Abstract
Paired-stimuli (S1-S2) procedures have long been used to assess auditory processing in psychosis. Such studies have shown aberrant evoked responses (ERPs) following long (S1 response) and/or short (S2 response) inter-stimulus intervals. The historical tendency from paired stimuli outcomes in the schizophrenia (SZ) literature is for (i) response to the first stimulus (S1) to be smaller among SZ, and (ii) response to the second stimulus (S2) to be larger among SZ in relation to the size of their S1. An interpretation of these two findings is that SZ have poor auditory response suppression to redundant stimuli (“poor gating”). The present study sought to determine if the reported S1 and S2 effects in SZ (smaller S1 and larger S2 in relation to S1 magnitude) require the paired-stimuli presentation format. Participants (18 schizophrenia and 17 healthy persons) were administered the equivalent of S1 (after a 4.5-sec ISI – “long ISI”) and S2 (after a 500-ms ISI – “short ISI”) stimuli under four conditions (traditional paired long and short, randomly interleaved long and short, block of long, block of short). Neural activity differences were consistent between-groups independent of condition: (i) schizophrenia cases had greater activity in the pre-stimulus to very early post-stimulus period, (ii) healthy persons had greater M100 activity to long ISI stimuli, and (iii) healthy persons had greater activity after the M50/M100 evoked fields (recovery phase) following short ISI stimuli. Simple early auditory processing in psychosis may be largely independent of stimulus presentation condition, an outcome that may help re-frame future translational studies. Traditional paired-stimuli auditory neural response effects may not require the paired-stimuli format.
Keywords: Schizophrenia, Psychosis, Auditory paired stimuli, Sensory gating, Magnetoencephalography, MEG, EEG, ERP
Introduction
Paired-stimuli paradigms are used to study auditory processing abnormalities in psychosis (de Wilde et al., 2007; Hamm et al., 2014). In the typical paradigm, identical stimuli (S1-S2) are presented in close temporal proximity (500-ms), with pairs separated by long intervals (e.g. 10-sec). Normally, neural responses within the first 100 to 200-ms following S2 are smaller than those following S1. Sensory filtering or ‘gating’ abnormalities are presumed if response magnitudes are more similar between the two stimuli. A smaller S1-S2 difference is a historically significant and widely replicated finding among psychosis cases (de Wilde et al., 2007; Nagamoto et al., 1991; Olincy et al., 2010).
Two assumptions of sensory processing theories for interpreting differences between S1-S2 response magnitudes are: (i) S1-S2 differences are largely determined by differences at S2, not S1; and (ii) the stimulus configuration (pairing of S2 500-ms after S1) provides a necessary expectation, peculiar to the traditional paired-stimuli configuration. The available evidence, however, compels a careful consideration of these assumptions. The majority of group effects in psychosis studies are associated with three sensory neural response features. First, preparation for stimulus presentation (enhanced activity pre-stimulus) differs between healthy persons and psychosis cases (Ethridge et al., 2011). There is an elevation of background brain activity in certain psychosis cases (Rolls et al., 2008; Thomas et al., 2019), including schizophrenia (Blumenfeld and Clementz, 2001; Hamm et al., 2014), which lowers neural signal-to-noise ratio for any stimulus processed against this elevated background. Lower signal-to-noise may mean compromised signal fidelity with accompanying reduced ability to parse stimulus salience (Ethridge et al., 2011; Hudgens-Haney et al., 2017, 2018). Second, reduced amplitude M100/N100, analogous to lower S1 magnitudes in paired-stimuli paradigms, is one of the most replicated findings in schizophrenia research (Rosburg et al., 2008). In the absence of any other effects, lower S1 amplitudes across stimulus conditions could account for the historical reports of smaller S1-S2 amplitude differences among psychosis cases in paired stimuli studies. Third, recovery from stimulation (the period after sensory registration and before the next stimulus when there is no obvious evoked response) differs between psychosis and healthy subjects (Brenner et al., 2009; Clementz and Blumenfeld, 2001; Hamm et al., 2014; Johannesen et al., 2005, 2013; Smith et al., 2010). In paired-stimuli paradigms, there is a 500-ms window between S1 and S2. Psychosis and healthy groups recovering from S1, leading up to S2, at different rates (Hamm et al., 2014; Parker et al., 2020; Popov et al., 2011). This difference could contribute to the historical report of sensory gating differences in psychosis because S2 is occurring against a different neural background in psychosis compared to healthy persons.
