Abstract
Osteoporosis is a systemic skeletal disease that is associated with negative physical and psychosocial consequences, so understanding the effective strategies that can be used in the prevention of osteoporosis is especially important. The aim of this study was to integrative review the published interventional of nonpharmacological studies regarding the prevention and treatment of osteoporosis among menopausal women. In this systematic review, databases such as PubMed, PsycInfo, Web of Science (ISI), Scopus, ScienceDirect, EmBase, Cochrane library, Google scholar, and Iranian databases, such as Scientific Information Database and Magiran, were searched. The latest search was performed between “November 2020 and December 2020” separately by two researchers and then double-checked by them. The quality of the included studies was assessed using the Jadad score calculation tool. Twenty eight randomized controlled trials and quasi-experimental studies were included in this current study. The quality assessment indicated that 19 studies had acceptable (good) methodological quality and also 9 studies had weak methodological quality. The main results of this study were classified in three main categories such as exercise or physical activity training (n = 15), educational sessions (n = 11), and other interventions (n = 2). The results of most included studies showed that nonpharmacological strategies such as physical activity and educational interventions are considered as the appropriate actions to prevention of osteoporosis among menopausal women so implementing these strategies can be a good alternative for women with contraindication of hormone therapy or therapeutic treatment.
Keywords: Education, exercise, menopause, osteoporosis, program
Introduction
According to the definition of the World Health Organization (WHO), menopause is defined as the cessation of menstrual bleeding following the stopping of ovarian follicle activity nearly continuous 12 months at the age of about 50 years.[1] Menopausal transition occurs over several years and is considered as a dynamic period in which women experience predictable changes in their menstrual cycle.[2] With the onset of menopausal period, the decrease of bone density increases significantly so that in the first 5–10 years of starting menopause, women lose approximately 25%–30% of their trabecular bone and also 10%–15% of their cortical bone reserves,[3] so strongly believed that postmenopausal women are severely at the increased risk of osteoporosis and its complications.[4]
Osteoporosis is defined as a systemic skeletal disease associated with a low bone mass and susceptibility to the fractures. Osteoporosis as a silent problem, has affected millions of individuals all over the world.[5] According to the International Osteoporosis Foundation, it is also a serious and growing problem in the Middle-East regions[6] so that based on an Iranian published study, 9.4% of men and 32.4% of women affected by osteoporosis and its complications.[7] The most common concerns in osteoporosis is fractures that prevalently occurs in the hip joint, sites which usually with stand the body weight. This issue is especially serious and fatal in the older ages.[8]
Osteoporosis is associated with negative psychosocial consequences, such as the loss of ability to perform social roles due to the pain and the deformed limbs, decreased social interactions that lead to loneliness, isolation, and depression.[9] Unlike the misconception of individuals regarding osteoporosis, it is not only a part of the natural aging process,[10] but also is one of the diseases that is affected by diet, lifestyle and can be prevented with adequate nutritional and physical activities.[11] Due to high morbidity, the importance of its preventing is considerably noticed among the health care providers and the need for preventive strategic plans in this area is significantly necessary issue.[12]
Recently for chronic diseases such as osteoporosis, the health care systems have been presented the preventive strategies such as education and self-care management,[13] however initiating self-care and self-preventive programs and increasing self-confidence in dealing with this disease, will be much better than providing services by specialists.[14]
Literature review showed that inadequate knowledge regarding osteoporosis and the lack of training related to preventive strategies in this regard during the menopausal period are among the most important causes of higher prevalence of this disease and its complications.[15] So education regarding the preventive strategies is a highly desirable and economically, cost-effective approach.[16]
According to the WHO declaration, the absolute and the relative increase in the elderly population and also increased unhealthy habits lead to an intense increase in the prevalence of osteoporosis and osteoporotic fractures.[17] Therefore, since the literature review showed that the best approach to prevent the osteoporosis has not been systematically addressed in the published studies, this study aimed to systematically review the nonpharmacological strategies performed regarding the prevention of the osteoporosis in menopausal women.
Materials and Methods
Design
This study is a systematic review that was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for the review of the published interventional of nonpharmacological studies regarding the prevention of the osteoporosis among the menopausal women.
Inclusion and exclusion criteria
We included each type of trials study that limited only to English and Persian languages. In the term of the type of intervention, we included studies with nonpharmacological approaches such as physical activity, educational strategies, nutritional management interventions for osteoporosis prevention, and other clinical trials which assessed the different pharmacological medications such as hormonal drugs in the prevention or the treatment of the osteoporosis were excluded from this study. Furthermore, studies which investigate the preventive strategies for osteoporosis in other periods such as reproductive aged or with no refer to the menopausal status were removed in the screening. Furthermore, studies which presented in congress and those their full-texts were not available and also we achieved only to their abstract were excluded from this systematic review.
Literature search and search strategy
The research question was formulated through PICOCS (participants, interventions, comparators and outcomes, context, study design) strategy. A comprehensive literature search was conducted in the electronic databases (PubMed, PsycInfo, Web of Science (ISI), Scopus, ScienceDirect, Embase, Cochrane library, Google scholar, and Iranian databases, such as Scientific Information Database and Magiran), separately by two researchers (The first and second author) and then double-checked by them. To identify more relevant articles, reference lists of included studies were also searched manually. Two researchers (The first and second author) independently screened the titles and abstracts of the included studies, and in cases which obviously relevant to our study aim, the full text was obtained and reviewed for further assessment according to the inclusion and exclusion criteria. The articles were screened for duplicates and other irrelevant studies under guidance of the second author.
The electronic search strategy is available in Appendix 1. Endnote software was applied to data management.
Data extraction and analysis
The full texts of the included studies were read carefully and the required information was extracted and summarized in the descriptive tables and cross-checked. Possible disagreements were resolved by discussion between the three authors (corresponding author, first and second author) [Table 1].
Table 1.
Characteristics of included studies
| First author, country | Publication year | Type of trial/blinding | Sample size in each groups | Age groups (years) | Primary outcome |
|---|---|---|---|---|---|
| Gonzalo-Encabo,[22] Canada | 2019 | Two-center, two-arm, RCT | High dose aerobic group (n=192) Moderate dose aerobic group (n=187) |
50-74 | BMD and content in postmenopausal women |
| Cleghorn,[25] Australia | 2001 | Open crossover trial | Milk first (n=56) Usual diet first (n=59) |
>50 | Reduces bone loss in women who are within 5 years of the menopause |
| Ciaschini,[24] Canada | 2010 | RCT | Patient education and patient-specific recommendations (n=101) Control group (n=100) |
≥55 | Implementation of appropriate osteoporosis management |
| Karakiriou,[41] Greece | 2012 | RCT | Exercise group (n=10) Vibration group (n=13) Control group (n=9) |
46-62 | BMD and muscle strength program |
| Chien,[42] Taiwan | 2000 | Quasi-experimental | Exercise group (n=22) Control group (n=21) |
48-65 | Enhancing physical fitness and BMD |
| Chan,[23] Hong Kong | 2004 | Randomized, prospective trial | Exercise group (n=67) Sedentary control group (n=65) |
N/A | BMD assessment |
| De Oliveira,[26] Brazil | 2018 | RCT | Vibration group (n=17) Pilates group (n=17) Control group (n=17) |
