Abstract
Objectives
To determine antimicrobial stewardship (AMS) programme practices in Asian secondary- and tertiary-care hospitals.
Methods
AMS programme team members within 349 hospitals from 10 countries (Cambodia, India, Indonesia, Japan, Malaysia, Pakistan, the Philippines, Taiwan, Thailand and Vietnam) completed a questionnaire via a web-based survey link. The survey contained questions as to whether 12 core components deemed essential for AMS programmes were implemented.
Results
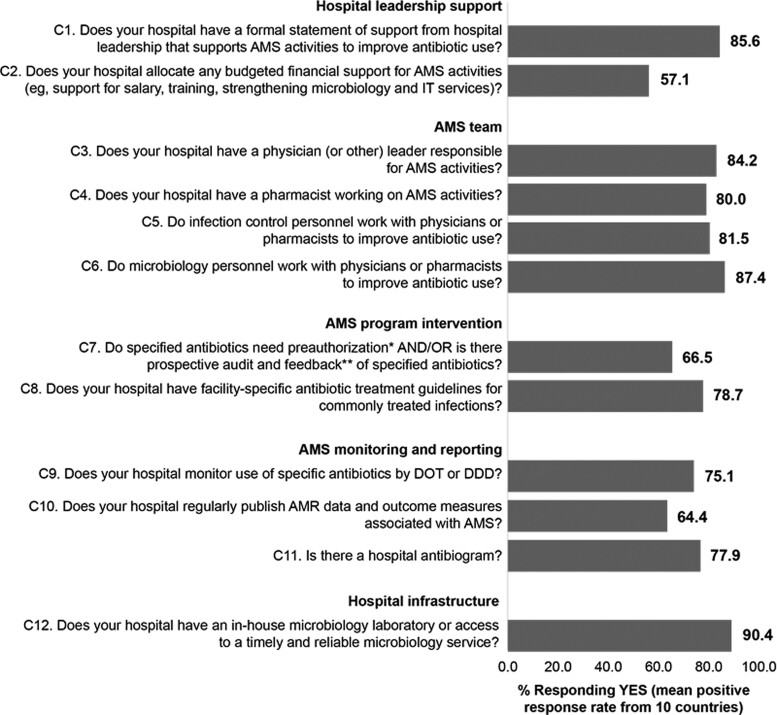
Overall, 47 (13.5%) hospitals fulfilled all core AMS programme components. There was a mean positive response rate (PRR) of 85.6% for the responding countries in relation to a formal hospital leadership statement of support for AMS activities, but this was not matched by budgeted financial support for AMS activities (mean PRR 57.1%). Mean PRRs were ≥80.0% for the core AMS team comprising a physician or other leader responsible for AMS activities, a pharmacist and infection control and microbiology personnel. Most hospitals had access to a timely and reliable microbiology service (mean PRR 90.4%). Facility-specific antibiotic treatment guidelines for common infections (mean PRR 78.7%) were in place more often than pre-authorization and/or prospective audit and feedback systems (mean PRR 66.5%). In terms of AMS monitoring and reporting, PRRs of monitoring specific antibiotic use, regularly publishing AMS outcome measures, and the existence of a hospital antibiogram were 75.1%, 64.4% and 77.9%, respectively.
Conclusions
Most hospitals participating in this survey did not have AMS programmes fulfilling the requirements for gold standard AMS programmes in hospital settings. Urgent action is required to address AMS funding and resourcing deficits.
Introduction
Antimicrobial resistance (AMR) represents a serious threat to global public health,1 and is a particularly urgent issue in Asia.2,3 Low- and middle-income countries are common in Asia and share a disproportionate burden of AMR.2,4 Misuse and overuse of antibiotics are driving a high prevalence of AMR in the region.5–9
Antimicrobial stewardship (AMS) is a coordinated set of interventions designed to improve the appropriate use of antimicrobial agents.10,11 Effective hospital AMS programmes are essential to reducing the emergence of AMR, and can offset or reduce costs while improving patient outcomes.12–18 There is consensus that hospital AMS programmes comprising a core set of components should be introduced to ensure optimal antibiotic prescribing. Gold standard AMS programme core components include hospital administration support, an appropriately trained AMS team, AMS programme goals and carefully planned interventions, a structured reporting system and adequate hospital infrastructure.10,11,19–21 Implementation of AMS programmes has, however, been inconsistent across countries and regions, and programmes often lack core components, particularly in low- and middle-income countries.2,4,22–24 Surveys conducted in hospitals in Asian countries, including high-income countries such as Japan, upper middle-income countries such as Malaysia and Thailand, and low- and middle-income countries such as India, Indonesia and Pakistan indicate that much work is required to improve AMS programmes across the region.3,23–36 One such survey, conducted in tertiary-care hospitals in central Thailand, has demonstrated that assessment of core AMS programme components can provide a useful gap analysis to help inform the optimization of AMS programmes.25
Here, we report the results of a similar survey of core AMS practices in secondary and tertiary acute-care hospitals within the Asian region. As part of ongoing efforts to implement successful AMS programmes in hospitals across Asia, these data will help to identify gaps in the implementation of components considered essential for effective hospital AMS programmes, thereby providing opportunities to improve AMS programmes.
Methods
Survey conduct
The survey targeted secondary and tertiary acute-care hospitals from 10 Asian countries: Cambodia, India, Indonesia, Japan, Malaysia, Pakistan, Philippines, Taiwan, Thailand and Vietnam. Within each country, we aimed to select hospitals that best represent local AMS practice across different regions and states. The following types of centre were excluded: primary care hospitals, hospitals without an intensive-care unit, ministries and government offices, public health centres, nursing and home care facilities.
Selected hospitals were invited to participate in the survey, which was delivered via a web-based survey link. The survey was to be completed only by staff involved in the hospital’s AMS programme. Respondents were encouraged to complete the survey based on a team discussion led by the head of the AMS programme or infection control team. Only one completed survey was accepted from each hospital. Responses were collected from 10 April 2020 to 9 April 2021.
