Abstract
Nicotine exposure is associated with regional changes in brain nicotinic acetylcholine receptors subtype expression patterns as a function of dose and age at the time of exposure. Moreover, nicotine dependence is associated with changes in brain circuit functional connectivity, but the relationship between such connectivity and concomitant regional distribution changes in nicotinic acetylcholine receptor subtypes following nicotine exposure is not understood. Although smoking typically begins in adolescence, developmental changes in brain circuits and nicotinic acetylcholine receptors following chronic nicotine exposure remain minimally investigated. Here, we combined in vitro nicotinic acetylcholine receptor autoradiography with resting state functional magnetic resonance imaging to measure changes in [3H]nicotine binding and α4ß2 subtype nicotinic acetylcholine receptor binding and circuit connectivity across the brain in adolescent (postnatal Day 33) and adult (postnatal Day 68) rats exposed to 6 weeks of nicotine administration (0, 1.2 and 4.8 mg/kg/day). Chronic nicotine exposure increased nicotinic acetylcholine receptor levels and induced discrete, developmental stage changes in regional nicotinic acetylcholine receptor subtype distribution. These effects were most pronounced in striatal, thalamic and cortical regions when nicotine was administered during adolescence but not in adults. Using these regional receptor changes as seeds, resting state functional magnetic resonance imaging identified dysregulations in cortico-striatal-thalamic-cortical circuits that were also dysregulated following adolescent nicotine exposure. Thus, nicotine-induced increases in cortical, striatal and thalamic nicotinic acetylcholine receptors during adolescence modifies processing and brain circuits within cortico-striatal-thalamic-cortical loops, which are known to be crucial for multisensory integration, action selection and motor output, and may alter the developmental trajectory of the adolescent brain. This unique multimodal study significantly advances our understanding of nicotine dependence and its effects on the adolescent brain.
Keywords: nicotine, adolescence, fMRI, brain circuits, nAChR autoradiography
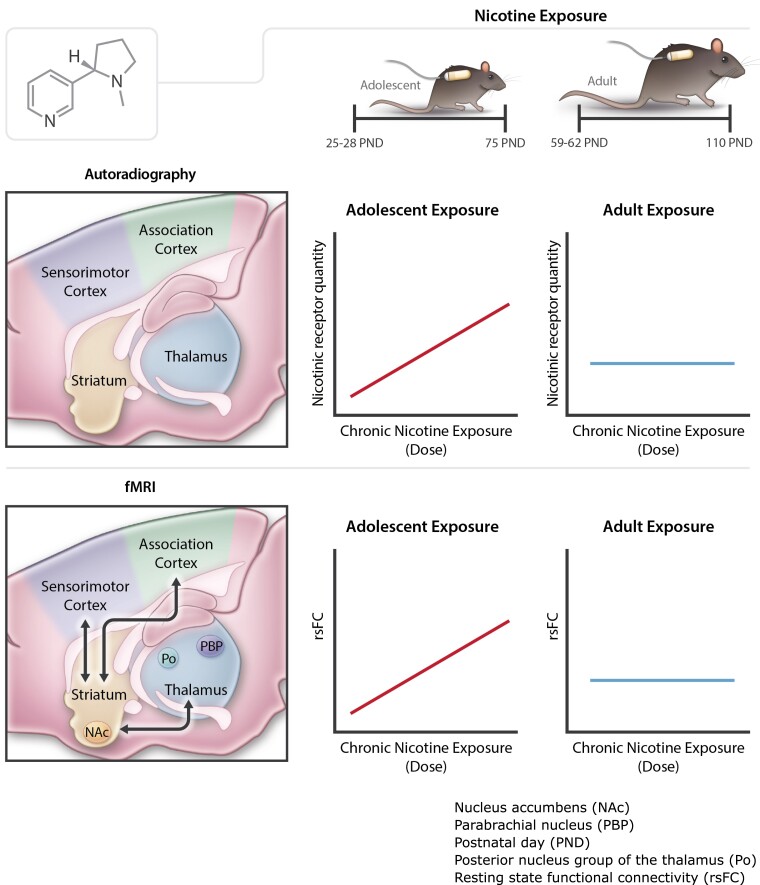
Keeley et al. present a multimodal study assessing changes in nicotinic receptor subtype expression and resting-state functional connectivity in adolescents and adults after chronic nicotine administration. Following exposure, differences in receptor density and resting-state functional connectivity were found in adolescents but not adults, particularly in the cortical, striatal and thalamic regions.
Graphical Abstract
Graphical abstract.
Introduction
Cigarette smoking and subsequent nicotine dependence is the leading cause of preventable death in the USA, with 90% of smokers starting to smoke during adolescence.1 With the recent swell of e-cigarette use among teenagers,2 and evidence that nicotine vaping increases the likelihood of subsequent cigarette smoking,3 understanding nicotine use and abuse in adolescence is critical to understanding, preventing and treating nicotine dependence and addiction.
Adolescence is an evolutionarily conserved developmental period4 characterized by anatomical and functional alterations of cortical, striatal, thalamic and other brain circuits,5,6 many of which overlap with those implicated in addiction.7 Changes in these circuits in adolescence may underlie increases in vulnerability to substance use disorders.8 This developmental vulnerability has been observed in both humans and in rodent laboratory models of nicotine exposure, where adolescents generally exhibit greater sensitivity to nicotine’s rewarding effects but less sensitivity to its aversive consequences, including the nicotine withdrawal syndrome.9–11
Nicotine binds to endogenous nicotinic acetylcholine receptors (nAChRs),4 pentameric ligand-gated ion channels12 whose unique channel subunit composition determines ligand affinity, ion selectivity and sensitivity. The major nAChR subtype in the mammalian brain is the α4β2, which are localized throughout the brain.13 They differ from other subtypes in terms of their sensitivity,14–17 and regulation following agonist exposure.18–27 Behaviourally, β2 subunits are critical for nicotine-induced reinforcement28,29 and nicotine withdrawal.30 However, a mechanistic understanding of how differences in nAChR regulation patterns following chronic nicotine exposure are involved in the developmental sensitivity to nicotine, and to nicotine dependence liability, remains limited due to the need to use radioactivity to measure receptors in humans using positron emission tomography (PET)14 or research design limitations inherent to preclinical models.31 Linking these regional changes in receptor density to circuit-level functional changes, and subsequently to their roles in mediating behaviours related to nicotine dependence may require a different experimental approach.
Resting state functional connectivity (rsFC) has been widely applied as a measure of functional circuit coherent activity.32 Due to its non-invasive measurement, rsFC represents a unique tool for bridging the gap between human and rodent studies, as the identical blood oxygen level dependent (BOLD) MRI signal is measurable in the same way across species.33 For example, across both humans and rodents, the severity of nicotine dependence has been shown to be negatively associated with the connectivity strength between the striatum and cingulate cortex,34–37 whereas rsFC strength of an insular-frontal module is associated with an increased risk to develop nicotine dependence.38 Nevertheless, how changes in nAChR densities as a function of chronic nicotine exposure relate to these and other circuit-level changes, especially with respect to the increased vulnerability of adolescents, remain to be studied.
Here, using a rat model of chronic nicotine exposure, we investigated changes in nAChR density throughout the brain following nicotine administration and subsequently how these effects relate to functional changes in associated brain circuits, providing a unique, integrative approach from receptors to circuits. Using a combined, within subject multimodal experimental design, we administered one of three doses of nicotine for 6 weeks to two aged cohorts (spanning the entirety of adolescent development and adulthood) and measured [3H]nicotine to label the majority of nAChR subtypes and the specific α4β2 nAChR subtype using in vitro receptor autoradiography and MRI BOLD signal to quantify rsFC. Identified changes in regional nAChR receptor subtype binding density specific to adolescent exposure to nicotine (i.e. a significant interaction effect) were subsequently used as ‘seeds’ in a whole brain rsFC analytical framework to identify the functional circuit correlates of these receptor changes.
