate levels in dorsal ACC when creatine scaled values were used, although they were not significant when using levels in institutional units (7). Similarly, we found no significant difference in dorsal ACC Glx or glutamate levels in institutional units in antipsychotic-naïve patients compared with HCs, but we found borderline significant lower glutamate/creatine in patients in this study. Because total creatine did not differ between patients and HCs (Table S4), this might be explained by the fact that total creatine is assessed in the same acquisition as glutamate and other metabolites, whereas water is assessed separately. Thus, the glutamate/creatine ratio might be less influenced by, for example, motion and therefore may be able to capture minor group differences. However, it is also likely that we did not place our voxel where the glutamatergic disturbances are most pronounced given that two recent studies of medicated first-episode patients found lower glutamate levels in more dorsal regions of ACC (54,74).
Glx and GABA levels in dorsal ACC were correlated in patients and HCs, as was also previously found in a group of minimally treated patients (4).
Strengths of the current study are a large group of lifetime antipsychotic-naïve patients, short duration between MR scanning and cognitive testing, and assessment of both Glx and GABA. However, limitations should also be addressed. First, we used single voxel MRS limited by measures in a predefined area. Second, the MEGAPRESS sequence used did not suppress macromolecules that therefore contributed to the GABA signal in both patients and HCs (75). Third, the finding of a correlation between glutamate and GABA levels within the same voxel could be spurious (76). Fourth, preclinical studies are needed to clarify the relationship between MRS measures and glutamate involved in neurotransmission and metabolism. Finally, we chose 3 cognitive domains based on previous findings, but other cognitive tests may be associated with glutamate and GABA levels as well.
To conclude, our findings suggest that higher resting Glx levels in dorsal ACC are associated with improved cognitive function in both antipsychotic-naïve patients and HCs. Moreover, lower GABA levels in dorsal ACC seem to be involved in the pathophysiology of psychotic illness and higher thalamic glutamatergic levels in the pathophysiology of schizophrenia. Although the exact relationship among 1H-MRS measures of glutamatergic metabolites, neurotransmission, and metabolism remains to be established, the findings imply that glutamate-modulating compounds might be able to improve cognitive deficits in first-episode patients.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | NA | |||
| Bacterial or Viral Strain | NA | |||
| Biological Sample | NA | |||
| Cell Line | NA | |||
| Chemical Compound or Drug | NA | |||
| Commercial Assay Or Kit | NA | |||
| Deposited Data; Public Database | NA | |||
| Genetic Reagent | NA | |||
| Organism/Strain | NA | |||
| Peptide, Recombinant Protein | NA | |||
| Recombinant DNA | NA | |||
| Sequence-Based Reagent | NA | |||
| Software; Algorithm | NA | |||
| Transfected Construct | NA | |||
| Other |
ACKNOWLEDGMENTS AND DISCLOSURES
Funding for the study was provided by a Ph.D. grant from the Faculty of Health and Medical Sciences, University of Copenhagen (to KBB), Ph.D. grants and a postdoctoral grant from the Mental Health Services in the Capital Region of Denmark (to KJ, AS, KT, and MØN), a Ph.D. grant from the Faculty of Social Sciences, University of Copenhagen (to MBT), an independent grant from the Lundbeck Foundation (Grant No. R155-2013-16337) to the Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (to BYG), support from the Mental Health Services, Capital Region of Denmark (to BYG), and a grant from the Gangsted Foundation (to BYG). RAEE received support from the National Institute of Health (Grant Nos. P41EB015909, R01EB016089, R01EB023963, R01MH106564, and R21MH098228). The funding sources played no further role in the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication.
We thank Peter Williamson and Jean Théberge for advice during the planning phase of the study and for providing the 1H-MRS voxel placement from their previous studies, Mark Mikkelsen for providing a modified version of Gannet to assess total creatine, and Mikkel Erlang for assistance with producing the figures.
Preliminary results of this study were presented on a poster in March 2017 at the 16th International Congress on Schizophrenia Research, San Diego.
BYG is the head of the Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research, which is partially financed by an independent grant from the Lundbeck Foundation (Grant No. R155-2013-16337), which is based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. Her group has also received a research grant from Lundbeck A/S for an additional independent investigator-initiated study. All grants are the property of, and administrated by, the Mental Health Services. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
ClinicalTrials.gov: The Pan European Collaboration on Antipsychotic Naïve Schizophrenia II (PECANSII); https://www.clinicaltrials.gov/ct2/show/NCT02339844?term=Glenth%C3%B8j&cond=Schizophrenia&rank=7; NCT02339844.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2020.06.027.
Contributor Information
Kirsten Borup Bojesen, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark.
Brian Villumsen Broberg, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark.