In this paper, we preliminarily evaluate these three neural features through manipulation of stimulus presentation conditions. Under the strongest version of the sensory gating interpretation of S1-S2 differences in psychosis, paired-stimuli pairings are required to obtain the historically reported effect. The expectation during the typical paired stimuli presentation format is a long ISI stimulus (S1) followed by a short ISI stimulus (S2), and that S2 reduction is a consequence of the irrelevance of S2 given that it is always the same as S1 (see also (Brenner et al., 2009)). To help clarify whether S1-S2 response magnitudes in psychosis are at least partially independent of the stimulus sequence expectation on the part of participants, we used the following manipulations (see Fig. 1): (i) a traditional S1-S2 paired-stimuli paradigm (i.e., S1 occurred after a long temporal interval and S2 occurred after a short temporal interval, on every trial); (ii) a mixed run in which “S1” and “S2” stimuli were randomly interleaved (i.e., long and short intervals between stimuli were randomly determined for every trial); (iii) a block of stimuli with longer interstimulus intervals (effectively all “S1”), and (iv) a block of stimuli with shorter interstimulus intervals (effectively all “S2). To the extent that background, S1, S2, and stimulus recovery magnitudes between psychosis and healthy subjects can be captured independent of the S1-S2 pairing, this will inform future theorizing and translational studies using the “sensory gating” paradigm.
Fig. 1.
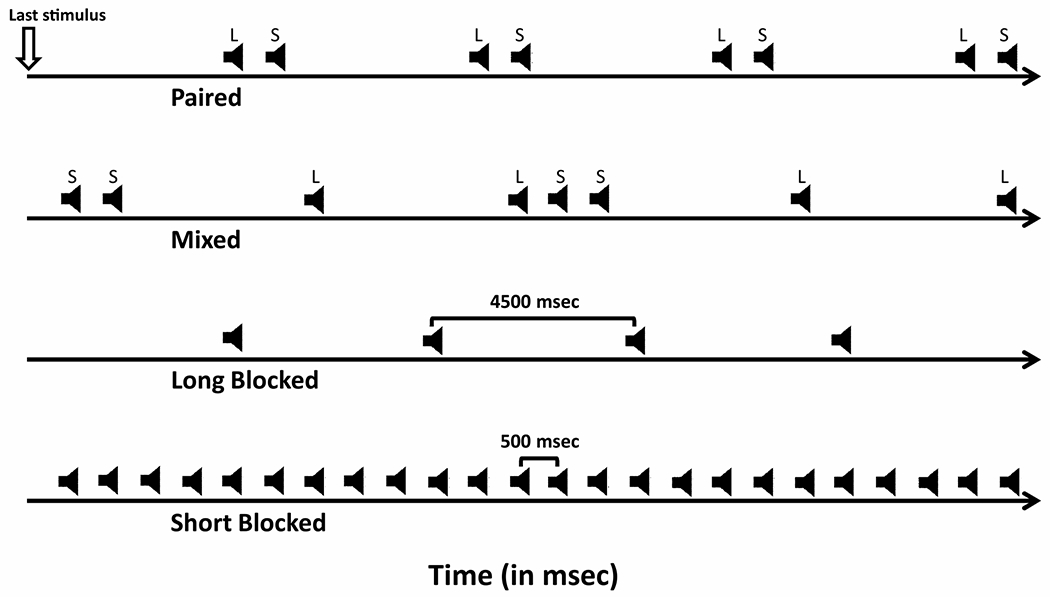
Subjects completed four conditions (the four rows from top to bottom) of a (i) traditional paired-stimuli paradigm; (ii) a mixed run with 0.5 s and 4.5 s ISI randomly interleaved, (iii) 4.5 s ISI (long blocked), and (iv) 0.5 s ISI (short blocked). Time runs along the x-axis. Speaker icons indicate delivery of an auditory stimulus during the particular condition types.
Method
Participants were 18 right-handed chronic outpatients with DSM-IV (American Psychiatric Association et al., 1994) schizophrenia (Median Age = 36yrs, 25th-75th %tile = 28–44yrs; 6 females) and 17 right-handed healthy subjects (Median Age = 37yrs, 25th-75th %tile = 28–46yrs; 10 females). Participants with schizophrenia were clinically stable on antipsychotic medications (12 on second generation; 6 on first generation). Subjects were interviewed with the SCID (First, 2004) by two Ph.D.-level psychologists to either verify their clinical diagnosis (schizophrenia) or rule out Axis I disorders (healthy subjects). Participants were absent of neurological hard signs, clinically confounding treatments, history of head trauma and current psychoactive substance use disorders. Healthy persons had no evidence, by self-report, of psychosis in either their first- or second-degree biological relatives. The project was approved by the UGA Institutional Review Board and all subjects provided written informed consent before testing. We collected neural activity data in the magnetoencephalography (MEG) environment, which is excellent for measuring cortical responses to auditory stimuli (Sams and Hari, 1991). MEG also has the advantage over EEG of measuring neural signals with minimal distortion because intervening tissues (skull, scalp) have little effect on the signals measured at the sensors in relation to the “true” response generated by activated neurons (Supek and Aine, 2014).
