Abstract
Lesion size, cellular infiltration, and tissue parasitism in the footpads of BALB/c mice infected with Leishmania major were all dramatically inhibited during acute but not chronic infection with Toxoplasma gondii. Similarly, acute but not chronic toxoplasmosis at the time of infection with L. major had a strong inhibitory effect on development of acquired immune responses mediated by Th2 lymphocytes. In contrast, no major changes in Leishmania-specific Th1-mediated responses were observed in mice coinfected with T. gondii.
Infection with Leishmania major, an obligatory intracellular parasite in mammals that multiplies in macrophages (21), leads to highly polarized Th1 and Th2 immune responses in resistant (C57BL/6) and susceptible (BALB/c) mice, respectively (26). The resistance is determined by the host’s ability to produce high levels of gamma interferon (IFN-γ) and low levels of interleukin 4 (IL-4), culminating in the release of reactive nitrogen intermediates that are highly effective in controlling Leishmania replication (10, 12, 28, 30, 32). In contrast, T cells from susceptible mice fail to produce high levels of IFN-γ and reactive nitrogen intermediate release by macrophages, allowing the spread of the parasite and leading to visceralization and death. This difference in the immune response and disease outcome has been attributed to the fact that C57BL/6 and BALB/c mice bias their immune responses to Th1 and Th2, respectively (15).
In contrast, infection with the intracellular protozoan Toxoplasma gondii, which can infect and replicate in any nucleated cell from the vertebrate host, induces a highly polarized Th1 response, independent of the host genetic background (e.g., BALB/c and C57BL/6) (1). The ability of T. gondii to trigger a Th1 response, instead of the expected Th2 response, in the BALB/c mouse can be attributed to the ability of this parasite to trigger the synthesis of overwhelming levels of IL-12 and IFN-γ during early stages of infection (7, 8). Thus, for the early containment of parasite replication, the IFN-γ synthesis (initially by NK cells and later by Th1 lymphocytes) leading to macrophage activation is crucial (11).
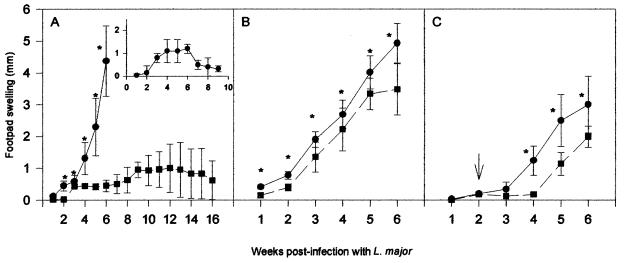
In the present study, we show that susceptible BALB/c mice previously infected with T. gondii become protected against lesion development in the footpad from infection with L. major. The basis of this T. gondii-induced protection was evaluated. As shown in Fig. 1A, animals infected with L. major (106 promastigotes) alone developed intense footpad swelling, and all had to be sacrificed at 7 weeks of infection, due to the size of the footpad lesion. Interestingly, infection with T. gondii 5 days prior to challenge with L. major resulted in protection against lesion development in BALB/c mice. These results were similar to the ones observed in C57BL/6-resistant mice (Fig. 1A, insert). Even at 16 weeks postinfection, the dually infected animals showed no major lesions in the footpads infected with L. major. In contrast, we found normal development of lesions when BALB/c mice were coinfected with L. major during chronic toxoplasmosis (Fig. 1B). Furthermore, when T. gondii cysts were given 2 weeks after infection with L. major, we observed only a small delay in footpad lesion development induced by L. major infection (Fig. 1C).
FIG. 1.