40-70 | BMD assessment |
| Estok,[27] USA | 2007 | RCT | DXA scan group (n=101) Control group (n=102) |
50-65 | Change general knowledge of osteoporosis and increase the calcium intake and increased weight-bearing exercise |
| Kemmler,[44] Germany | 2005 | Quasi-experimental | Exercise group (n=86) Control group (n=51) |
48-60 | Osteoporosis prevention |
| Feldstein,[28] USA | 2006 | RCT | EMR message (n=101) Usual care (n=101) EMR reminder+patient reminder (n=109) |
50-89 | Increase guideline-recommended osteoporosis care postfracture |
| Francis,[29] Australia | 2009 | A wait list controlled trial | Education group (n=103) Control group (n=95) |
>40 | Changed knowledge and health directed behavior |
| Going,[38] USA | 2003 | RCT | Exercise group (n=142) No exercise or control group (n=124) |
40-65 | Changes in BMD level |
| Ha,[48] China | 2014 | Quasi-experimental | Education group (n=23) control group (n=23) |
≥50 | Knowledge about osteoporosis, dietary calcium intake and the importance of physical activity |
| Kemmler,[43] Germany | 2002 | Quasi-experimental | Exercise group (n=86) Control group (n=51) |
N/A | Physical fitness, and change the BMD, and parameters related to quality of life |
| Kulp,[30] USA | 2004 | RCT | Educational video group (n=98) Control group (n=97) |
3580 | Patient behavior |
| Laslett,[31] Australia | 2011 | Quasi-experimental | OPSMC group (n=75) One session educational course group (n=71) |
≥50 | Osteoporosis knowledge and dietary calcium |
| Kemmler,[32] Germany | 2017 | Quasi-experimental | Exercise group (n=86) Control group (n=51) |
N/A (early postmenopausal women) | Total clinical fracture rate |
| Shu,[33] USA | 2009 | Cluster RCT | Educational group (n=972) Control group (primary care physicians) (n=875) |
≥65 | Improving the management of osteoporosis (initiation of BMD testing and pharmacotherapy for osteoporosis) |
| Oh,[35] Korea | 2014 | RCT | TLM group (n=21) Control group (receiving an educational booklet) (n=20) |
≥45 | Improve bone health (changes in knowledge, self-efficacy, and health behaviors concerning bone health) |
| Rolnick,[34] USA | 2001 | RCT | Education only group (n=301) Education plus BMD group (n=207) Control group (n=187) |
45-65 | BMD testing, initiation of lifestyle changes and pharmaceutical treatment |
| Sedlak,[46] USA | 2005 | Quasi-experimental | Tailored intervention (n=23) Control group (n=101) |
50-65 | Increases in knowledge of osteoporosis, health beliefs, or osteoporosis-prevention behaviors |
| Shakil,[47] USA | 2010 | Quasi-experimental | Educational seminar regarding osteoporosis (n=61) | ≥40 | Awareness of osteoporosis |
| Rafiq,[37] Pakistan | 2018 | Quasi-experimental | Treated by medication and weight bearing exercises (n=137) Medication alone (n=137) |
40-94 | Change in T-score |
| Newstead,[36] USA | 2004 | RCT | Jumping exercise (n=23) Control group (n=26) |
50-65 | Changes in BMD level |
| Ilona,[45] Romania | 2010 | Quasi-experimental | Medication, diet and exercises program (n=23) Control group (only diet and medication) (n=23) |
43-65 | BMD on the lumbar spine |
| Shirazi,[39] Iran | 2007 | RCT | TTM-based exercise education program (n=61) control group (n=55) | 40-65 | Enhancing Physical activity and strength training, muscle mass and bone density |
| Vanaky,[49] Iran | 2015 | RCT | Water exercise group (n=10) Control group (n=10) |
50-70 | BMD of the lumbar spine |
| Barzanjeh,[40] Iran | 2017 | Quasi experimental | Strength training program in water (n=15) Control group (n=15) |
50-65 | Strength training in water on BMD of the lumbar spine and femoral neck in postmenopausal women |
|
| |||||
| First author, country | Type of intervention | Duration of intervention in each session | Outcome measurement | Time of outcome measurement | Results |
|
| |||||
| Gonzalo-Encabo,[18] Canada | Aerobic exercise | 30-60 min/sessions of aerobic exercise | BHQ Canadian Diet History Questionnaire |
24 months | At 12 months, mean BMD among women in the high dose group was significantly higher than that of women randomized to the moderate dose group (P=0.02). The mean difference between groups remained statistically significant at 24 months (P=0.04) |
| Cleghorn,[19] Australia | Supplement of calcium-fortified milk versus usual diets | N/A | XR-36 Quickscan DEXA Fasting and 24-h urine samples |
2 years | The rate of bone loss from the spine was 1.76% points less in women with taking the milk supplement compared to usual diet (P=0.006) |
| Ciaschini,[20] Canada | Education | N/A | A brief OPTQoL | 6 and 12 months | More individuals in the intervention group were taking calcium and vitamin compared to the usual care group (P<0.05) |
| Karakiriou,[21] Greece | Vibration and exercise training | 3 days a week per session (15 min) | DEXA Serum osteocalcin by radioimmunoassay HPLC |
6 months | The BMD of L2_L4 increased in the exercise group (P<0.05), remained steady in the vibration group, and decreased in the control group (P<0.05) |
| Chien,[22] Taiwan | Aerobic exercise program | 50 min | Interviewer-administered Physical activity questionnaire, 3 days food frequency questionnaire |
24 weeks | Aerobics combined with high-impact exercise at a moderate intensity was effective in offsetting the decline in BMD in intervention group (P<0.05) |
| Chan,[23] Hong Kong | Programmed TCC exercise |
45 min a day for 5 days a week | DEXA Multislice pQCT |
12 months | General bone loss in both TCC and sedentary control subjects at all measured skeletal sites, but with a reportedly slower rate in the TCC group. A significant 2.6-3.6-foldretardation of bone loss (P=0.01) was found in both trabecularand cortical compartments of the distal tibia in the TCC group compared with the control group |
| De Oliveira,[24] Brazil | Whole-body vibration versus pilates exercise | 3 times a week for totaling 78 sessions | Dual-energy x-ray absorptiometry | 6 months | Significant mean differences between vibration (P=0.018) and pilates (P=0.012) versus control, for the BMD of the lumbar spine and trochanter in postmenopausal women |
| Estok,[25] USA | DEXA scan | 15 min for DEXA | Osteoporosis knowledge test Osteoporosis Health Belief Scale The Osteoporosis Self-Efficacy Scale |
6 and 12 months | The experimental manipulation had a direct positive effect (P<0.05) on calcium intake at6 months, and indirectly at 12 months. Providing DXA results did not relate to change in exercise |
| Kemmler,[26] Germany | Group exercise session | Four sessions per week (65-70 min each session) | DXA at the lumbar spine (L1-4) A detailed baseline questionnaire Individual 5-d dietary records |
38 months | After 38 months, significant differences between intervention and control groups were observed for the BMD at the lumbar spine, the femoral neck, body composition and menopausal symptoms (P<0.001) |
| Feldstein,[27] USA | EMR message or electronic reminder to the provider plus an educational letter mailed to the patient | 2-3 min per patient | EMR message or electronic reminder | 3 and 6 months | The effect of provider advice combined with patient education was not significantly different from provider advice alone (P=0.88) |
| Francis,[28] Australia | Education and self-management course | 2-2.5 h session | OKAT HeiQ OSES |
6 weeks | At 6-week follow-up, the intervention group showed a significant increase in osteoporosis knowledge (P<0.001) and a larger increase in health-directed behavior (P<0.05) compared with the control group |
| Going,[29] USA | Exercise sessions included stretching, balance and aerobic weight-bearing activity, weightlifting, an additional weight-bearing circuit of moderate impact activities | 3 days per week for approximately 10 min | DEXA The form of calcium citrate |
8 and 12 months | Trochanteric BMD was significantly increased approximately 1.0% in women who exercised and used calcium without HRT compared to a negligible change in women who used HRT and did not exercise |
| Ha,[30] China | Educational self-efficacy | 6 weekly 1-h sessions | The Chinese FFQ The Chinese Version of the IPAQLC |
3-months | Participants in the educational group had significant improvement in osteoporosis (P<0.001), self-efficacy (P=0.003), dietary calcium intake (P=0.002), level of physical activity (P=0.011) compared to the control group at the 3-month follow-up |
| Kemmler,[31] Germany | Exercise training | Joint exercise session 65-70 min Warm-up/endurance sequence: 15 min Jumping sequence: 1 min |
Schnell-trainer-dynamometer A Schnell M-3 isometric tester Exercise-specific tests A stepwise treadmill test up to voluntary maximum, -flexibility tests DXA |
14 months | There were significant differences between exercise and control groups regarding changes of bone density (P<0.001), maximum isometric strength and quality of life parameters such as lower back pain |
