Abstract
BACKGROUND & AIMS:
Controlled attenuation parameter (CAP) measurements using M probe have been reported to be lower than those of the XL-probe in detection of hepatic steatosis. However, there has been no direct comparison of CAP with the M vs the XL probe in patients with nonalcoholic fatty liver disease (NAFLD). We compared CAP with the M vs the XL probe for quantification of hepatic fat content, using magnetic resonance imaging proton density fat fraction (MRI-PDFF) as the standard.
METHODS:
We performed a prospective study of 100 adults (mean body mass index [BMI], 30.6 ± 4.7 kg/m2) with and without NAFLD, assessed by CAP with the M probe and XL probe on the same day, at a single research center, from November 2017 through November 2018. We then measured the MRI-PDFF as the reference standard. Outcomes were presence of hepatic steatosis, defined as MRI-PDFF ≥ 5%, and detection of hepatic fat content ≥ 10%, defined as MRI-PDFF ≥ 10%. We performed area under the receiver operating characteristic curve (AUROC) analyses to assess the diagnostic accuracy of CAP for each probe in detection of hepatic steatosis (MRI-PDFF ≥ 5%) and of hepatic fat content ≥ 10%.
RESULTS:
Of the study participants, 68% had an MRI-PDFF of 5% or more and 48% had an MRI-PDFF of 10% or more. The mean CAP measured by the M probe (310 ± 62 db/m) was significantly lower than by the X probe (317 ± 63 db/m) (P = −007). When M probe was used in participants with BMIs <30 kg/ m2 and XL probe in participants with BMIs ≥30 kg/m2, the CAP measured by the M probe (312 ± 51.4 db/m) remained significantly lower than that of the XL probe (345 ± 47.6 db/m) (P =−0035.), when the MRI-PDFF was above 5%. The optimal threshold of CAP for the detection of MRI-PDFF≥5%, was 294 db/mwith the M probe and 307 db/mwith the XL probe. The optimal threshold of CAP for the detection of MRI-PDFF ≥ 10%, was 311 db/m with the M probe and 322 db/m with the XL probe. For only the XL probe, CAP measurements with an interquartile range below 30 dB/m detected an MRI-PDFF≥5% with a lower AUROC (0.97; 95% CI, 0.80–1.00) than CAP measurements with an interquartile range above 30 dB/m (AUROC, 0.82; 95% CI, 0.71–0.90) (P = .0129).
CONCLUSIONS:
In an analysis of the same patients using CAP with the M probe and XL probe, with MRI-PDFF as the standard, we found that the M probe under-quantifies CAP values compared with the XL probe, independent of BMI. The type of probe should be considered when interpreting CAP data from patients with NAFLD.
Keywords: FibroScan, Liver Fat, NASH, Diagnostic
Nonalcoholic fatty liver disease (NAFLD) is now recognized as the most frequent liver disease in Western countries, affecting approximately one-third of the adult population in Western countries.1 Despite its growing epidemic, NAFLD remains largely underdiagnosed, even though it is likely to progress toward nonalcoholic steatohepatitis, liver fibrosis, or cirrhosis. In order to improve the detection and screening of NAFLD, noninvasive and widely available techniques that can clinically assess hepatic fat content are needed.
Although liver biopsy is considered as the reference method for the diagnosis of NAFLD, it is impracticable for the screening at the level of the population because of its cost and risk related to this invasive procedure, and is limited by significant inter- and intraobserver variability.2,3 Magnetic resonance imaging (MRI) that measures the proton density fat fraction (MRI-PDFF) has emerged as the leading noninvasive modality for hepatic fat content in NAFLD in terms of accuracy, precision, and reproducibility.4–7 However, like liver biopsies, MRI is expensive and not routinely accessible in many centers.
Currently, conventional ultrasonography is widely used as first-line assessment of hepatic steatosis. However, it is limited by a lack of quantitative accuracy, has a lack of sensitivity in low hepatic fat content, and is operator dependent.8 The controlled attenuation parameter (CAP) acquired by FibroScan (Echosens, Paris, France) is another ultrasound-based modality allowing rapid, noninvasive, bedside assessment of hepatic steatosis.9 The development of the XL probe has reduced the failure rate observed with the M probe, especially in patients with higher body mass index (BMI).10,11 Recent studies have suggested that the same cutoff can be used for the interpretation of the liver stiffness measurement using either XL or M probe in NAFLD.12,13 However, the impact of the type of probe on the measurement of CAP in NAFLD is not clear.