Materials and methods
For additional details, please see the Supplementary material.
Subjects
Male Sprague-Dawley rats (Charles River Laboratories, Kingston, NY, USA) arrived at the National Institute on Drug Abuse Intramural Research Program (NIDA-IRP) between the ages of postnatal day (p) 25–28 for the adolescent and p59–62 for the adult cohorts. All procedures were conducted during the light phase and in accordance to approved protocols by the Animal Care and Use Committee of the NIDA-IRP.
Surgery
Rats were weighed daily, and their weights and relative weight gains across the study can be found in Supplementary Fig. 1. Using aseptic techniques, rats were implanted with subcutaneous osmotic minipumps (Model #2002, Alzet, Cupertino, CA, USA) preloaded with either saline (SAL) or nicotine hydrogen bitartrate salt [dissolved in saline (pH ∼ 7.4) calculated as the free base to deliver either 1.2 mg/kg/d (low nicotine; LN) or 4.8 mg/kg/d (high nicotine; HN)]. These nicotine doses were chosen as they produce nicotine withdrawal symptoms in adult rats39,40 and are comparable to doses that produce blood nicotine concentrations similar to those observed in human smokers.31,41–45 The first implantation occurred at p33 and p68 for the adolescent and adult cohort, respectively. Pumps were replaced every 14 days for a total of 42 days of nicotine exposure in each cohort; in an attempt to maintain a relatively constant dosage, nicotine concentrations were adjusted prior to each surgery to account for weight gain. There were six experimental groups (total rats: n = 66): SAL (n = 9), LN (n = 10) and HN (n = 10) starting in the adolescent period (p33), and SAL (n = 13), LN (n = 12) and HN (n = 12) in the adult period (p68)—all animals were assigned to groups in a quasi-random fashion.
Functional magnetic resonance imaging acquisition
Longitudinal neuroimaging measures were collected starting in adolescence at p33 and adulthood at p68, and rats were scanned every 14 days; only imaging data collected on the final scan (p75 for rats beginning in adolescence and p110 for rats beginning in adulthood) were analysed here to match the timing of the terminal autoradiography measurements (see Supplementary material for details).
Brain collection and processing for autoradiography
After 42 days of nicotine administration, and immediately after the last imaging data acquisition, rats were anaesthetized with 5% isoflurane, rapidly decapitated and brains immediately harvested, flash frozen on dry ice and stored at −80°C. Brains were subsequently sectioned coronally (1:3; 20 µm) using a cryostat (−17°C) and mounted onto Superfrost Plus slides (Fisher Scientific, Newark, DE, USA), which were stored at −80°C.
Frozen sections were thawed for 1–2 h at room temperature prior to ligand binding procedures using [3H]nicotine (specific activity: 79.8 Ci/mmol, Perkin Elmer, Waltham, USA) as a marker for total nicotinic receptors and [3H]A-85380 (specific activity: 18 Ci/mmol, Novandi Chemistry, Sweden) for α4ß2 receptor specific subtype, which were adapted from previous protocols.46,47
Autoradiography quantification
Autoradiograms were analysed using ImageJ-Fiji.48 Binding quantification was determined by comparing greyscale values from anatomically defined regions of interest (ROIs) to standard curves constructed from 14C standards specific to each phosphor screen. A wire frame rat atlas including 91 a priori ROIs was created from a standard rat stereotaxic atlas,49 imported as overlays into ImageJ-Fiji and fit onto corresponding brain sections in the autoradiograms. Median grey scale values from each region were averaged across two to three sections spanning most of the anterior–posterior plane. To calculate nicotine and α4ß2 nAChR specific binding, non-specific binding was subtracted from total binding values, and this value was averaged across sections for each ROI. Binding levels are expressed in fmol/g by dividing the calibrated activity values (nCi/g) by the specific activity of the radioligand (Ci/mmol), which was corrected for radioactive decay and estimated chemical degradation (calculated using the data on the technical data supplied by the manufacturer).
Statistical analysis
Autoradiography statistical analysis—using RStudio 1.2.5033,50 individual two-way ANOVAs (nicotine dose × exposure age) were conducted for each ROI, followed by a pairwise t-test with Bonferroni correction for multiple comparisons. Significance was set to pcorrected < 0.05 throughout.
Functional magnetic resonance imaging data analysis—functional MRI data were processed using analysis of functional neuro-images (AFNI),51,52 and the FMRIB software library (FSL) package.53 Functional images were preprocessed using a standard pipeline, which included skull stripping, temporal SNR (tSNR) estimation, motion correction, registration, ICA denoizing, slice timing correction, band pass filtering (0.01–0.1 Hz), regression of average signals from cerebral spinal fluid and white matter and spatial blurring (FWHM of smooth kernel = 0.6 mm).54
Brain regions identified to have significant changes in nAChR autoradiography as a function of adolescent exposure to nicotine were used as seeds to identify the functional circuit consequences of changes in nAChR binding. The same atlas49 was used to anatomically define seed regions. Seed-based, whole brain rsFC analyses between the mean beta weight values in the seed ROI and all other brain voxels were performed using 3dMVM to test for the interaction between age (adolescent versus adult) and nicotine dose (0, 1.2 and 4.8 mg/kg/d). Spatial autocorrelation function (ACF), an indicator of smoothness, was estimated using the 3dFWHMx function in AFNI and the average ACF across rats was used as a smoothing parameter in the 3dClustSim function to estimate the probability of false positive clusters. The significance threshold was set to pcorrected < 0.05 (puncorrected < 0.01, cluster size > 7 voxels).
Data availability
Raw data are available upon request to the corresponding author.
Results
Autoradiography: nicotine dose × age interaction
Representative autoradiograms of [3H]nicotine total and [3H]A-85380 α4ß2 binding from all experimental groups and their non-specific binding (lower panels) can be found in Supplementary Fig. 2. Since the nicotine dose (SAL, LN and HN) × age (adult, adolescent) interaction was the main contrast of interest herein, we address that below and then briefly describe the main effects of nicotine and main effects of age analyses at the end of Results section. Full details are given in Supplementary Tables 1–4 and results section.