| Kulp,[32] USA | Education | Intervention group viewed an educational video for 10 min/once before their physician `encounter | Researcher made questionnaire for preventing bone loss (taking calcium and Vitamin D supplements, eating calcium-rich foods, and performing weight-bearing exercise) | 3 months | Women in the intervention group in comparison with control group started taking calcium supplements (26.5% versus 4.9%; P<0.001), started taking Vitamin D supplements (20.6% versus 6.6%; P=0.02), started a program of weight-bearing exercise (13.3% versus 1.7%; P=0.03), and started hormone therapy (8% versus 1%; P=0.04) |
| Laslett,[33] Australia | Education/nutritional management | 2.5 h, once a week for 4 consecutive weeks | OKAT Dietary calcium intake frequency questionnaire OSES Community healthy activities model program for seniors |
3 months | Osteoporosis knowledge and calcium from food increased after 3 months in both groups (P<0.01). Use of osteoporosis medications increased between baseline and 3 months in the OPSMC group while decreasing in the one-session group (P=0.039). There were no differences between the groups or over time in physical activity, calcium or exercise self-efficacy |
| Kemmler,[34] Germany | Physical activity | Two group classes of 60 to 65 min and two home training sessions of 20 to 25 min for 49 to 50 weeks a year | Pain frequency and intensity of the lower back 10-year hard CHD risk (myocardial infarction, coronary death) Frequency of fracture assessment plus structured interviews |
16 years | The ratio for clinical overall fractures was significantly lower in the exercise group 0.47 (95% CI: 0.24-0.92; P=0.03) |
| Shu,[35] USA | Education | 3 months | MPR (the ratio of available medication to the total number of days studied) | 10 months | There were no significant differences between the intervention group with 74% median MPRs (interquartile range [IQR], 19%-93%) and control group with 73% (IQR, 0%-93%) (P=0.18) |
| Oh,[36] Korea | Education/exercise/Vitamin D supplementation | 24-session, 2 times a week for 3 months) | DXA Bone biomarkers in serum and urine 27-item true-false test Osteoporosis self-efficacy scale Food frequency questionnaire The Korean Society of bone metabolism Anthropometrics, blood pressure, and pulse rate |
12 weeks | The intervention group compared with the control group showed significant increases in knowledge (P=0.019) and self-efficacy (P<0.01) and improvement in diet and regular exercise (P=0.005) after 12 weeks |
| Rolnick,[37] USA | Education | A 2 h educational session | DEXA Questionnaire of self-reported changes in health behaviors SCORE |
6 months | There were no significant differences in behavior except with regard to pharmaceutical therapy; subjects with education plus BMD were three times more likely than those receiving education only to report starting hormone replacement therapy (P=0.004). Low BMD scores were associated with increasing Vitamin D intake (P=0.03) and starting medication (P=0.001). Women in the intervention groups were significantly more likely to report modifying their diet (P<0.001), calcium (P<0.01), and Vitamin D intake (P<0.0001) than women in the control group |
| Sedlak,[38] USA | Education | N/A | OPBS OKT OHBS OSES DXA T-score |
6 months | There was no difference in knowledge between groups. Daily calcium intake increased in both groups, but, there was no significant difference between the groups in daily calcium intake. Weight-bearing exercise behaviors decreased from 96.04 min to 59.2 min in the tailored group but increased slightly in the nontailored group from 81.47 to 87.26 min of exercise |
| Shakil,[39] USA | Education | N/A | OKAT | 2 weeks | There was a significant difference (paired t60=−9.5, P<0.01) between the before and after the intervention |
| Rafiq,[40] Pakistan | Education/physical activity | 3 months (3 session per week), 5-10 min of warm-up exercise, 20 min of progressive weight bearing exercise, 15 min of resistance exercise with large muscle group, 5 min of stretching and balance | DEXA | 3 months | The DEXA scan median values after treatment were changed to 3.00 (0) for exercises and medication group and 2.00 (1) for medication group |
| Newstead,[41] USA | Physical activity | 2 days per week at 25-200 jumps per session | DXA Urine NTX Serum bone specific ALK PHOS |
12 months | There was not a significant difference between two groups in BMD score (P=0.51) and biomarkers of bone turnover (P=0.221) |
| Ilona,[42] Romania | Physical activity | 1 h exercise program twice a week for 12 months | T-score on the lumbar spine (the lumbar spine (L1-L4) DEXA |
12 months | The exercise group demonstrated a significant gain compared with the control group in T-score (30.3% versus 21.83%;) and spine BMD (12.56% versus 6.5%) |
| Shirazi,[43] Iran | Physical activity | 12 weeks (30-45 min three - time a week) | IPAQ 1RM SEBTs |
12 weeks after intervention | Significant improvements in physical activity (P<0.005), muscle strength (P<0.0001), dynamic balance (P<0.0001) and static balance (P<0.0001) were noted in the training group but not in control group |
| Vanaky,[44] Iran | Physical activity | 12 weeks 60 min that increased gradually to 90 min during 12 weeks | DXA | 12 weeks | There was a significant differences between pretest and posttest of bone density in experimental group (P=0.048) while this difference was not significant for the control group (P=0.872) |
| Barzanjeh,[45] Iran | Physical activity | 12-month strength training program in water, 3 times a week (Monday, Wednesday, and Friday), for 50 min | Bone densitometry of l2 and l3 vertebrae and femoral neck | 12 months | The strength training in the water had a significant effect on bone mineral density of L2-L3 vertebra (P=0/000) and bone mineral density of the femur (P=0/000) in postmenopausal women |
RCT=Randomized controlled trials, EMR=Electronic medical record, N/A=Not available, DXA=Dual X-ray absorptiometry, TLM=Therapeutic lifestyle modification, BMD=Bone mineral density, TCC=Tai Chi Chun, BHQ=Baseline Health Questionnaire, DEXA=Dual-energy X-ray densitometer, OPTQoL=Osteoporosis-targeted Quality of Life, HPLC=High-performance liquid chromatography, pQCT=Peripheral quantitative computed tomography, OKAT=Osteoporosis knowledge assessment test, HeiQ=Health Education Impact Questionnaire, OSES=The Osteoporosis Self-Efficacy Scale, FFQ=Food Frequency Questionnaire, IPAQLC=International physical activity questionnaire, long form, MPR=Medication possession ratio, SCORE=Simple calculated osteoporosis risk estimation, OPBS=Osteoporosis-preventing behaviors survey, OKT=Osteoporosis knowledge test, OHBS=The Osteoporosis Health Belief Scale, DEXA=Dual energy X-ray absorptiometry, NTX=N-telopeptide, ALK PHOS=Alkaline phosphatase, IPAQ=International Physical Activity Questionnaire, 1RM=One-repetition maximum, SEBTs=Star-excursion balance tests, HRT=Hormone replacement therapy, TTM=Trans theoretical model
Assessment of methodological quality
The research team decided to assess the methodological quality (risk of bias) of the trials through the modified Jadad Scale.[18,19] This validated tool is being widely used to evaluate the quality of the randomized controlled trials (RCTs). It is also comprised of two sections. The first section includes three direct statements such as “description of randomization of the study with appropriate methods,” “description of the double-blind study,” and “description of withdrawals and dropouts.” For the first statement, one point is assigned to a study if randomization has been mentioned and if the method of randomization has not been mentioned, an additional point can be awarded to this statement. For the second statement, if the study has mentioned “blinding,” one point is allocated and an additional point is considered provided that the appropriate method of blinding has been declared in the study. For the third statement, if withdrawals or dropouts have been described in the study, one point is given to this statement. The overall score of the first section of the Jadad Scale ranges from 0 to 5 and a higher score indicates a high-quality study.[19,20]
The second section of the modified Jadad Scale contains three additional statements about “a clear description of inclusion and exclusion criteria,” “a description of research method used to assess adverse effects,” and “a description of statistical analysis methods.” If the three statements have been cited in studies, they can receive one point; otherwise; the score of zero is considered. Overall scoring of this tool for each article can range from 0 (as the lowest quality) to eight (as the highest quality). Studies with scores of 4–8 can thus represent good to excellent (i.e., high-quality) and those with scores of 0–3 can have poor or low quality[20,21] [Table 2].
Table 2.
Quality assessment of included studies by the modified Jadad scale
| Was the approaches of statistical analysis described? | Was the method used to assess adverse effects described? | Was there a clear description of the inclusion/exclusion criteria? | Was there a description of withdrawals and drop outs? | Was the method of blinding appropriate? | Was the study described as blinding? | Was the method of randomization appropriate? | Was the study described as randomized? | |
|---|---|---|---|---|---|---|---|---|
| Paola Gonzalo-Encabo, (2019) |

