Abstract
Objectives
Black persons in the United States are more likely to suffer from social inequality. Chronic stress caused by social inequality and racial discrimination results in weathering of the body that causes physiological dysregulation and biological age being higher than chronological age (accelerated aging). Depression has been linked to both racial discrimination and accelerated aging and accelerated aging has been demonstrated to be higher in Black than White persons, on average. However, we know little about accelerated aging across the life course in Black Americans.
Methods
We used mixed-effects growth models to measure biological age acceleration, measured with cardiometabolic markers, over a 20-year period in Black participants of the Coronary Artery Risk Development in Young Adults Study who were aged 27–42 years at analytic baseline. We included an interaction between depressive symptoms and time to determine whether risk of depression was associated with a faster rate of biological aging.
Results
We found that the rate of biological aging increased over a 20-year span and that those at risk for depression had a faster rate of biological aging than those not at risk. We also found that various social factors were associated with biological age acceleration over time.
Discussion
Given the known association between perceived racial discrimination and depressive symptoms, we provide a novel instance of the long-term effects of social inequality. Specifically, biological age acceleration, a marker of physiological dysregulation, is associated with time among Black persons and more strongly associated among those with depressive symptoms.
Keywords: Biological age acceleration, CARDIA, Depression symptoms, High-effort coping, Social support
In the United States those groups who suffer the most from social inequality are racial and ethnic minorities, sexual and gender minorities, and those of lower socioeconomic status. Various research has demonstrated that different measures of social inequality are linked to disparate health outcomes among Black persons (Williams & Jackson, 2005) including discrimination (Gee, 2008), neighborhood factors (Roux & Mair, 2010), education (Assari, 2020), and income (Kahn & Fazio, 2005). The health profile of Black persons in the United States clearly shows that stress works to worsen chronic conditions (Bruce et al., 2015). Racial health disparities indicating poorer status for Black populations generally begin around midlife including disparities in optimal cardiovascular health (Brown et al., 2018), self-rated health (Beck et al., 2014), measures of cumulative health disadvantage such as allostatic load (Geronimus et al., 2006), and accelerated health declines (Thorpe et al., 2016). These phenomena have been repeatedly linked to the effects of stress from racial and economic inequality (Beck et al., 2014; Cooper, 2001; Geronimus et al., 2006).
The Weathering Hypothesis
Posited by Geronimus (1992), the weathering hypothesis states that stress, resulting from social adversity, wears the body down and ages it prematurely, resulting in diseases and consequences of aging appearing earlier than one would expect at a given chronological age. Weathering of the body, characterized by more rapid aging, has been demonstrated by using multiple measures of premature aging and wear and tear including allostatic load (Geronimus et al., 2006), epigenetic age (Liu et al., 2019), and biological age (Forrester et al., 2020, 2021; Levine & Crimmins, 2014) and has shown more rapid aging and worse outcomes for Black persons when compared to White persons. Chronic race-related stress is detrimental due to the development of vigilance behaviors that are thought to cause disruption of the hypothalamic-pituitary-adrenal axis (HPA axis), which leads to increased vulnerability to physical and mental chronic diseases such as cardiovascular disease and depression (Carter et al., 2019; Williams & Jackson, 2005). Racism as a general stressor has been repeatedly linked with depression, poor mental health, and psychological distress (Paradies et al., 2015). One consequence of living in a racist society is constant race-related stress that affects mental health, which in turn affects physiological markers resulting in weathering through more rapid biological aging.
Depressive Symptoms and Physiological Dysregulation
Major depressive disorder has been linked to increased accelerated biological aging at the cellular level (O’Donovan et al., 2012; Verhoeven et al., 2014; Wolkowitz et al., 2010) via epigenetic age measured by DNA methylation (Han et al., 2018). Both major depressive disorder and higher depressive symptoms have been linked to increased physiological dysregulation measured by allostatic load (McEwen, 2003) and inflammatory markers often used in measures of physiological dysregulation such as C-reactive protein (Copeland et al., 2012). Less often studied is the association between depressive symptoms and accelerated aging as measured by clinical biomarkers. The use of clinical biomarkers in research on stress and health is important because the clinical biomarkers unlike DNA methylation are proximal to the disease process. Further, multiple biomarkers provide an overview of multiple bodily systems rather than just the cardiovascular system, for example. We have shown that increased depressive symptoms predict higher biological age, and that this association is stronger in Black populations than in White populations (Forrester et al., 2019). Depression and physiological dysregulation have also been linked to coping methods and social factors. Prior research has shown that John Henryism, a form of high-effort coping which can result in physiological risk factors such as high blood pressure, is associated with higher levels of depression (Matthews et al., 2013). Therefore, it is likely that depression and a physiological measure of the effects of stress on health will be consistently worse for those who endorse high John Henryism. Similarly, social strain, particularly from close family and friends is detrimental for mental health, whereas feeling high neighborhood cohesion and having social support are considered favorable for mental health (Echeverría et al., 2008; Rook, 2015). All the aforementioned studies of depression and physiological dysregulation have been cross-sectional and, when focused on racial differences, comparative in nature. These studies are informative and provide evidence for the weathering hypothesis and the relationship between depression and physiological dysregulation. We build on this body of evidence in proposing a longitudinal study of these relationships.
Importance of Within-Racial Group Analyses and Longitudinal Data
There are currently no publications that empirically extend the weathering hypothesis using accelerated biological aging longitudinally, that is, examine the rate of change in biological aging across the lifetime in a Black sample; nor are there any studies that further test the rate of biological aging, measured through clinical biomarkers, as it relates to social factors thought to affect weathering. The likely reasons for this are twofold—(a) there are some, but few, diverse longitudinal studies with biological data at multiple time points; and (b) comparing African Americans to Whites is the default, though not always the best option (Whitfield et al., 2008). The first reason requires a shift in funding and recruitment strategies, while the second reason requires a shift in thinking. What benefit can be obtained by looking at weathering longitudinally in a Black sample? The few studies that include only Black participants have found variations in associations between contextual factors and health within African Americans (Barber et al., 2016b; Gebreab et al., 2012; Hickson et al., 2012; Min et al., 2017; Ong et al., 2017; Szanton et al., 2010) with some specifically looking at measures of cumulative biological risk (Barber et al., 2016a, 2016b; Hickson et al., 2012; Ong et al., 2017) and accelerated epigenetic aging (Simons et al., 2020). These studies have shown variations in cross-sectional associations but lack the longitudinal data to examine rate or trajectories of change.
Longitudinal examination of biological aging can provide the ability to look at variation in patterns among African American populations, the aim of this paper. Much of the work around weathering and rate/pace of biological aging has been done in White populations (Belsky et al., 2020) and the work that has been done in Black populations has been cross-sectional and comparative.