[3H]nicotine nAChR binding
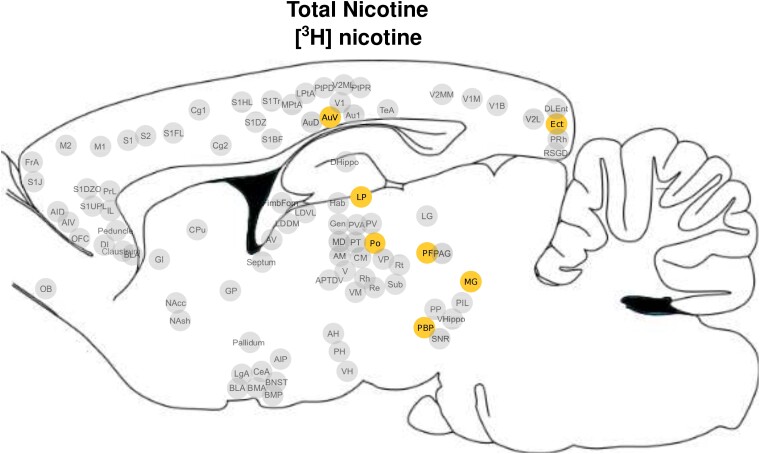
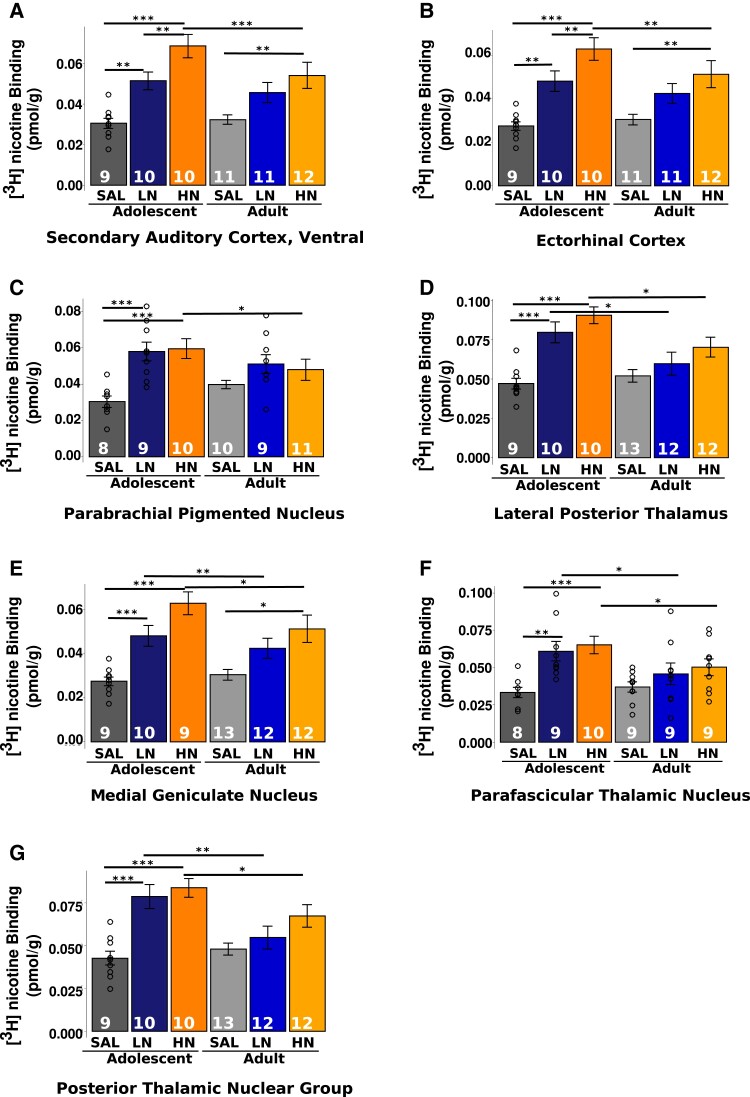
Subregions of the thalamus [lateral posterior nucleus (LP), medial geniculate nucleus (MG), parafascicular nucleus (PF) and posterior nucleus group (Po)], the parabrachial nucleus (PBP), association [ectorhinal cortex (Ect)] and sensorimotor cortices [secondary auditory cortex, ventral area (AuV); Fig. 1] demonstrated a significant nicotine dose × age interaction, such that increased [3H]nicotine nAChR binding density was seen in the adolescent cohort, regardless of nicotine dose; this effect was not observed among adult rats (all pcorrected < 0.05; Fig. 2A–G; Supplementary Table 5). Further, HN administration induced greater binding across all these regions in adolescents when compared with adults. Of these regions, only AuV and Ect displayed dose-dependent increases in binding in adolescent but not adult-exposed rats. Furthermore, all identified thalamic subregions demonstrated increased binding in both the LN and HN exposed adolescents when compared with their adult counterparts.
Figure 1.
Brain regions with a significant nicotine dose × age interaction in [3H]nicotine receptor binding. Pictorial representation of the seven ROIs that demonstrated significant nicotine dose × exposure age interaction effects for [3H]nicotine receptor binding. A list of abbreviations used in this figure can be found in the Supplementary material.
Figure 2.
Graphs of [3H]nicotine receptor binding in ROIs with a significant nicotine dose × age interaction. (A–G) Graphical depiction of the regional dose × age interactions shown in Fig. 1. Individual two-way ANOVAs were conducted followed by pairwise t-tests with Bonferroni correction for multiple comparisons. Bars represent mean ± SEM, and individual data points represent receptor binding of a brain region for an individual rat. * P < 0.05. ** P < 0.01. *** P < 0.001.
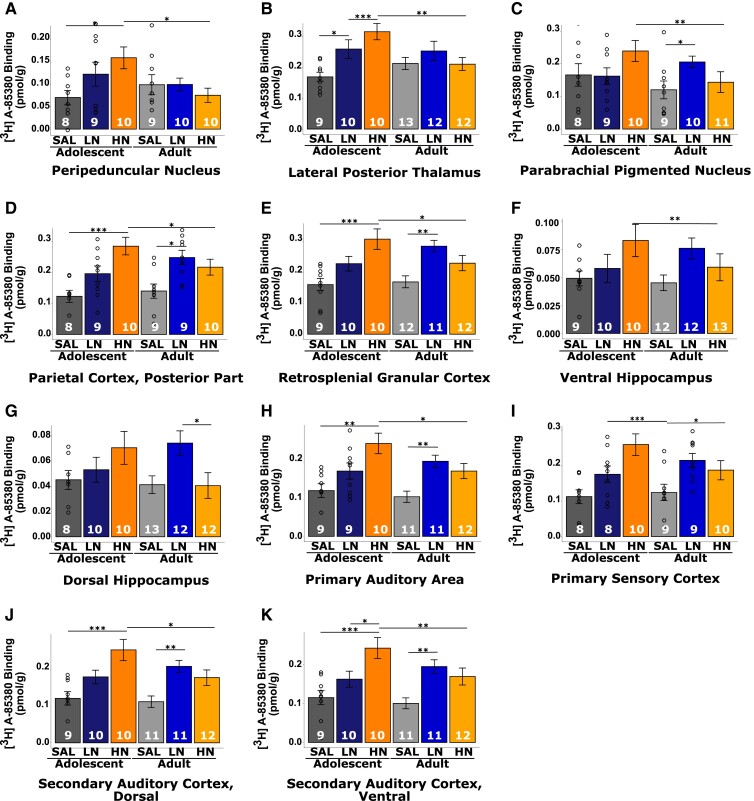
α4β2 nAChR binding
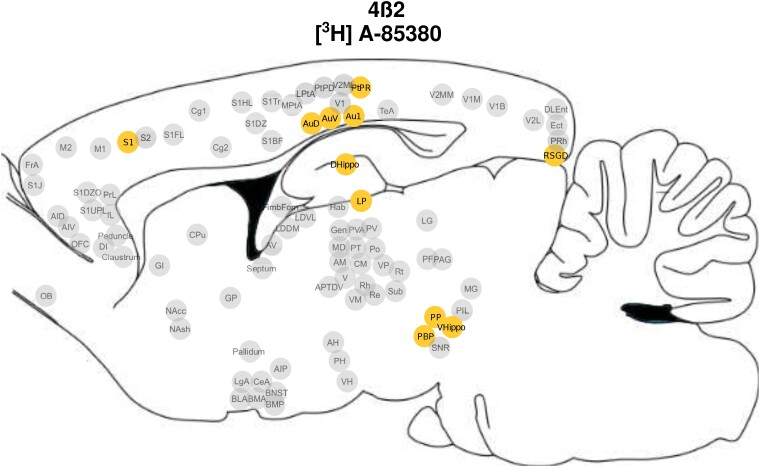
Across multiple regions, there was a significant nicotine dose × age interaction such that in adolescents, nicotine administration changed [3H]A-85380 binding across subregions of the sensorimotor [primary auditory cortex (Au1), secondary auditory cortex, dorsal area (AuD), AuV and primary somatosensory cortex (S1)] and association (parietal cortex, postural rostral part (PtPR) and retrosplenial cortex (RSGD) cortices, PBP, dorsal (dHP) and ventral hippocampus (vHP), and thalamus (LP and peripeduncular nucleus (PP) (Fig. 3; Supplementary Table 6). α4β2 receptor binding was elevated in the HN- when compared with their SAL-exposed adolescent counterparts in Au1, AuD, AuV and all measured subregions of the association cortex and the thalamus (pcorrected < 0.05; Fig. 4A–B, D–E, H–K). Finally, in all these regions except dHP, rats exposed to HN during adolescence had increased receptor density when compared with their HN adult exposed counterparts (pcorrected < 0.05; Fig. 2A–F, H–K). dHP displayed a unique pattern of α4β2 binding, with elevated receptor density observed only in adults exposed to LN when compared with the age matched SAL exposed counterparts (Fig. 2G).