|

|

|

|

|

|

|

|
| David Cleghorn (2001) |

|

|

|

|

|

|

|

|
| Patricia M Ciaschini (2010) |

|

|

|

|

|

|

|

|
| Styliani K. Karakiriou (2012) |

|

|

|

|

|

|

|

|
| M. Y. Chien (2000) |

|

|

|

|

|

|

|

|
| Kaiming Chan (2004) |

|

|

|

|

|

|

|

|
| de Oliveira (2018) |

|

|

|

|

|

|

|

|
| Patricia J. Estok (2007) |

|

|

|

|

|

|

|

|
| Wolfgang Kemmler (2005) |

|

|

|

|

|

|

|

|
| Adrianne Feldstein (2006) |

|

|

|

|

|

|

|

|
| K. L. Francis (2009) |

|

|

|

|

|

|

|

|
| Scott Going (2003) |

|

|

|

|

|

|

|

|
| Mei Ha (2014) |

|

|

|

|

|

|

|

|
| Wolfgang Kemmler (2002) |

|

|

|

|

|

|

|

|
| Kulp JL (2004) |

|

|

|

|

|

|

|

|
| Laslett LL (2011) |

|

|

|

|

|

|

|

|
| Kemmler W (2017) |

|

|

|

|

|

|

|

|
| Shu AD-H (2009) |

|

|

|

|

|

|

|

|
| Oh EG (2014) |

|

|

|

|

|

|

|

|
| Rolnick SJ (2001) |

|

|

|

|

|

|

|

|
| Sedlak CA (2005) |

|

|

|

|

|

|

|

|
| Shakil A (2010) |

|

|

|

|

|

|

|

|
| Rafiq S (2018) |

|

|

|

|

|

|

|

|
| Newstead A (2004) |

|

|

|

|

|

|

|

|
| Ilona I (2010) |

|

|

|

|

|

|

|

|
| Shirazi K (2007) |

|

|

|

|

|

|

|

|
| Vanaky B (2015) |

|

|

|

|

|

|

|

|
| Barzanjeh SP (2017) |

|

|

|

|

|

|

|

|
 =Yes,
=Yes,
 =No,
=No,
 =No describe
=No describe
Results
Search results
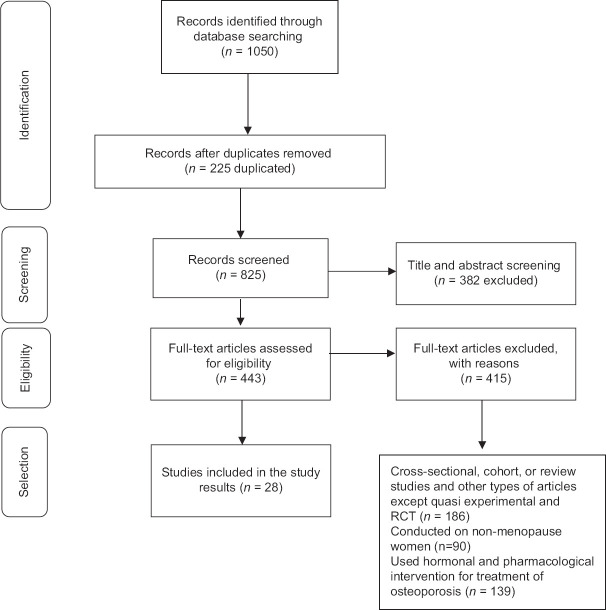
The search resulted in 1050 articles. Finally, 28 articles were systematically reviewed [Figure 1].
Figure 1.
PRISMA flow diagram
Risk of bias assessment
Based on the modified Jadad scale tool, 19 articles had acceptable or good quality[20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] while the quality of 9 articles was poor[41,42,43,44,45,46,47,48,49] [Table 2].
Description of included studies
The 28 studies included, 39,032 menopausal women and the sample sizes varied from 20 to 1847 individuals. Based on the 28 studies, the participants were 40–94 years, and in three studies, the age groups of participants were not determined.[23,32,43] Primary outcome in included studies was the bone mineral density (BMD) level assessment or testing or enhancing the physical fitness,[22,23,26,33,34,36,38,39,40,41,42,43] change in T-score (an indicator of bone density),[37] clinical fracture rate and recommendation for the postfracture care,[38,32] change in the osteoporosis knowledge level, the level of awareness and the behavior and the prevention,[27,29,30,31,44,46,47,48] and improvement the bone health and the osteoporosis management.[24,25,35] The duration of the treatment in the included studies was from 6 weeks to 38 months. The outcome measurement tools and results of included studies were indicated in details in Table 1.
Reporting the interventions regarding the prevention of the osteoporosis
The interventions of the included studies were systematically classified in three main categories which were reported as bellow:
Exercise or physical activity training
Fifteen included studies investigated the use of the different types of exercises in the prevention of the osteoporosis among postmenopausal women.
In the study by de Oliveira et al.,[26] 51 postmenopausal women were randomized into three groups: vibration, pilates, and control groups. The interventions were performed three times a week for 6 months, totaling 78 sessions. The results showed that there were significant mean differences between groups so that vibration versus control group regarding the areal BMD (body mass density) of the lumbar spine (P = 0.018) and trochanter (P = 0.012, d = 1.03) and pilates versus control group regarding the for the areal BMD of the lumbar spine (P = 0.008) and trochanter (P = 0.005). Hence, it is concluded that three weekly pilates or whole-body vibration had the equal effect on BMD among postmenopausal women.
In the study by Going et al.,[38] 320 healthy postmenopausal women were randomized to the exercise or the control groups (no exercise). All women received 800 mg calcium citrate supplements daily and performed exercises such as supervised aerobic, weight-bearing, and weight-lifting exercise, three times a week in exercise facilities and followed for 12 months. Results of this study showed that trochanteric BMD was significantly increased approximately 1.0% in women who exercised and used calcium without HRT compared to insignificant change in women who used HRT and did not exercise (P < 0.02).
In the study by Newstead et al.,[36] 53 postmenopausal women in the intervention group was included of 2 days per week at 25–200 jumps per session, and BMD of the sites such as femoral neck, total hip, and lumbar spine was measured at baseline and 12 months after beginning of the intervention through DXA (Dual X-ray Absorptiometry). Results of this study indicated that jumping exercises did not improve the BMD and biomarkers of the bone turnover compared to the control group.
In the study of Ilona et al.,[45] 46 postmenopausal women were randomized into the intervention and the control groups. The intervention group received multiple therapies based on the medication, diet, and the high impacts exercises program. They showed that after 12-month follow-up, although both groups exhibited significant improvements in T-score (−0,79 vs. −0,42 mean variation), and BMD in lumbar spine (P < 0.001), however, the exercise group indicated a significant gain in T-score and spine BMD compared to the control group.
In the study by Chan et al.,[23] 132 postmenopausal women were randomized into the TCC exercise group (45 min per day for 5 days in a week during 12 months) and sedentary control group. The BMD in participants was measured in the lumbar spine and proximal femur and in the distal tibia through multislice PQCT (Peripheral Quantitative Computed Tomography). The results showed that although BMD measurements indicate a general bone loss in both TCC and sedentary control in all measured skeletal regions, however in the TCC group is reported partially slower. Furthermore, a significant delay in bone loss in both trabecular and cortical sites of the distal tibia was seen in TCC group compared to control group (P < 0.01).
In the study by Kemmler et al.,[43] 59 early postmenopausal women with osteopenia without any medication or diseases which effect on their bone status participated in the intensive exercise training for 2 sessions a week and 41 women were considered for participating in the control group. Participants of both group received calcium and Vitamin D. The results of this study represented that there were a significant differences between exercise and control groups regarding the changes of bone density (P < 0.001), maximum isometric strength (P < 0.001), and quality of life issues such as lower back pain.
In another study by Kemmler et al.,[32] 137 early postmenopausal women with osteopenia randomized in the exercise group (two supervised groups and two home training sessions per week) and control group (continued their physical activity level). The results of this study showed that the risk of fracture was significantly lower in the intervention group (P < 0.03). Furthermore, another study by this author[44] which performed to investigate the effect of multipurpose exercise training on bone, blood lipids, physical fitness, and menopausal symptoms in postmenopausal women with osteopenia for 38 months, had been showed a significant differences between intervention and control groups regarding the BMD at the lumbar spine, the femoral neck, body composition and menopausal symptoms (P < 0.001).
