Introduction
Water, sanitation, and hygiene (WASH) services are described in global action plans as necessary to curb antimicrobial resistance (AMR), despite a lack of supporting data.1,2 WASH services are thought to interrupt environmental transmission of antimicrobial resistant bacteria by reducing fecal contamination of the environment (i.e., by sanitation) and fecal exposures (i.e., by drinking water treatment, hygiene).2 Further, WASH services reduce the disease burden attributable to enteric pathogens, which decreases antibiotic use and associated AMR selective pressure.2 Extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-E. coli) are recommended as a proxy for the global AMR threat, in part because ESBL-E. coli infections increase morbidity, mortality, and treatment costs3; are pervasive in humans, animals, and environmental compartments4; and confer resistance to critically important antimicrobials.
In this study, we evaluated the impact of a cluster-randomized controlled trial of in-line drinking water chlorination on ESBL-E. coli fecal carriage among Bangladeshi children. The trial previously demonstrated that chlorination significantly reduced pediatric diarrheal disease, antibiotic use, illness-related expenditures, and E. coli prevalence and concentrations in drinking water.5
Materials and Methods
We analyzed, double-blind, 479 fecal samples of children of age following their enrollment in a cluster-randomized controlled trial of in-line water chlorination at their primary drinking source in two low-income communities in Bangladesh (Dhaka and Tongi) between July 2015 and December 2016.5 The intervention ( fecal samples) included children whose primary water source was amended to include a passive chlorine dosing device; within the active control (), the device provided vitamin C.5 Fecal samples were collected once per child, a mean of 9.3 () months after enrollment in the study, representing the length of time children were exposed to the intervention. Prevalence and concentration of the ESBL-E. coli and ESBL–Klebsiella, Enterobacter, Shigella, and Citrobacter (ESBL-KESC) groups in fecal samples collected after the intervention were compared between the intervention and control children. The difference and associated significance in the carriage were determined using a modified Poisson regression. Impacts on concentrations were determined using multiple linear regression.