For the typical paired-stimuli task, there is a stimulus following a long interval (S1 - which here occurred after 4.5-sec because 3-sec is sufficient to obtain auditory evoked response differences in psychosis; (Shelley et al., 1999) and another stimulus following a short interval (S2–500 ms after S1). We tested the possibility that it is the interval between stimuli (long vs short), rather than stimulus context (paired-stimuli paradigm) that largely accounts for the traditional S1-S2 psychosis effects. Every subject completed four conditions: (i) 120 trials of a traditional paired-stimuli paradigm as described above; (ii) a mixed run with 0.5 s and 4.5 s ISI randomly interleaved, 120 of each ISI, (iii) 120 trials of 4.5 s ISI (long blocked), and (iv) 120 trials of only 0.5 s ISI (short blocked). Fig. 1 provides a schematic of the four conditions.
MEG recordings were obtained using a 143 channel CTF OMEGA whole head system (CTF/VSM Medtech Ltd., Coquitlam, BC, Canada). MEG data were recorded continuously, sampled at 600 Hz, with an analog filter bandpass of 0.6–300 Hz. An inflatable air bladder was fitted to the subject’s head (like a stocking cap) to encourage head stabilization throughout. Three head localization coils (positioned at the nasion, and left and right preauricular points) and Ag–AgCl electrodes (positioned at the outer canthi of each eye, and above and below the left eye for recording of horizontal and vertical eye movements, respectively) were affixed prior to testing. Head position relative to sensor locations was measured at the beginning and end of testing, with no participant moving more than 3 mm in any plane.
Data were pre-processed following previously published procedures, including adjustment for cardiac, muscle, and ocular artifacts (Gao et al., 2007; Hamm et al., 2011; Hayrynen et al., 2016). Prior to analyses, each subject’s evoked fields were standardized over all time points and sensors, yielding evoked fields in a common neural response space that could be compared across subjects and between groups. Evoked fields butterfly plots, averaging over conditions, along with M100 topographies, are shown in Fig. 2, which illustrate typical signal-to-noise. Signal-to noise for short interval responses is insufficient for reliable individual source estimates (Fuchs et al., 2017; Wagner et al., 2004). Instead, we calculated magnetic global field power (mGFP), a measure of variance of signal at each point in time (Ahonen et al., 2016; Lehmann and Skrandies, 1980, 1984). Like a root mean square measure, larger mGFP (like at the time of the M100) indicates a stronger signal; smaller mGFP (like at the time of the M50) indicates a weaker signal. Figs. 3 and 4 show mGFP for long and short ISI stimuli by condition. Data were segmented into 10-ms bins from 45-ms pre-stimulus to 295-ms post-stimulus. To quantify group (healthy, SZ) by condition (paired, mixed, blocked) effects as a function of long versus short ISI we used mixed model ANOVAs on mGFP over time prior to baseline adjustment (which captures pre-stimulus and stimulus recovery activity more effectively; (Ethridge et al., 2011)) and then after baseline adjustment (the typical evoked response quantification approach). These tests were followed by source estimates (sLoreta in Brainstorm; (Tadel et al., 2011), on grand averages, at times of significant effects to illustrate the approximate distribution of brain activities that differentiated healthy and SZ groups. We selected sLoreta versus least-square minimum norm to obtain source estimates for three main reasons: (i) sLoreta has lower localization errors, (ii) we were not interested in the strength of the estimated sources, only their locations, for which sLoreta is well suited, and (iii) sLoreta works well under low signal-to-noise conditions, which is a particular concern for estimating sources in response to short ISI (“S2”) stimuli (Pascual-Marqui, 2002; Pascual-Marqui et al., 2018; Wagner et al., 2004, 2007).
Fig. 2.
