Abstract
Context
Idiopathic central precocious puberty (iCPP) is defined by the premature reactivation of the hypothalamic-pituitary-gonadal axis with normal magnetic resonance imaging scan of the central nervous system, causing the development of secondary sexual characteristics before age 8 years in girls and 9 years in boys. MKRN3 loss of function variants now represent the most common genetic cause of iCPP.
Objective
This work aims to document the clinical course of puberty in 8 families harboring pathogenic MKRN3 variants.
Methods
This is an observational case series study of patients with CPP due to MKRN3 variants followed in a single center.
Results
Genetic analysis of MKRN3 was carried out in 28 unrelated patients with iCPP and a family history of paternal inheritance or no/unavailable maternal inheritance, particularly in case of very early and rapidly evolving CPP. We identified 6 novel and 2 recently described variants in the MKRN3 gene in 9 girls, 1 boy, and their family members. These mutations were all predicted to be deleterious by in silico prediction programs
Conclusion
We have identified 6 novel MKRN3 mutations in children with CPP. An MKRN3 loss of function should be considered after careful history pinpointing paternally inherited CPP. A family segregation study allowed the detection of an MKRN3 variant in 2 young brothers still prepubertal, raising the question of screening and management of asymptomatic prepubertal family members.
Keywords: precocious puberty, MKRN3, familial CPP, genetics of puberty
Puberty is defined by the development of secondary sex characteristics and achievement of fertility. It results from the reactivation of the hypothalamic-pituitary-gonadal axis, but the precise mechanisms leading to its onset are still not fully known, involving genetic, environmental, nutritional, and socioeconomic factors. Puberty is considered precocious if it starts before age 8 years in girls and 9 years in boys. Central precocious puberty (CPP) can be caused by a central nervous system lesion or classified as idiopathic (iCPP), with approximately 27.5% of familial cases [1]. A lot of studies have been conducted in an attempt to find the genetic variations that can lead to CPP. The first description of a monogenic form of CPP was an activating KISS1R mutation [2], followed by the report of a KISS1 variant [3]. These genes were likely candidates as key players in congenital hypogonadotropic hypogonadism, but they turned out to be rarely involved in CPP. No further case has been described to date despite a large screening. In 2013, Abreu et al [4] used an unprecedented approach by performing whole-exome sequencing in 15 families with CPP. This study led to the discovery of heterozygous loss-of-function mutations of the makorin RING finger 3 gene (MKRN3) as a cause of familial CPP. MKRN3 is located on chromosome 15q11.2 in the Prader-Willi syndrome critical region. It is a maternally imprinted gene, therefore only the paternally inherited allele is expressed, and the mutated allele must be inherited from the father to lead to CPP. MKRN3 encodes the makorin ring-finger protein 3 with a potential effect on gonadotropin-releasing hormone (GnRH) secretion. The MKRN3 protein is composed of 3 C3H1 zinc fingers, the MKRN3 type Cys-His region, and 1 C3H4 RING finger, responsible for ubiquitine ligase activity [4]. Since this publication, various other variants have been described, making MKRN3 loss-of-function mutations the main genetic cause of CPP to date [5, 6]. These findings suggest that MKRN3 acts to inhibit puberty initiation, and therefore support a current hypothesis of a predominantly inhibitory network that maintains quiescence of GnRH secretion during infancy, followed by a release of this inhibition together with the activation of an excitatory network allowing the restoration of the pulsatile secretion of GnRH, leading to the initiation of puberty.
In an elegant publication in 2020, Abreu et al [7] demonstrated that this action of MKRN3 is made, at least in part, by repressing KISS1 and TAC3 transcription. Another important finding about the putative mechanism of action of MKRN3 was described by Li and colleagues [8], who demonstrated the importance of MBD3 (methyl-CpG-DNA binding protein 3) ubiquitination by MKRN3 to silence GNRH1.
Recently, a publication identified a paternally inherited DLK1 deletion in a family with CPP [9]. Subsequently, 2 other publications reported loss-of-function mutations in this paternally expressed imprinted gene in CPP patients [10, 11]. This discovery emphasized the importance of genomic imprinting in the mechanism of puberty onset.
In this study, we report the clinical course of puberty in 10 patients from 8 unrelated families harboring distinct pathogenic MKRN3 variants. Six variants are novel and 2 have been recently reported [5, 12–14].