We detected and enumerated ESBL-E. coli and ESBL-KESC groups directly from fecal samples using CHROMID ESBL agar (bioMérieux). Using short-read metagenomic sequencing, we determined occurrence and relative abundance of beta-lactamase (bla) genes in a subset () of fecal samples. We sequenced a subset () of ESBL-E. coli isolates. The protocol for the original trial was approved by the review committees at the International Center for Diarrheal Diseases Research, Bangladesh (protocol 14022) and Stanford University (protocol 30456), and included consent for future analyses.5 E. coli genomes are archived at the National Center for Biotechnology Information (NCBI), BioProject PRJNA705080. Metagenomes are archived as NCBI Bioproject PRJNA706606. Supporting information on methods, including the CONsolidated Standards of Reporting Trials (CONSORT) 2010 checklist, are available at https://doi.org/10.17605/OSF.IO/9NGT8.
Results and Discussion
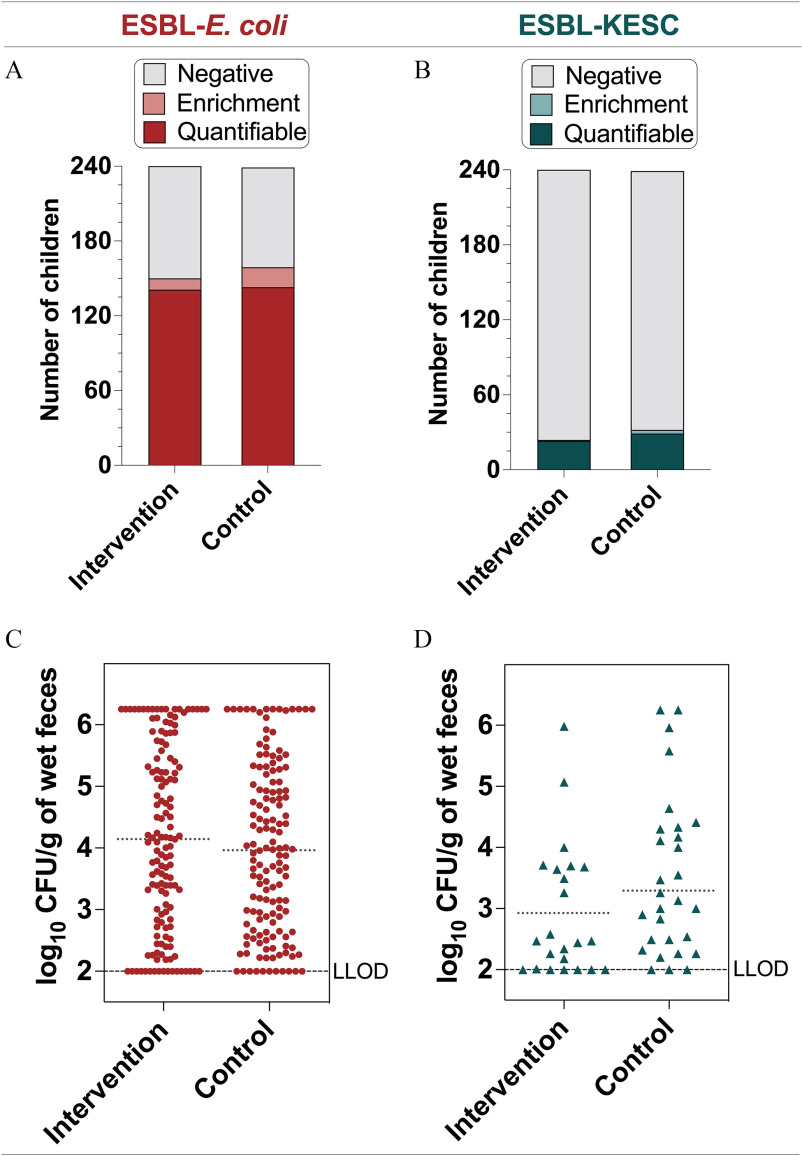
In-line drinking water chlorination did not significantly reduce fecal carriage or concentrations of ESBL-E. coli or ESBL-KESC in Bangladeshi children, despite previous efficacy against diarrheal disease and antibiotic use.5 Specifically, ESBL-E. coli prevalence was 4.0% (67% vs. 63%) and ESBL-KESC was 3.4% (13% vs. 10%) higher in the control than in the intervention group, but the differences were not statistically significant when controlling for study site and participant age (Figure 1, Table 1; ). Notably, 9 of 479 (2%) samples were removed from analysis because no date of birth was reported. Relative risk (RR) [95% confidence interval (CI)] of the intervention for ESBL-E. coli was 0.98 (0.78, 1.23) and for ESBL-KESC was 0.76 (0.44, 1.29) (Table 1). ESBL-E. coli and ESBL-KESC concentrations were also not statistically significantly different when controlling for study site and participant age (Figure 1, Table 1). ESBL-E. coli accounted for the median 4% of all E. coli ().
Figure 1.
Fecal carriage rates of (A) ESBL-E. coli and (B) ESBL-KESC in the intervention () and control () groups. The term negative was assigned to samples where no growth was observed after direct plating onto CHROMID ESBL agar or after the enrichment step; enrichment corresponds to samples with presumptive ESBL-E. coli colonies after the enrichment step; and quantifiable corresponds to samples with presumptive ESBL-E. coli colonies after direct plating onto CHROMID ESBL agar. Concentrations of (C) ESBL-E. coli and (D) ESBL-KESC in the intervention and control groups among samples with direct positive cultures (quantifiable). The dotted horizontal line is the mean log10 CFU/g-wet feces in the intervention and control groups; the LLOD is indicated. Note: CFU, colony forming units; ESBL, extended-spectrum beta-lactamase-producing; KESC, the Klebsiella spp., Enterobacter spp., Serratia spp., and Citrobacter spp. group; LLOD, lower limit of detection.
Table 1.
Impact of the drinking water chlorination intervention on children’s carriage and concentrations of ESBL-E. coli and ESBL-KESC controlling for study site and age ( for all models).