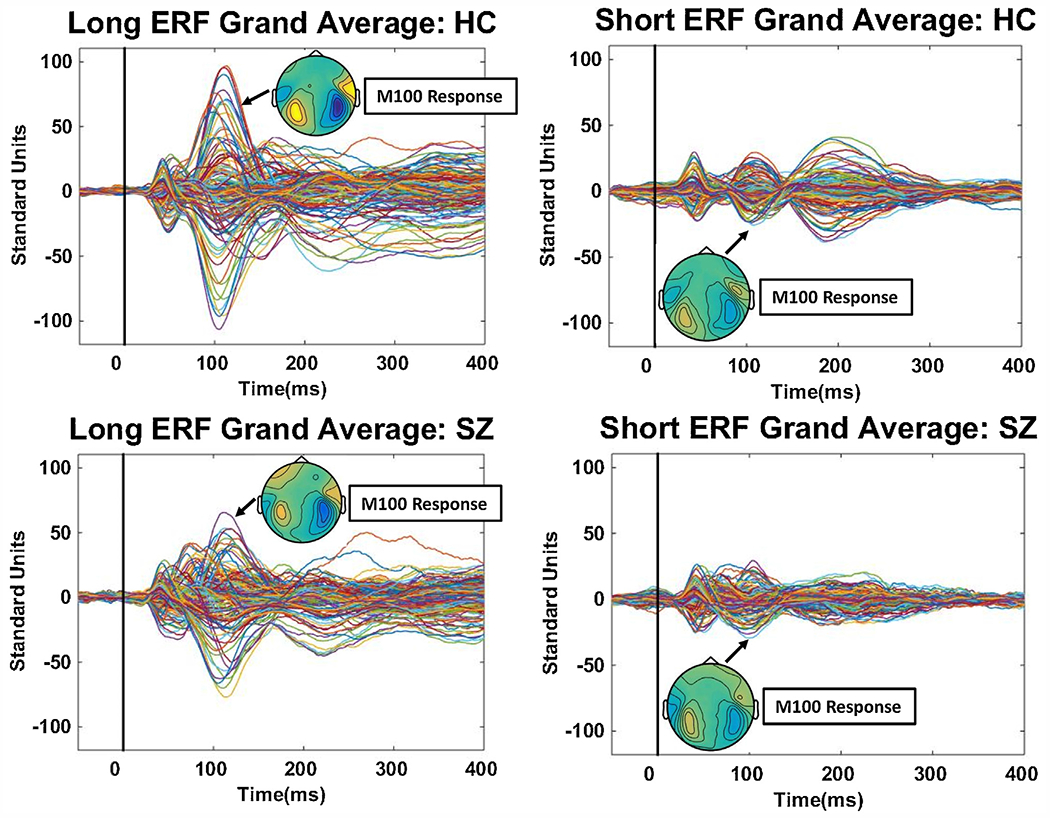
Grand averaged butterfly plots (all 143 MEG sensors; sensors have different colored lines) of the evoked magnetic fields in response to long ISI (left panels) and short ISI (right panels) stimuli. Stimuli occurred at time 0 on the x-axis. The plots have been averaged over all contexts for healthy persons (top panels) and schizophrenia subjects (bottom panels). The inserted top-down fields topography of the M100 is shown for each grand average, and they illustrate the expected dipolar configuration over left and right auditory cortices for these binaural stimuli.
Fig. 3.
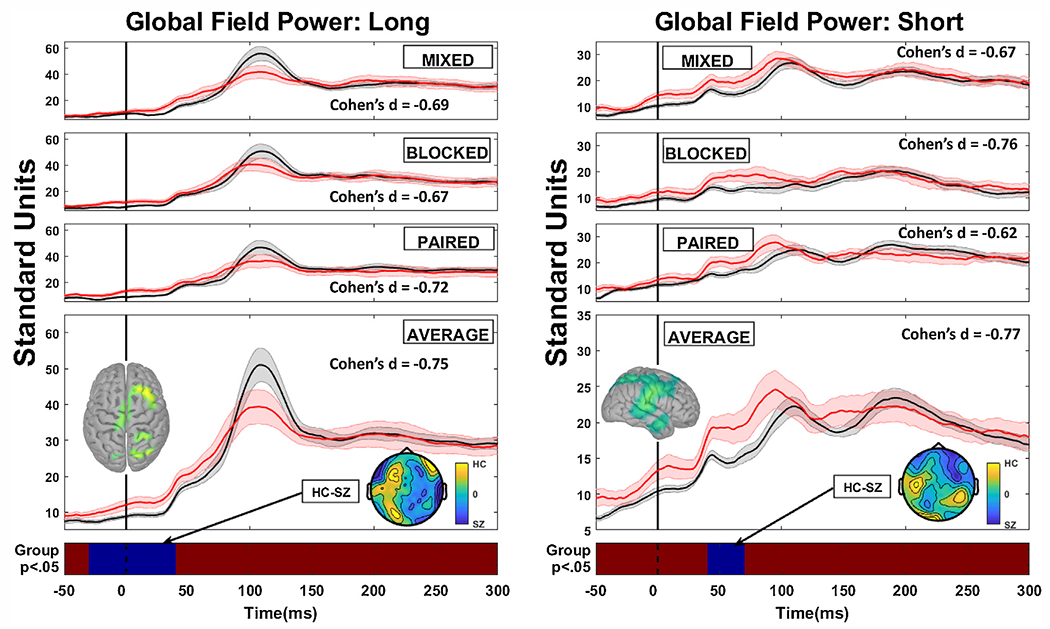
Grand average magnetic global field power plots prior to baseline adjustment for healthy (black lines) and schizophrenia cases (red lines) as a function of context (mixed condition is when ISIs were randomly interleaved; blocked condition when one ISI only is presented in series; paired condition is the typical paired-stimuli configuration with long followed by short ISI repeatedly). Long duration plots are in the left hand panels and short duration plots are in the right hand panels. The grand average over all contexts is shown in the bottom panel, along with the regions of statically significant group comparison differences (blue regions) against the non-significant red strip at the bottom (there were no significant interactions so only the main effect of group is displayed). Stimuli occurred at time 0 on the x-axis. The inserted top-down fields topographies below the grand average line plots illustrate the main effect difference between healthy and schizophrenia cases. Effect sizes are shown for the region of the statistically significant