Birgitte Fagerlund, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark; Faculty of Health and Medical Sciences, and Department of Psychology, Faculty of Social Sciences, University of Copenhagen, Glostrup, Denmark.
Kasper Jessen, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark.
Marie Bjerregaard Thomas, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark.
Anne Sigvard, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Social Sciences, University of Copenhagen, Glostrup, Denmark.
Karen Tangmose, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Social Sciences, University of Copenhagen, Glostrup, Denmark.
Mette Ødegaard Nielsen, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Social Sciences, University of Copenhagen, Glostrup, Denmark.
Gitte Saltoft Andersen, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark.
Henrik Bo Wiberg Larsson, Functional Imaging Unit, Department of Clinical Physiology and Nuclear Medicine, Rigshospitalet Glostrup, University of Copenhagen, Glostrup, Denmark.
Richard A.E. Edden, Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, F.M. Kirby Research Center for Functional Brain Imaging, Baltimore, Maryland.
Egill Rostrup, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark.
Birte Yding Glenthøj, Center for Neuropsychiatric Schizophrenia Research and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, Mental Health Center Glostrup, University of Copenhagen, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Social Sciences, University of Copenhagen, Glostrup, Denmark.
REFERENCES
- 1.Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, et al. (2010): 1H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry 15:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. (2002): Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 159:1944–1946. [DOI] [PubMed] [Google Scholar]
- 3.Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. (2007): Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry 191:325–334. [DOI] [PubMed] [Google Scholar]
- 4.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. (2012): Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69:449–459. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Zhu Y, Song Z, Mei L, Zhang J, Chen T, et al. (2015): Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls. Shanghai Arch Psychiatry 27:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, Leon-Ortiz P, Rodriguez-Mayoral O, Jung-Cook H, et al. (2018): Prefrontal and Striatal Gamma-Aminobutyric Acid Levels and the Effect of Antipsychotic Treatment in First-Episode Psychosis Patients. Biol Psychiatry 83:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bojesen KB, Ebdrup BH, Jessen K, Sigvard A, Tangmose K, Edden RAE, et al. (2020): Treatment response after 6 and 26 weeks is related to baseline glutamate and GABA levels in antipsychotic-naive patients with psychosis. Psychol Med 50:2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama N, Theberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RW, et al. (2011): Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry 198:448–456. [DOI] [PubMed] [Google Scholar]
- 9.Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, et al. (2018): Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: A multicentre 1H-MRS study (OPTiMiSE). Mol Psychiatry 23:2145–2155. [DOI] [PubMed] [Google Scholar]
- 10.Borgan FR, Jauhar S, McCutcheon RA, Pepper FS, Rogdaki M, Lythgoe DJ, et al. (2019): Glutamate levels in the anterior cingulate cortex in un-medicated first episode psychosis: A proton magnetic resonance spectroscopy study. Sci Rep 9:8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Tang Y, Zhang T, Cui H, Xu L, Zeng B, et al. (2016): Reduced γ-aminobutyric acid and glutamate+glutamine levels in drug-naive patients with first-episode schizophrenia but not in those at ultrahigh risk. Neural Plast 2016:3915703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, et al. (2013): Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: A longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gomez-Cruz G, Leon-Ortiz P, et al. (2016): Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: A proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr Bull 42:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsson A, Waters N, Carlsson ML (1999): Neurotransmitter interactions in schizophrenia—Therapeutic implications. Eur Arch Psychiatry Clin Neurosci 249(suppl 4):37–43. [DOI] [PubMed] [Google Scholar]
- 15.Williamson PC, Allman JM (2012): A framework for interpreting functional networks in schizophrenia. Front Hum Neurosci 6:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwata Y, Nakajima S, Plitman E, Mihashi Y, Caravaggio F, Chung JK, et al. (2018): Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: A systematic review and meta-analysis of 1H-MRS studies. Prog Neuropsychopharmacol Biol Psychiatry 86:340–352. [DOI] [PubMed] [Google Scholar]
- 17.Legind CS, Broberg BV, Mandl RCW, Brouwer R, Anhoj SJ, Hilker R, et al. (2019): Heritability of cerebral glutamate levels and their association with schizophrenia spectrum disorders: A 1[H]-spectroscopy twin study. Neuropsychopharmacology 44:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis DA, Curley AA, Glausier JR, Volk DW (2012): Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Burgos G, Fish KN, Lewis DA (2011): GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast 2011:723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. (1994): Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214. [DOI] [PubMed] [Google Scholar]