Chan et al14 reported a significantly lower value of CAP measurements with the M probe compared with the XL probe in Asian patients with NAFLD using a categorical histological grade of steatosis from liver biopsies. However, to really provide a relevant comparison of CAP measurement for the detection of hepatic steatosis, a reference method using a quantitative modality should be used. We have previously determined the optimal threshold of CAP using either M or Xl probe for the detection of the presence of NAFLD using MRI-PDFF as the gold standard.15 However, head-to-head comparison of consecutive measurements of CAP with both the M and XL probes vs MRI-PDFF as reference performed the same day in patients with and without NAFLD has not been reported yet.
Therefore, we conducted a prospective study including well-characterized American adults with and without NAFLD who underwent CAP measurement using both M and XL probes for the quantification of liver fat content and MRI-PDFF assessment as the gold standard.
Materials and Methods
Study Participant and Design
This is a prospective study designed to compare CAP measurement using M vs the XL probe for the detection of hepatic steatosis in participants prospectively recruited and screened for the presence of hepatic steatosis, using MRI-PDFF as reference. All participants underwent MRI-PDFF and CAP measurements using a FibroScan with both M and XL probes. We followed the Standards for Reporting of Diagnostic Accuracy guidelines in this study of CAP in detecting hepatic steatosis (Supplementary Table 1).
Study participants were recruited at the NAFLD Research Center at the University of California, San Diego (UCSD) between November 2017 and November 2018; 113 potential participants with risk factors of NAFLD were screened, and 105 participants complied with the study protocol and underwent MRI-PDFF and CAP assessment with both the M and XL probes; 100 participants were included in the final analysis with MRI-PDFF assessment using both the M and XL probes (Supplementary Figure 1). All participants underwent a careful evaluation for other causes of hepatic steatosis and liver disease and were invited for a clinical research visit with standardized history, physical and anthropometric exam, fasting biochemical testing, transient elastography, and CAP assessment at the UCSD NAFLD Research Center16–19 and advanced MRI-based phenotyping at the UCSD MR3T Research Laboratory. This study was compliant with the Health Insurance Portability and Accountability Act, informed written consent was obtained from all patients, and the study was approved by the UCSD Institutional Review Board (no. 171333). Please see the Supplementary Material for detailed clinical research evaluation inclusion and exclusion criteria.
Outcome Measures
The primary outcome was the presence of hepatic steatosis, defined as MRI-PDFF ≥ 5%. The secondary outcome was the detection of hepatic fat content ≥ 10%, defined as MRI-PDFF ≥ 10%, as this threshold is used in several therapeutic trials as inclusion criteria.
CAP Measurement
CAP measurement was performed by a trained technician blinded to clinical and MRI results, using the FibroScan 502 Touch model (M probe; XL probe; Echosens). Detailed methods have been previously described in Caussy et al.15 All participants underwent 2 consecutives measurement of CAP by the same FibroScan using both M (3.5 MHz) and XL (2.5 MHz) probe. Please see the Supplementary Material.
Magnetic Resonance Imaging
MRI-PDFF advanced MRI-based phenotyping was performed at the UCSD MR3T Research Laboratory using the 3T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI), with all participants in the supine position. MRI-PDFF was used to measure hepatic steatosis, defined as MRI-PDFF ≥ 5%. The details of the MRI protocol have been previously described in Caussy et al.19 The image analysts were blinded to all clinical and biochemical data.
Rationale for Using MRI-PDFF for the Quantification of Hepatic Steatosis as Gold Standard
The rationale for using MRI-PDFF as a gold standard can be summarized by the following points. First, to really provide a relevant comparison of CAP measurement with the M vs the XL probe for the quantitative detection of hepatic steatosis, a reference method using a quantitative modality should be used. MRI-PDFF is a highly precise, accurate, and reproducible quantitative noninvasive biomarker for the quantification of liver fat content.20,21 Second, to be able to compare CAP measurement with the M vs the XL probe for the detection of NAFLD, participants with NAFLD (MRI-PDFF ≥ 5%) and without NAFLD (MRI-PDFF<5%) are needed, and it would have been unethical to perform a liver biopsy in normal participants, who did not have a clinical indication of performing a liver biopsy.