main effect for individual contexts (even thought here were no significant interactions) as well as for the grand average over contexts. The inserted top-down source topographies above the grand average line plots illustrate the source configuration for the region of statistically significant difference.
Fig. 4.
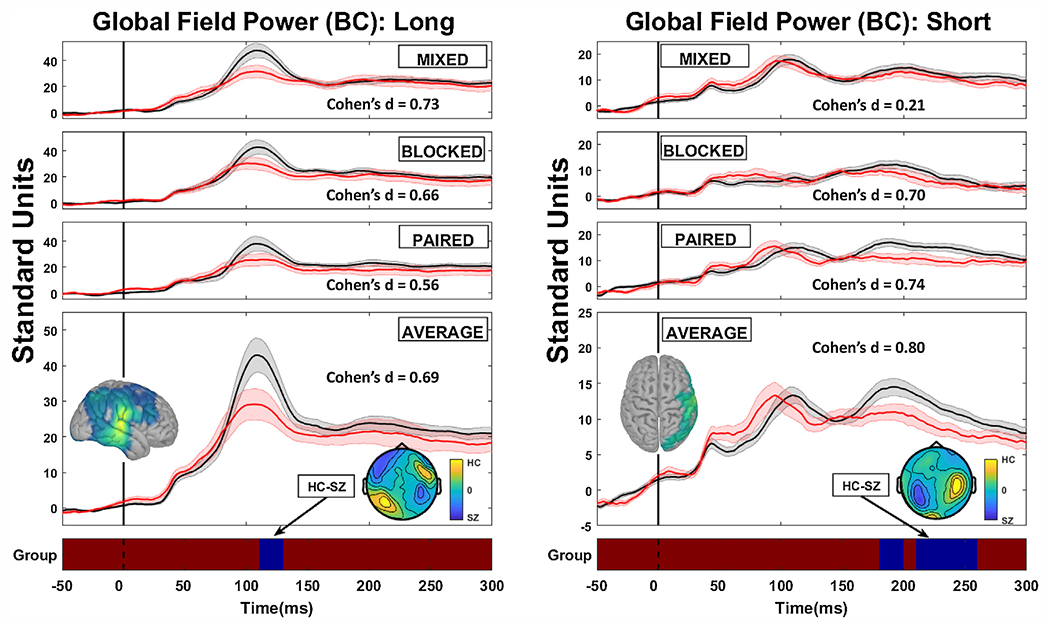
Grand average magnetic global field power plots following baseline adjustment for healthy (black lines) and schizophrenia cases (red lines) as a function of context (mixed condition is when ISIs were randomly interleaved; blocked condition when one ISI only is presented in series; paired condition is the typical paired-stimuli configuration with long followed by short ISI repeatedly). Long duration plots are in the left hand panels and short duration plots are in the right hand panels. The grand average over all contexts is shown in the bottom panel, along with the regions of statically significant group comparison differences (blue regions) against the non-significant red strip at the bottom (there were no significant interactions so only the main effect of group is displayed). Stimuli occurred at time 0 on the x-axis. The inserted top-down fields topographies below the grand average line plots illustrate the main effect difference between healthy and schizophrenia cases. Effect sizes are shown for the region of the statistically significant main effect for individual contexts (even thought here were no significant interactions) as well as for the grand average over contexts. The inserted top-down source topographies above the grand average line plots illustrate the source configuration for the region of statistically significant difference.