Purpose of This Study
The purpose of this study is twofold—to create a first-of-its-kind baseline for rate of biological aging in Black persons that can be built upon in the future; and then to examine the longitudinal relationship between biological aging and depressive symptoms. Part of our understanding of this relationship will come from understanding mitigating and exacerbating factors that can be points of future intervention such as high-effort coping, social support, social stress, neighborhood cohesion, and social ties. We hypothesize that biological age acceleration will increase over time and that being at risk for depression will be associated with faster accelerated aging. We further hypothesize negative social factors (e.g., high social strain, high John Henryism, racial discrimination) will be associated with faster accelerated aging while positive social factors (e.g., social support, neighborhood cohesion) will be associated with a slower rate of accelerated aging.
Method
Data
The data for this study come from the Coronary Artery Risk in Young Adults Study (CARDIA), a multicenter longitudinal study of 5,115 Black or White individuals aged 18–30 in 1985–1986. Following the baseline examination at Year 0 (Y0) in 1985–1986, follow-up examinations were conducted at Y2, Y5, Y7, Y10, Y15, Y20, Y25, and Y30 (2015–2016). Interim phone or mail contacts to ascertain vital status and hospitalizations were conducted yearly. At baseline, CARDIA participants had to be free of long-term disease and disability and were selected by random sampling after stratification so that there would be approximately equal numbers of Black and White persons, men and women, ages 18–24 and 25–30 and ≤12 and >12 years of education at each of the four CARDIA field centers in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. All participants provided informed consent and institutional review board approval was obtained at each field center (University of Alabama at Birmingham, Northwestern University, University of Minnesota, and Kaiser Permanente of Northern California).
Sample
The baseline sample included 2,637 Black participants. We excluded participants who were pregnant (n = 34) or were missing biological or depression symptom data at Y10 (n = 891), Y20 (n = 502), and Y30 (n = 362). The majority of those missing at each time point were missing due to overall study attrition.
Measures
Biological age/accelerated aging
Accelerated aging (AccA) was defined as the difference between biological age (BA) and chronological age (CA; AccA = BA − CA) so that a positive value indicates that a person is biologically older than their chronological age and conversely a negative value indicates that a person is biologically younger than their chronological age. Biological age was calculated with the Klemera & Doubal Method (KDM; Klemera & Doubal, 2006). The KDM is based on a series of regressions of each biomarker on chronological age in standardized units of the biomarker to establish the association of each biomarker with chronological age. The reasoning in this method is that biological aging represents a divergence from what one would expect at a particular chronological age in the general population (Klemera & Doubal, 2006). In order to calculate biological age so that it represents the approximate chronological age at which a person in the general population would have the combination of biomarkers corresponding to the biological age, we used a reference data set from National Health and Nutrition Examination Survey (NHANES) to calculate the biological age parameters and applied the parameters to the CARDIA data set (Levine, 2013). We used NHANES participants from 2007 (the first year that spirometry data were included) through 2010 that were nonpregnant and aged between 30 and 75 years. We ran the series of regressions required by KDM and calculated the parameters and then used the parameters along with the CARDIA participants’ chronological ages to create the final biological age. We used 4-year weights provided by NHANES to ensure that our results are representative of the general population.
We selected six biomarkers based on general knowledge of their association with aging, availability in CARDIA, and significant association with chronological age in CARDIA: total and high-density lipoprotein (HDL) cholesterol (mg/dl), glucose (mg/dl), body mass index, forced expiratory volume in 1 s (FEV1/h2; liters), and mean arterial pressure (MAP; MAP = 2 × diastolic bp + systolic bp/3; mmHg). We used the same biomarker combination that we have used in our other work (Forrester et al., 2021) utilizing biological age acceleration with the exception of C-reactive protein, because it was not available at all three time points. We used biomarkers from the Y10, Y20, and Y30 exams.
Depressive symptoms
Depressive symptoms were measured with the Center for Epidemiological Studies—Depression (CES-D) scale (Radloff, 1977), a 20-question scale that asks about frequency of experiencing symptoms of depression (e.g., sleep changes, weight changes, “feeling blue”). Responses range from 0 (none of the time) to 3 (most or all of the time) for a total score range of 0–60. We dichotomized depression symptoms as “at risk for depression” (CES-D score ≥ 16) and “not at risk for depression” (CES-D score < 16). The cutoff of 16 has a sensitivity of 0.95 and specificity of 0.29 in the general population. We used CES-D score from the Y10, Y20, and Y30 exams.
John Henryism
The John Henryism scale, a measure of active coping at Y0, is a scale with a set of 12 statements that describe self-determination. Possible responses range from “Completely true” (1) to “Completely false” (5) with a score range of 12–60. We used a similar method to other studies including CARDIA studies and used the median score as the cut point for “low” versus “high.” The median score for the sample was 50.
Racial discrimination
Racial discrimination was measured with the racial attribution section of the Experiences of Discrimination Scale (Williams et al., 1997) at Y30. The scale asks about experiencing discrimination due to race or skin color in seven domains (i.e., school, getting a job, getting housing, at work, at home, getting medical care, and in public). We modeled discrimination categorically to enable discrimination-stratified mixed modeling since we did not have discrimination at Y10 − Y30 to include it in the mixed modeling. The discrimination variable was defined as endorsing no domains, less than three domains, and endorsing three or more domains. The race subscale had good internal consistency (α = 0.80).
Neighborhood environment variables
Neighborhood cohesion.—
Neighborhood cohesion was measured at Y20 and was measured by asking how strongly participants agreed or disagreed with the follow statements about their neighborhood: (1) People around here are willing to help; (2) This is a close-knit neighborhood; (3) People in this neighborhood can be trusted; (4) People in this neighborhood don’t generally get along with each other; and (5) People in this neighborhood do not share the same values. Answer values ranged from 1 (strongly agree) to 5 (strongly disagree). Questions 1–3 were reverse-coded so that a higher score indicated higher neighborhood cohesion. All items were summed, with an overall score range of 5–25 and then modeled discretely using tertiles (Whitaker et al., 2019).
Neighborhood resources.—
Neighborhood resources was measured at Y30 and was measured by asking questions related to physical activity resources in the participant’s neighborhood. Yes/no questions were asked regarding the availability of each of the following in the respondent’s neighborhood: (1) an exercise facility; (2) a park; (3) sidewalks; (4) walking and/or bike paths; and (5) public transportation. A “yes” answer was given a “1” while a “no” answer was given a “0.” Responses were summed with a possible score range of 1–5 and modeled in four score categories based on the sampling distribution: 0–2, 3, 4, and 5 (Whitaker et al., 2019).
Social variables
Social ties.—
Overall number of social relationships was measured at Y20 by the mean of reported number of close friends and close relatives (available answer categories were 0, 1–2, 3–5, 6–9, or 10+). We collapsed overall mean scores into four groups representing 0–2, 2.5, 3–5, and 6 or more social ties. Each of the social variables was categorized based on the sample distribution as has been done in previous CARDIA studies analyzing social variables and biological risk (Seeman et al., 2014) to allow for stratification.