Course of lesion development in BALB/c mice infected with L. major (circles) or with both L. major and T. gondii (squares). The animals were infected intraperitoneally with 20 cysts of the ME-49 strain derived from the brains of C57BL/6 mice chronically infected with T. gondii and challenged in the hind footpads with 106 stationary forms of the WHO MHOM/80/Friedlin strain of L. major during acute (A) or chronic (B) toxoplasmosis. (C) Mice were previously infected with L. major and, after 14 days, were challenged with T. gondii. The animals in the different experiments were monitored for 7 weeks, except for animals infected with T. gondii 5 days prior to infection with L. major, which were monitored for 16 weeks (A). The insert in panel A shows a curve of footpad swelling of resistant C57BL/6 mice infected with 106 stationary forms of L. major. Each point represents the mean (± standard deviation) lesion size for 10 mice. Asterisks indicate that differences are statistically significant (P < 0.05), as evaluated by Students’ t test. Results from one representative experiment of two separately performed are shown.
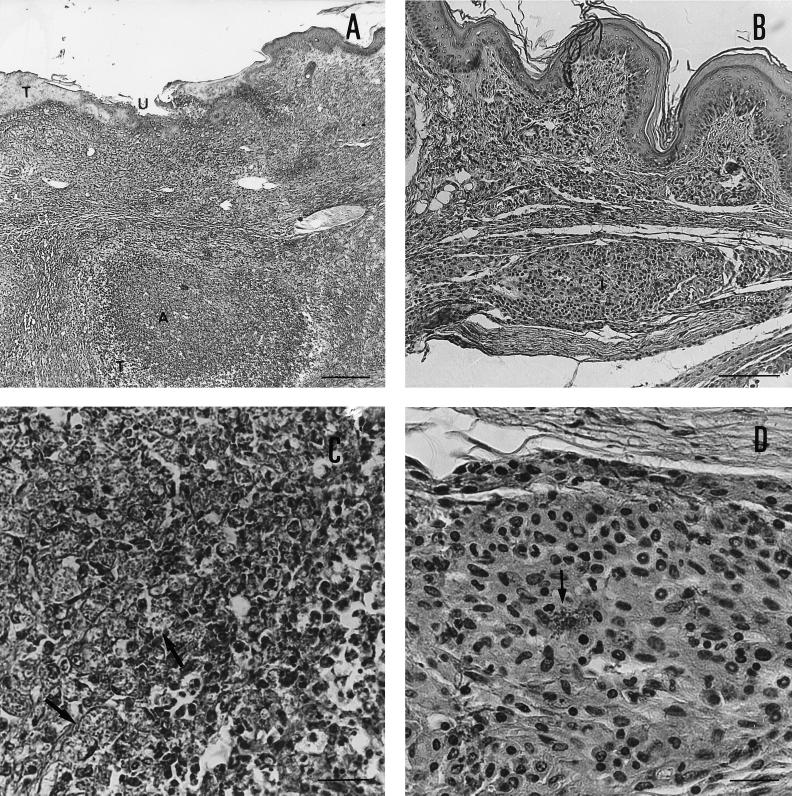
The results presented in Fig. 2 illustrate the tissue pathology observed in mice infected with L. major alone (Fig. 2A and C) or infected with L. major 5 days postinfection with the ME-49 strain of T. gondii (Fig. 2B and D). BALB/c mice infected with L. major alone showed an intense diffuse inflammatory infiltrate, tissue necrosis, ulceration areas, abscess formation (Fig. 2A) and intense tissue parasitism (Fig. 2C) in their footpads. In contrast, as observed in C57BL/6 mice infected with L. major alone (data not shown), dually infected mice showed a small inflammatory infiltrate with little tissue destruction, well-delimited nodules (Fig. 2B) with lymphocytes, plasma cells, few polymorphonuclear cells, many noninfected macrophages, and few infected ones (Fig. 2D). No necrotic areas, ulceration, or abscess formation was present in the latter group.
FIG. 2.