Materials and Methods
Patients
We reported patients with confirmed CPP followed in the Paediatric Endocrinology Clinic of the Hôpital Universitaire des Enfants Reine Fabiola from 2015 to 2021, and harboring pathogenic MKRN3 variants, but normal KISS1 and KISS1R. Patients were not screened for DLK1 mutations. CPP was diagnosed on the basis of the presence of clinical signs of pubertal development before age 8 years in girls and 9 years in boys with advanced bone age (BA) and pubertal basal and/or peak luteinizing hormone (LH) levels after stimulation by GnRH. Patients underwent genetic testing because of a family history suggesting paternal inheritance, the presence of a sibling with CPP or a pubertal onset before age 7 years, and no maternal history. However, it is important to note that the analysis was not part of a protocol and was therefore not systematic. We considered CPP as familial if at least one first- or second-degree relative had documented or reported CPP. BA was determined by the Greulich and Pyle method. We proposed family segregation analysis.
Hormone Assays
Serum levels of LH, follicle-stimulating hormone (FSH), and estradiol or testosterone (T) were measured by immunochemiluminometric assay (Roche Cobas 8000 E801 module). The lower limit detection was 0.3 mUI/mL and the upper limit was 200 mUI/mL both for LH and FSH. The coefficient of variation ranged from 1.98% to 2% for LH and 1.61 to 1.66% for FSH for all assays. GnRH stimulation test was performed using 100 μg of synthetic GnRH administered by intravenous bolus, and serum levels of LH and FSH were measured at 0, 30, 45, and 60 minutes. We considered as pubertal a basal LH level greater than 0.3 U/L and/or a GnRH-stimulated LH peak greater than 5 U/L.
Genetic Analysis
Genomic DNA was isolated from peripheral blood leukocytes of the index case patients and their relatives when possible. Written informed consent for this genetic analysis was obtained from all the members tested or from the parents of children who had not reached legal majority. The coding region and intronic borders of the MKRN3 gene (GRCh37; NM_005664.3) were analyzed using the Sanger sequencing method [15].