Questionnaire design
A questionnaire (Table S1, available as Supplementary data at JAC online) was developed by the study steering committee [expert infectious disease (ID) clinicians and researchers from Asia]. The questionnaire was designed primarily to determine which core components of AMS programmes are not yet in place and need to be addressed. The survey was available in English and, at the request of the Japanese investigator, translated into Japanese.
Elements of hospital AMS programmes relating to hospital leadership support, AMS programme team membership and training, AMS programme interventions, AMS monitoring and reporting, and hospital infrastructure were subdivided into yes/no questions about 12 core components (tagged ‘C’) considered essential to characterize an AMS programme, and 27 supplementary components (tagged ‘S’) considered to be additional or optional. These questions were modelled on a consensus statement on AMS programmes for Asian acute-care hospitals.19 They are similar to the Transatlantic Taskforce on Antimicrobial Resistance set of core and supplementary indicators for hospital AMS programmes (developed by a multidisciplinary expert panel through a modified Delphi process and consensus meeting),21 and the US CDC checklist for core elements of hospital AMS programmes.20 Question C12 (pertaining to accessibility of microbiology services) was deemed inappropriate for Japan, where reliable microbiology services are generally easily accessible, and was therefore not included in the Japanese survey. The steering committee members also developed an additional set of questions on challenges faced when implementing AMS programmes for inclusion in the survey. Possible responses were ‘very much a challenge’, ‘sometimes a challenge’, ‘not an issue’ or ‘not sure’. Collected data also included general hospital characteristics and the background of the personnel filling in the survey.
Statistical analysis
Descriptive statistics were reported. Positive response rates (PRRs) were calculated based on the total number of ‘yes’ or ‘no’ responses to questions. Blank answer fields or ‘not applicable’ responses were excluded. Mean PRRs for core AMS elements were calculated for the 10 countries. Overall and individual country PRRs were also calculated for all core and supplementary AMS components.
Proportions of hospitals facing challenges were calculated based on the total number of responses. Blank answer fields were excluded.
Ethics
Apart from Malaysia, ethics committee approval was not required because the study was non-interventional and did not collect patient data. In Malaysia, the study required approval by the Medical Research and Ethics Committee (approved 11 November 2020; reference no. NMRR-20-1861-55498). As required by the National Medical Research Register, all Malaysian respondents provided informed consent before participating in the survey.
No financial incentives were provided for participation.
Results
Surveys were circulated to 561 hospitals within the 10 targeted countries, and responses were received from a total of 349 hospitals (62.2% response rate; Table S2). Overall, 206 (59.0%) of the responding hospitals provided tertiary-level care and 157 (45.0%) were private hospitals (Table 1). Indonesia had the highest proportion of private hospital respondents (41/42, 97.6%), followed by India (56/95, 58.9%). In contrast, Malaysian respondents were predominantly from public hospitals (62/66, 93.9%), and Vietnamese respondents were exclusively from public hospitals (4/4). There were 200 hospitals (57.3%) with ≥1 ID specialist, including ≥69.0% of hospitals from Cambodia, Indonesia, Japan, the Philippines, Taiwan, Thailand and Vietnam. ID specialists were available in 35.8% to 41.9% of hospitals in India, Malaysia and Pakistan. Most hospitals had microbiology laboratories (90.8%) and infection control processes (93.7%) in place.
Table 1.
Characteristics of responding hospitals in each surveyed country
| Hospital characteristic, n (%) | Cambodia (n = 2) | India (n = 95) | Indonesia (n = 42) | Japan (n = 16) | Malaysia (n = 66) | Pakistan (n = 31) | Philippines (n = 4) | Taiwan (n = 57) | Thailand (n = 32) | Vietnam (n = 4) | Overall (n = 349) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level of care | |||||||||||
| Secondary | 1 (50.0) | 24 (25.3) | 29 (69.0) | 3 (18.8) | 42 (63.6) | 8 (25.8) | 0 | 24 (42.1) | 10 (31.3) | 2 (50.0) | 141 (41.0) |
| Tertiary | 1 (50.0) | 71 (74.7) | 13 (31.0) | 13 (81.3) | 24 (36.4) | 23 (74.2) | 4 (100.0) | 33 (57.9) | 22 (68.8) | 2 (50.0) | 206 (59.0) |
| Type of hospitala | |||||||||||
| Private | 1 (50.0) | 56 (58.9) | 41 (97.6) | 2 (12.5)b | 1 (1.5) | 13 (41.9) | 2 (50.0) | 31 (54.4) | 9 (28.1) | 1 (25.0) | 157 (45.0)b |
| Public | 1 (50.0) | 24 (25.3) | 0 | 3 (18.8) | 62 (93.9) | 16 (51.6) | 2 (50.0) | 9 (15.9) | 14 (42.8) | 4 (100.0) | 135 (38.7) |
| Military | 0 | 2 (2.1) | 0 | 0 | 0 | 1 (3.2) | 0 | 5 (8.8) | 1 (3.1) | 0 | 9 (2.6) |
| University-affiliated | 0 | 13 (13.7) | 3 (7.1) | 11 (68.8) | 3 (4.5) | 4 (12.9) | 0 | 12 (21.1) | 11 (34.4) | 0 | 57 (16.3) |
| Medical school-affiliated | 2 (100.0) | 53 (55.8) | 9 (21.4) | 5 (31.3) | 25 (37.9) | 16 (51.6) | 3 (75.0) | 29 (50.9) | 19 (59.4) | 3 (75.0) | 164 (47.0) |
| Hospital resources | |||||||||||
| ID specialist | 2 (100.0) | 34 (35.8) | 29 (69.0) | 14 (87.5) | 25 (37.9) | 13 (41.9) | 4 (100.0) | 50 (87.7) | 25 (78.1) | 4 (100.0) | 200 (57.3) |
| Epidemiologist | 1 (50.0) | 30 (31.6) | 9 (21.4) | 1 (6.3) | 4 (6.1) | 7 (22.6) | 2 (50.0) | 24 (42.1) | 13 (40.6) | 3 (75.0) | 94 (26.9) |
| Infection control | 2 (100.0) | 87 (91.6) | 37 (88.1) | 14 (87.5) | 65 (98.5) | 29 (93.5) | 4 (100.0) | 54 (94.7) | 31 (96.9) | 4 (100.0) | 327 (93.7) |
| Microbiology lab | 2 (100.0) | 93 (97.9) | 32 (76.2) | 15 (93.8) | 63 (95.5) | 31 (100.0) | 4 (100.0) | 45 (78.9) | 28 (87.5) | 4 (100.0) | 317 (90.8) |
May not total 100% due to inclusion of some hospitals in >1 category.