Histopathology of BALB/c mouse footpad infected with L. major alone (A and C) or infected with T. gondii 5 days earlier to infection with L. major (B and D). Groups of animals were sacrificed 7 weeks after infection with L. major, and the infected footpads were fixed, included in paraffin, cut, and stained with hematoxylin and eosin. Footpads from BALB/c mice infected with L. major alone (A and C) showed an intense and diffuse inflammatory infiltrate, tissue necrosis (T), ulceration areas (U), and abscess formation (A). (A) Hematoxylin and eosin; original magnification, ×52.8; 1.05 cm = 200 μM). An intense tissue parasitism (C) is observed in the footpads of mice infected with L. major alone. (C) Hematoxylin and eosin; original magnification, ×528; 1.05 cm = 20 μM). Arrows indicate replicating amastigotes inside macrophages. In contrast, dually infected mice at 7 weeks postinfection with L. major showed a small inflammatory infiltrate with little tissue destruction and well-delimited inflammatory foci (I). No necrotic areas, ulceration, or abscess formation was present in the footpads of dually infected animals (B) Hematoxylin and eosin; original magnification, ×132; 1.32 cm = 100 μM). An inflammatory focus, at higher magnitude, shows the presence of lymphocytes, plasma cells, few polymorphonuclear cells, many noninfected macrophages, and few infected macrophages (arrows).
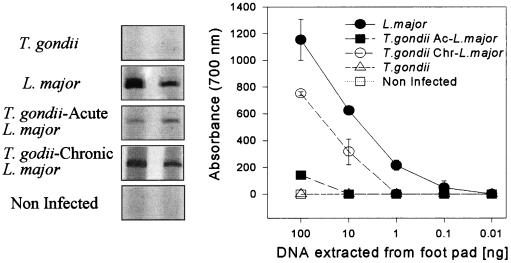
In order to measure the tissue parasitism, we used as a template in PCR, DNA extracted from the footpads of animals infected with L. major. The PCR was performed in a volume of 25 μl containing GP63 (22) primers 5′ GTGCGCACGTGAACTGG 3′ (sense, nucleotides 499 to 517) and 5′ CACCCGCAGTAGTTGTAG 3′ (antisense, nucleotides 917 to 934) and different concentrations of footpad DNA, as indicated in the right panel of Fig. 3. The PCR was performed with a 30-cycle program, and the product was electrophoresed in a 6% polyacrylamide gel developed by silver staining (23). PCR specific for L. major GP-63 DNA (Fig. 3) showed a small difference of tissue parasitism in footpads from mice infected only with L. major and mice chronically infected with T. gondii and coinfected with L. major. Our PCR yielded 100 to 1,000 times less GP63-specific product, when DNA extracted from footpad tissue of BALB/c mice infected with L. major 5 days after infection with T. gondii was used as a template, compared with that from BALB/c mice infected with L. major alone.
FIG. 3.
PCR analysis of gp63 expression in footpads from mice infected with L. major alone, during the acute (T. gondii Ac-L. major) or chronic (T. gondii Chr- L. major) phase of T. gondii infection. Mice were infected intraperitoneally or not with 20 cysts of T. gondii and challenged 5 days (acute) or 30 days (chronic) later with 106 stationary forms of L. major. At the 7th week of L. major infection, mice were sacrificed, and the footpads were used as a DNA source employed in a GP63-specific PCR analysis. The left panel shows the PCR products of a reaction using 100 ng of footpad DNA and primers specific for L. major GP63. Each curve represents the average (± standard deviation) of two animals, indicating the intensity of the GP63-specific PCR product employing different concentrations of footpad DNA, as measured by densitometric analysis. No PCR products were found in samples obtained from uninfected animals or animals infected with T. gondii alone. Similar results were found in two different experiments.