Statement of Ethics
The parents and relatives of the index case patients have given their written informed consent to publish their case and the case of their children. The study was approved by the ethical committee of the Hôpital Universitaire des Enfants Reine Fabiola.
Results
Genetic analysis of MKRN3 was carried out in 28 unrelated patients with iCPP and a family history of paternal inheritance or absent maternal inheritance of CPP.
We identified 10 patients carrying a pathogenic MKRN3 variant. Fourteen relatives of 5 probands agreed to undergo genetic analysis.
Clinical Features of Central Precocious Puberty Patients With MKRN3 Variant
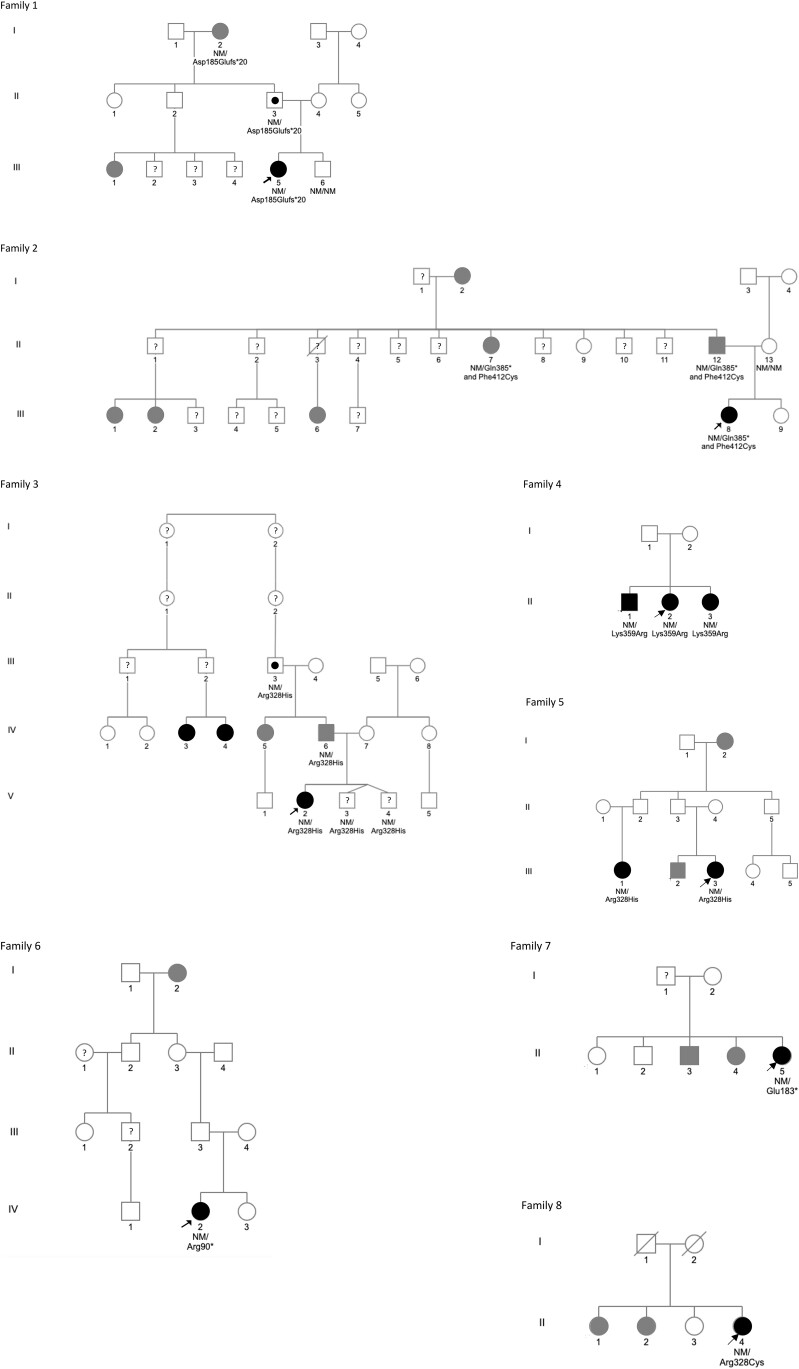
Nine patients from 8 families with MKRN3 mutations were girls, and they all presented with a history of isolated thelarche before age 8 years (median 7.0, range, 6.0-7.3 years). The boy was seen for the first time at age 11.7 years at Tanner stage 4 and with a serum T in the adult range. BA was advanced in all cases (median 2.2, range, 1.3-4.2 years). The diagnosis of CPP was confirmed by GnRH stimulation test, with median basal LH levels of 2.7 IU/L (range, 0.1-20.3 IU/L) and GnRH-stimulated levels of 22 IU/L (range, 9.2 to > 200 IU/L). The median basal and GnRH-stimulated FSH levels were 4.9 IU/L (range, 2.1-8.7 IU/L) and 13.6 IU/L (range, 10.1-53.2 IU/L), respectively. The 9-year-old girl from family 1 had menarche 8 days after the GnRH stimulation test and has not been treated with GnRH agonists. Her adult height is within her target height range. The boy from family 4 was not treated either and is still growing. The 8 other girls are currently on GnRH analogues (Fig. 1, Table 1).
Figure 1.
Pedigrees of the 8 families with MKRN3 pathogenic variants. Arrows: proband; square: male; circle: female; black symbols: clinically affected individuals; gray symbols: self-reported central precocious puberty; question mark: unknown phenotype/very young patient; black dot: asymptomatic carriers; NM: nonmutated allele; roman numerals: generation; arabic numerals: individuals in each generation.
Table 1.