Includes one semipublic hospital.
Gaps in AMS programme core components
Overall, 47 of 349 hospitals (13.5%) fulfilled all 12 core AMS programme components (Table 2). Taiwan had the most hospitals fulfilling all core AMS components (17/57, 29.8%), followed by Malaysia (9/66, 13.6%).
Table 2.
Positive responses to AMS programme core component questions from responding hospitals in each surveyed country
| Core AMS programme component, n (%)a | Cambodia (n = 2) | India (n = 95) |
Indonesia (n = 42) | Japan (n = 16) |
Malaysia (n = 66) | Pakistan (n = 31) | Philippines (n = 4) | Taiwan (n = 57) | Thailand (n = 32) | Vietnam (n = 4) | Overall (n = 349) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fulfilled all components | 0 | 6/95 (6.3) | 6/42 (14.3) | N/Ab | 9/66 (13.6) | 3/31 (9.7) | 2/4 (50.0) | 17/57 (29.8) | 4/32 (12.5) | 0 | 47/349 (13.5) |
| Hospital leadership support | |||||||||||
| Formal statement of support | 2/2 (100) | 65/93 (69.9) | 38/42 (90.5) | 14/16 (87.5) | 62/66 (93.9) | 27/31 (87.1) | 4/4 (100.0) | 52/56 (92.9) | 19/32 (59.4) | 3/4 (75.0) | 286/346 (82.7) |
| Budgeted financial support | 1/2 (50.0) | 48/92 (52.2) | 26/42 (61.9) | 6/16 (37.5) | 30/66 (45.5) | 15/31 (48.4) | 3/4 (75.0) | 38/55 (69.1) | 10/32 (31.3) | 4/4 (100.0) | 181/344 (52.6) |
| AMS team | |||||||||||
| Physician (or other) leader | 2/2 (100.0) | 53/95 (55.8) | 36/42 (85.7) | 16/16 (100.0) | 64/66 (97.0) | 19/31 (61.3) | 4/4 (100.0) | 49/57 (86.0) | 26/32 (81.3) | 3/4 (75.0) | 272/349 (77.9) |
| Pharmacist | 2/2 (100.0) | 40/95 (42.1) | 38/42 (90.5) | 16/16 (100.0) | 66/66 (100.0) | 19/31 (61.3) | 4/4 (100.0) | 49/56 (87.5) | 22/32 (68.8) | 2/4 (50.0) | 258/348 (74.1) |
| IC staff | 1/2 (50.0) | 72/92 (78.3) | 33/40 (82.5) | 13/16 (81.3) | 63/66 (95.5) | 23/29 (79.3) | 3/3 (100.0) | 47/57 (82.5) | 29/32 (90.6) | 3/4 (75.0) | 287/341 (84.2) |
| Microbiology staff | 2/2 (100.0) | 86/93 (92.5) | 29/39 (74.4) | 13/16 (81.3) | 64/66 (97.0) | 24/31 (77.4) | 3/3 (100.0) | 42/57 (73.7) | 25/32 (78.1) | 4/4 (100.0) | 292/343 (85.1) |
| AMS Interventions | |||||||||||
| Pre-authorization and/or PAF | 0 | 49/93 (52.7) | 18/39 (46.2) | 14/16 (87.5) | 63/66 (95.5) | 22/31 (71.0) | 3/3 (100.0) | 49/54 (90.7) | 23/32 (71.9) | 2/4 (50.0) | 243/340 (71.5) |
| Facility-specific treatment guidelines | 2/2 (100.0) | 69/93 (74.2) | 25/39 (64.1) | 12/16 (75.0) | 38/66 (57.6) | 24/31 (77.4) | 3/3 (100.0) | 46/54 (85.2) | 17/32 (53.1) | 4/4 (100.0) | 240/340 (70.6) |
| AMS monitoring and reporting | |||||||||||
| Monitor specific antibiotic use by DOT or DDD | 2/2 (100.0) | 37/88 (42.0) | 33/38 (86.8) | 13/16 (81.3) | 65/66 (98.5) | 19/31 (61.3) | 2/3 (66.7) | 52/53 (98.1) | 20/30 (66.7) | 2/4 (50.0) | 245/331 (74.0) |
| Regularly publish AMR data and AMS outcome measures | 1/2 (50.0) | 42/88 (47.7) | 22/38 (57.9) | 15/16 (93.8) | 41/66 (62.1) | 11/31 (35.3) | 3/3 (100.0) | 46/53 (86.8) | 18/30 (60.0) | 2/4 (50.0) | 201/331 (60.7) |
| Hospital antibiogram | 2/2 (100.0) | 69/88 (78.4) | 24/38 (63.2) | 14/16 (87.5) | 49/66 (74.2) | 15/31 (48.4) | 3/3 (100.0) | 51/54 (94.4) | 25/30 (83.3) | 2/4 (50.0) | 254/332 (76.5) |
| Hospital infrastructure | |||||||||||
| Timely/reliable microbiology service | 2/2 (100.0) | 83/87 (95.4) | 27/38 (71.1) | NAb | 57/66 (86.4) | 28/31 (90.3) | 3/3 (100.0) | 49/54 (90.7) | 24/30 (80.0) | 4/4 (100.0) | 277/315 (87.9) |
DDD, defined daily dose; DOT, days of therapy; IC, infection control; NA, not applicable; PAF, prospective audit and feedback.