Results
Prior to baseline adjustment, there were two significant group main effects (see Fig. 3): (i) in response to long ISI stimuli from just pre-stimulus up until shortly after stimulus presentation (minus 25-ms to 45-ms), average F(1,33) = 5.06, p < .047, average Cohen’s d = −0.75 and (ii) in response to short ISI stimuli in the M50 time range (45-ms to 75-ms), average F(1,33) = 5.34, p < .049; average Cohen’s d = −0.77. SZ had greater mGFP activity in both time ranges. There were no significant interactions involving stimulus presentation condition. Magnetic fields difference plots and sLoreta solutions on the unadjusted data indicate that (i) greater SZ activity during the long ISI stimuli preparatory period was associated with sources in mostly right hemisphere frontal and parietal cortices; and (ii) greater SZ activity in response to short ISI stimuli in the M50 time range was associated with sources in mainly right auditory cortex and supramarginal gyrus.
Following baseline adjustment, there were two significant group main effects (see Fig. 4): (i) in response to long ISI stimuli, in the M100 time range (115-ms to 135-ms), healthy had greater mGFP than SZ, average F (1,33) = 4.43, p < .048; average Cohen’s d = 0.69, and (ii) in response to short ISI stimuli, in the time range of the late part of the M200 (225-ms to 265-ms), healthy had greater mGFP than SZ, average F(1,33) = 4.85, p < .045; average Cohen’s d = 0.80. There were no significant interactions involving stimulus presentation condition. Magnetic fields difference plots and sLoreta solutions on the baseline adjusted data indicate that (i) in response to long ISI stimuli healthy had greater M100 activity mainly in right primary auditory and temporo-parieto-occipital junction; and (ii) in response to short ISI stimuli healthy had greater activation in the later M200 time range in temporal, inferior frontal, and parietal cortices.
Discussion
This project provides novel information about the interpretation of traditional paired-stimuli paradigm outcomes in psychosis and other severe psychiatric syndromes. Analyses of neuromagnetic responses showed replication of the three previously mentioned effects: preparation for stimulation, response to the first stimulus (called S1, which occurs after a long temporal delay), and recovery from and in anticipation of impending stimuli. The similarity of effects across stimulation conditions indicates they may be mostly related to fundamental auditory sensory processing functions rather than peculiar to the stimulus expectation associated with the traditional paired-stimuli format (an S1 after a long temporal delay and an S2 after a brief temporal delay).
First, the importance of preparatory effects was evident in two outcomes: (i) there was a baseline offset difference (SZ > healthy) in response to long ISI stimuli, replicating other studies using the same and different paradigms (Clementz and Blumenfeld, 2001; Ethridge et al., 2011; Hamm et al., 2014; Hudgens-Haney et al., 2018); and (ii) there was a difference in M50 amplitude to the short ISI stimuli (SZ > healthy) that disappeared after adjusting for pre-stimulus activity, also recapitulating previous findings (Ethridge et al., 2011; Hudgens-Haney et al., 2018). Both of these outcomes may be related to exuberant intrinsic activity observed among a subset of psychosis cases (Clementz et al., 2016). Second, the importance for group differentiation of the M/N100 response to auditory stimuli after longer (>3 s) but not shorter stimulus delays after baseline adjustment replicates previous reports (Clementz and Blumenfeld, 2001; Johannesen et al., 2005, 2013; Shelley et al., 1999). This outcome also implicates problems with basic sensory registration of salient stimuli among at least a subset of psychosis cases (Clementz et al., 2016, 2008). Third, difference in later stimulus processing, especially in recovery from stimulation has been previously reported in psychosis (Ethridge et al., 2011). Because recovery functions between psychosis and healthy subjects differ, the interpretation of group sensory processing differences to closely spaced stimuli are especially complicated (Wang et al., 2010), and may require paradigmatic manipulations to properly parse the relevant disrupted neural operations.
It is assumed that the ‘condition-test’ format of the paired-stimuli task is essential to assessing auditory stimulus processing deviations in psychosis. The outcomes of the present project, however, indicate that inter-stimulus interval, rather than expectation of an S1 followed 500-ms later by an S2 may be more relevant for indexing critical fundamental psychosis-relevant auditory sensory processing deviations. These outcomes require replication and extension in larger and more diverse psychosis samples, including in psychosis Biotypes (Clementz et al., 2016). We had a mixed group of schizophrenia cases here so it is uncertain whether specific neurobiological types may have unique sensory profiles across stimulation conditions. If variation in stimulus context, however, is largely irrelevant for assessing at least some aspects of basic psychosis-relevant sensory processing deviations, this would be an important advance in useful knowledge supporting novel translational investigations.