In the study by Chien et al.,[42] 22 women were assigned nonrandomly to intervention group for 6-month exercise program and 21 women were considered for control group. The results indicated that the BMD level of the L2–L4 and the femoral neck in the exercise group significantly increased 2% (P > 0.05) and 6.8% (P < 0.05), respectively, in intervention group compared to decrease 2.3% (P < 0.05) and 1.5% in control group (P > 0.05).
In the study by Gonzalo-Encabo et al.,[22] 400 women were randomized to moderate dose group (150 min per week) and high dose group (300 min per week) aerobic exercises. The total BMD was measured at baseline, 12 months after the beginning of intervention. The results of this study showed that the mean BMD among women in the high dose group was estimated significantly higher than women assigned into the moderate group (P < 0.02).
In the study by Karakiriou et al.,[41] 32 healthy postmenopausal women were assigned to exercise, vibration, and control groups. The exercise group participated in a supervised program of strength training for 2 days a week and the vibration group performed vibration training 3 days a week. The BMD of the lumbar spine (L2_L4) was assessed and muscle strength in baseline and also 6 months after the beginning of the intervention. The BMD of L2_L4 increased in the exercise group (P < 0.05), remained steady in the vibration group, and decreased in the control group (P < 0.05).
In the study by Rafiq et al.,[37] 274 patients randomly assigned to medication and weight-bearing exercises and only medication group. Furthermore, DEXA scan was performed to determine the T-score before and after the intervention. The results showed that the median score of DEXA was increased in both groups. However, the physical activity along with medication is more effective in the treatment of menopausal osteoporosis compared to only prescribing medication.
In Karimzadeh Shirazi et al. study,[39] the effects of a Trans Theoretical Model (TTM)-based osteoporosis preventive physical activity education on improving the muscle strength and balance among women were examined. In this study, participants were randomly assigned to the 12-week TTM-based exercise education program and control group. The results of this study showed that the intervention had a significant effect on women's progress in physical activity, muscle strength, and also dynamic balance compared to the control group (P < 0.001).
In the study by Vanaky et al.,[49] 20 postmenopausal women were equal randomly assigned to weight-bearing water aerobic exercise and control group and the intervention was followed 60–90 min three times weekly for 12 weeks by participants. The results of study revealed that there was significant differences in the pretest and posttest bone density assessment of the women in the intervention group (P < 0.05) while this difference among control group was not significant (P > 0.05).
In the study by Barzanjeh et al.,[40] 30 postmenopausal women with osteoporosis were assigned in the 12-month strength training program in water, three times a week and control group. The results of this study showed that this interventional program had a significant effect on BMD of L2–L3 spine and femoral neck (P < 0.001). Furthermore, the intervention had a significant effect on the percentage of L2–L3 spine and femoral T-score (P < 0.001).
Educational interventions
Eleven included studies investigated the use of different types of educational interventions in the prevention of osteoporosis among postmenopausal women.
In the study by Shu et al.,[33] 972 patients and 436 primary care physicians were randomized to the intervention group (educational interventions to increase the adherence to the osteoporosis medications) and 875 patients into the control group. For a 3-month period, randomly primary care physicians received face-to-face education by trained pharmacists, while patients received letters and automated telephone calls and control group received no education and the adherence to the medication was assessed through the medication possession ratio (MPR) and other measurement during 10 months of intervention. The results of this study showed that no significant differences in the median MPRs were 74% for the intervention group compared to 73% for the control group (P = 0.18). The median times to medication discontinuation after the intervention were 85 days in the intervention group compared to 79 days for the control group.
In the study by Shakil et al.,[47] a health education intervention was performed among the intervention group and the results were assessed by pre and posttest. The results indicated a significant increase in the osteoporosis knowledge regarding adequate calcium intake and the risk factor of the osteoporosis after postintervention (P < 0.01).
In the study by Feldstein et al.,[28] 311 patients who suffered a fracture and had not received BMD measurement or medication for osteoporosis and 159 health-care providers were participated in this study. The intervention was included the clinical guidelines advice which delivered to primary care providers through electronic medical record message or electronic reminder only or both to the primary care providers and the educational letter mailed to the patients. The results showed there was not a significant difference between providers advices combined with the patient's education compared to the primary care providers alone (P = 0.88).
In the study by Francis et al.,[29] 198 postmenopausal women were randomized to intervention (self-management course to improve the osteoporosis knowledge, self-efficacy, and self-management skills behavior) and control groups. The intervention was performed four times weekly for 2 h. The results of this study showed that after 6-week follow-up, the osteoporosis knowledge (P < 0.001) and the health-related behaviors (P < 0.05) were significantly increased in the intervention group compared to the control group.
In study by Kulp et al.,[30] which performed to evaluate the effectiveness of the educational video on improving the knowledge and the preventive health behaviors regarding osteoporosis. After the educational video session and 3 months later, questionnaire was completed by participants. The results of this study showed that after 3-month follow-up, in the intervention group, there was a significant change in the participant's health behavior such as calcium supplements (P < 0.001) and Vitamin D (P = 0.02) intake, and starting the weight-bearing exercises (P = 0.04) compared to the control group.
In the study by Laslett et al.,[31] 146 women were nonrandomly assigned to the intervention (osteoporosis prevention and self-management course) and the control groups. In this study, participants completed the questionnaire at baseline and 3 months later. Results showed that the osteoporosis knowledge and calcium intake from nutrition were increased significantly in both groups compared to the baseline (P < 0.01). There were no differences between two groups regarding the physical activity, the calcium, or the exercise self-efficacy.
In the study by Ha et al.,[48] 46 participants with type 2 diabetes were assigned equally to the intervention and the control groups. The intervention group received 1 h educational sessions for 6 weeks and the control group only received standard care. They showed that the osteoporosis knowledge (P < 0.001), self-efficacy (P = 0.003), dietary calcium intake (P = 0.002), and the level of physical activity (P = 0.011) were significantly increased in the intervention compared to the control group.