Statistical Analyses
Comparison between CAP using M and XL probes were performed using paired nonparametric Wilcoxon test. Receiver-operating characteristic curve analyses were used to assess the diagnostic accuracy of CAP for each probe for the detection of hepatic steatosis (MRI-PDFF ≥ 5%) and of hepatic fat content ≥ 10%. The optimal thresholds maximizing both sensitivity and specificity of each modality were determined using the Youden index.22 Area under the receiver-operating characteristic curve (AUROCs) in an independent subgroup according to the interquartile range (IQR) of CAP were compared using Hanley and McNeil method.23
Sample Size Estimation
We proceeded based on a previous study showing a mean difference of CAP measurement between the M and XL probes of 41 dB/m with a standard deviation of 60 and assuming a correlation between M and XL probe of 0.50.15 A projected sample size of 84 participants is needed to detect a significant difference between M and XL probe using a paired t test with a power of 0.80 and alpha of 0.05. Therefore, this study including 100 participants had enough power to detect significant difference between CAP measured using the M probe vs using the XL probe.
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), SPSS V.21 (IBM, Armonk, NY), or MedCalc V.18.11 (Ostend, Belgium). A 2-tailed P value ≤.05 was considered statistically significant.
Results
Study Participants
A total of 100 participants with or without NAFLD with MRI-PDFF assessment and CAP measurements using both M and XL probes were included in the analysis (Table 1). The majority of the participants (66%) had an MRI and CAP with the M and XL probes the same day and the mean time between MRI and CAP assessment was 11.3 (95% confidence interval [CI], 6.2–16.5) days. Both CAP measurements with the M and XL probes were significantly correlated, with hepatic fat content assessed by MRI-PDFF (Supplementary Figure 2).
Table 1.
Baseline Characteristics of the Cohort Stratified by the Presence of NAFLD (MRI-PDFF ≥5%)
| Characteristics | Overall (N = 100) | Non-NAFLD MRI-PDFF <5% (n = 32) | NAFLD MRI-PDFF ≥5% (n = 68) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 57.5 (13.2) | 57.0 (16.3) | 57.7 (11.6) | .803 |
| Female | 56 (56) | 14 (43.8) | 42 (61.8) | .075 |
| White | 42 (42) | 14 (43.8) | 28 (41.2) | .624 |
| Hispanic or Latino | 36 (36) | 11 (34.4) | 25 (36.8) | .971 |
| Clinical | ||||
| Type 2 diabetes | 56 (56) | 16 (50) | 40 (58.8) | .407 |
| BMI, kg/m2 | 30.6 (4.7) | 28.4 (5.1) | 31.6 (4.2) | <.001a |
| Waist circumference, cm | 103.0 (15.6) | 97.8 (15.0) | 105 (15.4) | .004a |
| Obesity | 55 (55) | 12 (37.5) | 43 (63.2) | .010a |
| Biological data | ||||
| AST, U/L | 37.4 (34.3) | 26.7 (10.2) | 42.4 (40.2) | .031a |
| ALT, U/L | 45.3 (57.2) | 27.5 (20.8) | 53.7 (66.3) | .018a |
| Alkaline phosphatase, U/L | 81.7 (27.9) | 85.5 (34.9) | 79.8 (24.0) | .339 |
| GGT, U/L | 57.6 (64.7) | 51.7 (71.3) | 60.9 (61.1) | .377 |
| Total bilirubin, mg/dL | 0.50 (0.25) | 0.48 (0.21) | 0.5 (0.3) | .446 |
| Direct bilirubin, mg/dL | 0.22 (0.13) | 0.20 (0.0) | 0.23 (0.15) | .338 |
| Albumin, g/dL | 4.45 (0.27) | 4.42 (0.27) | 4.5 (0.3) | .572 |
| Glucose, mg/dL | 125.6 (49.7) | 116.3 (45.1) | 129.9 (51.4) | .843 |
| Hemoglobin A1c, % | 6.7 (1.8) | 6.5 (1.8) | 6.8 (1.7) | .302 |
| Triglycerides, mg/dL | 156.5 (76.8) | 128.6 (73.6) | 170.1 (75.3) | .028a |
| Total cholesterol, mg/dL | 186.9 (40.5) | 187.2 (41.8) | 186.7 (40.3) | .814 |
| HDL-cholesterol, mg/dL | 47.1 (12.0) | 54.5 (12.0) | 43.5 (10.3) | .001a |
| LDL-cholesterol, mg/dL | 108.3 (35.1) | 107.6 (33.2) | 108.6 (36.3) | .776 |