Social support.—
Social support was measured at Y20 by asking how much family and friends care about you, how much can you rely upon them to help with a problem, how much they understand the way you feel about things, and how much you can open up to them. Available response categories were “1—not at all,” “2—a little,” “3—some,” and “4—a lot.” A summary score that reflected the average social support was created and then collapsed into four categories that reflected the distribution of scores as well as the clustering around the original four answers. The categories were 1–1.5 (reflecting “none to less than a little”), 1.75–2.25 (reflecting “a little”), 2.5–3 (reflecting “some”), and 3.25 or more (reflecting “more than some to a lot”).
Social strain.—
Social strain was measured at Y20 by asking how often friends or family get on your nerves, criticize you, let you down when you’re counting on them, and make too many demands of you. The social strain questions had the same response categories as the social support questions and were modeled in the same way.
Covariates
Sex/gender, age at Y10, and study site were included as covariates in all models. Sex/gender was self-reported as male or female. Age at analytic baseline (Y10) was included in all models. Study site indicated at which center the participant had their study baseline (Y0) interviews conducted.
Statistical Analysis
The outcome for all models was difference between biological age and chronological age (AccA). We measured 20-year change in accelerated aging using a mixed effects growth model with time as the only predictor. The model took the form
where ΔAccAij is change in the accelerated age measure from baseline for individual “i” at time “j,” γ 1 estimates 20-year change in accelerated aging for at-risk participants, and µ 0i and µ 1i are the random intercepts and slopes estimated for each participant “i.” We tested if being at risk for depression was associated with faster accelerated aging also using a mixed effects growth model. The model included main effects terms for follow-up time and risk for depression, an interaction term tested differential effect of follow-up time by risk of depression, and covariates for sex, age at baseline, and study site and took the form
where ΔAccAij, γ 1, and µ 0i and µ 1i are interpreted as above. γ 2 estimates any baseline difference in accelerated aging between those at risk for depression and those not at risk for depression, γ 3 estimates the difference in 20-year change in accelerated aging between those at risk for depression and those not at risk for depression, and χ is a vector of covariates. γ 3 tested our central hypothesis that endorsing enough symptoms to be considered “at risk” for depression would be associated with faster accelerated aging over time.
We were also interested in the effect of different forms of coping and exacerbation on the relationship between being at risk for depression and change in accelerated aging. We were unable to include interaction terms because we did not have the coping measures at each time point. We completed the analysis above stratified by the levels of our coping/exacerbation variables of interest (John Henryism, racial discrimination, neighborhood cohesion, neighborhood resources, social ties, social support, and social strain) to determine the difference in 20-year change in accelerated aging between those at risk for depression and those not at risk by level of each variable. Analyses were completed using Stata16 (StataCorp, 2019) and RStudio (RStudio Team, 2020).
Results
Sample
Table 1 provides summary statistics of accelerated aging and biological markers. Participants included in the sample were 62% female and were on average 34, 44, and 54 years of age chronologically at exams Y10, Y20, and Y30, respectively. Participant’s mean biological ages were calculated as 38, 51, and 60 years at exams Y10, Y20, and Y30, respectively (Table 1). Accelerated aging increased significantly (p < .01) on average by more than 3 years between Y10 and Y20 but then significantly decreased slightly on average by 1.2 (p < .01) years between Y20 and Y30. Most biomarkers changed as expected (got worse) with time except for HDL cholesterol which improved over time and FEV in 1 s which improved from Y10 to Y20 but then decreased, as would be expected with age. All biomarker changes were significant at the p < .001 level. The proportion of participants who were at risk for depression decreased significantly from Year 10 to Year 20 (p < .01) and decreased from Year 20 to Year 30, but not significantly so (p = .857).
Table 1.
Summary Statistics for Accelerated Aging and Biomarkers for Black Participants: by Examination Year: CARDIA, 1995–2015
| Year10 | Year20 | Year30 | |
|---|---|---|---|
| Age (years): mean (SD) | 34.3 (3.7) | 44.4 (3.7) | 54.4 (3.7) |
| Biological age: mean (SD) | 37.8 (5.2) | 51.3 (7.9) | 60.1 (6.6) |
| Accelerated aging (years): mean (SD) | 3.4 (4.2) | 6.9 (7.1) | 5.7 (5.8) |
| At risk for depressiona: N (%) | 221 (25.8) | 170 (19.8) | 152 (17.7) |
| Cholesterol (mg/dl): mean (SD) | 176.7 (32.9) | 183.7 (34.1) | 189.1 (38.5) |
| High-density lipoprotein cholesterol (mg/dl) | 50.8 (13.0) | 54.2 (15.7) | 59.3 (17.9) |
| Glucose (mg/dl): mean (SD) | 87.8 (14.8) | 99.3 (26.6) | 106.0 (37.8) |
| Forced expiratory: volume/height2 (liters): mean (SD) | 1.1 (0.2) | 2.7 (0.6) | 0.8 (0.2) |
| Mean arterial pressure (mmHg): mean (SD) | 86.8 (10.0) | 91.1 (12.4) | 92.6 (12.3) |
| Body mass index: mean (SD) | 29.0 (6.5) | 31.4 (7.1) | 32.3 (7.2) |
Notes: N = 858. CARDIA = Coronary Artery Risk in Young Adults Study; SD = standard deviation.
aAt risk for depression = Center for Epidemiological Studies—Depression score > 16.
Rate of Biological Age Acceleration
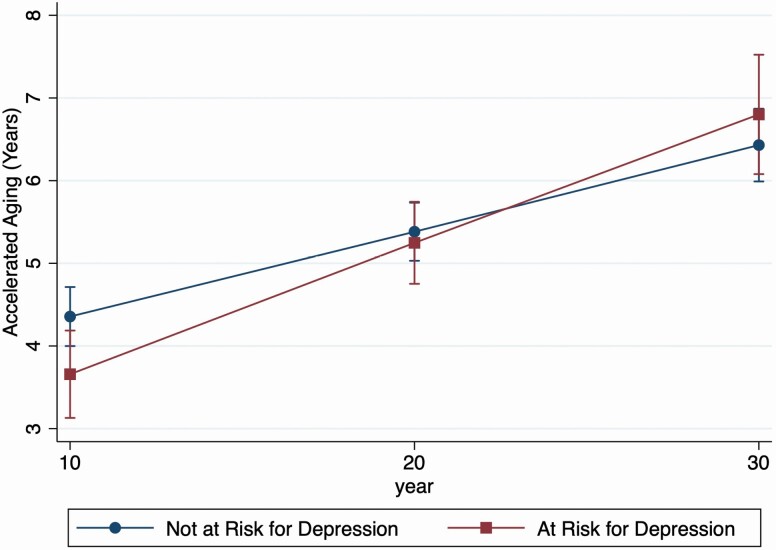
Figure 1 shows the predicted rate of biological age acceleration based on the growth models. Over the 20-year time span participant’s biological age acceleration increased on average by 1.2 years meaning that participants were 1.2 years old biologically than chronologically on average (95% CI: 1.10, 1.14). Being at risk for depression (CES-D score ≥ 16) was associated with a lower mean biological age acceleration of nearly 0.96 years (95% CI: −1.84, −0.07), when the interaction between risk for depression and time was not included. When a term modeling the interaction between being at risk for depression and time was added to the model, being at risk for depression was associated with a faster biological age acceleration of 0.05 years over time (95% CI: 0.01, 0.10). Those at risk for depression start out with a lower biological age acceleration than those not at risk but between Y20 and Y30 the smoothest lines representing this association cross such that those at risk for depression have a slightly higher biological age acceleration than those not at risk for depression by Y30 (Figure 1).