Figure 3.
Brain regions with a significant nicotine dose × age interaction in [3H]A-85380 binding. Pictorial representation of the 11 regions of interest that demonstrated significant nicotine dose × exposure age interaction effects for [3H]A-85380 binding, reflecting the α4ß2 nAChR subtype. A list of abbreviations used in this figure can be found in the Supplementary material.
Figure 4.
Graphs of [3H]A-85380 binding in ROIs with a significant nicotine dose × age interaction. (A–K) Graphical depiction of the regional dose × age interactions shown in Fig. 3. Individual two-way ANOVAs were conducted followed by pairwise t-tests with Bonferroni correction for multiple comparisons. Bars represent mean ± SEM, and individual data points represent receptor binding of a brain region for an individual rat. * P < 0.05. ** P < 0.01. *** P < 0.001.
Functional connectivity
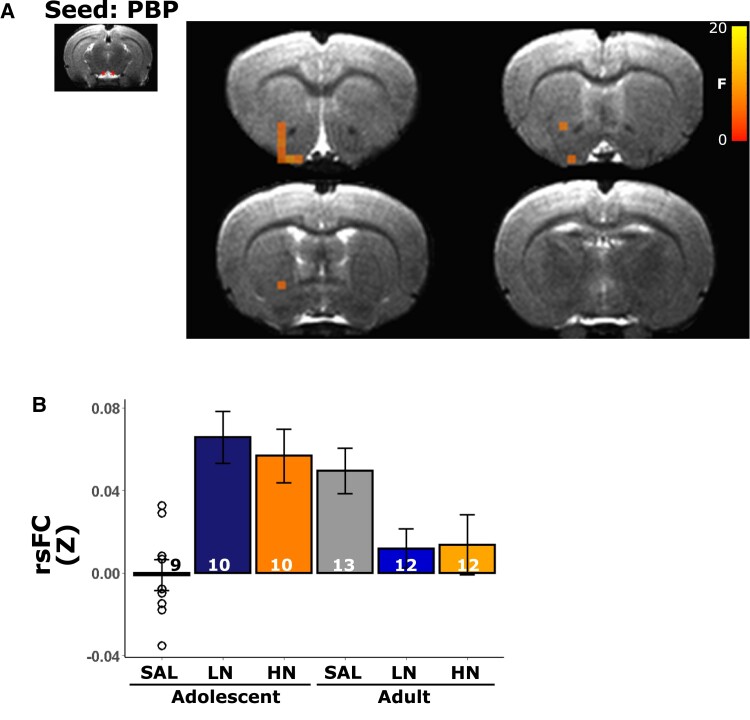
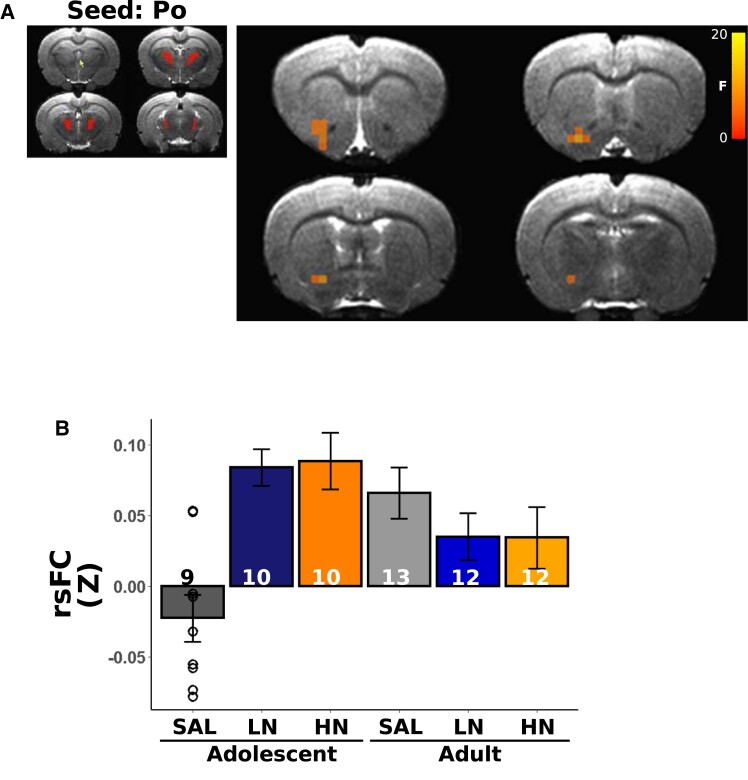
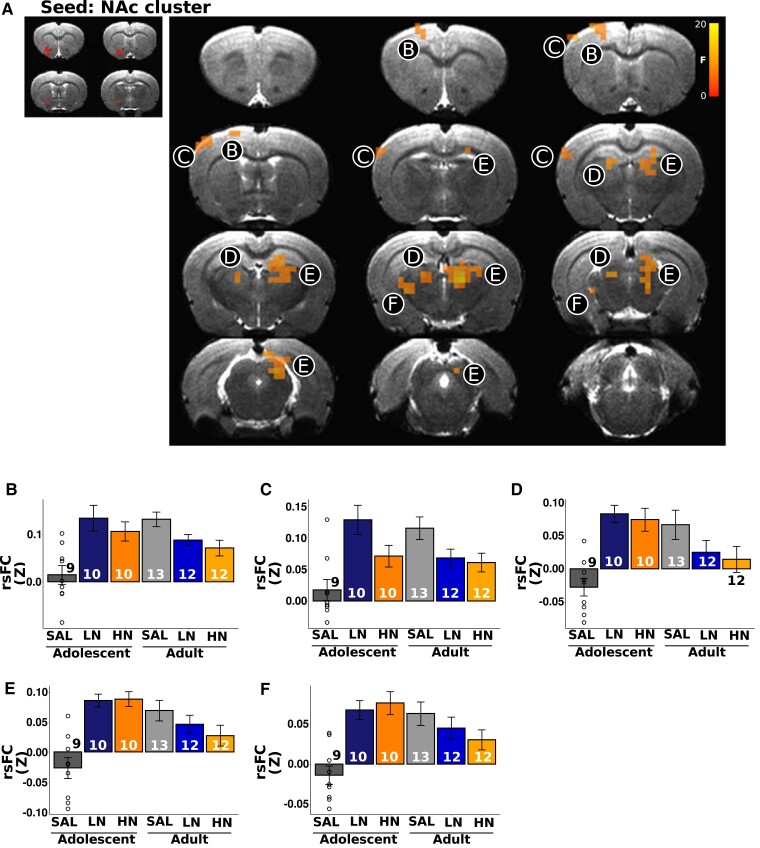
We next asked whether circuits anchored in the regions highlighted above that showed significant nAChR density age × dose interactions also demonstrated modified functional circuit strength. Using these 16 regions as ‘seeds’ (the DLEnt region was not included in this analysis due to poor temporal signal to noise ratio), whole brain rsFC analyses revealed only two regions whose connectivity changed as a function of adolescent nicotine exposure: PBP (Fig. 5A), which shows a significant age × dose interaction for both [3H]nicotine and α4ß2 nAChR binding, and Po (Fig. 6A), which shows a significant age × dose interaction only for [3H]nicotine nAChR binding. In these areas, functional connectivity strength between the PBP and portions of the nucleus accumbens (NAc) showed greater connectivity in adolescent nicotine-exposed rats, whereas rats exposed to nicotine as adults showed decreased connectivity (Fig. 5B; note that bar graphs are for visual presentation and do not represent statistical contrasts). Notably, the change in connectivity between PBP and portions of the NAc with adolescent nicotine exposure paralleled the observed changes in [3H]nicotine and α4ß2 nAChR binding, i.e. as the dose of nicotine increased among adolescents, both binding and connectivity increased; no such parallel was observed in adults, regardless of exposure to nicotine. In an exploratory follow-up analysis, we next placed a seed in this FC identified NAc region but observed no significant differences as a function of the age × dose interaction. Functional connectivity using the Po as a seed, whose [3H]nicotine nAChR binding similarly changed as a function of age × dose, identified a Po-NAc circuit (Fig. 6A) with the same pattern of connectivity changes as the PBP seed. Compared with the adult cohort, adolescent exposure to nicotine increased connectivity between these two regions whereas adult exposure to nicotine blunted connectivity versus the adolescent group (Fig. 6B). Once again, these FC findings in adolescents parallel the effects on [3H]nicotine nAChR binding; i.e. as the dose of nicotine increased among adolescents, [3H]nicotine nAChR binding increased. Finally, in an exploratory analysis, seeding this NAc region revealed changes in connectivity (Fig. 7A) with multiple regions of the sensorimotor cortex (Fig. 7B and C) and thalamus (Fig. 7D and E). Most regions followed a similar pattern, such that nicotine administration in adolescence increased circuit strength between striatal and thalamic and thalamic and cortical regions whereas connectivity was decreased in adult rats exposed equally to nicotine (Fig. 7F). These data suggest that chronic nicotine-induced binding changes had profound, but spatially limited effects on functional circuits in adolescent exposure to nicotine.