| Outcomes | Constant | Intervention (Ref: control) | Dhaka (Ref: Tongi) | Age (16–30 months) (Ref: age months) | Age () (Ref: age months) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RR (95% CI) or | Pr () or Pr () | RR (95% CI) or | Pr () or Pr () | RR (95% CI) or | Pr () or Pr () | RR (95% CI) or | Pr () or Pr () | RR (95% CI) or | Pr () or Pr () | |
| ESBL-E.coli carriage | 0.61 (0.45, 0.82)a | 0.001a | 0.98 (0.78, 1.23) | 0.85 | 0.78 (0.61, 0.98)a | 0.04a | 1.07 (0.76, 1.54) | 0.69 | 1.29 (0.96, 1.78) | 0.10 |
| ESBL-KESC carriage | 0.13 (0.06, 0.24)a | a | 0.76 (0.44, 1.28) | 0.31 | 0.83 (0.47, 1.44) | 0.52 | 1.47 (0.69, 3.27) | 0.33 | 1.03 (0.52, 2.25) | 0.93 |
| ESBL-E.coli concentration | a | a | 0.41 | a | 0.01a | 0.49 | 0.23 | |||
| ESBL-KESC concentration | a | a | 0.08 | 0.42 | 0.05 | 0.95 | ||||
Note: The difference and associated significance in the carriage between the intervention and the control group following exposure to the drinking water intervention were determined using modified Poisson regression. Impacts on concentrations were determined using multiple linear regression. Constant estimates refers to the average prevalence of ESBL-E. coli carriage (or other outcomes) when all variables are at their reference levels (e.g., prevalence in the control group, Tongi study site, among children months of age). Adj, adjusted; AIC, Akaike information criterion; CI, confidence interval; ESBL, extended-spectrum beta-lactamase-producing; KESC, Klebsiella spp., Enterobacter spp., Serratia spp., and Citrobacter spp. group; Ref, reference; RR, relative risk; SE, standard error.
Variables are statistically significant as defined at .
Analysis of other factors influencing ESBL-E. coli and ESBL-KESC prevalence and concentration in children identified significant influences of study site (Tongi) and number of households in a compound () for ESBL-E. coli and use of antibiotics in the previous 2 months for ESBL-KESC (supporting information including full results are available at https://doi.org/10.17605/OSF.IO/9NGT8). Gender, age, study enrollment duration, treatment center visits, and people in the household had no significant effect.
The intervention had no impact on the relative abundance or occurrence of any bla gene or allele in the child fecal metagenomes or occurrence of any bla allele in the E. coli isolates. In fecal metagenomes, blaCfxA ( of 95, or 94%), blaACI (75%), blaTEM (72%), and blaOXA (60%) were detected. ESBL-encoding genes were also found in children without culturable ESBL-E. coli.
In the genomes of the 96 sequenced ESBL-E. coli, we identified 50 unique sequence types (STs), including ST38 (12.8%) and ST131 (11.6%), which are associated with extraintestinal infections.6 In almost all (99%) of ESBL-E. coli we detected an ESBL gene, with blaCTX-M-15 as the most prevalent (90%). Genes conferring resistance to macrolides (73%), quinolones (48%), tetracyclines (37%), and trimethoprim (48%) were common.
The lack of a significant effect of chlorination on ESBL-E. coli carriage stands in contrast to the impact chlorination had on diarrheal disease. Environmental interventions targeting a single exposure route, even one associated with a substantial portion of enteric pathogen transmission, may be insufficient to reduce AMR in regions of high AMR prevalence and multiple concurrent exposure routes.4,7
High AMR prevalence offers increased opportunities for transmission, which may limit intervention efficacy. Although it was not significant, we observed a meaningful reduction in prevalence of ESBL-KESC carriage, which was detected in only 12% () of all children. The lower prevalence of ESBL-KESC compared with ESBL-E. coli (observed in 65% of 479 children) is similar to the 9% observed for diarrheal disease, which the intervention significantly reduced.5 ESBL-KESC was also further reduced in Dhaka [ (95% CI: 0.23, 1.28)], where the intervention was more effective against diarrheal disease, than in Tongi [ (95% CI: 0.45, 1.71)]. Although ESBL-E. coli is considered an AMR indicator organism, other indicators with lower prevalence—such as ESBL-KESC—may provide useful insight in evaluations of interventions. Investigations of the resistome and mobilome may further aid in identification of intervention impacts.7
A lack of an observed impact of water chlorination on ESBL-E. coli carriage may also be attributed to the longer duration of carriership (estimated at 1.1 y) relative to other enteric pathogens (typically ).8,9 Interventions that interrupt exposures but do not directly reduce carriage may not impact prevalence until there has been sufficient loss of carriage.
A major limitation of the study was power. Increased sample size may have benefited our secondary analysis examining reduction in ESBL-KESC prevalence, which, despite a meaningful effect size, was not significant. Additional limitations were the open enrollment study design and limited duration of the intervention prior to stool collection (). However, subgroup analysis on enrollment duration showed no substantial difference in impact compared with that observed for the entire cohort (supporting information; https://doi.org/10.17605/OSF.IO/9NGT8).