Figure 1.
Predicted rate of biological age acceleration by risk of depression: Coronary Artery Risk in Young Adults Study (CARDIA), 1995–2015.
Biological Age Acceleration and Coping/Exacerbation Factors
John Henryism
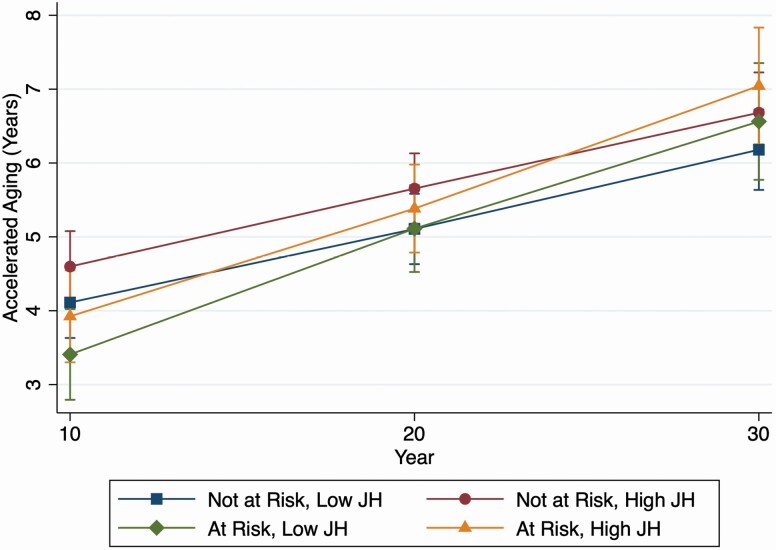
Figure 2 shows the rate of biological age acceleration by risk of depression and level of John Henryism. Mean biological age acceleration over follow-up was similar for those who reported high and low John Henryism at baseline (Y0; 0.12 years vs 0.11 years, respectively). Biological age acceleration models including depression risk and depression risk over time, stratified by level of John Henryism at baseline, showed significantly faster biological age acceleration over time for those at risk of depression among those who reported high John Henryism (0.07 years, 95% CI: 0.01, 0.14) but a nonsignificant faster biological age acceleration for those who reported low John Henryism (0.03 years, 95% CI: −0.02, 0.09; Figure 2). Baseline average age acceleration by depression status followed a similar pattern as in Figure 1.
Figure 2.
Predicted rate of biological age acceleration by risk of depression and level of John Henryism score: Coronary Artery Risk in Young Adults Study (CARDIA), 1995–2015.
Racial discrimination
Mean biological age acceleration over follow-up decreased as exposure to racial discrimination increased (none: 0.14 years, 95% CI: 0.11–0.16; <3 domains: 0.11 years, 95% CI: 0.08, 0.14; ≥3 domains: 0.09 years, 95% CI: 0.06, 0.12). There was no significant biological age acceleration for those at risk of depression compared to those not at risk for depression by number of discrimination domains endorsed.
Neighborhood cohesion
Mean biological age acceleration over follow-up was similar by tertile of neighborhood cohesion, though it was slightly higher among those in the lowest tertile (low: 0.14 years, 95% CI: 0.11, 1.18; middle: 0.11 years, 95% CI: 0.08, 0.15; high: 0.10 years, 95% CI: 0.08, 0.13). There were no significant interactions between time and depression risk by level of neighborhood cohesion. The highest tertile of neighborhood cohesion was the only one to show slower biological age acceleration over time for those at risk of depression but the interaction was not significant (−0.11 years, 95% CI: −0.36, 0.14).
Neighborhood resources
Mean biological age acceleration over follow-up did not change by quartile of neighborhood resources (~0.11-year increase for each quartile). Biological age acceleration was significantly faster for those at risk of depression among those in the lowest (0.10 years, 95% CI: 0.001, 0.21) and highest (0.13 years, 95% CI: 0.06, 0.21) quartiles of reported neighborhood resources.
Social ties
Mean biological age acceleration over follow-up decreased slightly as number of social ties increased (Table 2). There were no significant associations between time and depression risk based on number of social ties.
Table 2.
Mean Biological Age Acceleration Over Follow-Up by Exacerbation and Mitigation Factors: CARDIA, 1995–2015
| N | Mean change in biological age accelerationa (95% CI) | |
|---|---|---|
| John Henryism | ||
| Low | 431 | 0.11 (0.09, 0.13) |
| High | 427 | 0.12 (0.10, 0.15) |
| Racial discrimination | ||
| None | 315 | 0.14 (0.11, 0.17) |
| Less than three domains | 231 | 0.11 (0.08, 0.14) |
| Three or more domains | 304 | 0.09 (0.06, 0.12) |
| Neighborhood cohesion | ||
| Low | 177 | 0.14 (0.11, 0.18) |
| Medium | 274 | 0.11 (0.08, 0.15) |
| High | 389 | 0.10 (0.08, 0.13) |
| Neighborhood resources | ||
| First quartile | 155 | 0.11 (0.07, 0.15) |
| Second quartile | 184 | 0.12 (0.08, 0.15) |
| Third quartile | 252 | 0.11 (0.08, 0.14) |
| Fourth quartile | 261 | 0.12 (0.09, 0.15) |
| Social ties | ||
| 0–2 | 200 | 0.14 (0.10, 0.18) |
| 2.5 | 173 | 0.11 (0.08, 0.16) |
| 3–5 | 194 | 0.11 (0.07, 0.14) |
| 6 or more | 302 | 0.11 (0.08, 0.14) |
| Social support | ||
| None—a little | 15 | 0.15 (0.03, 0.29) |
| A little | 96 | 0.14 (0.08, 0.19) |
| Some | 128 | 0.13 (0.09, 0.17) |
| More than some—a lot | 605 | 0.11 (0.09, 0.13) |
| Social strain | ||
| None—a little | 225 | 0.10 (0.06, 0.13) |
| A little | 432 | 0.11 (0.09, 0.14) |
| Some | 119 | 0.12 (0.08, 0.17) |
| More than some—a lot | 68 | 0.15 (0.10, 0.21) |
Notes: CARDIA = Coronary Artery Risk in Young Adults Study; CI = confidence interval.
aBiological age acceleration is the difference between biological age and chronological age over follow-up.