- 21.Jentsch JD, Tran A, Le D, Youngren KD, Roth RH (1997): Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology 17:92–99. [DOI] [PubMed] [Google Scholar]
- 22.Moghaddam B, Adams BW (1998): Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352. [DOI] [PubMed] [Google Scholar]
- 23.Amitai N, Semenova S, Markou A (2007): Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 193:521–537. [DOI] [PubMed] [Google Scholar]
- 24.Greco B, Invernizzi RW, Carli M (2005): Phencyclidine-induced impairment in attention and response control depends on the background genotype of mice: Reversal by the mGLU(2/3) receptor agonist LY379268. Psychopharmacology (Berl) 179:68–76. [DOI] [PubMed] [Google Scholar]
- 25.Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001): Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cereb Cortex 11:837–848. [DOI] [PubMed] [Google Scholar]
- 26.Heilbronner SR, Hayden BY (2016): Dorsal anterior cingulate cortex: A bottom-up view. Annu Rev Neurosci 39:149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt J, Dawson N, Morris BJ, Grent-’t-Jong T, Roux F, Uhlhaas PJ (2017): Thalamo-cortical communication, glutamatergic neurotransmission and neural oscillations: A unique window into the origins of ScZ? Schizophr Res 180:4–12. [DOI] [PubMed] [Google Scholar]
- 28.Dempster K, Norman R, Theberge J, Densmore M, Schaefer B, Williamson P (2015): Glutamatergic metabolite correlations with neuropsychological tests in first episode schizophrenia. Psychiatry Res 233:180–185. [DOI] [PubMed] [Google Scholar]
- 29.Ohrmann P, Kugel H, Bauer J, Siegmund A, Kolkebeck K, Suslow T, et al. (2008): Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res 106:156–163. [DOI] [PubMed] [Google Scholar]
- 30.Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, et al. (2010): Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: A preliminary study. NeuroImage 49:2783–2790. [DOI] [PubMed] [Google Scholar]
- 31.Marsman A, Mandl RC, Klomp DW, Bohlken MM, Boer VO, Andreychenko A, et al. (2014): GABA and glutamate in schizophrenia: A 7 T 1H-MRS study. NeuroImage Clin 6:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto N, Yoshimura R, Kakeda S, Moriya J, Hayashi K, Ikenouchi-Sugita A, et al. (2009): Associations between plasma levels of 3-methoxy-4-hydroxyphenylglycol (MHPG) and negative symptoms or cognitive impairments in early-stage schizophrenia. Hum Psychopharmacol 24:639–645. [DOI] [PubMed] [Google Scholar]
- 33.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. (2016): Medial frontal GABA is lower in older schizophrenia: A MEGA-PRESS with macromolecule suppression study. Mol Psychiatry 21:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. (2013): In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull 39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A, et al. (2007): Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: A proton magnetic resonance spectroscopy study. J Psychiatr Res 41:625–634. [DOI] [PubMed] [Google Scholar]
- 36.Jessen K, Mandl RCW, Fagerlund B, Bojesen KB, Raghava JM, Obaid HG, et al. (2019): Patterns of cortical structures and cognition in antipsychotic-naive patients with first-episode schizophrenia: A partial least squares correlation analysis. Biol Psychiatry Cogn Neurosci Neuroimaging 4:444–453. [DOI] [PubMed] [Google Scholar]
- 37.Fagerlund B, Pantelis C, Jepsen JRM, Raghava JM, Rostrup E, Thomas MB, et al. (2020): Differential effects of age at illness onset on verbal memory functions in antipsychotic-naive schizophrenia patients aged 12–43 years [published online ahead of print Mar 11]. Psychol Med. [DOI] [PubMed] [Google Scholar]
- 38.Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. (1990): SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 47:589–593. [DOI] [PubMed] [Google Scholar]
- 39.Kay SR, Fiszbein A, Opler LA (1987): The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- 40.Guy W (1976): Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology, revised. Bethesda, MD: National Institute of Mental Health. [Google Scholar]
- 41.American Psychiatric Association (1994): Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Press. [Google Scholar]
- 42.Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R (2000): Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 101:323–329. [PubMed] [Google Scholar]
- 43.Sahakian BJ, Owen AM (1992): Computerized assessment in neuropsychiatry using CANTAB: Discussion paper. J R Soc Med 85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 44.Lowe C, Rabbitt P (1998): Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: Theoretical and practical issues. Neuropsychologia 36:915–923. [DOI] [PubMed] [Google Scholar]
- 45.Nelson HE, O’Connell A (1978): Dementia: The estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex 14:234–244. [DOI] [PubMed] [Google Scholar]
- 46.Bojesen KB, Andersen KA, Rasmussen SN, Baandrup L, Madsen LM, Glenthoj BY, et al. (2018): Glutamate Levels and Resting Cerebral Blood Flow in Anterior Cingulate Cortex Are Associated at Rest and Immediately Following Infusion of S-Ketamine in Healthy Volunteers. Front Psychiatry 9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R (1998): Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266–272. [DOI] [PubMed] [Google Scholar]
- 48.Provencher SW (1993): Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679. [DOI] [PubMed] [Google Scholar]