Not all hospitals responded to all questions; PRRs were based on completed responses to the relevant question (i.e. excluded any hospitals that did not respond to the question).
Question C12 was not included in the Japanese survey.
Hospital leadership support for AMS
Despite a mean PRR of 85.6% for the 10 responding countries in relation to a formal statement of support for AMS activities from hospital leadership, the mean PRR for budgeted financial support for AMS activities was 57.1% (Figure 1). Except for Vietnam, discrepancies between proportions of hospitals with a formal statement of support for AMS versus budgeted financial support were observed in all countries (Table 2). In Cambodia, Japan, Malaysia and Pakistan, for example, there was a formal statement of support for AMS in ≥87.1% of hospitals, but ≤50.0% of hospitals had allocated budgeted financial support for AMS activities.
Figure 1.
Core AMS components and corresponding mean PRR from the 10 surveyed countries. *Specified antibiotics need to be approved by a physician or pharmacist before dispensing or ≤48 hours after dispensing. **A physician or pharmacist reviews courses of therapy and provides suggestions for use of specified antibiotics ≤48 hours after prescription. DDD, defined daily dose; DOT, days of therapy; IT, information technology.
AMS team and ID training
Mean PRRs for the 10 responding countries were ≥80.0% in terms of the core AMS team comprising at least a physician or other leader responsible for AMS activities, a pharmacist and infection control and microbiology personnel (Figure 1). Countries with a physician or other leader responsible for AMS activities in >80.0% of responding hospitals were Cambodia, Indonesia, Japan, Malaysia, the Philippines, Taiwan and Thailand, with relatively low PRRs occurring in India (53/95, 55.8%) and Pakistan (19/31, 61.3%) (Table 2). Except for India, Pakistan, Thailand and Vietnam, ≥ 87.5% of responding hospitals in other countries had pharmacists working on AMS activities. Other staff working on AMS teams to improve antibiotic use included infection control staff in ≥75.0% of hospitals from each country except Cambodia (50.0%), and microbiology staff in ≥73.7% of hospitals from each country (Table 2).
AMS programme interventions
The mean PRR for the 10 responding countries was 66.5% for implementation of pre-authorization and/or prospective audit and feedback systems (Figure 1). These systems were being used in ≥87.5% of hospitals in Japan, Malaysia, the Philippines and Taiwan (Table 2). The countries with the lowest proportions of responding hospitals using pre-authorization and/or prospective audit and feedback systems were Cambodia (0/2, 0%), Indonesia (18/39, 46.2%), Vietnam (2/4, 50.0%) and India (49/93, 52.7%).
Facility-specific antibiotic treatment guidelines were available in 53.1% (Thailand) to 100% (Cambodia, the Philippines, Vietnam) of responding hospitals (Table 2). The mean PPR for this core AMS component was 78.7% (Figure 1).
AMS monitoring and reporting
Monitoring use of specific antibiotics by days of therapy or defined daily dose occurred in ≥81.3% of responding hospitals from Cambodia, Indonesia, Japan, Malaysia and Taiwan (Table 2), but the lower PRR from other countries led to a mean PRR of 75.1% (Figure 1). Indian hospitals had the lowest PRR in relation to such antibiotic monitoring (37/88, 42.0%).
The mean PRR for the 10 countries was 64.4% for regular publishing of AMR data and AMS outcome measures (Figure 1). As shown in Table 2, this occurred in 93.8% (15/16) of Japanese hospitals and 86.8% (46/53) of Taiwanese hospitals, as well as all responding hospitals in the Philippines. AMS data and outcome measures were published regularly in ≤50% of responding hospitals from Cambodia, India, Pakistan and Vietnam.
Hospital antibiograms were available in ≥83.3% of responding hospitals from several countries, including Japan, Thailand and Taiwan. Pakistan and Vietnam were the only countries in which antibiograms were available in ≤50.0% of responding hospitals.
Microbiology services
The mean PRR for the 10 responding countries was 90.4% for access to a timely and reliable microbiology service (Figure 1). Except for Indonesia (71.1% PRR), ≥ 80.0% of hospitals from all countries had an in-house microbiology laboratory or access to a timely and reliable microbiology service (Table 2).
Gaps in AMS programme supplementary components
Responses to supplementary component questions (Table S3) showed that 68.0% of AMS leaders and 54.3% of pharmacists working on AMS activities had specialized ID training. Guidelines for de-escalation of broad-spectrum antibiotics and intravenous-to-oral conversion of antibiotics were available in 49.4% and 54.7% of hospitals, respectively. Among hospitals with antibiograms, 92.1% had them regularly updated. Rapid diagnostic testing and selective susceptibility reporting were used in 72.2% and 85.7% of hospitals with access to reliable microbiology services, respectively. Many hospitals did not have IT systems to support the AMS programme: 48.8% had the IT capability to gather and analyse AMS data, 64.7% used electronic health records and 56.0% used computerized physician order entry. Educational activities on improving antibiotic prescribing were provided for clinicians and other relevant staff in 77.0% of hospitals overall. Such educational activities were provided in ≥86.4% of hospitals from Japan, Malaysia and Taiwan. Overall, only 45.7% of hospitals that provided such educational activities made them mandatory and certified, most commonly in Japan (76.9%) and Taiwan (84.6%).