Social support
Mean biological age acceleration over follow-up decreased as amount of social support increased such that “none to less than a little” social support (0.15 years, 95% CI: 0.03, 0.29) had a larger increase than those with “a little” social support (0.14 years, 95% CI: 0.08, 0.19) who had a larger increase than those with “some” support (0.13 years, 95% CI: 0.09, 0.17) who had a larger increase than those who reported “more than some to a lot” of social support (0.11 years, 95% CI: 0.09, 0.13). The only significant change in rate of biological age acceleration for those at risk of depression among levels of reported social support was among those who reported “more than some to a lot” of social support (0.06 years, 95% CI: 0.01, 0.12).
Social strain
Mean biological age acceleration over time increased as amount of social strain increased. Those with “none to less than a little” social strain had mean biological age acceleration of 0.10 years (95% CI: 0.06, 0.13) and the mean increased with amount of social strain—“a little” (0.11 years, 95% CI: 0.09, 0.14), “some” (0.12 years, 95% CI: 0.08, 0.17), “more than some to a lot” (0.15 years, 95% CI: 0.10, 0.21). The only significant change in rate of biological change among those at risk for depression was a faster change for those who reported “a little” social strain (0.08 years, 95% CI: 0.07, 0.003, 0.14).
Discussion
This is the first study to measure rate of biological age acceleration in a sample of Black persons and to test predictors of rate of biological age acceleration. We hypothesized that biological age acceleration would increase with time and that being at risk for depression would be associated with faster accelerated aging compared to not being at risk for depression. Further, we hypothesized that those at risk for depression who engage in high-effort coping and have high social strain would have a faster rate of biological accelerated aging than those not at risk for depression and who do not engage in high-effort coping. We also hypothesized that high social strain would be associated with faster biological age acceleration while more social ties, neighborhood cohesion, social support, and neighborhood resources would be associated with slower biological age acceleration. Our results showed support for some of our hypotheses.
Our findings regarding biological age acceleration over time are not unexpected based on previous serial cross-sectional work indicating that odds of a higher allostatic load score, a measure of physiological dysregulation, increased by 10-year age group and was higher among Black persons compared to White persons at each level (Geronimus et al., 2006). These findings are also in line with our previous work studying cross-sectional accelerated aging in the CARDIA cohort (Forrester et al., 2019). However, they extend our previous work by allowing us to estimate the rate of biological age acceleration in a Black sample and create a “baseline” of sorts for Black biological aging. Our and others’ prior work has shown that depression and depressive symptoms appear to be associated with increased biological age acceleration (Forrester et al., 2019, 2021). Our analysis showed that not only are depression symptoms associated with an increase in biological age acceleration, but when interacted with time they are associated with a faster rate of biological age acceleration. This indicates that chronic depressive symptoms over time are associated with faster rate of biological aging beyond the increase in biological age acceleration that we see at one point in time, lending support to the cumulative nature of biological age acceleration. These findings are especially important in a Black sample because extensive research has shown an association between racial discrimination and depression symptoms (Paradies et al., 2015; Williams, 2018). It is likely that the stress of living in a racist society gives rise to depression symptoms. There are various mechanisms that have been suggested, such as excess cortisol from dysregulation of the HPA axis which results in further dysregulation of biological indicators of chronic disease such as cardiovascular and metabolic markers. Beyond the effects of depression alone we were interested in how social and coping factors might mitigate or exacerbate this effect. An understanding of these factors is particularly important for intervention work.
Our results showed that the use of high-effort coping, termed John Henrysim, was not differentially associated with accelerated biological aging on its own such that rate of biological age acceleration was similar for both those who scored high and low on the John Henryism scale. However, when looking at rate of biological age acceleration for those at risk of depression compared to those not at risk for depression by John Henryism status, we found that rate of biological age acceleration was significantly faster for those at risk of depression only in the high-effort coping group. This rate was slightly faster than the rate for the full sample. Hudson and colleagues (2016) found that John Henryism was associated with increased odds of having a major depressive episode over the lifetime. Other studies have found similar results regarding John Henryism and depression (Matthews et al., 2013; Neighbors et al., 2007) while others have found that among Black women John Henryism did not negatively affect mental (Bronder et al., 2014) or physical (McKetney & Ragland, 1996) health. Research has shown that other maladaptive coping strategies such as eating comfort foods and using substances can preserve mental health but harm physiological health (Mezuk et al., 2013). Although we did not have the ability to specifically test that hypothesis, future research should take this into account. Our results indicate that high-effort coping is detrimental to both mental and physical health and could be a target for future intervention. Future interventions might consider employing stress management strategies. Our results for other mitigating and exacerbating factors were less clear.
Social Factors
The association between the social variables and rate of biological age acceleration were mixed. The rate of biological age acceleration appeared to be slower among those who reported more social support compared to those who reported less social support. Similarly, the rate of biological age acceleration appeared to be faster among those who reported more social strain compared to those who reported less social strain. There was no clear difference in rate of biological age acceleration by number of social ties. Our previous work showed that when comparing Black and White persons, social participation, measured by number of social groups one belongs to, was associated with significantly less accelerated aging among Black persons (Forrester et al., 2019). Although there has not been much research done regarding social support and its effect on accelerated biological aging, there is a large body of research on the benefits of social relationships to health behaviors and overall health (Howick et al., 2019) and especially in the Black community (Krause, 2002; Tang et al., 2008). The association between depression risk and accelerated biological aging by social factor groups was not expected. Lincoln and colleagues (2005) found that social support was associated with decreased depression symptoms using a similar social support and strain scale, but we were unable to find any clear relationship between social support and strain and the rate of accelerated biological aging for those at risk for depression compared to those not at risk for depression. Rook (2015) demonstrated that much of the evidence available indicates that health-related effects of negative social interactions are more influential than for positive social interactions and this is evident in HPA axis function. As such, one would expect that social strain would be associated with both depression and accelerated biological aging. The nature of depression symptoms may complicate the relationship because depression symptoms could cause fewer social relationships or skew a person’s perceptions of the ones that they have. Similarly, if we consider depression symptoms to be a reaction to or at least partially due to the strain of being a Black person in a racist society, social relationships may be complicated by the timing of our measurements. Because we only modeled social relationships at Year 20 and depression symptoms are presumably a response to a lifetime of inequality, we may not have been able to adequately capture the effects of social relationships and especially social relationships that are important in Black communities. In reference to Black communities, we modeled neighborhood cohesion and neighborhood resources as possible mitigating factors of the rate of biological aging and depression risk.