Given the extensive support for WASH investments to combat AMR,1,2,10 there is a clear need to identify conditions under which interventions will be effective. Gathering such evidence requires: a) defining meaningful reductions in AMR carriage; b) identifying interventions with the potential to achieve these reductions (such as those effective against diarrheal disease); c) granting sufficient exposure to the intervention to allow loss of AMR carriage, which may be longer than needed for diarrheal reductions; and d) evaluating a sufficient sample size.
Acknowledgments
M.C.M., A.J.P., and T.R.J. conceived and supervised the study; M.C.M., M.A.I., V.F.L., S.P.L., A.J.P., and T.R.J. developed the proposal, planned experiments, and secured the funding; J.M.S., S.S., S.P.L., and A.J.P. collected and provided samples; M.C.M., E.E.G., L.T., L.C., and T.N. processed samples; M.C.M., T.R.J., and M.L.N. analyzed and interpreted data; M.C.M., E.E.G., and T.R.J. wrote the original draft; all authors contributed to writing the final draft.
This work was funded by the Thrasher Research Fund (#14205). The funder had no role in data collection, data analysis, data interpretation, or writing of this report. M.L.N. was supported by NIH award KL2TR002545 and the Stuart B. Levy Center for Integrated Management of Antimicrobial Resistance at Tufts (Levy CIMAR), a collaboration of Tufts Medical Center and the Tufts University Office of the Vice Provost for Research (OVPR) Research and Scholarship Strategic Plan (RSSP).
References
- 1.WHO (World Health Organization), FAO (Food and Agriculture Organization of the United Nations), OIE (World Organisation for Animal Health). 2020. Technical Brief on Water, Sanitation, Hygiene and Wastewater Management to Prevent Infections and Reduce the Spread of Antimicrobial Resistance. Geneva, Switzerland: WHO. https://apps.who.int/iris/rest/bitstreams/1279113/retrieve [accessed 1 April 2022]. [Google Scholar]
- 2.Nadimpalli ML, Marks SJ, Montealegre MC, Gilman RH, Pajuelo MJ, Saito M, et al. 2020. Urban informal settlements as hotspots of antimicrobial resistance and the need to curb environmental transmission. Nat Microbiol 5(6):787–795, PMID: , 10.1038/s41564-020-0722-0. [DOI] [PubMed] [Google Scholar]
- 3.Schwaber MJ, Carmeli Y. 2007. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 60(5):913–920, PMID: , 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 4.Rousham EK, Asaduzzaman M, Mozmader TIMAU, Amin MB, Rahman M, Hossain MI, et al. 2021. Human colonization with extended-spectrum beta-lactamase-producing E. coli in relation to animal and environmental exposures in Bangladesh: an observational One Health study. Environ Health Perspect 129(3):37001, PMID: , 10.1289/EHP7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickering AJ, Crider Y, Sultana S, Swarthout J, Goddard FG, Anjerul Islam S, et al. 2019. Effect of in-line drinking water chlorination at the point of collection on child diarrhoea in urban Bangladesh: a double-blind, cluster-randomised controlled trial. Lancet Glob Health 7(9):e1247–e1256, PMID: , 10.1016/S2214-109X(19)30315-8. [DOI] [PubMed] [Google Scholar]
- 6.Alghoribi MF, Gibreel TM, Farnham G, Al Johani SM, Balkhy HH, Upton M. 2015. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J Antimicrob Chemother 70(10):2757–2762, PMID: , 10.1093/jac/dkv188. [DOI] [PubMed] [Google Scholar]
- 7.Nadimpalli ML, Lanza VF, Montealegre MC, Sultana S, Fuhrmeister ER, Worby CJ, et al. 2022. Drinking water chlorination has minor effects on the intestinal flora and resistomes of Bangladeshi children. Nat Microbiol 7(5):620–629, PMID: , 10.1038/s41564-022-01101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julian TR. 2016. Environmental transmission of diarrheal pathogens in low and middle income countries. Environ Sci Process Impacts 18(8):944–955, PMID: , 10.1039/c6em00222f. [DOI] [PubMed] [Google Scholar]
- 9.Teunis PFM, Evers EG, Hengeveld PD, Dierikx CM, Wielders CCH, van Duijkeren E. 2018. Time to acquire and lose carriership of ESBL/pAmpC producing E. coli in humans in the Netherlands. PLoS One 13(3):e0193834, PMID: , 10.1371/journal.pone.0193834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. 2015. Global Action Plan on Antimicrobial Resistance. https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 [accessed 1 April 2022].