Clinical and hormonal data of affected children with central precocious puberty carrying an MKRN3 pathogenic variant
| Family / Index case |
Sex | Family ancestry | Age at pubertal onset, y | Age at diagnosis, y | Tanner stage at diagnosis | BMI, SDS | ΔBA-CA, y | Basal LH/ poststimulated LH, IU/L |
Basal FSH/ poststimulated FSH, IU/L |
E2, ng/L T, nmol/L |
Ht at diagnosis, SDS | ΔHt-TH, SDS | FH, SDS | ΔFH – TH, SDS | MKRN3 mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1/
III-5 |
F | Belgium | 7.0 | 9.0 | A2P4M4 | 0.7 | 3 | 20.3 >200 |
6.7 53.2 |
170 | 1.8 | 2.3 | −1 | 0.5 | c.555_556delCA, p.(Asp185Glufs*20) Frameshift |
|
2/
III-8 |
F | Belgium | 7.3 | 7.6 | A1P1M2 | 0 | 1.9 | 0.1 9.2 |
2.1 14.6 |
<5 | 2 | 1.2 | / | / | c.1153C > T, p.(Gln385*) Nonsense c.1235T > G, p.(Phe412Cys) Missense |
|
3/
V-2 |
F | Belgium | 6.4 | 6.5 | A1P1M2 | 0.5 | 1.3 | 0.8 15.3 |
4.5 10.4 |
<25 | 0.9 | 1.7 | / | / | c.983G > A, p.(Arg328His) Missense |
|
4/
II-1 |
M | Cameroon | ? | 11.7 | A3P3G4 | 1.2 | 2 | 2.8 40 |
4.9 11.2 |
15.7 | 0.4 | 1 | / | / | c.1076A > G, p.(Lys359Ar) Missense |
|
4/
II-2 |
F | Cameroon | 6.8 | 7.2 | A2P2M2 | 1.3 | 2.8 | 2.9 16.4 |
8.7 15.4 |
45 | 1.5 | 2.1 | / | / | c.1076A > G, p.(Lys359Ar) Missense |
|
4/
II-3 |
F | Cameroon | 6.8 | 7.2 | A1P1M2 | 0 | 1.5 | <1 13.9 |
2.4 17.3 |
<25 | 0.1 | 0.7 | / | / | c.1076A > G, p.(Lys359Ar) Missense |
|
5/
III-3 |
F | Belgium | 7.3 | 8.3 | A1P1M3-4 | 1.6 | 2.1 | 3.4 40.1 |
4.7 10.1 |
29 | 1.5 | 0.2 | 0.1 | 1.2 | c.983G > A, p.(Arg328His) Missense |
|
6/
IV-2 |
F | Belgium | 7 | 8.7 | A2P3M3 | 0.3 | 2.3 | 2.6 23.4 |
5.4 11.4 |
46 | 0.9 | 0.5 | / | / | c.268C > T, p.(Arg90*) Nonsense |
|
7/
II-5 |
F | Morocco | 8.4 | 8.7 | A2P2M3 | 1.1 | 2.3 | 3.1 104.8 |
5.5 33.8 |
12 | −0.8 | 0.3 | / | / | c.547G > T, p.(Glu183*) Nonsense |
|
8/
II-4 |
F | Angola | 6 | 6.8 | A1P1M3 | 1.6 | 4.2 | 1.64 20.6 |
4.86 12.6 |
52 | 2.7 | 2.4 | / | / | c.982C > T, p.(Arg328Cys) Missense |
Abbreviations: BA, bone age; CA, chronological age; E2, estradiol; F, female; FH, final height; FSH, follicle-stimulating hormone; LH, luteinizing hormone; M, male; SDS, SD score; T, testosterone; TH, target height.
MKRN3 Variants
We identified 6 novel and 2 recently described [5, 12–14] variants in the MKRN3 gene of 9 girls, 1 boy, and their family members. These mutations were all predicted to be deleterious by in silico prediction programs (Fig. 2, Table 1, Table 2). In family 1, the variant p.(Asp185Glufs*20) was a frameshift mutation between 2 zinc finger motifs C3H1. In family 2, genetic analysis showed 2 variants: The first one p.(Gln385*) was a nonsense mutation with a premature interruption of the open reading frame by a stop codon between the C3HC4 RING finger and a C3H1 zinc finger motif. The second one on the same allele p.(Phe412Cys) was a missense mutation located in a zinc finger domain and predicted to be deleterious by 3 different in silico prediction programs (SIFT, Mutation Taster, and PolyPhen2), but probably not responsible for the phenotype of our patient given the location of the previous variant. Both variants were paternally inherited. In family 3, we identified a missense mutation p.(Arg328His) also located in a RING-type zinc finger domain and recently described [12–14]. Patient 4, her affected brother, and her affected sister carried a missense mutation p.(Lys359Arg) located also in the RING-type zinc finger domain with contradictory in silico predictions but assumed to be deleterious by 2 programs (Mutation Taster, PolyPhen2). The proband from family 5 carried the same mutation as in family 3. In family 6 and in family 7, we identified a nonsense mutation with a premature interruption of the open reading frame by a stop codon (Arg90* and Glu183*, respectively). In family 8, the variant was a missense mutation in the RING-type zinc finger domain p.(Arg328Cys) already described in other patients [5, 13, 14].
Figure 2.