Neighborhood Factors
We found that rate of biological age acceleration was slightly slower as neighborhood cohesion increased, but that there was not much difference in rate of biological age acceleration by amount of neighborhood resources. Our findings on neighborhood cohesion are consistent with other studies showing that neighborhood cohesion is beneficial to outcomes including inflammatory markers (Neergheen et al., 2019). Although we did not find that neighborhood resources were differentially associated with the rate of biological aging, we did find that among those with the lowest and highest neighborhood resources rate of biological age acceleration was faster for those at risk of depression compared to those not a risk for depression. Although seemingly contradictory, these results are not surprising. One might expect persons from neighborhoods with fewer resources to have a faster rate of biological aging when at risk for depression considering that neighborhood’s characteristics are associated with perceived stress in Black communities (Henderson et al., 2016). One might be less likely to initially think that persons from higher-resourced neighborhoods would show a similar interaction but the likelihood of higher-resourced neighborhoods being predominately White might explain this finding (Landrine & Corral, 2009). Racial composition of neighborhoods affects what and how many resources are available in a neighborhood with predominately Black neighborhoods having fewer resources. When White residents begin moving into a neighborhood and bringing more resources with them the result is often not positive for Black residents (Gaskin et al., 2014). Specifically, when large numbers of White residents move in Black residents are often displaced or “priced-out,” a process known as gentrification (Smith et al., 2020). Those that do stay may choose not to use the resources available and/or may become more stressed through increased surveillance of Black residents both by White residents and by the police. Although an exploration of specific neighborhood characteristics and neighborhood racial composition is beyond the scope of this analysis, future research would benefit from a more in-depth look to explore this possibility.
Discrimination
We found that biological age acceleration appeared to decrease as endorsement of discrimination domains increased. Although seemingly contrary this is not an unexpected result. Our previous analysis (Forrester et al., 2019) also showed that there was not a significant relationship between reported perceived discrimination and accelerated biological aging cross-sectionally. Although we cannot say conclusively why, there are a few possible explanations. As this is a measure of ever having been discriminated against it may be that the discrimination happened a long time ago and while they remember it, it no longer affects them as saliently. There is also the possibility that those who report not having experienced discrimination have not been in the position to be discriminated against (e.g., not spending much time in White spaces such as neighborhoods and jobs) and have higher accelerated aging from effects of structural racism such as segregation rather than discrimination.
Our work should be considered in light of a few limitations. Although we were able to cover a 20-year span we were only able to look at biomarker measurements at 10-year intervals as opposed to 5-year or 2-year intervals, which may have given us a more precise measurement of rate of biological age acceleration. As we did not have repeated measurements for the social and neighborhood variables, we were unable to look at how changes in these variables affected changes in rate of biological age acceleration by risk of depression. This may account for the lack of associations. We were unable to look at specific coping measures because CARDIA does not have a coping scale. Although diet and exercise data are available it was beyond the scope of this paper and should be examined in future research. The number of hypotheses we tested may have made our analysis susceptible to multiple comparisons and power was low for some of the stratified analyses. Finally, the CARDIA sample was very healthy at baseline, which may make our results less generalizable to populations that were less healthy earlier in the life course (18–30 years of age).
In a first of its kind, our analysis provides a baseline for rate of biological age acceleration in a sample of Black persons. Our findings regarding faster biological aging for those at risk of depression emphasize the importance of understanding the causes of depressive symptoms in Black populations as well as the pathways by which depression accelerates biological aging. In the absence of eliminating social inequality and stressors from society, we should at least focus on helping those who suffer from the most inequality find empirically tested ways to cope that will not be detrimental to their physiological health.
Contributor Information
Sarah N Forrester, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Keith E Whitfield, University of Nevada, Las Vegas, Las Vegas, Nevada, USA.
Catarina I Kiefe, Department of Population and Quantitative Health Sciences, University of Massachusetts Chan Medical School, Worcester, Massachusetts, USA.
Roland J Thorpe, Jr, Department of Health, Behavior, and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Funding
This work was supported by the University of Massachusetts Center for Clinical and Translational Science (KL2TR001455 to S. N. Forrester); the National Institute on Minority Health and Health Disparities (U54MD000214 to R. J. Thorpe); and the National Institute on Aging (K02AG059140 and P30AG059298 to R. J. Thorpe and R01AG054363 to R. J. Thorpe and K. E. Whitfield). The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI).
Conflict of Interest
None declared.
Author Contributions
S. N. Forrester planned the study, completed data analysis, and wrote the paper. K. E. Whitfield contributed to writing the paper and conceptualization of the study; C. I. Kiefe helped plan the study, assisted with getting the data, and revised the paper; R. J. Thorpe Jr. contributed to conceptualization of the study, writing the paper, and revising the paper.
References
- Assari, S. (2020). Understanding America: Unequal economic returns of years of schooling in whites and blacks. World Journal of Educational Research (Los Angeles, Calif.), 7(2), 78–92. doi: 10.22158/wjer.v7n2p78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, S., Hickson, D. A., Kawachi, I., Subramanian, S. V., & Earls, F. (2016a). Double-jeopardy: The joint impact of neighborhood disadvantage and low social cohesion on cumulative risk of disease among African American men and women in the Jackson Heart Study. Social Science & Medicine (1982), 153, 107–115. doi: 10.1016/j.socscimed.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, S., Hickson, D. A., Kawachi, I., Subramanian, S. V., & Earls, F. (2016b). Neighborhood disadvantage and cumulative biological risk among a socioeconomically diverse sample of African American adults: An examination in the Jackson Heart Study. Journal of Racial and Ethnic Health Disparities, 3(3), 444–456. doi: 10.1007/s40615-015-0157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A. N., Finch, B. K., Lin, S. F., Hummer, R. A., & Masters, R. K. (2014). Racial disparities in self-rated health: Trends, explanatory factors, and the changing role of socio-demographics. Social Science & Medicine (1982), 104, 163–177. doi: 10.1016/j.socscimed.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky, D. W., Caspi, A., Arseneault, L., Baccarelli, A., Corcoran, D. L., Gao, X., Hannon, E., Harrington, H. L., Rasmussen, L. J. H., Houts, R., Huffman, K., Kraus, W. E., Kwon, D., Mill, J., Pieper, C. F., Prinz, J., Poulton, R., Schwartz, J., Sugden, K., ... Moffitt, T. E. (2020). Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife, 9, e54870. doi: 10.1101/2020.02.05.927434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronder, E. C., Speight, S. L., Witherspoon, K. M., & Thomas, A. J. (2014). John Henryism, depression, and perceived social support in Black women. Journal of Black Psychology, 40(2), 115–137. doi: 10.1177/0095798412474466 [DOI] [Google Scholar]