Figure 5.
Parabrachial nucleus (PBP) seed identified the functional circuit consequences of changes in nAChR binding. (A) Using the PBP as a ‘seed’, we identified a cluster within the NAc whose connectivity changed as a function of the exposure age × dose interaction. (B) Extracted rsFC Z values reveal higher PBP-NAc connectivity in adolescent nicotine exposed rats, comparable to adult saline exposed rats. The significance threshold was set to pcorrected < 0.05 (puncorrected < 0.01, cluster size > 7 voxels). Note that extracted data are for illustrative purposes only and no statistical comparisons were performed between groups. Bars represent mean ± SEM, and individual data points represent rsFC of a brain region for an individual rat.
Figure 6.
Posterior nucleus group of the thalamus (Po) seed identified the functional circuit consequences of changes in nAChR binding. (A) Using the Po as a ‘seed’, we identified a cluster within the NAc whose connectivity changed as a function of the exposure age × dose interaction. (B) Extracted rsFC Z values reveal higher Po-NAc connectivity in adolescent nicotine exposed rats, comparable to that observed in adult saline exposed rats. The significance threshold was set to pcorrected < 0.05 (puncorrected < 0.01, cluster size > 7 voxels). Note that extracted data are for illustrative purposes only and no statistical comparisons were performed between groups. Bars represent mean ± SEM, and individual data points represent rsFC of a brain region for an individual rat.
Figure 7.
The identified NAc cluster seed identified the functional circuit consequences of changes in nAChR binding. (A) In an exploratory analysis using the NAc identified in Fig. 6A as a ‘seed’, we observed changes in connectivity as a function of the exposure age × dose interaction across the sensorimotor cortex (B+C) and thalamus (D, E and F). All regions showed heightened connectivity with adolescent exposure to nicotine, comparable to that observed in adult saline exposed rats. The significance threshold was set to pcorrected < 0.05 (puncorrected < 0.01, cluster size > 7 voxels). Note that extracted data are for illustrative purposes only, and no statistical comparisons were performed between groups. Bars represent mean ± SEM, and individual data points represent rsFC of a brain region for an individual rat.
Autoradiography: main effect of nicotine and age
Since the nicotine dose × age interaction on rsFC was the a priori focus of this experiment, the receptor binding main effects of nicotine dose and age were analysed (and reported below for completeness), but they did not subsequently serve as functional connectivity seeds.
nAChR binding
A dose-dependent increase in nAChR density was observed in most subregions of the frontal, sensorimotor and association cortices and all insular subregions, such that rats exposed to HN had greater [3H]nicotine binding than those exposed to LN who in turn, had greater binding than the SAL group (Supplementary Fig. 3A and Table 1; pcorrected < 0.05). Effects for [3H]nicotine binding were widely spatially distributed, with 32 of 91 ROIs showing significantly higher nAChR density in adolescent compared with adults rats (all pcorrected < 0.05; Supplementary Fig. 3C and Table 3), notably in regions such as the cingulate cortex, claustrum, auditory cortex, and select association cortices, striatal, hippocampal, amygdala, extended amygdala and thalamic regions.
α4β2 nAChR binding
In all frontal, amygdalar, association and sensorimotor subregions measured and specifically in the ventral pallidum, the majority of the insula and select medial and posterior thalamic subregions, rats exposed to nicotine had significantly greater α4β2 density than those exposed to SAL (Supplementary Fig. 3Band Table 2; pcorrected < 0.05). In contrast to [3H]nicotine receptor binding, a smaller subset of ROIs displayed significantly elevated α4ß2 nAChR binding in adolescent rats, including select striatal and thalamic regions as well as the peduncle (all pcorrected < 0.05; Supplementary Fig. 3Dand Table 4).
Discussion
Interaction analyses between nicotine dose and drug exposure age revealed that high doses of nicotine administered to adolescents (but not adults) caused elevated [3H]nicotine and α4ß2 nAChR binding in portions of the association and sensorimotor cortices and the thalamus and elevated α4ß2 binding in the ventral hippocampus. These dose × age differences in [3H]nicotine and α4ß2 nAChR density were accompanied by changes in select striatal and thalamic regional rsFC circuits, such that circuit connectivity strength between striatal-thalamic and striatal-cortical brain regions increased as a function of adolescent, but not adult exposure to nicotine.
Adolescent exposure to a high nicotine dose increases [3H]nicotine and α4ß2 nAChR upregulation in the cortex, striatum and thalamus and increases striatal-thalamic-cortical connectivity
Adolescent-specific increases in nAChR as a result of nicotine exposure has been observed previously, along with concomitant longer lasting effects upon the removal of nicotine.55 Notably, adolescents can also tolerate greater doses of nicotine than adults.56,57 Here, we observed binding and functional connectivity changes within and between the cortex, striatum and thalamus, suggesting that nicotine modulates cortico-striatal-thalamic-cortical (CSTC) circuit loops. In CSTC circuits, neural activity is transmitted from multiple cortical regions through the striatum and converges within the thalamus, which then projects back onto cortical subregions to close the loop,58,59 nAChRs can as regulators at CSTC loops.60–66 Acquisition, integration and execution of context-appropriate behavioural responses requires synthesis of emotional, cognitive and motor functions, which are mediated by functionally and anatomically segregated parallel CSTC pathways, such that integration occurs within each of these anatomically distinct brain regions.67–69 CTSC circuit strength is at least partially developmental in origin.70,71 For example, anatomical tracts connecting the cortex and thalamus undergo synaptic pruning during adolescence,72 and cholinergic modulation within the thalamus plays a crucial role in cortical plasticity, sensory processing and arousal.73,74 Notably, CSTC circuit structure, function and connectivity are altered in multiple neuropsychiatric diseases,75 including substance use disorder76–79
Here, we propose that adolescent nicotine exposure modifies cholinergic processing, partially mediated and expressed by alterations in nAChRs, primarily α4ß2 nAChRs, which concomitantly results in changes in functional connectivity between CSTC circuit nodes. Nicotine-induced increases in cortical, striatal and thalamic nAChRs during adolescence are associated with an imbalance in CSTC loops, which are crucial for multisensory processing, action selection and motor output, and may fundamentally alter the developmental trajectory of the adolescent brain with potentially important behavioural consequences. Indeed, changes in thalamic function and connectivity with the striatum and cortex may modulate the processing of learned smoking-related cues, which are known to induce craving and, via negative reinforcement, repeated drug use.80,81 As such, the changes in observed functional connectivity strength may mediate multiple elements of smoking behaviour. For example, connectivity between the ventral striatum and cingulate cortex has been shown to be inversely related to the severity of nicotine dependence in both humans and rodents.34–37 Critically, the observed effects of nicotine on CSTC circuits are likely directly due to nicotine exposure and not resultant from any individual differences in predispositions to develop nicotine dependence, since we observed no overlap between the CSTC circuits identified here and previously identified insular circuits in rats, measured before nicotine exposure, that predict nicotine dependence severity.38 Further research is needed to examine potential behavioural effects associated with these nicotine-induced receptor and circuit changes.