- 49.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ (2014): Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 40:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E (2011): In vivo detection of GABA and glutamate with MEGA-PRESS: Reproducibility and gender effects. J Magn Reson Imaging 33:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE (2013): Glutamate in schizophrenia: A focused review and meta-analysis of 1H-MRS studies. Schizophr Bull 39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falkenberg LE, Westerhausen R, Craven AR, Johnsen E, Kroken RA, Berg EML, et al. (2014): Impact of glutamate levels on neuronal response and cognitive abilities in schizophrenia. NeuroImage Clin 4:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bustillo JR, Chen H, Gasparovic C, Mullins P, Caprihan A, Qualls C, et al. (2011): Glutamate as a marker of cognitive function in schizophrenia: A proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry 69:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang AM, Pradhan S, Coughlin JM, Trivedi A, DuBois SL, Crawford JL, et al. (2019): Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 76:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bustillo JR, Jones T, Chen H, Lemke N, Abbott C, Qualls C, et al. (2017): Glutamatergic and neuronal dysfunction in gray and white matter: A spectroscopic imaging study in a large schizophrenia sample. Schizophr Bull 43:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rusch N, Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Ebert D, et al. (2008): Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res 99:155–163. [DOI] [PubMed] [Google Scholar]
- 57.Rowland LM, Pradhan S, Korenic S, Wijtenburg SA, Hong LE, Edden RA, et al. (2016): Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl Psychiatry 6:e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnsten AF, Girgis RR, Gray DL, Mailman RB (2017): Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiatry 81:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fagerlund B, Pinborg LH, Mortensen EL, Friberg L, Baare WF, Gade A, et al. (2013): Relationship of frontal D2/3 binding potentials to cognition: A study of antipsychotic-naive schizophrenia patients. Int J Neuropsychopharmacol 16:23–36. [DOI] [PubMed] [Google Scholar]
- 60.Taylor R, Neufeld RW, Schaefer B, Densmore M, Rajakumar N, Osuch EA, et al. (2015): Functional magnetic resonance spectroscopy of glutamate in schizophrenia and major depressive disorder: Anterior cingulate activity during a color-word Stroop task. NPJ Schizophr 1:15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL (2011): 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed 24:943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979): Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205:929–932. [DOI] [PubMed] [Google Scholar]
- 63.Goldman-Rakic PS (1995): Cellular basis of working memory. Neuron 14:477–485. [DOI] [PubMed] [Google Scholar]
- 64.Sesack SR, Carr DB, Omelchenko N, Pinto A (2003): Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci 1003:36–52. [DOI] [PubMed] [Google Scholar]
- 65.Arnsten AF, Wang M, Paspalas CD (2015): Dopamine’s actions in primate prefrontal cortex: Challenges for treating cognitive disorders. Pharmacol Rev 67:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dienel SJ, Enwright JF 3rd, Hoftman GD, Lewis DA (2020): Markers of glutamate and GABA neurotransmission in the prefrontal cortex of schizophrenia subjects: Disease effects differ across anatomical levels of resolution. Schizophr Res 217:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chudasama Y, Bussey TJ, Muir JL (2001): Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci 14:1009–1020. [DOI] [PubMed] [Google Scholar]
- 68.Floresco SB, Braaksma DN, Phillips AG (1999): Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci 19:11061–11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baxter MG (2013): Mediodorsal thalamus and cognition in non-human primates. Front Syst Neurosci 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunt PR, Aggleton JP (1998): Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: A deficit in shifting response rules. J Neurosci 18:10045–10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giguere M, Goldman-Rakic PS (1988): Mediodorsal nucleus: Areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 277:195–213. [DOI] [PubMed] [Google Scholar]
- 72.Wenneberg C, Nordentoft M, Rostrup E, Glenthoj LB, Bojesen KB, Fagerlund B, et al. (2020): Cerebral glutamate and gamma-aminobutyric acid levels in individuals at ultra-high risk for psychosis and the association with clinical symptoms and cognition. Biol Psychiatry Cogn Neurosci Neuroimaging 5:569–579. [DOI] [PubMed] [Google Scholar]
- 73.Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K (2009): Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model. Hum Brain Mapp 30:2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reid MA, Salibi N, White DM, Gawne TJ, Denney TS, Lahti AC (2019): 7T proton magnetic resonance spectroscopy of the anterior cingulate cortex in first-episode schizophrenia. Schizophr Bull 45:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aufhaus E, Weber-Fahr W, Sack M, Tunc-Skarka N, Oberthuer G, Hoerst M, et al. (2013): Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: A MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med 69:317–320. [DOI] [PubMed] [Google Scholar]
- 76.Maddock R (2014): The problem of spurious correlations between pairs of brain metabolite values measured in the same voxel with magnetic resonance spectroscopy. JAMA Psychiatry 71:338–339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