- Brown, A. F., Liang, L. J., Vassar, S. D., Escarce, J. J., Merkin, S. S., Cheng, E., Richards, A., Seeman, T., & Longstreth, W. T., Jr. (2018). Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Annals of Internal Medicine, 168(8), 541–549. doi: 10.7326/M17-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, M. A., Griffith, D. M., & Thorpe, R. J., Jr. (2015). Stress and the kidney. Advances in Chronic Kidney Disease, 22(1), 46–53. doi: 10.1053/j.ackd.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, S. E., Ong, M. L., Simons, R. L., Gibbons, F. X., Lei, M. K., & Beach, S. R. H. (2019). The effect of early discrimination on accelerated aging among African Americans. Health Psychology, 38(11), 1010–1013. doi: 10.1037/hea0000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, R. S. (2001). Social inequality, ethnicity and cardiovascular disease. International Journal of Epidemiology, 30(Suppl. 1), S48–S52. doi: 10.1093/ije/30.suppl_1.s48 [DOI] [PubMed] [Google Scholar]
- Copeland, W. E., Shanahan, L., Worthman, C., Angold, A., & Costello, E. J. (2012). Cumulative depression episodes predict later C-reactive protein levels: A prospective analysis. Biological Psychiatry, 71(1), 15–21. doi: 10.1016/j.biopsych.2011.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría, S., Diez-Roux, A. V., Shea, S., Borrell, L. N., & Jackson, S. (2008). Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: The Multi-Ethnic Study of Atherosclerosis. Health & Place, 14(4), 853–865. doi: 10.1016/j.healthplace.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Forrester, S., Jacobs, D., Zmora, R., Schreiner, P., Roger, V., & Kiefe, C. I. (2019). Racial differences in weathering and its associations with psychosocial stress: The CARDIA study. SSM - Population Health, 7, 100319. doi: 10.1016/j.ssmph.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, S. N., Zmora, R., Schreiner, P. J., Jacobs, D. R., Jr., Roger, V. L., Thorpe, R. J., Jr., & Kiefe, C. I. (2020). Racial differences in the association of accelerated aging with future cardiovascular events and all-cause mortality: The coronary artery risk development in young adults study, 2007–2018. Ethnicity & Health, 1–13. doi: 10.1080/13557858.2020.1839021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, S. N., Zmora, R., Schreiner, P. J., Jacobs, D. R., Jr., Roger, V. L., Thorpe, R. J., Jr., & Kiefe, C. I. (2021). Accelerated aging: A marker for social factors resulting in cardiovascular events? SSM - Population Health, 13, 100733. doi: 10.1016/j.ssmph.2021.100733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin, D. J., Thorpe, R. J., Jr., McGinty, E. E., Bower, K., Rohde, C., Young, J. H., LaVeist, T. A., & Dubay, L. (2014). Disparities in diabetes: The nexus of race, poverty, and place. American Journal of Public Health, 104(11), 2147–2155. doi: 10.2105/AJPH.2013.301420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreab, S. Y., Diez-Roux, A. V., Hickson, D. A., Boykin, S., Sims, M., Sarpong, D. F., Taylor, H. A., & Wyatt, S. B. (2012). The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: The Jackson Heart Study. Social Science & Medicine (1982), 75(9), 1697–1707. doi: 10.1016/j.socscimed.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, G. C. (2008). A multilevel analysis of the relationship between institutional and individual racial discrimination and health status. American Journal of Public Health, 98(Supplement_1), S48–S56. doi: 10.2105/ajph.92.4.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus, A. T. (1992). The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethnicity & Disease, 2(3), 207–221. https://europepmc.org/article/med/1467758 [PubMed] [Google Scholar]
- Geronimus, A. T., Hicken, M., Keene, D., & Bound, J. (2006). “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96(5), 826–833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. K. M., Aghajani, M., Clark, S. L., Chan, R. F., Hattab, M. W., Shabalin, A. A., Zhao, M., Kumar, G., Xie, L. Y., Jansen, R., Milaneschi, Y., Dean, B., Aberg, K. A., van den Oord, E. J. C. G., & Penninx, B. W. J. H. (2018). Epigenetic aging in major depressive disorder. The American Journal of Psychiatry, 175(8), 774–782. doi: 10.1176/appi.ajp.2018.17060595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, H., Child, S., Moore, S., Moore, J. B., & Kaczynski, A. T. (2016). The influence of neighborhood aesthetics, safety, and social cohesion on perceived stress in disadvantaged communities. American Journal of Community Psychology, 58(1-2), 80–88. doi: 10.1002/ajcp.12081 [DOI] [PubMed] [Google Scholar]
- Hickson, D. A., Diez Roux, A. V., Gebreab, S. Y., Wyatt, S. B., Dubbert, P. M., Sarpong, D. F., Sims, M., & Taylor, H. A. (2012). Social patterning of cumulative biological risk by education and income among African Americans. American Journal of Public Health, 102(7), 1362–1369. doi: 10.2105/AJPH.2011.300444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howick, J., Kelly, P., & Kelly, M. (2019). Establishing a causal link between social relationships and health using the Bradford Hill Guidelines. SSM—Population Health, 8, 100402. doi: 10.1016/j.ssmph.2019.100402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, D. L., Neighbors, H. W., Geronimus, A. T., & Jackson, J. S. (2016). Racial discrimination, John Henryism, and depression among African Americans. The Journal of Black Psychology, 42(3), 221–243. doi: 10.1177/0095798414567757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, J. R., & Fazio, E. M. (2005). Economic status over the life course and racial disparities in health. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60(Spec No. 2), 76–84. doi: 10.1093/geronb/60.special_issue_2.s76 [DOI] [PubMed] [Google Scholar]
- Klemera, P., & Doubal, S. (2006). A new approach to the concept and computation of biological age. Mechanisms of Ageing and Development, 127(3), 240–248. doi: 10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Krause, N. (2002). Exploring race differences in a comprehensive battery of church-based social support measures. Review of Religious Research, 44(2), 126–149. doi: 10.2307/3512512 [DOI] [Google Scholar]
- Landrine, H., & Corral, I. (2009). Separate and unequal: Residential segregation and black health disparities. Ethnicity & Disease, 19(2), 179–184. https://ethndis.org/priorarchives/ethn-19-02-179.pdf [PubMed] [Google Scholar]