Deficits in the expression of goal-directed behaviours emerge from damage to a combination of nodes within CSTC circuits.82 With the distinct roles of these CSTC loops in behaviours such as reward, cognition and motor processes related to dependence and addiction,83 discrete targeting of CSTC loops that mediate each of these behavioural outputs may help reveal the complex aetiology of drug dependence and addiction. For example, direct manipulation of the identified Po-NAc-sensorimotor cortical circuits using targeted chemogenetic or optogenetic techniques in animal models of adolescent nicotine dependence and their relationship to nicotine dependence behaviour may elucidate the role of these circuits in mediating nicotine dependence and potentially provide insight into circuits to be targeted for treatment. Disentangling the distinct, parallel and combinatorial roles of the CSTC circuits in addiction-related behaviours requires a careful consideration of the anatomical connectivity between these brain regions and their association with functional connectivity strength. Further, we highlight here effects that are specific to adolescent nicotine exposure (i.e. those effects observed solely in adolescents exposed to nicotine, identified through the dose × age interaction effects) and not the pharmacological effects of nicotine administration (i.e. those effects observed regardless of the age of exposure, identified through the main effects of nicotine dose) or developmental changes (i.e. those effects observed dependent on the age of the rat, identified through the main effects of age) in nAChRs per se. It is critical to note that the identified changes are in the number of binding sites, which does not necessarily reflect functional susceptibility. Given that the vast majority of smokers begin smoking during the adolescent period,1 we provide insight into those regions and circuits that are susceptible to nicotine only in adolescence and not simply global, widespread changes that occur with nicotine exposure.
Chronic nicotine administration increases nAChR binding
Central cholinergic transmission predominantly acts by altering neuronal excitability, changing presynaptic release of neurotransmitters and synchronizing the activity of neurons; in essence, CNS acetylcholine acts predominantly as a neuromodulator of synaptic activity.84 It bears noting that the global effect of increased nAChR binding as a function of chronic nicotine exposure observed here is consistent with the extant literature85,86 and was likely driven by changes in the α4ß2 subtypes, since they comprise the majority of nAChR in the mammalian brain.87 The consequences of global upregulation of nAChR as a result of nicotine administration have been discussed extensively elsewhere (for example,88,89) and here we highlight some overlapping changes in ROI known to be implicated in nicotine dependence and addiction.
Increased [3H]nicotine and α4ß2 nAChR binding was observed in insular and cingulate cortices. Significant increases in α4ß2 nAChR binding in the cingulate (and many other brain regions) were described previously by Doura et al.,31 using a comparable dose of nicotine; the insula was not measured in this study. However, Cano et al.90 did not find any significant differences in α4ß2 nAChR binding in the brain of adult or adolescent rats that were exposed to chronic nicotine, although they used a single low dose of nicotine (1.5 mg/kg/day) and drug exposure was limited to only 10 days. Taken together with our findings, the results of these prior studies suggest that chronic nicotine exposure leads to widespread increases in α4ß2 nAChR binding in the brain in both adolescent and adult rats and that the extent of such changes depends on both the dose of nicotine and the duration of exposure.
The insula, together with cingulate cortices, are key components of the salience network (SN).91,92 This large scale network is thought to help shift an individual’s attention to homeostatically relevant stimuli,93 is engaged during smoking cue processing94 and is functionally and structurally altered in smokers.95–102 Insular, prefrontal and cingulate cortex grey matter density are decreased in smokers compared with non-smokers, and the extent of this decrease correlates with lifetime cigarette exposure.102 Functional connectivity with these SN hubs are associated with nicotine dependence severity in humans34,36 and can predict the severity of nicotine dependence in rats.37,38 Results from human imaging studies in combination with the increases in receptor binding in key cortical areas implicated in smoking observed herein suggest that nicotine-induced nAChR upregulation and selective grey matter volume decreases may be especially linked in regional components of the SN. We did not explicitly investigate changes in insular functional connectivity herein, since there was no significant exposure age × dose interaction, the main thrust of this study. That said, nicotine dependence is often co-morbid with other neuropsychiatric diseases,103 which are developmentally dependent,104,105 and disruption in SN processing and function have been proposed as a transdiagnostic marker of neuropsychiatric disease.106
Limitations
Although this multimodal study significantly advances our understanding of the effects of chronic nicotine exposure specifically in the adolescent brain, there are a number of limitations that should be mentioned. Since nicotine dependence related behaviours and somatic withdrawal signs are not consistently observed in adolescent rats,11 this absence of behavioural correlates of dependence precludes linking changes in receptor binding with overt behavioural signs of dependence severity. Furthermore, we examined only a subset of nAChR subunits, used a narrow range of nicotine doses, and our measurement period occurred after 6 weeks of nicotine exposure, such that we only captured the chronic effects of nicotine and not those effects that may have changed rapidly following initial nicotine exposure prior to tolerance formation and receptor re-regulation. To this point, polymorphisms in the α5 nAChR gene have been shown to modulate cingulate based circuits in humans.35 In addition, our nicotine administration method was not ecologically valid, since the drug was administered passively and continuously, which differs substantially from normal nicotine administration in humans,107 and our experiments included only male rats, limiting any translational interpretations to only male smokers. Further, given the substantial weight gain observed in the first 2 weeks of adolescence, our dosage of nicotine was likely lower than what was calculated. Notably, we only examined changes in connectivity associated with age × dose interaction differences in receptor binding to minimize the number of comparisons for statistical significance correction. As such, additional functional circuit changes likely exist in the absence of testing herein. It is important to point out that we do not fully understand the nAChR binding selectivity profiles of [3H]nicotine and [3H]A-85380 in rats. [3H]nicotine is thought to also bind, although at low levels, to heteromeric subunits containing α3 and α6.108 In contrast, [3H]A85380 is expected to label only α4β2.109 Accordingly, we cannot be completely certain the extent to which they will bind to different receptor populations in the rat brain. Finally, our connectivity measures were collected in anaesthetized animals, which might not reflect the awake, unanaesthetized state.
Conclusions
Using a model of adolescent nicotine exposure, we observed dose, developmental stage and regional nicotine-dependent changes to specific nAChR subtype expression, especially for the α4ß2 subtype, as well as changes in functional connectivity in CSTC loop regions. These observations can help distinguish those circuit changes observed cross-sectionally in human studies that are resultant from nicotine exposure and not predispositional in nature. Most current smoking pharmacotherapies attempt to modulate nAChR function, so it remains imperative to understand the effects of nicotine on this receptor system for the purpose of understanding, preventing and treating smoking addiction and nicotine dependence.