In the study by Ciaschini et al.,[24] which evaluate the effect of a multifaceted community-based care program to improve the evidence-based management among patients, 201 eligible patients were assigned to the intervention (facilitated BMD testing and patient education and specific recommendations for osteoporosis treatment) and the control groups. The results of this study showed that the pharmacological treatment consumption, calcium, and Vitamin D intake were significantly increased among the intervention group compared to the control group.
In the study by Rolnick et al.,[34] 508 women with no history of osteoporosis prevention treatment were randomly assigned to education class regarding osteoporosis and educational class plus BMD test. The results the study showed that women in the intervention groups were significantly more possible to modify their diet (P < 0.001), calcium (P < 0.01), and Vitamin D intake (P < 0.001) compared to the control group. Furthermore, low BMD scores were associated with increasing Vitamin D intake (P = 0.03) and starting medication for osteoporosis (P = 0.001).
In the pilot RCT study by Sedlak et al.,[46] the treatment group received a tailored nursing intervention; the control group received no care. The result of this study showed that there was no difference in the knowledge regarding osteoporosis between groups. Daily calcium intake increased in both the intervention and the control groups, and there were no differences between groups regarding daily calcium intake. Furthermore, weight-bearing exercise behaviors decreased from in the intervention group but slightly increased in the control group.
In study by Oh et al.,[35] 41 women were randomly assigned to the intervention and the control groups. The intervention group received a 24 sessions therapeutic lifestyle modification program included exercise education, consumption calcium and Vitamin D supplements for 12 weeks. The results of this study indicated that the knowledge and self-efficacy of the participants in the intervention group increased significantly compared to the control group and their diet and exercise status improved effectively after 12-week intervention.
Other interventions
In the study by Cleghorn et al.,[25] 115 menopausal women (<5-year postmenopausal women) randomly assigned to this 2-year crossover trial so that in the 1st year, study groups included Group 1: Supplement of 3 L of calcium-fortified milk weekly in and Group 2: Usual diets and in the 2nd year, groups were reversed. The results of this study showed that each women received calcium-fortified milk compared to her own control, the rate of bone loss from the spine was 1.76% points decreased (P = 0.006). Furthermore, the fasting urine level of two markers of bone resorption in some women of milk group were significantly lower compared to them in the usual diet (P = 0.03).
In the study by Estok et al.,[27] the effect of weight-bearing exercise in the postmenopausal women on receiving personal knowledge of BMD, general knowledge of the osteoporosis, health beliefs, and osteoporosis prevention behavior was investigated. The results of this study showed that weight-bearing exercise had a significant positive effect on the calcium intake and women's information about this issue that they had osteopenia or osteoporosis and more likely to change in daily calcium intake than those with normal bone density (P < 0.05).
Discussion
This systematic review reflected on published nonpharmacological interventional studies with an emphasis on the prevention of the osteoporosis among menopausal women. Accordingly, the review of the related literature showed that different interventions had been conducted on the prevention of the osteoporosis among menopausal women worldwide.
Most of the clinical trial results which were studied in this study indicated the positive effect of physical activity on increasing bone density, which in this regard is consistent with many studies that have shown the relationship between physical activity and bone density in the menopausal women.[50,51] Exercise and physical activity are recommended as nonpharmacological interventions to increase bone density at a young age and prevent bone loss in middle age. In the elderly, exercise also plays an important role in increasing the bone density, preventing falls, and the possible fractures.[52,53] While in this regard, Gusi et al. (2006) who studied the effect of 2-month physical exercises on obese postmenopausal BMD, showed that exercises had no positive effect on the hip and spines (L2–L4).[54] Furthermore, individuals who had insufficient physical activity were at higher risk for osteoporosis.[55] In fact, performing higher physical activities associated with stronger and denser bones responses. Overall the weight-bearing bones are mainly located in the legs and are more used in activities such as walking, brisk walking, and climbing stairs, which in turn can increase BMD in menopausal women.[56] According to the researches by the International Osteoporosis Federation, the best physical activity to combat osteoporosis included exercise involves applying weight to the bones such as tennis, mountaineering, volleyball, and aerobics. On the other hand, the intensity of exercise is one of the important factors that increase the BMD, so that to arrive this aim, physical activity with an intensity of 70%–90% of the heart rate and at least 3–5 times per week for 45 min to are needed to improve bones density.[57] Due to the included studies had different characteristics of interventions regarding the duration and, the type of the intervention, and the intensity of exercise, it is not simply possible to comment on the best type of physical activity for the prevention and the treatment of osteoporosis in postmenopausal women.[22,23,41,43] Exercise is probably effective in preventing and treating of the osteoporosis by affecting the process of the bone formation and regeneration under the influence of the systemic hormones and pressures on different areas of the body.[58]
In connection with the educational interventions studied in this study, despite the fact that most of the studies were showed the positive effects of the interventions, they pointed to the level of awareness and ability of postmenopausal women to use calcium and increase bone mineral density. However, the studies used different methods of education. For this reason, determining the best educational method is not recognizable. The studies also examined a variety of implications. The results of this systematic review study also are consistent with the findings of a systematic review performed in 2014,[59] which examined the effectiveness of the multifaceted group osteoporosis training in RCT and observational studies. In this study authors reported that a group training interventions may have a positive effect on the lifestyle changes, the knowledge, and the quality of life in participated individuals, but no clear conclusions can be drawn from the included interventions. In contrast, based on the results of a systematic review based on RCTs, quasi-experimental studies, and comparative studies on professional health education, eight out of nine studies showed that performed interventions improved the patient's adherence to osteoporosis drugs.[5]
Limitation and recommendation