- Levine, M. E. (2013). Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 68(6), 667–674. doi: 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, M. E., & Crimmins, E. M. (2014). Evidence of accelerated aging among African Americans and its implications for mortality. Social Science & Medicine (1982), 118, 27–32. doi: 10.1016/j.socscimed.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln, K. D., Chatters, L. M., & Taylor, R. J. (2005). Social support, traumatic events, and depressive symptoms among African Americans. Journal of Marriage and the Family, 67(3), 754–766. doi: 10.1111/j.1741-3737.2005.00167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., Chen, B. H., Assimes, T. L., Ferrucci, L., Horvath, S., & Levine, M. E. (2019). The role of epigenetic aging in education and racial/ethnic mortality disparities among older U.S. women. Psychoneuroendocrinology, 104, 18–24. doi: 10.1016/j.psyneuen.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, D. D., Hammond, W. P., Cole-Lewis, Y., Nuru-Jeter, A., & Melvin, T. (2013). Racial discrimination and depressive symptoms among African-American men: The mediating and moderating roles of masculine self-reliance and John Henryism. Psychology of Men & Masculinity, 14(1), 35–46. doi: 10.1037/a0028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. doi: 10.1016/s0006-3223(03)00177-x [DOI] [PubMed] [Google Scholar]
- McKetney, E. C., & Ragland, D. R. (1996). John Henryism, education, and blood pressure in young adults. The CARDIA study. Coronary Artery Risk Development in Young Adults Study. American Journal of Epidemiology, 143(8), 787–791. doi: 10.1093/oxfordjournals.aje.a008816 [DOI] [PubMed] [Google Scholar]
- Mezuk, B., Abdou, C. M., Hudson, D., Kershaw, K. N., Rafferty, J. A., Lee, H., & Jackson, J. S. (2013). “White Box” epidemiology and the social neuroscience of health behaviors: The environmental affordances model. Society and Mental Health, 3(2), 79–95. doi: 10.1177/2156869313480892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, Y. I., Anugu, P., Butler, K. R., Hartley, T. A., Mwasongwe, S., Norwood, A. F., Sims, M., Wang, W., Winters, K. P., Correa, A. (2017). Cardiovascular disease burden and socioeconomic correlates: Findings from the Jackson Heart Study. Journal of the American Heart Association, 6(8), e004416. doi: 10.1161/jaha.116.004416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neergheen, V. L., Topel, M., Van Dyke, M. E., Sullivan, S., Pemu, P. E., Gibbons, G. H., Vaccarino, V., Quyyumi, A. A., & Lewis, T. T. (2019). Neighborhood social cohesion is associated with lower levels of interleukin-6 in African American women. Brain, Behavior, and Immunity, 76, 28–36. doi: 10.1016/j.bbi.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors, H. W., Njai, R., & Jackson, J. S. (2007). Race, ethnicity, John Henryism, and depressive symptoms: The national survey of American life adult reinterview. Research in Human Development, 4(1–2), 71–87. doi: 10.1080/15427600701481004 [DOI] [Google Scholar]
- O’Donovan, A., Tomiyama, A. J., Lin, J., Puterman, E., Adler, N. E., Kemeny, M., Wolkowitz, O. M., Blackburn, E. H., & Epel, E. S. (2012). Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity, 26(4), 573–579. doi: 10.1016/j.bbi.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, A. D., Williams, D. R., Nwizu, U., & Gruenewald, T. L. (2017). Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cultural Diversity & Ethnic Minority Psychology, 23(1), 27–35. doi: 10.1037/cdp0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies, Y., Ben, J., Denson, N., Elias, A., Priest, N., Pieterse, A., Gupta, A., Kelaher, M., & Gee, G. (2015). Racism as a determinant of health: A systematic review and meta-analysis. PLoS One, 10(9), e0138511. doi: 10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rook, K. S. (2015). Social networks in later life: Weighing positive and negative effects on health and well-being. Current Directions in Psychological Science, 24(1), 45–51. doi: 10.1177/0963721414551364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, A. V. D., & Mair, C. (2010). Neighborhoods and health. Annals of the New York Academy of Sciences, 1186, 125–145. doi: 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
- RStudio Team . (2020). RStudio: Integrated development for R. RStudio. http://www.rstudio.com/ [Google Scholar]
- Seeman, T. E., Gruenewald, T. L., Cohen, S., Williams, D. R., & Matthews, K. A. (2014). Social relationships and their biological correlates: Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychoneuroendocrinology, 43, 126–138. doi: 10.1016/j.psyneuen.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Simons, R. L., Lei, M. K., Klopack, E., Beach, S. R. H., Gibbons, F. X., & Philibert, R. A. (2020). The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Social Science & Medicine (1982), 282, 113169. doi: 10.1016/j.socscimed.2020.113169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. S., Breakstone, H., Dean, L. T., & Thorpe, R. J., Jr. (2020). Impacts of gentrification on health in the US: A systematic review of the literature. Journal of Urban Health, 97(6), 845–856. doi: 10.1007/s11524-020-00448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp . (2019). Stata Statistical Software: Release 16. StataCorp LLC. https://www.stata-press.com/data/r16/u.html [Google Scholar]
- Szanton, S. L., Thorpe, R. J., & Whitfield, K. (2010). Life-course financial strain and health in African-Americans. Social Science & Medicine, 71(2), 259–265. doi: 10.1016/j.socscimed.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T. S., Brown, M. B., Funnell, M. M., & Anderson, R. M. (2008). Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. The Diabetes Educator, 34(2), 266–276. doi: 10.1177/0145721708315680 [DOI] [PubMed] [Google Scholar]
- Thorpe, R. J., Jr., Fesahazion, R. G., Parker, L., Wilder, T., Rooks, R. N., Bowie, J. V., Bell, C. N., Szanton, S. L., & LaVeist, T. A. (2016). Accelerated health declines among African Americans in the USA. Journal of Urban Health, 93(5), 808–819. doi: 10.1007/s11524-016-0075-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven, J. E., Révész, D., Epel, E. S., Lin, J., Wolkowitz, O. M., & Penninx, B. W. (2014). Major depressive disorder and accelerated cellular aging: Results from a large psychiatric cohort study. Molecular Psychiatry, 19(8), 895–901. doi: 10.1038/mp.2013.151 [DOI] [PubMed] [Google Scholar]
- Whitaker, K. M., Xiao, Q., Pettee Gabriel, K., Gordon Larsen, P., Jacobs, D. R., Jr., Sidney, S., Reis, J. P., Barone Gibbs, B., Sternfeld, B., & Kershaw, K. (2019). Perceived and objective characteristics of the neighborhood environment are associated with accelerometer-measured sedentary time and physical activity, the CARDIA Study. Preventive Medicine, 123, 242–249. doi: 10.1016/j.ypmed.2019.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield, K. E., Allaire, J. C., Belue, R., & Edwards, C. L. (2008). Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63(5), P301–P308. doi: 10.1093/geronb/63.5.p301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. R. (2018). Stress and the mental health of populations of color: Advancing our understanding of race-related stressors. Journal of Health and Social Behavior, 59(4), 466–485. doi: 10.1177/0022146518814251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. R., & Jackson, P. B. (2005). Social sources of racial disparities in health. Health Affairs (Project Hope), 24(2), 325–334. doi: 10.1377/hlthaff.24.2.325 [DOI] [PubMed] [Google Scholar]
- Williams, D. R., Yu, Y., Jackson, J. S., & Anderson, N. B. (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2(3), 335–351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Wolkowitz, O. M., Epel, E. S., Reus, V. I., & Mellon, S. H. (2010). Depression gets old fast: Do stress and depression accelerate cell aging? Depression and Anxiety, 27(4), 327–338. doi: 10.1002/da.20686 [DOI] [PubMed] [Google Scholar]