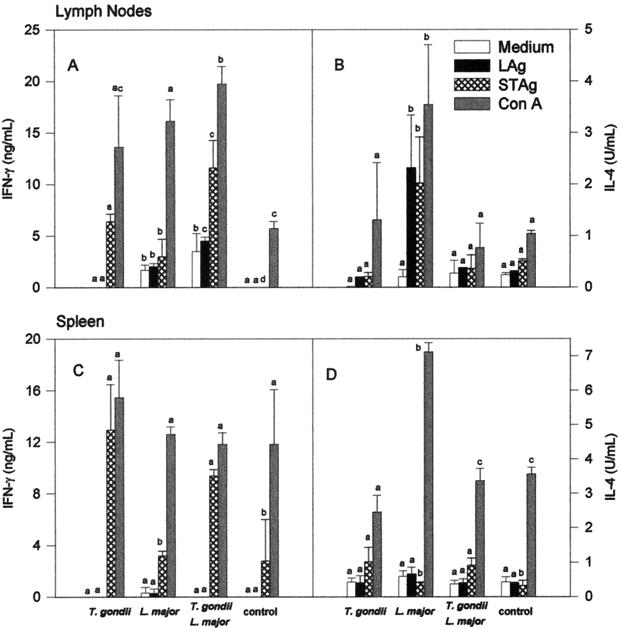
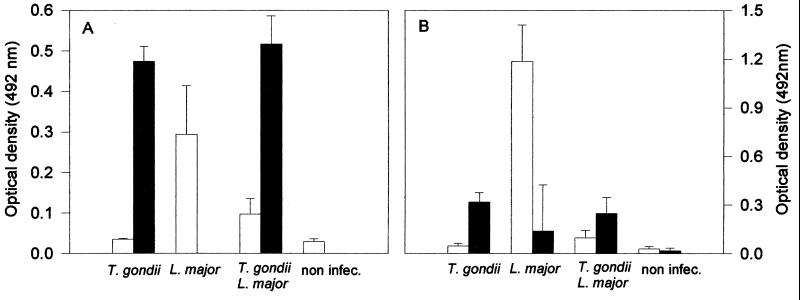
In order to compare the immune responses in mice infected with L. major alone versus those in dually infected animals, lymph node and spleen cells from mice were collected at 7 weeks postinfection with L. major, and IL-4 and IFN-γ responses were evaluated after lymphocyte stimulation with soluble tachyzoite antigens (STAg) (5), Leishmania antigens (LAg) (31), or mitogen (concanavalin A [ConA]). Our ex vivo experiments show that lymph node but not spleen cells from animals infected with L. major alone produced high levels of IL-4 after stimulation with LAg (Fig. 4). Interestingly, STAg triggered the synthesis of IL-4 by lymph node cells from mice infected only with L. major. Denkers et al. (2) have shown a superantigen activity in STAg. Thus, we assume that STAg is activating, in a nonspecific manner, T cells from BALB/c mice that have differentiated into Th2 lymphocytes after infection with L. major. Infection with T. gondii 5 days prior to infection with L. major had a major modulatory activity on IL-4 synthesis induced by parasite antigens (Fig. 4B) or mitogen (Fig. 4B and D). Intriguingly, coinfection with T. gondii did not result in a corresponding enhancement of IFN-γ synthesis by lymph node or spleen cells from mice infected with L. major (Fig. 4A and C).
FIG. 4.
IFN-γ and IL-4 production by spleen and draining lymph node cells from BALB/c mice infected with L. major alone or coinfected with T. gondii. Mice were intraperitoneally infected or not with 20 cysts of T. gondii and challenged 5 days later with 106 stationary forms of L. major. Seven weeks later, the mice were sacrificed. Lymph node and spleen cells were harvested and cultured for 72 h. The culture was done in medium alone (white bars) or in the presence of LAg (black bars), STAg (crosshatched bars), or ConA (gray bars). IFN-γ and IL-4 were assayed in the culture supernatants by capture enzyme-linked immunosorbent assay. Bars represent means (± standard deviations) of four mice per group. Different letters indicate that differences are statistically significant (P < 0.05) by nonparametric Kruskal-Wallis’ test, when comparing cytokine responses from animals from different groups by lymph node or spleen cells stimulated with a single stimulus (i.e., medium, LAG, STAg, or ConA).