The limitations of this study included the limitation of the research community to the postmenopausal women, not mentioning the type of menopause (early, late and natural), the impossibility of analysis according to the type of menopause. Future studies are recommended in different types of postmenopausal women. Another limitation is that only the articles written in Persian and English languages were selected and included in the present systematic review. A comprehensive review of osteoporosis prevention methods (physical activity, educational interventions, and other nonpharmacological methods) is one of the strengths of this study.
Conclusion
The results of the most included studies showed that nonpharmacological strategies are considered as the appropriate actions to prevention of the osteoporosis among the menopausal women so implementing these strategies can be a good alternative for women with contraindication of hormone therapy or therapeutic management. Furthermore, due to the other positive effects of exercise and also the uncomplicated nature of most the physical exercises, it is recommended that postmenopausal women follow a regular physical activity program after consulting with their physicians.
Financial support and sponsorship
This study has been approved and financial support by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.FNM.REC.1399.046).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Tehran University of Medical Sciences for financial support.
Appendix
Appendix 1.
Electronic search strategy
| Database: PubMed, Web of Science , Scopus, ScienceDirect, Embase, Cochrane library, Google scholar, SID, and Magiran |
| Date of latest search: November 2020 to December 2020 |
| Example of Search strategy used for PubMed: (“clinical trial” OR “randomized controlled trial” OR “quasi-experimental” OR “pilot randomized controlled trial” OR “nonrandomized trial” OR “interventional studies”) AND (“postmenopausal women” OR “postmenopause” OR “menopausal aged women” OR “menopause women” OR “menopausal period”) AND (“nonpharmacological interventions” OR “nonpharmacological care” OR “lifestyle modifications” OR “educational program” OR “patient education” OR“ physical activity education” OR “exercise training” OR “supplement therapy” OR “calcium intake” OR “nutritional management”) AND (“osteoporosis” OR “bone density” OR “osteoporosis prevention” OR” osteoporosis management”) |
References
- 1.Sherman S. Defining the menopausal transition. Am J Med. 2005;118(Suppl 12B):3–7. doi: 10.1016/j.amjmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop+10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–68. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soltani A, Sedaghat M, Adibi H, Hamidi Z, Shenazandi H, Khalilifar A, et al. Risk factor analysis of osteoporosis in women referred to bone densitometry unit of Endocrinology and Metabolism Research Center of Tehran University of Medical Sciences. Iran South Med J. 2002;5:82–91. [Google Scholar]
- 4.Tsao LI. Relieving discomforts: The help-seeking experiences of Chinese perimenopausal women in Taiwan. J Adv Nurs. 2002;39:580–8. doi: 10.1046/j.1365-2648.2002.02327.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith CA. A systematic review of healthcare professional-led education for patients with osteoporosis or those at high risk for the disease. Orthop Nurs. 2010;29:119–32. doi: 10.1097/NOR.0b013e3181d24414. [DOI] [PubMed] [Google Scholar]
- 6.El-Hajj Fuleihan G, Adib G, Nauroy L. Switzerland: An International Osteoporosis Foundation (IOF) Publication; 2011. The middle east & Africa regional audit, epidemiology, costs & burden of osteoporosis in 2011. [Google Scholar]
- 7.Ardeshir Larijani MB, Aaf S, Pazhouhi M, Bastan Hagh MH, Mirfeizi SZ, Dashti R, et al. Bone mineral density variations in 20-69 yr. Iran South Med J. 2002;5:41–9. population of Tehran/Iran. [Google Scholar]
- 8.Prada G, Fita I, Nacu R, Ignat I, Petrescu R, Jugravu O, et al. Risk factors for complications of osteoporosis in older people. Eur Geriatr Med. 2013;4:S25. [Google Scholar]
- 9.Gold DT, Shipp KM, Lyles KW. Managing patients with complications of osteoporosis. Endocrinol Metab Clin North Am. 1998;27:485–96. doi: 10.1016/s0889-8529(05)70018-9. [DOI] [PubMed] [Google Scholar]
- 10.Magee JA, Stuberg WA, Schmutte GT. Bone health knowledge, self-efficacy, and behaviors in adolescent females. Pediatr Phys Ther. 2008;20:160–6. doi: 10.1097/PEP.0b013e3181705814. [DOI] [PubMed] [Google Scholar]
- 11.Stránský M, Ryšavá L. Nutrition as prevention and treatment of osteoporosis. Physiol Res. 2009;58(Suppl 1):S7–11. doi: 10.33549/physiolres.931858. [DOI] [PubMed] [Google Scholar]
- 12.Larijani B, Tehrani MM, Hamidi Z, Soltani A, Pajouhi M. Osteoporosis, prevention, diagnosis and treatment. J Reprod Infertil. 2005;6:5–25. [Google Scholar]
- 13.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569–72. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mithal A, Dhingra V, Lau E. Beijing China: An International Osteoporosis Foundation (IOF) Publication; 2009. The Asian audit: Epidemiology, costs and burden of osteoporosis in Asia. [Google Scholar]
- 16.Chan MF, Ko CY. Osteoporosis prevention education programme for women. J Adv Nurs. 2006;54:159–70. doi: 10.1111/j.1365-2648.2006.03804.x. [DOI] [PubMed] [Google Scholar]
- 17.de Souza MP. Osteoporosis diagnosis and treatment. Rev Bras Ortop. 2010;45:220–9. doi: 10.1016/S2255-4971(15)30361-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern S, Douglas M. Evidence-based Obstetric Anesthesia. Oxford, UK: Blackwell Publishing Ltd; 2005. Jadad scale for reporting randomized controlled trials. [Google Scholar]
- 19.Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4:79–88. doi: 10.2174/157488709788186021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord. 2001;12:232–6. doi: 10.1159/000051263. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalo-Encabo P, McNeil J, Boyne DJ, Courneya KS, Friedenreich CM. Dose-response effects of exercise on bone mineral density and content in post-menopausal women. Scand J Med Sci Sports. 2019;29:1121–9. doi: 10.1111/sms.13443. [DOI] [PubMed] [Google Scholar]
- 23.Chan K, Qin L, Lau M, Woo J, Au S, Choy W, et al. A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Arch Phys Med Rehabil. 2004;85:717–22. doi: 10.1016/j.apmr.2003.08.091. [DOI] [PubMed] [Google Scholar]
- 24.Ciaschini PM, Straus SE, Dolovich LR, Goeree RA, Leung KM, Woods CR, et al. Community based intervention to optimize osteoporosis management: Randomized controlled trial. BMC Geriatr. 2010;10:60. doi: 10.1186/1471-2318-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleghorn DB, O’Loughlin PD, Schroeder BJ, Nordin BE. An open, crossover trial of calcium-fortified milk in prevention of early postmenopausal bone loss. Med J Aust. 2001;175:242–5. doi: 10.5694/j.1326-5377.2001.tb143554.x. [DOI] [PubMed] [Google Scholar]
- 26.de Oliveira LC, de Oliveira RG, de Almeida Pires-Oliveira DA. Effects of whole-body vibration versus Pilates exercise on bone mineral density in postmenopausal women: A randomized and controlled clinical trial. J Geriatr Phys Ther. 2019;42:E23–31. doi: 10.1519/JPT.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 27.Estok PJ, Sedlak CA, Doheny MO, Hall R. Structural model for osteoporosis preventing behavior in postmenopausal women. Nurs Res. 2007;56:148–58. doi: 10.1097/01.NNR.0000270031.64810.0c. [DOI] [PubMed] [Google Scholar]