The strengths of our study include the technology used for auditory neural processing assessment (MEG), which is ideally suited for this type of investigation (Supek and Aine, 2014), and the use of multiple means for assessing auditory processing to physically identical stimuli (“S1” and “S2” in different conditions, although S1 always had the same long ISI and S2 always had the same short ISI). The only parameter that changed was the expectation on the part of the subject of when an S1 versus an S2 would occur. Among limitations, most patients were medicated, so the effect of treatment cannot be excluded. In other projects with larger samples, however, we have not found large proportions of variance in ERP responses associated with medication status (Clementz et al., 2016; see also Hamilton et al., 2019a, 2019b).
Funding source
NIMH R01 MH057886.
Footnotes
Declaration of Competing Interest
None of the authors have any conflicts of interest to report.
References
- Ahonen L, Huotilainen M, Brattico E, 2016. Within- and between-session replicability of cognitive brain processes: an MEG study with an N-back task. Physiol. Behav 158, 43–53. 10.1016/j.physbeh.2016.02.006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, American Psychiatric Association, Task Force on DSM-IV, 1994. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed. American Psychiatric Association. [Google Scholar]
- Blumenfeld LD, Clementz BA, 2001. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clin. Neurophysiol 112 (9), 1650–1659. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O’Donnell BF, Hetrick WP, 2009. Event-related potential abnormalities in schizophrenia: a failure to “gate in” salient information? Schizophr. Res 113 (2–3), 332–338. 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, 2001. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp. Brain Res 139 (4), 377–390. 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Wang J, Keil A, 2008. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J. Neurosci 28 (50), 13411–13418. 10.1523/JNEUROSCI.4095-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, 2016. Identification of distinct psychosis biotypes using brain-based biomarkers. Am. J. Psychiatry 173 (4), 373–384. 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH, 2007. A meta-analysis of P50 studies in patients with schizophrenia and relatives: differences in methodology between research groups. Schizophr. Res 97 (1–3), 137–151. 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Ethridge L, Moratti S, Gao Y, Keil A, Clementz BA, 2011.Sustained versus transient brain responses in schizophrenia: the role of intrinsic neural activity. Schizophr. Res 133 (1–3), 106–111. 10.1016/j.schres.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MBG, 2004. The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). M.. In: Segal MJHDL (Ed.), Comprehensive Handbook of Psychological Assessment, Vol. 2. Personality Assessment. John Wiley & Sons, Inc., pp. 134–143. [Google Scholar]
- Fuchs M, Kastner J, Tech R, Wagner M, Gasca F, 2017. MEG and EEG dipole clusters from extended cortical sources. Biomed. Eng. Lett 7 (3), 185–191. 10.1007/s13534-017-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Boyd M, Poon L, Clementz BA, 2007. Age-associated hemispheric asymmetry reduction on the auditory M100 to nonverbal stimuli. Brain Imaging Behav. 1 (3–4), 93–101. [Google Scholar]
- Hamilton HK, Roach BJ, Bachman PM, Belger A, Carrion RE, Duncan E, Johannesen JK, Light GA, Niznikiewicz MA, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Cannon TD, Mathalon DH, 2019a. Association between P300 responses to auditory oddball stimuli and clinical outcomes in the psychosis risk syndrome. JAMA Psychiatry 10.1001/jamapsychiatry.2019.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, Woods SW, Roach BJ, Llerena K, McGlashan TH, Srihari VH, Ford JM, Mathalon DH, 2019b. Auditory and Visual Oddball Stimulus Processing Deficits in Schizophrenia and the Psychosis Risk Syndrome: Forecasting Psychosis Risk With P300. Schizophr. Bull 45 (5), 1068–1080. 10.1093/schbul/sby167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NA, Sponheim SR, Clementz BA, 2011. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol. Psychiatry 69 (10), 989–996. 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Boutros NN, Keshavan MS, Sweeney JA, Pearlson GD, Tamminga CA, Clementz BA, 2014. Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum. Psychophysiology 51 (4), 348–357. 10.1111/psyp.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayrynen LK, Hamm JP, Sponheim SR, Clementz BA, 2016. Frequency-specific disruptions of neuronal oscillations reveal aberrant auditory processing in schizophrenia. Psychophysiology 53 (6), 786–795. 10.1111/psyp.12635. [DOI] [PubMed] [Google Scholar]