Schematic structure of the MKRN3 human protein with the variants identified in our families. The MKRN3 protein is composed of 3 C3H zinc fingers, the MKRN-type Cys-His region, and 1 C3HC4 RING finger. The numbers indicate the amino acid positions in the protein. The arrows indicate the location of the variants identified in the study.
Table 2.
MKRN3 variants identified in patients with central precocious puberty and pathogenicity evaluation
| Family | MKRN3 variant | MAF Gnom AD | Clinvar | HGMD | Mutation Taster | Polyphen 2 | SIFT |
|---|---|---|---|---|---|---|---|
| 1 | c.555_556delCA, p.(Asp185Glufs*20) | Absent | Absent | Absent | Disease causing | a | a |
| 2 | c.1153C > T, p.(Gln385*) | Absent | Absent | Absent | Disease causing | a | a |
| 2 | c.1235T > G, p.(Phe412Cys) | Absent | Absent | Absent | Disease causing | Probably damaging | Deleterious |
| 3 | c.983G > A, p.(Arg328His) | 8,79E-06 | Absent | Disease causing | Disease causing | Probably damaging | Deleterious |
| 4 | c.1076A > G, p.(Lys359Arg) | Absent | Absent | Absent | Disease causing | Probably damaging | Tolerated |
| 5 | c.983G > A, p.(Arg328His) | 8,79E-06 | Absent | Disease causing | Disease causing | Probably damaging | Deleterious |
| 6 | c.268C > T, p.(Arg90*) | Absent | Absent | Absent | Disease causing | a | a |
| 7 | c.547G > T, p.(Glu183*) | Absent | Absent | Absent | Disease causing | a | a |
| 8 | c.982C > T, p.(Arg328Cys) | Absent | Pathogenic | Disease causing | Disease causing | Probably damaging | Deleterious |
For frameshift and nonsense mutations, these tools were not appropriate.
Family Segregation Analysis
Genetic analysis was performed in 14 relatives of the patients.
Paternal inheritance is clinically documented in families 1, 2, 3, 5, and 6 and documented genotypically in family 1, family 2 and family 3 (Fig. 1, Table 3). In family 4, 3 siblings are affected carriers and paternal transmission is suspected given the normal pubertal age of the mother.
Table 3.
Clinical data of affected relatives of index cases
| Family, relative | Sex | Age at menarche (F)/pubertal onset (M), y | Final height, SDS | Target height, SDS | MKRN3 variant(s) | Other clinical information |
|---|---|---|---|---|---|---|
| 1, I-2 | F | 8 | −1.5 | Unknown | c.555_556delCA, p.(Asp185Glufs*20) |
/ |
| 1, III-1 | F | 8 | Unknown | Unknown | Unknown | / |
| 2, I-2 | F | 8 | −0.6 | Unknown | Unknown | / |
| 2, II-7 | F | 8 | −2.5 | −0.4 | c.1153C > T, p.(Gln385*) c.1235T > G, p.(Phe412Cys) |
/ |
| 2, II-12 | M | 9.5 | −0.3 (measured) | −0.4 | c.1153C > T, p.(Gln385*) c.1235T > G, p.(Phe412Cys) |
/ |
| 2, III-1 | F | 10 | −0.6 | Unknown | Unknown | / |
| 2, III-2 | F | 9 | Not reached yet | Unknown | Unknown | / |
| 2, III-6 | F | 9.5 | Not reached yet | Unknown | Unknown | / |
| 3, IV-3 | F | M2 at 6 | Unknown | −1 | Unknown | Treated with GnRHa |
| 3, IV-4 | F | M2 at 7.8 | Not reached yet | −1 | Unknown | Treated with GnRHa |
| 3, IV-5 | F | 6.5 | −2.8 | −1.5 | c.983G > A, p.(Arg328His) | Obesity |
| 3, IV-6 | M | 9 | −1.1 (measured) | −1.5 | c.983G > A, p.(Arg328His) | / |
| 5, I-2 | F | 9 | −1.6 | Unknown | Unknown | / |
| 5, III-1 | F | 9 | −1.5 | −1.1 | c.983G > A, p.(Arg328His) | Obesity |
| 7, II-3 | M | 9-10 | −1.6 | −1.3 | Unknown | 2 y of prednisone for necrotizing keratitis |
| 7, II-4 | F | 9 | −2.8 | −1.3 | Unknown | / |
Abbreviations: F, female; GnRHa, gonadotropin-releasing hormone agonist; M, male.