Experiments were also performed to analyze the in vivo effects of T. gondii infection on L. major-elicited humoral immune responses. STAg- and LAg-specific total immunoglobulin G (IgG) or IgG1 and IgG2a isotypes in sera of animals infected with L. major and/or T. gondii were measured as previously described (3). Our results support the conclusion of our previous experiments by showing that coinfection with T. gondii had a major inhibitory effect on the in vivo synthesis of Leishmania-specific IgG (Fig. 5A). More importantly, the results presented in Fig. 5B indicate that most of the inhibitory activity on Leishmania-specific IgG is due to inhibition of Ig from the IgG1 isotype, which is stimulated by IL-4. Again, no enhancement of Leishmania-specific IgG2a, an IgG isotype driven by Th1 lymphocytes, was observed.
FIG. 5.
Total IgG antibodies (A) specific for L. major (white bars) or T. gondii (black bars) antigens and (B) specific IgG1 (white bars) or IgG2a (black bars) isotypes against L. major in mice infected or not with T. gondii and challenged with L. major. Mice were infected with 20 cysts of T. gondii and 5 days later were challenged with 106 stationary forms of L. major in the hind footpads. Mice were sacrificed 7 weeks later, and sera were obtained for total IgG and IgG isotype assays by enzyme-linked immunosorbent assay. Bars represents means (± standard deviations) of eight mice per group. These data are pooled from two separate experiments.
Different studies have demonstrated that infection with T. gondii results in protection against different nonrelated pathogens, such as parasites (19, 20), bacteria (27), and viruses (6), as well as development of certain types of tumor cells (14). In most of these studies, it is suggested that activation of cells from an innate immune system, such as macrophages, rather than induction of cross-reactive immunity is responsible for the protective activity elicited by T. gondii infection.
In addition to leading to microbiostatic or microbicidal activity of macrophages, different studies have suggested that this early activation of the innate system has an important role in directing the differentiation of Th precursor cells to the Th1 phenotype (4, 16). However, this question has been difficult to evaluate during infection with T. gondii, because the two cytokines IL-12 and IFN-γ, which are crucial for driving T-cell differentiation to the Th1 phenotype, are also essential elements in resistance to T. gondii. Thus, in the absence of endogenous IL-12 or IFN-γ, animals infected with T. gondii succumb to infection in approximately 9 days (8), before the process of T-cell differentiation is completed.
In contrast, infection with L. major is an extremely interesting model with which to study T-cell differentiation (12, 16, 17, 30). First, L. major is a less virulent parasite and will take a much longer time to cause pathology and lethality, in the absence of endogenous IL-12 and IFN-γ (13). Second, the synthesis of IL-12 and/or IFN-γ is not high enough (or fast enough) to change the tendency of T cells from BALB/c mice to differentiate into Th2 cells. Interestingly, our results show that infection with T. gondii 5 days prior L. major infection makes BALB/c mice highly resistant to the latter parasite. A major question raised by these results is related to the mechanism of protection against L. major observed in mice coinfected with T. gondii. A major argument against cross-reactive protection is the fact that no protection was observed when BALB/c mice chronically infected with T. gondii were challenged with L. major. Furthermore, little or no cross-reactivity was observed when parasite antigens were used to stimulate T cells as well as to measure the levels of antiparasite antibody responses in serum.
In our previous studies (8, 9), we have shown that the peak of IL-12 and IFN-γ synthesis during acute infection with T. gondii occurs at approximately 5 to 8 days postinfection. Therefore, it is tempting to speculate that the protective effect of T. gondii infection is probably related to the overwhelming levels of IL-12 and IFN-γ synthesis during acute toxoplasmosis. It is noteworthy that the protective effect of T. gondii infection against L. major is equivalent to treatment with recombinant IL-12, if not more efficient. Protection persisted even up to 16 weeks of infection, when the animals were sacrificed. However, our results also show that after establishment of L. major infection, the challenge with T. gondii induced only a discreet delay in footpad swelling, similar to that observed after administration of IL-12 in late stages of infection with L. major (13, 24, 33).