- 28.Feldstein A, Elmer PJ, Smith DH, Herson M, Orwoll E, Chen C, et al. Electronic medical record reminder improves osteoporosis management after a fracture: A randomized, controlled trial. J Am Geriatr Soc. 2006;54:450–7. doi: 10.1111/j.1532-5415.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 29.Francis KL, Matthews BL, Van Mechelen W, Bennell KL, Osborne RH. Effectiveness of a community-based osteoporosis education and self-management course: A wait list controlled trial. Osteoporos Int. 2009;20:1563–70. doi: 10.1007/s00198-009-0834-0. [DOI] [PubMed] [Google Scholar]
- 30.Kulp JL, Rane S, Bachmann G. Impact of preventive osteoporosis education on patient behavior: Immediate and 3-month follow-up. Menopause. 2004;11:116–9. doi: 10.1097/01.GME.0000079221.19081.11. [DOI] [PubMed] [Google Scholar]
- 31.Laslett LL, Lynch J, Sullivan TR, McNeil JD. Osteoporosis education improves osteoporosis knowledge and dietary calcium: Comparison of a 4 week and a one-session education course. Int J Rheum Dis. 2011;14:239–47. doi: 10.1111/j.1756-185X.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 32.Kemmler W, Kohl M, von Stengel S. Long-term effects of exercise in postmenopausal women: 16-year results of the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS) Menopause. 2017;24:45–51. doi: 10.1097/GME.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 33.Shu AD, Stedman MR, Polinski JM, Jan SA, Patel M, Truppo C, et al. Adherence to osteoporosis medications after patient and physician brief education: Post hoc analysis of a randomized controlled trial. Am J Manag Care. 2009;15:417–24. [PMC free article] [PubMed] [Google Scholar]
- 34.Rolnick SJ, Kopher R, Jackson J, Fischer LR, Compo R. What is the impact of osteoporosis education and bone mineral density testing for postmenopausal women in a managed care setting? Menopause. 2001;8:141–8. doi: 10.1097/00042192-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Oh EG, Yoo JY, Lee JE, Hyun SS, Ko IS, Chu SH. Effects of a three-month therapeutic lifestyle modification program to improve bone health in postmenopausal Korean women in a rural community: A randomized controlled trial. Res Nurs Health. 2014;37:292–301. doi: 10.1002/nur.21608. [DOI] [PubMed] [Google Scholar]
- 36.Newstead A, Smith KI, Bruder J, Keller C. The effect of a jumping exercise intervention on bone mineral density in postmenopausal women. J Geriatr Phys Ther. 2004;27:47. [Google Scholar]
- 37.Rafiq S, Zia S, Ijaz MJ, Shahid HA, Adeel M. Role of weight-bearing exercises in the treatment of post-menopausal osteoporosis. J Coll Physicians Surg Pak. 2018;28:122–5. doi: 10.29271/jcpsp.2018.02.122. [DOI] [PubMed] [Google Scholar]
- 38.Going S, Lohman T, Houtkooper L, Metcalfe L, Flint-Wagner H, Blew R, et al. Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporos Int. 2003;14:637–43. doi: 10.1007/s00198-003-1436-x. [DOI] [PubMed] [Google Scholar]
- 39.Karimzadeh Shirazi K, Niknami SH, Heydarnia A. Effects of a TTM-based osteoporosis preventive physical activity education, on increasing muscle. Hakim Res J. 2007;10:34–42. [Google Scholar]
- 40.Barzanjeh SP, Nouie F, Mirzaei S. Effect of 12 months of strength training in water on bone mineral density of the lumbar spine and femoral neck in postmenopausal women with osteoporosis. Sci J Kurdistan Univ Med Sci. 2017;21:27–35. [Google Scholar]
- 41.Karakiriou SK, Douda HT, Smilios IG, Volaklis KA, Tokmakidis SP. Effects of vibration and exercise training on bone mineral density and muscle strength in post-menopausal women. Eur J Sport Sci. 2012;12:81–8. [Google Scholar]
- 42.Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS. Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int. 2000;67:443–8. doi: 10.1007/s002230001180. [DOI] [PubMed] [Google Scholar]
- 43.Kemmler W, Engelke K, Lauber D, Weineck J, Hensen J, Kalender WA. Exercise effects on fitness and bone mineral density in early postmenopausal women: 1-year EFOPS results. Med Sci Sports Exerc. 2002;34:2115–23. doi: 10.1097/00005768-200212000-00038. [DOI] [PubMed] [Google Scholar]
- 44.Kemmler W, von Stengel S, Weineck J, Lauber D, Kalender W, Engelke K. Exercise effects on menopausal risk factors of early postmenopausal women: 3-yr Erlangen fitness osteoporosis prevention study results. Med Sci Sports Exerc. 2005;37:194–203. doi: 10.1249/01.mss.0000152678.20239.76. [DOI] [PubMed] [Google Scholar]
- 45.Ilona I, Taina A, Mirela S, Eugenia R, Mihaela Z. The role of high-impacts exercises in improve bone mineral density in postmenopausal women with osteopenia or osteoporosis. Citius Altius Fortius. 2010;27:110. [Google Scholar]
- 46.Sedlak CA, Doheny MO, Estok PJ, Zeller RA. Tailored interventions to enhance osteoporosis prevention in women. Orthop Nurs. 2005;24:270–6. doi: 10.1097/00006416-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Shakil A, Gimpel NE, Rizvi H, Siddiqui Z, Ohagi E, Billmeier TM, et al. Awareness and prevention of osteoporosis among South Asian women. J Community Health. 2010;35:392–7. doi: 10.1007/s10900-010-9263-4. [DOI] [PubMed] [Google Scholar]
- 48.Ha M, Hu J, Petrini MA, McCoy TP. The effects of an educational self-efficacy intervention on osteoporosis prevention and diabetes self-management among adults with type 2 diabetes mellitus. Biol Res Nurs. 2014;16:357–67. doi: 10.1177/1099800413512019. [DOI] [PubMed] [Google Scholar]
- 49.Vanaky B, Sadeghi H, Piri M, Ramezani N. The effect of weight bearing water aerobic exercise on the bone density of the lumbar spine of 50-70 years old overweight women. Sci J Rehabil Med. 2015;4:46–52. [Google Scholar]
- 50.Kolbe-Alexander TL, Charlton KE, Lambert EV. Lifetime physical activity and determinants of estimated bone mineral density using calcaneal ultrasound in older South African adults. J Nutr Health Aging. 2004;8:521–30. [PubMed] [Google Scholar]
- 51.Nilsson M, Ohlsson C, Eriksson AL, Frändin K, Karlsson M, Ljunggren O, et al. Competitive physical activity early in life is associated with bone mineral density in elderly Swedish men. Osteoporos Int. 2008;19:1557–66. doi: 10.1007/s00198-008-0600-8. [DOI] [PubMed] [Google Scholar]
- 52.Karinkanta S, Heinonen A, Sievänen H, Uusi-Rasi K, Pasanen M, Ojala K, et al. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: Randomized, controlled trial. Osteoporos Int. 2007;18:453–62. doi: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- 53.Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gusi N, Raimundo A, Leal A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: A randomized controlled trial. BMC Musculoskelet Disord. 2006;7:92. doi: 10.1186/1471-2474-7-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo JE, Park HS. Prevalence and associated risk factors for osteoporosis in Korean men. Arch Osteoporos. 2018;13:88. doi: 10.1007/s11657-018-0506-9. [DOI] [PubMed] [Google Scholar]
- 56.Askari M, Lotfi MH, Owlia MB, Fallahzadeh H, Mohammadi M. Survey of osteoporosis risk factors (Review Article) J Sabzevar Univ Med Sci. 2018;25:854–63. [Google Scholar]
- 57.Zehnacker CH, Bemis-Dougherty A. Effect of weighted exercises on bone mineral density in post menopausal women. A systematic review. J Geriatr Phys Ther. 2007;30:79–88. doi: 10.1519/00139143-200708000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Alexandre C, Vico L. Pathophysiology of bone loss in disuse osteoporosis. Joint Bone Spine. 2011;78:572–6. doi: 10.1016/j.jbspin.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Jensen AL, Lomborg K, Wind G, Langdahl BL. Effectiveness and characteristics of multifaceted osteoporosis group education – A systematic review. Osteoporos Int. 2014;25:1209–24. doi: 10.1007/s00198-013-2573-5. [DOI] [PubMed] [Google Scholar]