The carriers twin brothers of patient V.2 of family 3 are young (aged 4 years) and have not shown signs of puberty yet.
In family 2, it is interesting to note that the paternal grandmother (family 2: I-2) reported a history of precocious puberty. Unfortunately, her DNA was not available. However, given the mode of transmission, her phenotype cannot be related to an MKRN3 variant. For individuals II-7 and II-12 of family 2, we assume that both mutations are in cis. Indeed, if both mutations were in trans, it would imply that patient III-8 inherited both variants from her father (her mother being negative for both variants). In turn, this would imply an uniparental disomy, which would lead to Angelman syndrome and this patient did not have it.
When possible, we collected clinical data from the relatives. Two of them had been followed in our center and were treated by GnRH agonists, but declined genetic analysis (family 3: IV-3, IV-4). The other 11 girls with reported CPP had had menarche at a median age of 8.5 years (range, 6.5-10.0 years). The most severely affected was the paternal aunt of index case number 3 (family 3: IV-5) with a menarche age of 6.5 years. The 2 fathers with a personal history of CPP (family 2: II-12 and family 3: IV-6) reported a pubertal onset at 9.5 years and 9 years, respectively. Regarding final heights, in the 3 families with enough data, girls were below their target height, while their brothers reached their target height (family 2: II-12; family 3: IV-6; family 7: II-3) (see Table 2).
Discussion
We identified 6 novel and 1 recently described MKRN3 pathogenic variants in 9 girls and 1 boy with iCPP: 4 missense mutations, 3 nonsense mutation, and 1 frameshift mutation. One of the girls presented with 2 mutations. This is the first study describing a family with 2 distinct pathogenic MKRN3 variants, probably located on the same allele. Segregation analysis was conducted in 14 family members, and clinical information about the relatives was collected. We identified 6 relatives also carrying an MKRN3 loss-of-function variant.
MKRN3 is an intronless gene located on chromosome 15q11-q13 in the Prader-Willi syndrome region, described for the first time in 1999 [16]. It is a maternally imprinted gene thus expressed only from the paternally inherited allele, coding for a zinc finger protein composed by 3 C3H1 zinc fingers, 1 motif rich in Cys and His residues and 1 C3H4 RING zinc finger. MKRN3 is a member of the makorin family of ubiquitin ligases and appears to have been acquired more recently than the other ubiquitin ligases, as it is conserved only in therian mammals. It is ubiquitously expressed with a predominant expression in the brain and lung of human fetal tissues and in adult testes. Abreu et al [4] also demonstrated a high level of MKRN3 messenger RNA in the arcuate nucleus of prepubertal mice, with a decrease in expression before puberty initiation. The mechanism by which MKRN3 loss of function leads to CPP is only partially understood. Recently, a study indicated that MKRN3 does not directly influence GNRH1 expression but may interact with several other proteins involved in pubertal onset [17]. Another study demonstrated that MKRN3 represses the promoter activity of human KISS1 and TAC3, 2 stimulators of GnRH secretion, probably by an ubiquitination-mediated mechanism [7]. MKRN3 also ubiquitinates MBD3, which epigenetically silences GNRH1 [8].
Since 2013, a total of 50 MKRN3 loss-of-function variants have been reported in the literature [4, 18–22]. There are 2 apparent hot spots. The first one is located in the loop structure between 2 C3H1 zinc finger motifs in the amino terminal region of the protein (between codons 122 and 238) and mainly housing frameshift mutations, as in patient 1. The second one concerns the C3HC4 domain (between codons 311 and 365) with mostly missense mutations, as in patient 3 (this variant was recently described) and 4. The 2 mutations present in patient 2 are located further downstream in the coding region.
In their review, Valadares et al [18] make the assumption that missense mutations are less severe, since the diagnosis of CPP is made later in these patients. Our findings are not consistent with this hypothesis given that the patients with a missense mutation were the youngest at the onset of puberty, with a frank poststimulated LH peak. The paternal aunt of patient 3, who is carrying the same missense mutation, also had a severe phenotype with menarche at 6.5 years. Seraphim et al [19] characterized the pathogenicity of the missense variants using Yasara software, which analyzes the effect of the mutation on the stability of the protein. Unfortunately, this type of analysis was not available to us.