AMS programme challenges
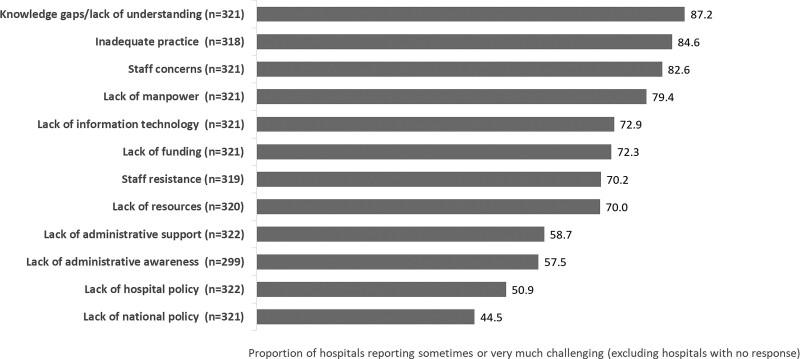
Some of the most commonly faced challenges when implementing AMS programmes were related to knowledge gaps and lack of understanding, and inadequate practice, which were considered sometimes or very much challenging by 87.2% and 84.6% of responding hospitals, respectively (Figure 2). Further to this, staff concerns that AMS strategies, such as antibiotic restriction, may lead to poor patient outcomes were considered sometimes or very much challenging by 82.6% of responding hospitals. Staff resistance to AMS programmes was a challenge for >60% of responding hospitals from all countries except Japan and the Philippines (Figure S1).
Figure 2.
Challenges faced when implementing hospital AMS programmes.
As shown in Figure 2, lack of funding and resources, including manpower and IT capabilities, were also common barriers to implementing AMS programmes in Asian hospitals. Challenges related to lack of resources and manpower were considered sometimes or very much challenging by >60% of responding hospitals in each of the surveyed countries (Figure S2).
Overall, almost 60% of hospitals indicated that there was a lack of administrative AMS awareness, and that lack of administrative support for AMS posed a challenge for the implementation of hospital AMS programmes (Figure 2).
Discussion
The results of our survey have shown that only 13.5% of 349 secondary- and tertiary-care hospitals surveyed across 10 Asian countries with wide-ranging economic development levels fulfilled all 12 AMS programme core components.
In a similar survey of tertiary-care hospitals in central Thailand that assessed the same AMS programme core components included in the current study, 60.0% of 45 surveyed hospitals fulfilled all 12 core components.25 In our survey, a much lower proportion of Thai hospitals (12.5%) had implemented all core AMS programme components. It is possible that, on average, the tertiary-care hospitals in the central Thailand survey had more experience with AMS programmes than the hospitals recruited from throughout Thailand in our survey, 31.3% of which were secondary-care hospitals as opposed to the more advanced tertiary level of care. Only 59.4% of the 32 Thai hospitals participating in our survey had a formal statement of AMS support from hospital leadership; such a statement was available for all hospitals in the central Thailand survey.25
Ultimately, resource constraints pose a barrier to the implementation of comprehensive AMS programmes in many Asian hospitals. Although a high proportion of hospitals participating in our survey had formal hospital leadership statements to support AMS, this was not reflected in the level of financial support for AMS activities. This was the case even in Japan (a high-income country), where there was no budgeted financial AMS support in 62.5% of 16 responding hospitals. Unsurprisingly, therefore, lack of funding and resources, including adequate manpower, IT and funding, were commonly identified as posing challenges for AMS programmes. To help overcome funding gaps, hospital administrators need to be provided with a credible business case to persuade them that funding an AMS programme is beneficial to the hospital.19,25 It is recommended that programmes start small and build capacity over time, gradually introducing AMS interventions by hospital unit or ward.19
Once formal approval and support have been obtained from hospital administration, an AMS team, ideally including an ID specialist and a clinical pharmacist (with ID training, if possible), infection control personnel and microbiology personnel, should be recruited.19 In resource-limited settings, the minimum personnel for an effective AMS team comprises an interested clinician, a pharmacist and a collaborating microbiologist.10,11,19 Overall, responding hospitals generally performed well in terms of core AMS team members, but some countries fell short in relation to ID specialists. India, Malaysia and Pakistan were the only countries in which <50% of responding hospitals employed ID specialists. A shortage of hospital ID specialists was also identified in an Asia–Pacific survey by Lee and colleagues.3 This survey, conducted in 2014, included a number of countries from our survey but, unlike our study, Pakistan (n = 0), India (n = 1) and Indonesia (n = 2) were not well represented.3 As seen in our survey, particularly in India and Pakistan—where only 55.8% and 61.3% of hospitals, respectively, had a physician responsible for AMS, and 42.1% and 61.3% of hospitals, respectively, had a pharmacist working on AMS—a number of hospitals may struggle to find the personnel to build a highly effective AMS team, particularly in low- and middle-income countries.4,19 Training more ID physicians and pharmacists would bolster the ability of hospitals to implement effective AMS programmes,24 but, if this is not feasible, hospitals should work within their available resources to create the most effective AMS team possible.19 A positive step in India was the recent National Medical Commission advisory for the establishment of AMS programme committees in all medical colleges and attached hospitals.