Supplementary Material
Abbreviations
- ACF =
autocorrelation function
- AFNI =
analysis of functional neuroimaging
- Au1 =
primary auditory cortex
- AuD =
secondary auditory cortex, dorsal area
- AuV =
secondary auditory cortex, ventral area
- BOLD =
blood oxygen level dependent
- CSTC =
cortico-striatal-thalamic-cortical
- dHP =
dorsal hippocampus
- Ect =
ectorhinal cortex
- FSL =
FMRIB Software Library
- FWHM =
full width half maximum
- HN =
high 4.8 mg/kg/d
- ICA =
independent component analysis
- LN =
low 1.2 mg/kg/d nicotine
- LP =
lateral posterior nucleus of the thalamus
- MG =
medial geniculate nucleus of the thalamus
- NAc =
nucleus accumbens
- nAChR =
nicotinic acetylcholine receptor
- P =
postnatal day
- PBP =
parabrachial nucleus
- PET =
positron emission tomography
- PF =
parafasicular nucleus group of the thalamus
- Po =
posterior nucleus group of the thalamus
- PP =
peripeduncular nucleus of the thalamus
- PtPR =
parietal cortex, postural rostral part
- ROI =
region of interest
- rsFC =
resting state functional connectivity
- rsfMRI =
resting state functional magnetic resonance imaging
- RSGD =
retrosplenial cortex
- S1 =
primary somatosensory cortex
- SAL =
saline
- s.c. =
subcutaneous
- tSNR =
temporal signal to noise ratio
- vHP =
ventral hippocampus
Contributor Information
Robin J Keeley, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
McKenzie E Prillaman, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Miranda Scarlata, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Antonia Vrana, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Pei-Jung Tsai, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Juan L Gomez, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Jordi Bonaventura, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA; Departament de Patologia Terapèutica Experimental, Institut de Neurociènes, Universitat de Barcelona, Gran Via de les Corts Catalanes, 585, 08007 Barcelona, Spain.
Hanbing Lu, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Michael Michaelides, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Elliot A Stein, National Institute on Drug Abuse, Intramural Research Program (NIDA-IRP), National Institutes of Health, Baltimore, MD 21224, USA.
Funding
Supported by the Intramural Research Program of National Institute on Drug Abuse/National Institutes of Health, Food and Drug Administration Center on Tobacco Products (grant # NDA 13001-001-00000 to E.A.S.) and a postdoctoral fellowship award from the Canadian Institutes of Health Research (CIHR) to R.J.K. (FRN 152478).
Competing interests
M.M. has received research funding from AstraZeneca, Redpin Therapeutics and Attune Neurosciences for work unrelated to this study. The authors have declared no other conflict of interests.
Supplementary material
Supplementary material is available at Brain Communications online.
References
- 1. CDCTobaccoFree . Youth and Tobacco Use. Centers for Disease Control and Prevention. Published February 28, 2019. Accessed May 28, 2019. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/index.htm
- 2. Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the future national survey results on drug use 1975-2018: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan; 2019. [Google Scholar]
- 3. Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA. 2015;314(7):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(Pt 16):3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52(2, Suppl 2):S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koob GF, Volkow ND. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spear LP. Neurobehavioral changes in adolescence. Curr Dir Psychol Sci. 2000;9(4):111–114. [Google Scholar]
- 9. O’Dell LE, Bruijnzeel AW, Smith RT, et al. Diminished nicotine withdrawal in adolescent rats: Implications for vulnerability to addiction. Psychopharmacology (Berl). 2006;186(4):612–619. [DOI] [PubMed] [Google Scholar]
- 10. Dannenhoffer CA, Spear LP. Age differences in conditioned place preferences and taste aversions to nicotine. Dev Psychobiol. 2016;58(5):660–666. [DOI] [PubMed] [Google Scholar]
- 11. Keeley RJ, Mayer TE, Hsu LM, Lu H, Yang Y, Stein EA. Differential expression of nicotine withdrawal as a function of developmental age in the rat. Pharmacol Biochem Behav. 2019;187:172802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Changeux JP. The nicotinic acetylcholine receptor: The founding father of the pentameric ligand-gated Ion channel superfamily. J Biol Chem. 2012;287(48):40207–40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Changeux JP. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nat Rev Neurosci. 2010;11(6):389–401. [DOI] [PubMed] [Google Scholar]
- 14. Brody AL, Mandelkern MA, London ED, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63(8):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dani JA, Radcliffe KA, Pidoplichko VI. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. Eur J Pharmacol. 2000;393(1):31–38. [DOI] [PubMed] [Google Scholar]
- 16. Feltz A, Trautmann A. Desensitization at the frog neuromuscular junction: A biphasic process. J Physiol. 1982;322(1):257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quick MW, Lester RAJ. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53(4):457–478. [DOI] [PubMed] [Google Scholar]
- 18. Auta J, Longone P, Guidotti A, Costa E. The regulation of hippocampal nicotinic acetylcholine receptors (nAChRs) after a protracted treatment with selective or nonselective nAChR agonists. J Mol Neurosci. 1999;13(1-2):31–45. [DOI] [PubMed] [Google Scholar]
- 19. Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21(6):1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christensen DZ, Mikkelsen JD, Hansen HH, Thomsen MS. Repeated administration of alpha7 nicotinic acetylcholine receptor (nAChR) agonists, but not positive allosteric modulators, increases alpha7 nAChR levels in the brain. J Neurochem. 2010;114(4):1205–1216. [DOI] [PubMed] [Google Scholar]
- 21. Kawai H, Berg DK. Nicotinic acetylcholine receptors containing alpha 7 subunits on rat cortical neurons do not undergo long-lasting inactivation even when up-regulated by chronic nicotine exposure. J Neurochem. 2001;78(6):1367–1378. [DOI] [PubMed] [Google Scholar]
- 22. Keyworth H, Georgiou P, Zanos P, et al. Wheel running during chronic nicotine exposure is protective against mecamylamine-precipitated withdrawal and up-regulates hippocampal α7 nACh receptors in mice. Br J Pharmacol. 2018;175(11):1928–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marks MJ, Pauly JR, Gross SD, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12(7):2765–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology (Berl). 2006;184(3-4):314–327. [DOI] [PubMed] [Google Scholar]
- 25. Sanderson EM, Drasdo AL, McCrea K, Wonnacott S. Upregulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res. 1993;617(2):349–352. [DOI] [PubMed] [Google Scholar]
- 26. Slotkin TA, Cousins MM, Seidler FJ. Administration of nicotine to adolescent rats evokes regionally selective upregulation of CNS alpha 7 nicotinic acetylcholine receptors. Brain Res. 2004;1030(1):159–163. [DOI] [PubMed] [Google Scholar]
- 27. Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl). 1999;141(2):145–153. [DOI] [PubMed] [Google Scholar]
- 28. Picciotto MR, Zoli M, Rimondini R, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. [DOI] [PubMed] [Google Scholar]
- 29. Zoli M. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18(5):1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325(1):302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. [DOI] [PubMed] [Google Scholar]
- 33. Sumiyoshi A, Keeley RJ, Lu H. Physiological considerations of functional magnetic resonance imaging in animal models. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;4(6):522–532. [DOI] [PubMed] [Google Scholar]
- 34. Hong LE, Gu H, Yang Y, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hong LE, Hodgkinson CA, Yang Y, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA. 2010;107(30):13509–13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li S, Yang Y, Hoffmann E, Tyndale RF, Stein EA. CYP2A6 Genetic variation alters striatal-cingulate circuits, network hubs, and executive processing in smokers. Biol Psychiatry. 2017;81(7):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keeley RJ, Hsu LM, Brynildsen JK, Lu H, Yang Y, Stein EA. Intrinsic differences in insular circuits moderate the negative association between nicotine dependence and cingulate-striatal connectivity strength. Neuropsychopharmacology. 2020;45(6):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hsu LM, Keeley RJ, Liang X, et al. Intrinsic insular-frontal networks predict future nicotine dependence severity. J Neurosci. 2019;39(25):5028–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brynildsen JK, Najar J, Hsu LM, et al. A novel method to induce nicotine dependence by intermittent drug delivery using osmotic minipumps. Pharmacol Biochem Behav. 2016;142:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malin DH, Lake JR, Newlin-Maultsby P, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43(3):779–784. [DOI] [PubMed] [Google Scholar]
- 41. Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867(1-2):29–39. [DOI] [PubMed] [Google Scholar]