Regarding the mode of inheritance, it is interesting to point out that, if the proband's father has inherited the variant from his mother, as in family 1, he will not experience CPP himself. In this situation, a family history going back to the third generation will be needed to demonstrate the familial character of CPP. This is difficult to obtain given that it is common for patients to have no information regarding their father's relatives pubertal timing. Consequently, it is likely that MKRN3 mutations are underdiagnosed among patients erroneously considered to have sporadic CPP (family 4, II-2). Since our case series is monocentric, the access to the patient history might have been more accurate and detailed, which facilitates the selection of patients for whom genetic analysis is recommended. Of note, among 28 requested genetic analysis, 10 came back positive for an MKRN3 variant. We suggest testing in case of paternal inheritance but also when family history is unreliable.
It is also important to note that all but one of the affected children were girls, and that more female than male individuals with a loss-of-function MKRN3 variant are described in the literature. This may be due to underdiagnosis of CPP in boys. Another hypothesis to explain this skewed sex ratio is that Y-bearing sperm cells carrying MKRN3 pathogenic variants could be less efficient in fertilizing the ovocyte [5]. This hypothesis is supported by data reporting fewer boys in affected families, which is not obvious in our small cohort.
Sexual dimorphism in the action of MKRN3 has also been suggested with a later age at pubertal onset in boys carrying the variant [13]. It is interesting to note that in our series, among the adults with clinical data and a pathogenic MKRN3 variant, the 3 women had an earlier onset of puberty and a final height below their target height compared to the affected men (family 2: II-12; family 3: IV-6; family 7: II-3). A recent study described a male patient with a history of untreated CPP and a pathogenic MKRN3 variant who eventually reached his target height [20]. Seraphim et al [19] also reported that 87.5% of male patients were diagnosed through familial screening, which might suggest that male CPP caused by MKRN3 mutations can be clinically subtle.
We proposed screening for family segregation the family members of affected patients, including the prepubertal siblings. As in 3 previous studies [5, 6, 23], we identified 2 very young boys carriers of MKRN3 variants in family 3. This allows a close clinical and biological follow-up to detect the early onset of pubertal signs, although this raises ethical questions. We have opted for clinical follow-up, with performance of an LH-releasing hormone test in case of clinical suspicion. Should they present with CPP, it is questionable whether systematic magnetic resonance imaging of the central nervous system is indicated in these patients. Finally, several authors have reported a decline in circulating MKRN3 levels before pubertal onset and through puberty in healthy children and children with precocious puberty [17, 24–26]. However, none of these individuals carried an MKRN3 variant. It would be interesting to study the evolution of MKRN3 level of the still-prepubescent children screened in our cohort.
In conclusion, in a single center, we have identified 6 novel MKRN3 pathogenic variants causing CPP, strengthening the evidence for the involvement of this gene in this condition. An MKRN3 defect should be considered after careful history pinpointing paternally inherited CPP or in case of nonmaternally inherited CPP. Indeed, one of the patients was initially considered as having sporadic precocious puberty, highlighting the interest of a broader screening, especially in case of an incomplete family history. Puberty started early in our patients and their affected relatives, particularly in girls, whose final height seemed more reduced, but only a few adults could be studied. We also identified 2 young and still asymptomatic siblings. The implementation of a specific monitoring protocol for these patients remains to be established.
Acknowledgments
We thank our study nurses, Lambert Leenders and Frédérique Schwilden, for their help in the management and treatment of newly diagnosed patients with CPP. We thank all the patients and their families for their contribution.
Abbreviations
- BA
bone age
- CCP
central precocious puberty
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- iCCP
idiopathic CCP
- LH
luteinizing hormone
- MKRN3
makorin RING finger 3 gene
- T
testosterone
Contributor Information
Caroline Gernay, Email: cgernay@chuliege.be, Paediatric Endocrinology Unit, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, 1020 Brussels, Belgium.
Cécile Brachet, Paediatric Endocrinology Unit, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, 1020 Brussels, Belgium.
Emese Boros, Paediatric Endocrinology Unit, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, 1020 Brussels, Belgium.
Sylvie Tenoutasse, Paediatric Endocrinology Unit, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, 1020 Brussels, Belgium.
Cécile Libioulle, Department of Genetics, Centre Hospitalier Universitaire du Sart-Tilman, Université de Liège, 4000 Liège, Belgium.
Claudine Heinrichs, Email: claudine.heinrichs@huderf.be, Paediatric Endocrinology Unit, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, 1020 Brussels, Belgium.