Although most hospitals in the current survey had access to a timely and reliable microbiology service, up to approximately 20% of these hospitals relied solely on conventional techniques for pathogen identification and susceptibility testing, and approximately 15% did not use selective susceptibility reporting. True diagnostic stewardship prioritizes diagnostics-guided therapy and use of optimal testing methods.37 Although it is important to strive towards strengthening laboratory capacity to deliver technologically advanced testing, optimal testing methods are unavailable in many settings.37 Cost is therefore likely to remain prohibitive for true diagnostic stewardship in many Asian hospitals, particularly in low- and middle-income countries.19,38 For example, selective antimicrobial susceptibility reporting would also be beneficial but it requires the specialized expertise of a clinical microbiologist and could be difficult to implement in many Asian hospitals.19
All hospital AMS programmes should include some form of prospective audit and/or formulary restriction to curb prescribing behaviours that promote AMR.19 The relatively low mean PRR of 66.5% for the responding countries in our survey suggests a wide gap in relation to these core interventions. This was of particular concern for hospitals in Cambodia, Indonesia, Vietnam and India. Results from the survey by Lee et al. also indicate that such interventions were not always implemented in Asia–Pacific hospitals.3 Formulary restriction and pre-authorization is likely to be less labour-intensive than prospective audit and feedback, and can be implemented on a small scale by evaluating antibiotic usage patterns and resistance trends.19 Interventions targeted at a single antibiotic agent or class thought to be misused may be more practical rather than wide-ranging formulary restriction in many resource-limited Asian hospitals.19,39–41 However, prospective audit and feedback is inherently better suited to the prescribing culture in Asian countries, where physicians tend to practice social bedside medicine and like to be personally involved in patient care.19
Facility-specific guidelines for infections commonly treated in hospitals can be adapted from pre-existing national or international guidelines, and compared with other core AMS interventions, require a relatively small amount of manpower and funding to develop.3,19 Encouragingly, facility-specific guidelines for antibiotic therapy of common infections were generally in place, with a mean PRR of 78.7% for our surveyed countries. Similarly, three quarters of respondents in the Asia–Pacific survey by Lee et al. reported having such guidelines in their institutions.3
In relation to the core components for AMS monitoring and reporting, publication of AMR data and AMS outcome measures was the widest gap in our survey. Regular reporting of AMS performance to prescribers and other stakeholders may encourage administrative support for AMS programmes, reduce prescriber resistance to AMS and assuage concerns that AMS strategies could lead to poor patient outcomes,19,24,42 all of which were common challenges for AMS programmes in our surveyed hospitals. Monitoring the use of specific antimicrobial agents and provision of hospital antibiograms are integral for effective AMS monitoring and reporting. India had the lowest PRR in relation to monitoring specific antibiotic use, followed by Vietnam and Pakistan. In line with this observation, tracking and reporting of antibiotic use and outcomes have previously been identified as particularly neglected core components of AMS programmes in Pakistani hospitals, and should therefore be prioritized.29,32 In our survey, the absence of hospital antibiograms was also of particular concern in Pakistan, as antibiograms were available in <50% of responding hospitals. Antibiograms are important tools for guiding empiric antibiotic therapy,43,44 and should therefore also be highlighted as a priority area for Pakistani and other Asian hospitals.
One of the limitations of our study is the very small number of respondents from certain countries: Cambodia (n = 2), the Philippines (n = 4) and Vietnam (n = 4). Responses from hospitals in these countries may not be representative of each country overall. Whereas the survey response rate for hospitals in the Philippines was low (33%), the small numbers of hospitals participating in the survey from Cambodia and Vietnam were a consequence of limited survey distribution networks in these countries rather than poor response rates (100% in both countries). In many countries, distribution of the survey during the COVID-19 pandemic may have contributed to low survey response rates, which could potentially have been improved with a less comprehensive, user-friendly survey (i.e. exclusion of supplementary component questions) translated into the languages of each participating country for ease of comprehension.
As well as being potentially susceptible to nonresponse bias, particularly in countries with response rates <60% (India, Indonesia, Philippines, Taiwan), our results may have been influenced by reliance on data reported by staff involved in the hospital AMS programmes. No pilot testing was performed to validate the survey questions.
In addition to differences in AMS programmes between countries, there are also large differences between facilities within countries.45 Another potential limitation of our study pertains to potential over-representation of relatively well-resourced tertiary hospitals, which may have been AMS centres of excellence within their countries. This may have contributed to some unexpectedly high rates of implementation of core AMS programme components relative to country income status. Furthermore, over half of the responding hospitals from Cambodia, India, Pakistan, the Philippines, Taiwan, Thailand and Vietnam were medical school-affiliated, which was found to be an independent factor associated with fulfilment of all core hospital AMS programme components in the previous survey of AMS programmes in central Thailand.25 However, although similar proportions of Thai hospitals in the central Thailand survey and our survey were medical school-affiliated (53.3% and 59.4%, respectively), a relatively low proportion of Thai hospitals had implemented all core AMS programme components in our survey (12.5% versus 60.0%), suggesting that other factors influenced the fulfilment of all core AMS programme components.
Another limitation of our study relates to the absence of questions and data regarding the outcomes of interventions, such as rates of multidrug-resistant bacterial infection and antimicrobial consumption and expenditure. Future studies focusing on outcome data are needed to help to determine which AMS programme core elements contribute to the success of AMS programmes in Asian hospitals.
Conclusion and recommendation
The results of our survey show that gaps in core components of hospital AMS programmes in a range of Asian countries relate to lack of funding, failure to implement necessary AMS interventions (prospective audit and/or formulary restriction) and failure to monitor and report AMS outcomes. Country- and hospital-specific solutions to funding and resourcing shortfalls are urgently needed to improve AMS programmes in Asian countries.
Supplementary Material
Acknowledgements
We would like to thank the Infection Control Society of Taiwan; the Malaysian Society of Infectious Diseases and Chemotherapy; the Japanese Society of Chemotherapy; the Philippine Hospital Infection Control Society; Dr Noor Amelia Abd Rasid of the Ministry of Health, Malaysia; and Dr Norazah Ahmad of the Institute for Medical Research, Malaysia, for their contribution to data collection; and the Director General of Health, Malaysia for granting permission to publish this article. We would also like to thank all hospital personnel who participated in this survey.
The authors acknowledge Weber Shandwick Hong Kong for their support in data collection and analysis, and for preparing the manuscript, which was funded by an unrestricted educational grant from Pfizer, Inc.
A summary of our findings has been presented at IDWeek 2021 and published in Open Forum Infect Dis 2021; 8: S57–8 as part of the IDWeek abstracts.
Contributor Information
Feng-Yee Chang, Department of Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei City, Taiwan.
Yin Ching Chuang, Medical Research Department, Chi Mei Medical Center, Tainan City, Taiwan.
Balaji Veeraraghavan, Department of Clinical Microbiology, Christian Medical College and Hospital, Vellore, India.
Anucha Apisarnthanarak, Department of Medicine, Thammasat University Hospital, Pathum Thani, Thailand.
Maria Fe Tayzon, Department of Medicine, Section of Infectious Diseases, Hospital Infection Control and Epidemiology Center, The Medical City, Pasig City, Philippines.