A recent study illustrated the ability of acute and chronic infection with T. gondii to enhance a Th1 response during vaccination with a nonparasite-related antigen (25). However, our data show that the change in lesion size, cytokine, and IgG isotype response to Leishmania antigens was only observed in mice acutely, but not chronically, infected with T. gondii. Furthermore, unexpectedly we found an insignificant enhancement of Leishmania-specific Th1 lymphocyte activity. In fact our major finding in terms of the cytokine synthesis of the dually infected animals was the complete suppression of IL-4 synthesis compared to that in animals infected with L. major alone. These findings were also confirmed by measurement of Leishmania-specific IgG isotypes. We found that the synthesis of Leishmania-specific IgG1, but not IgG2a, was suppressed in the dually infected mice.
Therefore, our study suggests that, at least at the level of Th cell differentiation, the major mechanism of action operating during acute toxoplasmosis, and possibly responsible for protection against immunopathology elicited by L. major, is the inhibition of Th precursor cells from developing into the Th2 phenotype. Consistent with this interpretation are the findings that if given 2 weeks postinfection with L. major, T. gondii becomes unable to protect against lesion development in the footpad. In fact, earlier reports indicate that although IL-4 does not appear to be sufficient to make C57BL/6 mice susceptible to infection with L. major (29), neutralizing monoclonal antibodies against IL-4 protect BALB/c mice against lesions caused by L. major, only if given in the first week of infection (18, 28).
Finally, our earlier studies demonstrate that infection with T. gondii in mice results in induction of persistent T-cell-mediated immunity characterized by production of high levels of IFN-γ and IL-2 as well as low levels of IL-4 and IL-5, when stimulated with tachyzoite antigens (5, 8). This induction of Th1 lymphocytes by T. gondii occurs even in BALB/c mice (5) that present a genetic propensity for the development of antigen-specific Th2 lymphocytes (15). Together, the results presented here suggest that inhibition of differentiation of Th precursor cell into Th2 lymphocytes during acute infection with T. gondii may be one important component for the development of parasite-specific, highly polarized Th1 immune responses that persist during chronic toxoplasmosis.
Acknowledgments
We thank Wagner Taffuri, Denise C. Cara, and Luiz Antônio R. Freitas for the analysis of histopathology data and helpful discussions. We also gratefully acknowledge Elaine Speziali and Mariléia C. Andrade for technical support in IgG isotype measurement. H.C.S. is grateful to Gilton Santiago for support and encouragement during this work.
This work was supported in part by FAPEMIG and CNPq (522.056/95-4). R.T.G. and L.Q.V. received a research fellowship from CNPq. H.C.S. is a medical student supported by FAPEMIG. M.A.P.O. is a graduate student and received a scholarship from CAPES.