- 42. Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. In: Henningfield JE, London ED, Pogun S, eds. Nicotine psychopharmacology. Handbook of Experimental Pharmacology. Springer; 2009:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fredriksson I, Adhikary S, Steensland P, et al. Prior exposure to alcohol has No effect on cocaine self-administration and relapse in rats: Evidence from a rat model that does not support the gateway hypothesis. Neuropsychopharmacology. 2017;42(5):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Isaac PF, Rand MJ. Cigarette smoking and plasma levels of nicotine. Nature. 1972;236(5345):308–310. [DOI] [PubMed] [Google Scholar]
- 45. Murrin LC, Ferrer JR, Zeng WY, Haley NJ. Nicotine administration to rats: Methodological considerations. Life Sci. 1987;40(17):1699–1708. [DOI] [PubMed] [Google Scholar]
- 46. Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5(5):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chin CL, Pauly JR, Surber BW, et al. Pharmacological MRI in awake rats predicts selective binding of α4β2 nicotinic receptors. Synapse. 2008;62(3):159–168. [DOI] [PubMed] [Google Scholar]
- 48. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: An open-source platform for biological-image analysis. Nat Met. 2012;9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Academic Press/Elsevier; 2007. [Google Scholar]
- 50. RStudio Team . RStudio: Integrated development for R. Published online2015. http://www.rstudio.com/
- 51. Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. [DOI] [PubMed] [Google Scholar]
- 52. Cox RW. AFNI: What a long strange trip it’s been. Neuroimage. 2012;62(2):743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 54. Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci USA. 2012;109(10):3979–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851(1-2):9–19. [DOI] [PubMed] [Google Scholar]
- 56. Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult Male mice. J Pharmacol Exp Ther. 2007;322(1):399–407. [DOI] [PubMed] [Google Scholar]
- 57. Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol. 2007;29(1):74–80. [DOI] [PubMed] [Google Scholar]
- 58. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and Cortex. Ann Rev Neurosci. 1986;9(1):357–381. [DOI] [PubMed] [Google Scholar]
- 59. Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26(32):8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dani JA, Balfour DJK. Historical and current perspective on tobacco use and nicotine addiction. Trends Neurosci. 2011;34(7):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gioanni Y, Rougeot C, Clarke PBS, Lepousé C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: Increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11(1):18–30. [DOI] [PubMed] [Google Scholar]
- 62. Chu ZG, Zhou FM, Hablitz JJ. Nicotinic acetylcholine receptor-mediated synaptic potentials in rat neocortex. Brain Res. 2000;887(2):399–405. [DOI] [PubMed] [Google Scholar]
- 63. Proulx E, Piva M, Tian MK, Bailey CDC, Lambe EK. Nicotinic acetylcholine receptors in attention circuitry: The role of layer VI neurons of prefrontal cortex. Cell Mol Life Sci. 2014;71(7):1225–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mameli-Engvall M, Evrard A, Pons S, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50(6):911–921. [DOI] [PubMed] [Google Scholar]
- 65. Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. α6-Containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus Accumbens. Neuropsychopharmacol. 2008;33(9):2158–2166. [DOI] [PubMed] [Google Scholar]
- 66. Pistillo F, Clementi F, Zoli M, Gotti C. Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: Focus on nicotine effects. Prog Neurobiol. 2015;124:1–27. [DOI] [PubMed] [Google Scholar]
- 67. Averbeck BB, Lehman J, Jacobson M, Haber SN. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci. 2014;34(29):9497–9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Choi EY, Tanimura Y, Vage PR, Yates EH, Haber SN. Convergence of prefrontal and parietal anatomical projections in a connectional hub in the striatum. Neuroimage. 2017;146:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Haber SN, Calzavara R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Res Bull. 2009;78(2-3):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keshavan MS, Giedd J, Lau JYF, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–558. [DOI] [PubMed] [Google Scholar]
- 72. Fair DA, Bathula D, Mills KL, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abudukeyoumu N, Hernandez-Flores T, Garcia-Munoz M, Arbuthnott GW. Cholinergic modulation of striatal microcircuits. Eur J Neurosci. 2019;49(5):604–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hohmann CF. A morphogenetic role for acetylcholine in mouse cerebral neocortex. Neurosci Biobehav Rev. 2003;27(4):351–363. [DOI] [PubMed] [Google Scholar]
- 75. Jacobs GR, Ameis SH, Ji JL, et al. Developmentally divergent sexual dimorphism in the cortico-striatal–thalamic–cortical psychosis risk pathway. Neuropsychopharmacology. 2019;44(9):1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Asensio S, Romero MJ, Romero FJ, et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64(5):397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug Alcohol Depend. 2006;84(3):231–239. [DOI] [PubMed] [Google Scholar]
- 78. Taylor SB, Lewis CR, Olive MF. The neurocircuitry of illicit psychostimulant addiction: Acute and chronic effects in humans. Subst Abuse Rehabil. 2013;4:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tomasi D, Volkow ND, Wang R, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS ONE. 2010;5(5):e10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. J Consult Clin Psychol. 1987;55(3):367–371. [DOI] [PubMed] [Google Scholar]
- 81. Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: Prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64(5):993–1002. [DOI] [PubMed] [Google Scholar]
- 82. Balleine BW, Morris RW, Leung BK. Thalamocortical integration of instrumental learning and performance and their disintegration in addiction. Brain Res. 2015;1628:104–116. [DOI] [PubMed] [Google Scholar]
- 83. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol. 1995;114(2):454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19(12):4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. Measuring nicotinic receptors with characteristics of α4β2, α3β2 and α3β4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82(3):468–481. [DOI] [PubMed] [Google Scholar]
- 88. Dani JA, Jenson D, Broussard JI, De Biasi M. Neurophysiology of nicotine addiction. J Addict Res Ther. 2011;Suppl 1(1):001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wittenberg RE, Wolfman SL, De Biasi M, Dani JA. Nicotinic acetylcholine receptors and nicotine addiction: A brief introduction. Neuropharmacology. 2020;177:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cano M, Reynaga DD, Belluzzi JD, Loughlin SE, Leslie F. Chronic exposure to cigarette smoke extract upregulates nicotinic receptor binding in adult and adolescent rats. Neuropharmacology. 2020;181:108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsai PJ, Keeley RJ, Carmack SA, et al. Converging structural and functional evidence for a rat salience network. Biol Psychiatry. 2020;88(11):867–878. [DOI] [PubMed] [Google Scholar]
- 93. Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74(7):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55(1):77–84. [DOI] [PubMed] [Google Scholar]
- 96. Carroll AJ, Sutherland MT, Salmeron BJ, Ross TJ, Stein EA. Greater externalizing personality traits predict less error-related insula and anterior cingulate cortex activity in acutely abstinent cigarette smokers. Addict Biol. 2015;20(2):377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fedota JR, Ding X, Matous AL, et al. Nicotine abstinence influences the calculation of salience in discrete insular circuits. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(2):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fedota JR, Matous AL, Salmeron BJ, Gu H, Ross TJ, Stein EA. Insula demonstrates a non-linear response to varying demand for cognitive control and weaker resting connectivity with the executive control network in smokers. Neuropsychopharmacology. 2016;41(10):2557–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fritz HC, Wittfeld K, Schmidt CO, et al. Current smoking and reduced gray matter volume—A voxel-based morphometry study. Neuropsychopharmacology. 2014;39(11):2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Morales AM, Ghahremani D, Kohno M, Hellemann GS, London ED. Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology. 2014;39(8):1816–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang X, Salmeron BJ, Ross TJ, et al. Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage. 2011;54(1):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hughes JR. Comorbidity and smoking. Nicotine Tob Res. 1999;1(Suppl_2):S149–S152. [DOI] [PubMed] [Google Scholar]
- 104. Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: A review of recent literature. Curr Opin Psychiatry. 2007;20(4):359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 106. McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23(4):581–587. [DOI] [PubMed] [Google Scholar]
- 108. Ross SA, Wong JYF, Clifford JJ, et al. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci. 2000;20(17):6431–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol. 2011;82(8):828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available upon request to the corresponding author.