- Hudgens-Haney ME, Ethridge LE, Knight JB, McDowell JE, Keedy SK, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA, Clementz BA, 2017. Intrinsic neural activity differences among psychotic illnesses. Psychophysiology 54 (8), 1223–1238. 10.1111/psyp.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgens-Haney ME, Ethridge LE, McDowell JE, Keedy SK, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA, Clementz BA, 2018. Psychosis subgroups differ in intrinsic neural activity but not task-specific processing. Schizophr. Res 195, 222–230. 10.1016/j.schres.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP, 2005. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr. Res 78 (2–3), 269–284. 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, O’Donnell BF, Shekhar A, McGrew JH, Hetrick WP, 2013. Diagnostic specificity of neurophysiological endophenotypes in schizophrenia and bipolar disorder. Schizophr. Bull 39 (6), 1219–1229. 10.1093/schbul/sbs093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W, 1980. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalogr. Clin. Neurophysiol 48 (6), 609–621. 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W, 1984. Spatial analysis of evoked potentials in man–a review. Prog. Neurobiol 23 (3), 227–250. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R, 1991. Gating of auditory response in schizophrenics and normal controls. Effects of recording site and stimulation interval on the P50 wave. Schizophr. Res 4 (1), 31–40. 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- Olincy A, Braff DL, Adler LE, Cadenhead KS, Calkins ME, Dobie DJ, Green M, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Wagner BD, Freedman R, 2010. Inhibition of the P50 cerebral evoked response to repeated auditory stimuli: results from the Consortium on Genetics of Schizophrenia. Schizophr. Res 119 (1–3), 175–182. 10.1016/j.schres.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DA, Trotti RL, McDowell JE, Keedy SK, Gershon ES, Ivleva EI, Pearlson GD, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, 2020. Auditory paired-stimuli responses across the psychosis and bipolar spectrum and their relationship to clinical features. Biomark. Neuropsychiatry 3100014 10.1016/j.bionps.2020.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, 2002. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol 24 (Suppl. D), 5–12. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Faber P, Kinoshita T, Kochi K, Milz P, Nishida K, Yoshimura M, 2018. Comparing EEG/MEG neuroimaging methods based on localization error, false positive activity, and false positive connectivity. bioRxiv 269753 10.1101/269753. [DOI] [Google Scholar]
- Popov T, Jordanov T, Weisz N, Elbert T, Rockstroh B, Miller GA,2011. Evoked and induced oscillatory activity contributes to abnormal auditory sensory gating in schizophrenia. Neuroimage 56 (1), 307–314. 10.1016/j.neuroimage.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G, 2008. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat. Rev. Neurosci 9 (9), 696–709. 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM, 2008. Reduced auditory evoked potential component N100 in schizophrenia–a critical review. Psychiatry Res. 161 (3), 259–274. 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Sams M, Hari R, 1991. Magnetoencephalography in the study of human auditory information processing. Ann. N. Y. Acad. Sci 620, 102–117. 10.1111/j.1749-6632.1991.tb51577.x. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC, 1999. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr. Res 37 (1), 65–79. [DOI] [PubMed] [Google Scholar]
- Smith AK, Edgar JC, Huang M, Lu BY, Thoma RJ, Hanlon FM, McHaffie G, Jones AP, Paz RD, Miller GA, Canive JM, 2010. Cognitive abilities and 50- and 100-msec paired-click processes in schizophrenia. Am. J. Psychiatry 167 (10), 1264–1275. 10.1176/appi.ajp.2010.09071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek S, Aine CJ, 2014. Magnetoencephalography: From Signals to Dynamic Cortical Networks [still Image]. Springer. [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM, 2011. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci 2011879716 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas O, Parker D, Trotti R, McDowell J, Gershon E, Sweeney J, Keshavan MS, Keedy SK, Ivleva E, Tamminga CA, Pearlson GD, Clementz BA, 2019. Intrinsic neural activity differences in psychosis biotypes: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium. Biomark. Neuropsychiatry 1100002 10.1016/j.bionps.2019.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Fuchs M, Kastner J, 2004. Evaluation of sLORETA in the presence of noise and multiple sources. Brain Topogr. 16 (4), 277–280. [DOI] [PubMed] [Google Scholar]
- Wagner M, Fuchs M, Kastner J, 2007. SWARM: sLORETA-weighted accurate minimum norm inverse solutions. Int. Congr. Ser 1300, 185–188. 10.1016/j.ics.2007.02.043. [DOI] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA, 2010. Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia. Cereb. Cortex 20 (7), 1749–1755. 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]