REFERENCES
- 1.Denkers E Y, Gazzinelli R T. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denkers E Y, Caspar P, Sher A. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor Vβ5-bearing CD8+ lymphocytes. J Exp Med. 1994;180:985–995. doi: 10.1084/jem.180.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faria A M C, Ficker S M, Speziali E, Menezes J S, Stransky B, Verdolin B A, Lahmann W M, Rodrigues V S, Vaz N M. Aging and immunoglobulin isotype patterns in oral tolerance. Braz J Med Biol Res. 1998;31:35–48. doi: 10.1590/s0100-879x1998000100005. [DOI] [PubMed] [Google Scholar]
- 4.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 5.Gazzinelli R T, Hakim F, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 6.Gazzinelli R T, Hartley J W, Fredrickson T N, Chattopadhyay S K, Sher A, Morse H C., III Opportunistic infections and retrovirus-induced immunodeficiency: studies of acute and chronic infections with Toxoplasma gondii in mice infected by LP-BM5 murine leukemia viruses. Infect Immun. 1992;60:4394–4401. doi: 10.1128/iai.60.10.4394-4401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzinelli R T, Hieny S, Wynn T, Wolf S, Sher A. IL-12 is required for the T-cell independent induction of IFN-γ by an intracellular parasite and induces resistance in T-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 9.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, Müller W, Trinchieri G, Sher A. In absence of endogenous IL-10 mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 10.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-γ stimulated macrophages by induction of tumor necrosis factor-α. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 11.Hayashi S, Chan C C, Gazzinelli R T, Roberge F G. Contribution of nitric oxide to host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- 12.Heinzel F P, Sadick M D, Holaday B J, Locksley R M. Reciprocal expression of IFN-γ or IL-12 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell 4subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzel F P, Rerko R M, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 14.Hibbs J H, Jr, Lambert L H, Jr, Remington J S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971;124:587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh C S, Macatonia S E, O’Garra A, Murphy K M. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Launois P, Maillard I, Pingel S, Swihart K G, Xénarios I, Acha-Orbea H, Diggelmann H, Locksley R M, MacDonald H R, Louis J A. IL-4 rapidly produced by Vβ4 Vα8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 17.Launois P, Swihart K G, Milon G, Louis J A. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 18.Lezama-Davila C M, Williams D M, Gallagher G, Alexander J. Cytokine control of Leishmania infection in the BALB/c mouse: enhancement and inhibition of parasite growth by local administration of IL-2 or IL-4 is species and time dependent. Parasite Immunol. 1992;14:37–48. doi: 10.1111/j.1365-3024.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud A A F, Warren K S, Strickland G T. Acquired resistance to infection with Schistosoma mansoni induced by Toxoplasma gondii. Nature. 1976;263:56–57. doi: 10.1038/263056a0. [DOI] [PubMed] [Google Scholar]
- 20.Mahmoud A A F, Strickland G T, Warren K S. Toxoplasmosis and host-parasite relationship in murine schistosomiasis mansoni. J Infect Dis. 1977;135:408–413. doi: 10.1093/infdis/135.3.408. [DOI] [PubMed] [Google Scholar]
- 21.Mauël J. Intracellular survival of protozoan parasites with special reference to Leishmania ssp., Toxoplasma gondii and Trypanosoma cruzi. Adv Parasitol. 1996;38:1–51. doi: 10.1016/s0065-308x(08)60032-9. [DOI] [PubMed] [Google Scholar]
- 22.Murray P J, Handman E, Glaser T A, Spithill T W. Leishmania major: expression and gene structure of glycoprotein 63 molecule in virulent and avirulent clones and strains. Exp Parasitol. 1990;71:294–304. doi: 10.1016/0014-4894(90)90034-a. [DOI] [PubMed] [Google Scholar]
- 23.Murta S, Gazzinelli R T, Brener Z, Romanha A J. Correlation of molecular markers and Trypanosoma cruzi strains naturally resistant and non-resistant to nitroheterocyclic derivatives. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Nabors G S, Afonso L C C, Farrell J P, Scott P. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and pentostan. Proc Natl Acad Sci USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen T D, Bigaignon G, Broeck J V, Vercammen M, Nguyen T N, Delmee M, Turneer M, Wolf S F, Coutelier J P. Acute and chronic phases of Toxoplasma gondii infection in mice modulate the host immune responses. Infect Immun. 1998;66:2991–2995. doi: 10.1128/iai.66.6.2991-2995.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiner S L, Locksley R M. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 27.Ruskin J, Remington J S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968;160:72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- 28.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon γ-independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadick M D, Street N, Mosmann T R, Locksley R M. Cytokine regulation of murine leishmaniasis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect Immun. 1991;59:4710–4714. doi: 10.1128/iai.59.12.4710-4714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott P. IFN-γ modulates the early development of Th1 and Th2 responses in murine model of cutaneous leishmaniasis. J Immunol. 1991;147:3149–3155. [PubMed] [Google Scholar]
- 32.Stenger S, Donhauser N, Thüring H, Röllinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1791–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]