Andrea L Kwa, Department of Pharmacy, Singapore General Hospital, Singapore, Singapore; Emerging Infectious Diseases Programme, Duke-NUS Medical School, Singapore, Singapore.
Cheng-Hsun Chiu, Department of Pediatrics, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Zakuan Zainy Deris, Department of Medical Microbiology and Parasitology, School of Medical Sciences/Hospital Universiti Sains Malaysia, USM Health Campus, Kubang Kerian, Kelantan, Malaysia.
Suraya Amir Husin, Medical Development Division, Ministry of Health, Putrajaya, Malaysia.
Hazimah Hashim, Pharmacy Practice and Development Division, Ministry of Health, Petaling Jaya, Malaysia.
Anis Karuniawati, Department of Microbiology, Medical Faculty, Universitas Indonesia, Jakarta, Indonesia.
Altaf Ahmed, Department of Pathology/Microbiology, Pakistan Kidney and Liver Institute, Lahore, Pakistan.
Tetsuya Matsumoto, Department of Infectious Diseases, International University of Health and Welfare, Chiba-ken, Japan.
Van Kinh Nguyen, Infectious Diseases Department, Hanoi Medical University, Hanoi, Vietnam.
Thi Thu Huong Dinh, Emergency Department - Infection Control, National Hospital for Tropical Diseases, Hanoi, Vietnam.
Funding
This study was supported by an unrestricted educational grant from Pfizer, Inc. to the Infection Control Society of Taiwan (grant no. 57186265). The sponsor had no role in the preparation, review or approval of the manuscript or in the decision to submit for publication.
Transparency declarations
The authors declare the following financial interests/personal relationships that may be considered as potential competing interests. T.M. and Z.Z.D. have received research grants and/or speaking fees from Merck Sharp & Dohme and Pfizer. All other authors: none to declare.
Supplementary data
Figures S1 and S2 and Tables S1 to S3 are available as Supplementary data at JAC online.
References
- 1. O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. https://amr-review.org/sites/default/files/160518_Finalpaper_with cover.pdf.
- 2. Kakkar AK, Shafiq N, Singh Get al. Antimicrobial stewardship programs in resource constrained environments: understanding and addressing the need of the systems. Front Public Health 2020; 8: 140. 10.3389/fpubh.2020.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee TH, Lye DC, Chung DRet al. Antimicrobial stewardship capacity and manpower needs in the Asia Pacific. Glob Antimicrob Resist 2021; 24: 387–94. 10.1016/j.jgar.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 4. Pierce J, Apisarnthanarak A, Schellack Net al. Global antimicrobial stewardship with a focus on low- and middle-income countries: a position statement for the International Society for Infectious Diseases. Int J Infect Dis 2020; 96: 621–9. 10.1016/j.ijid.2020.05.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu L-Y, Apisarnthanarak A, Khan Eet al. Carbapenem-resistant Acinetobacter baumannii and Enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev 2017; 30: 1–22. 10.1128/CMR.00042-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang C-I, Song J-H. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infect Chemother 2013; 45: 22–31. 10.3947/ic.2013.45.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai C-C, Lee K, Xiao Yet al. High burden of antimicrobial drug resistance in Asia. J Glob Antimicrob Resist 2014; 2: 141–7. 10.1016/j.jgar.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 8. Saleem AF, Pethani A. Antimicrobial stewardship—do we need it in Pakistan? J Pak Med Assoc 2020; 70: 2449–53. [DOI] [PubMed] [Google Scholar]
- 9. Suwantarat N, Carroll KC. Epidemiology and molecular characterization of multidrug-resistant gram-negative bacteria in Southeast Asia. Antimicrob Resist Infect Control 2016; 5: 15. 10.1186/s13756-016-0115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barlam TF, Cosgrove SE, Abbo LMet al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dellit TH, Owens RC, McGowan JEet al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. 10.1086/510393 [DOI] [PubMed] [Google Scholar]
- 12. Baur D, Gladstone BP, Burkert Fet al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and metaanalysis. Lancet Infect Dis 2017; 17: 990–1001. 10.1016/S1473-3099(17)30325-0 [DOI] [PubMed] [Google Scholar]
- 13. Davey P, Brown E, Charani E. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017; 2: CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honda H, Ohmagari N, Tokuda Yet al. Antimicrobial stewardship in inpatient settings in the Asia Pacific region: a systematic review and meta-analysis. Clin Infect Dis 2017; 64(Suppl 2): S119–26. 10.1093/cid/cix017 [DOI] [PubMed] [Google Scholar]
- 15. Karanika S, Paudel S, Grigoras Cet al. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother 2016; 60: 4840–52. 10.1128/AAC.00825-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nathwani D, Varghese D, Stephens Jet al. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control 2019; 8: 35. 10.1186/s13756-019-0471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuts EC, Hulscher MEJL, Mouton JWet al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–56. 10.1016/S1473-3099(16)00065-7 [DOI] [PubMed] [Google Scholar]
- 18. Van Dijck C, Vlieghe E, Cox JA. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ 2018; 96: 266–80. 10.2471/BLT.17.203448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Apisarnthanarak A, Kwa AL, Chiu CHet al. Antimicrobial stewardship for acute-care hospitals: an Asian perspective. Infect Control Hosp Epidemiol 2018; 39: 1237–45. 10.1017/ice.2018.188 [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . Core elements of hospital antibiotic stewardship programs. US Department of Health and Human Services, CDC; 2019. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf
- 21. Pollack LA, Plachouras D, Sinkowitz-Cochran Ret al. A concise set of structure and process indicators to assess and compare antimicrobial stewardship programs among EU and US hospitals: results from a multinational expert panel. Infect Control Hosp Epidemiol 2016; 37: 1201–11. 10.1017/ice.2016.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howard P, Pulcini C, Levy Hara Get al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother 2015; 70: 1245–55. 10.1093/jac/dku497 [DOI] [PubMed] [Google Scholar]
- 23. Mathew P, Ranjalkar J, Chandy SJ. Challenges in implementing antimicrobial stewardship programmes at secondary level hospitals in India: an exploratory study. Front Public Health 2020; 8: 493904. 10.3389/fpubh.2020.493904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel PK. Minding the gap: rethinking implementation of antimicrobial stewardship in India. Infect Control Hosp Epidemiol 2019; 40: 520–1. 10.1017/ice.2019.62 [DOI] [PubMed] [Google Scholar]
- 25. Apisarnthanarak A, Jantarathaneewat J, Wever DJ. Gap analysis on antimicrobial stewardship program in central Thailand. Infect Control Hosp Epidemiol 2019; 40: 1077–9. 10.1017/ice.2019.185 [DOI] [PubMed] [Google Scholar]
- 26. Herawati F, Jaelani AK, Wijono Het al. Antibiotic stewardship knowledge and belief differences among healthcare professionals in hospitals: A survey study. Heliyon 2021; 7: e07377. 10.1016/j.heliyon.2021.e07377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maeda M, Muraki Y, Kosaka Tet al. The first nationwide survey of antimicrobial stewardship programs conducted by the Japanese Society of Chemotherapy. J Infect Chemother 2019; 25: 83–8. 10.1016/j.jiac.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 28. Moriyama Y, Ishikane M, Kusama Yet al. Nationwide cross-sectional study of antimicrobial stewardship and antifungal stewardship programs in inpatient settings in Japan. BMC infect Dis 2021; 21: 355. 10.1186/s12879-021-06035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mubarak N, Khan AS, Zahid Tet al. Assessment of adherence to the core elements of hospital antibiotic stewardship programs: a survey of the tertiary care hospitals in Punjab. Pakistan. Antibiotics (Basel) 2021; 10: 906. 10.3390/antibiotics10080906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mushtaque M, Khalid F, Ishaqui AAet al. Hospital antibiotic stewardship programs—qualitative analysis of numerous hospitals in a developing country. Infect Prev Pract 2019; 1: 100025. 10.1016/j.infpip.2019.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen YTH, Truong DV, Huynh TPet al. Implementation status of antimicrobial stewardship programs in hospitals: a quantitative analysis study in Ho Chi Minh city, Vietnam. MedPharmRes 2020; 4: 34–9. 10.32895/UMP.MPR.4.2.5 [DOI] [Google Scholar]
- 32. Raheem M, Anwaar S, Aziz Zet al. Adherence to the core elements of outpatient antibiotic stewardship: a cross-sectional survey in the tertiary care hospitals of Punjab, Pakistan. Infect Drug Resist 2020; 13: 3833–41. 10.2147/IDR.S268574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saleh MS, Hong YH, Muda MRet al. Perception and practices of public hospital pharmacists towards the antimicrobial stewardship programme in the State of Selangor, Malaysia. Eur J Hosp Pharm 2020; 27: 173–7. 10.1136/ejhpharm-2018-001679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saleem Z, Hassali MA, Hashmi FKet al. Snapshot of antimicrobial stewardship programs in the hospitals of Pakistan: findings and implications. Heliyon 2019; 5: e02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah A, Vijayvargiya P, O’Horo JCet al. India Antimicrobial stewardship and resistance (INTEREST) 2018: a needs assessment survey. Infect Control Hosp Epidemiol 2021; 42: 616–8. 10.1017/ice.2020.1242 [DOI] [PubMed] [Google Scholar]
- 36. Shin J-H, Mizumo S, Okuno Tet al. Nationwide multicenter questionnaire surveys on countermeasures against antimicrobial resistance and infections in hospitals. BMC infect Dis 2021; 21: 234. 10.1186/s12879-021-05921-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel R, Fang FC. Diagnostic stewardship: opportunity for a laboratory–infectious diseases partnership. Clin Infect Dis 2018; 67: 799–801. 10.1093/cid/ciy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Apisarnthanarak A, Kim HB, Moore Let al. Rapid diagnostic testing for antimicrobial stewardship: utility in Asia Pacific. Infect Control Hosp Epidemiol 2021; 42: 864–8. 10.1017/ice.2021.149 [DOI] [PubMed] [Google Scholar]
- 39. Mitchell KF, Safdar N, Abad CL. Evaluating carbapenem restriction practices at a private hospital in Manila, Philippines as a strategy for antimicrobial stewardship. Arch Public Health 2019; 77: 31. 10.1186/s13690-019-0358-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nguyen-Thi HY, Nguyen D-A, Huynh P-Tet al. Impact of antimicrobial stewardship program on vancomycin usage: costs and outcomes at hospital for tropical diseases in Ho Chi Minh City, Vietnam. Risk Mang Health Policy 2021; 14: 2637–46. 10.2147/RMHP.S307744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teng CB, Ng TM, Tan MWet al. Safety and effectiveness of improving carbapenem use via prospective review and feedback in a multidisciplinary antimicrobial stewardship programme. Ann Acad Med Singap 2015; 44: 19–25. 10.47102/annals-acadmedsg.V44N1p19 [DOI] [PubMed] [Google Scholar]
- 42. Rupali P, Palanikmar P, Shathamurthy Pet al. Impact of an antimicrobial stewardship intervention in India: evaluation of post prescription review and feedback as a method of promoting optimal antimicrobial use in intensive care units of a tertiary care hospital. Infect Control Hosp Epidemiol 2019; 40: 512–9. 10.1017/ice.2019.29 [DOI] [PubMed] [Google Scholar]
- 43. Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2007; 27: 1306–12. 10.1592/phco.27.9.1306 [DOI] [PubMed] [Google Scholar]
- 44. Truong WR, Hidayat L, Bolaris MAet al. The antibiogram: key considerations for its development and utilization. JAC Antimicrob Resist 2021; 3: dlab060. 10.1093/jacamr/dlab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cox JA, Vlieghe E, Mendelson Met al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–8. 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.