Disclosures
The authors have nothing to disclose.
Data Availability
Some data sets generated and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89(4):1794–1800. [DOI] [PubMed] [Google Scholar]
- 2. Teles MG, Bianco SDC, Brito VN, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silveira LG, Noel SD, Silveira-Neto AP, et al. Mutations of the KISS1 gene in disorders of puberty. J Clin Endocrinol Metab. 2010;95(5):2276–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon D, Ba I, Mekhail N, et al. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol. 2016;174(1):1–8. [DOI] [PubMed] [Google Scholar]
- 6. Stecchini M, Macedo DB, Reis ACS, et al. Time course of central precocious puberty development caused by an MKRN3 gene mutation: a prismatic case. Horm Res Paediatr. 2016;86(2):126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130(8):4486–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li C, Lu W, Yang L, et al. MKRN3 regulates epigenetic switch of mammalian puberty via ubiquitination of MBD3. Natl Sci Rev. 2020;7(3):671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dauber A, Cunha-Silva M, Macedo DB, et al. Paternally inherited DLK1 deletion associated with familial central precocious puberty. J Clin Endocrinol Metab. 2017;102(5):1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomes LG, Cunha-Silva M, Crespo RP, et al. DLK1 is a novel link between reproduction and metabolism. J Clin Endocrinol Metab. 2019;104(6):2112–2120. [DOI] [PubMed] [Google Scholar]
- 11. Montenegro L, Labarta J, Piovesan M, et al. Novel genetic and biochemical findings of DLK1 in children with central precocious puberty: a Brazilian-Spanish study. J Clin Endocrinol Metab. 2020;105(10):3165–3172. [DOI] [PubMed] [Google Scholar]
- 12. Bessa DS, Maschietto M, Aylwin CF, et al. Methylome profiling of healthy and central precocious puberty girls. Clin Epigenetics. 2018;10(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bessa DS, Macedo DB, Brito VN, et al. High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology. 2017;105(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grandone A, Capristo C, Cirillo G, et al. Molecular screening of MKRN3, DLK1 and KCNK9 genes in girls with idiopathic central precocious puberty. Horm Res Paediatr. 2017;88(3-4):194–200. [DOI] [PubMed] [Google Scholar]
- 15. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74(12):5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jong MTC, Gray TA, Ji Y, et al. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8(5):783–793. [DOI] [PubMed] [Google Scholar]
- 17. Yellapragada V, Liu X, Lund C, et al. MKRN3 interacts with several proteins implicated in puberty timing but does not influence GNRH1 expression. Front Endocrinol. 2019;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valadares LP, Meireles CG, de Toledo IP, et al. MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. J Endocr Soc. 2019;3(5):979–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seraphim CE, Canton APM, Montenegro L, et al. Genotype-phenotype correlations in central precocious puberty caused by MKRN3 mutations. J Clin Endocrinol Metab. 2021;106(4):e1041-–e1050.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varimo T, Iivonen AP, Kansakoski J, et al. Familial central precocious puberty: two novel MKRN3 mutations. Pediatr Res. 2021;90(2):431–435. [DOI] [PubMed] [Google Scholar]
- 21. Liu M, Fan L, Gong CX. A novel heterozygous MKRN3 nonsense mutation in a Chinese girl with idiopathic central precocious puberty: a case report. Medicine (Baltimore). 2020;99(38):e22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yin X, Wang J, Han T, et al. A novel loss-of-function MKRN3 variant in a Chinese patient with familial precocious puberty. Front Genet. 2021;12:663746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grandone A, Cantelmi G, Cirillo G, et al. A case of familial central precocious puberty caused by a novel mutation in the makorin RING finger protein 3 gene. BMC Endocr Disord. 2015;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hagen CP, Sørensen K, Mieritz MG, Johannsen TH, Almstrup K, Juul A. Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab. 2015;100(5):1920–1926. [DOI] [PubMed] [Google Scholar]
- 25. Bush AS, Hagen CP, Almstrup K, Juul A. Circulating MKRN3 levels decline during puberty in healthy boys. J Clin Endocrinol Metab. 2016;101(6):2588–2593. [DOI] [PubMed] [Google Scholar]
- 26. Grandone A, Cirillo G, Sasso M, et al. MKRN3 levels in girls with central precocious puberty and correlation with sexual hormone levels: a pilot study. Endocrine. 2018;59(1):203–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data sets generated and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.