| Platelet count (109/L) | 249.3 (73.7) | 260.4 (84.1) | 243.8 (68.1) | .241 |
| Prothrombin time | 11.1 (1.3) | 10.9 (0.7) | 11.3 (1.6) | .648 |
| INR | 1.0 (0.1) | 1.0 (0.7) | 1.1 (0.1) | .770 |
| Clinical prediction rules | ||||
| AST/ALT ratio | 0.99 (0.35) | 1.12 (0.29) | 0.93 (0.35) | .004a |
| FIB-4 | 1.53 (1.08) | 1.40 (1.07) | 1.59 (1.09) | .443 |
| NAFLD fibrosis score | −1.183 (1.546) | −1.467 (1.987) | −1.040 (1.266) | .608 |
| Imaging data | ||||
| MRI-PDFF, % | 11.3 (9.3) | 2.1 (1.31) | 15.7 (8.1) | <.001a |
| MRE, kPa | 2.6 (1.1) | 2.3 (0.78) | 2.68 (1.28) | .454 |
| FibroScan M probe | ||||
| Liver stiffness | 6.6 (4.0) | 4.9 (2.9) | 7.5 (3.9) | .004a |
| IQR of TE | 1.0 (1.1) | 0.7 (1.1) | 1.2 (1.2) | .025a |
| IQR/M | 16 (11) | 15 (12) | 16 (10) | .137 |
| Unreliable liver stiffnessb | 3 (3) | 1 (3.1) | 2 (2.9) | 1.000 |
| CAP, dB/m | 310 (76) | 261 (84) | 323 (61) | <.001a |
| IQR of CAP | 24 (19) | 26 (24) | 22 (18) | .034a |
| FibroScan XL probe | ||||
| Liver stiffness | 5.4 (3.0) | 4.8 (3.5) | 5.8 (2.8) | .093a |
| IQR | 0.9 (0.8) | 0.8 (0.8) | 1.0 (0.8) | .566 |
| IQR/median | 18.0 (11) | 16 (13) | 18 (10) | .537 |
| Unreliable liver stiffnessb | 5 (5) | 2 (6.3) | 3 (4.4) | 1.000 |
| CAP, dB/m | 317 (76) | 259 (96) | 340 (64) | <.001a |
| IQR of CAP | 42.0 (27) | 46.5 (30) | 40.5 (29) | .007a |
Values are median (IQR) or n (%). The P value determined by comparing patients without NAFLD and with NAFLD using an independent-samples t test, Wilcoxon 2-sample test, or a chi-square or Fisher exact test, as appropriate.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; FIB-4, Fibrosis-4; GGT, γ-glutamyltransferase; HDL, high-density lipoprotein; INR, international normalized ratio; IQR, interquartile range; LDL, low-density lipoprotein; MRE, magnetic resonance elastography; MRI-PDFF, magnetic resonance imaging–proton density fat fraction; NAFLD, nonalcoholic fatty liver disease; TE, transient elastography.
P value <.05.
Unreliable liver stiffness was defined as number of valid measurement <10 and/or IQR/median >30%.29
Lower CAP Measurement Using the M Probe Compared With the XL Probe When Hepatic Fat Content Increases
The CAP measurement using the M probe was significantly lower compared with the CAP measurement using the XL probe: median CAP 310 ± 61.6 dB/m vs 317 ± 63.2 dB/m (P = .007) (Table 2). When stratified by hepatic fat content, CAP measurements using the M probe were significantly lower compared with measurements using the XL probe in NAFLD participants with MRI-PDFF ≥ 5%: median CAP 323.5 ± 46.5 dB/m vs 340.6 ± 52.1 dB/m (P = .017), whereas no statistical difference was observed in participants with low hepatic fat content (MRI-PDFF <5%): median CAP 261.5 ± 60.6 dB/m vs 259.5 ± 54.6 dB/m (P = .235) (Supplementary Figure 3). When the M and XL probes were used in participants with BMI <30 kg/m2 and ≥ 30 kg/m2, respectively, CAP measurement with the M probe remained significantly lower compared with the XL probe when MRI-PDFF increased above 5%: 312 ± 53 vs 345 ± 60 (P = .0035) (Figure 1).
Table 2.
CAP Measurement Using the M Probe Is Significantly Lower Compared With the XL Probe
| Overall (N = 100) |
MRI-PDFF <5% (n = 32) |
MRI-PDFF ≥5% (n = 68) |
MRI-PDFF ≥10% (n = 48) |
|
|---|---|---|---|---|
| M probe CAP | 310 (61.6) | 261.5 (60.6) | 323.50 (46.5) | 333.5 (33.7) |
| XL probe CAP | 317 (63.2) | 259.5 (54.6) | 340.63 (52.1) | 350.0 (35.5) |
| P value | .007a | .235 | .017a | .003a |
The P value determined using a paired nonparametric Wilcoxon test.
CAP, controlled attenuation parameter; MRI-PDFF, magnetic resonance imaging–proton density fat fraction.
P value <.05.
Figure 1.
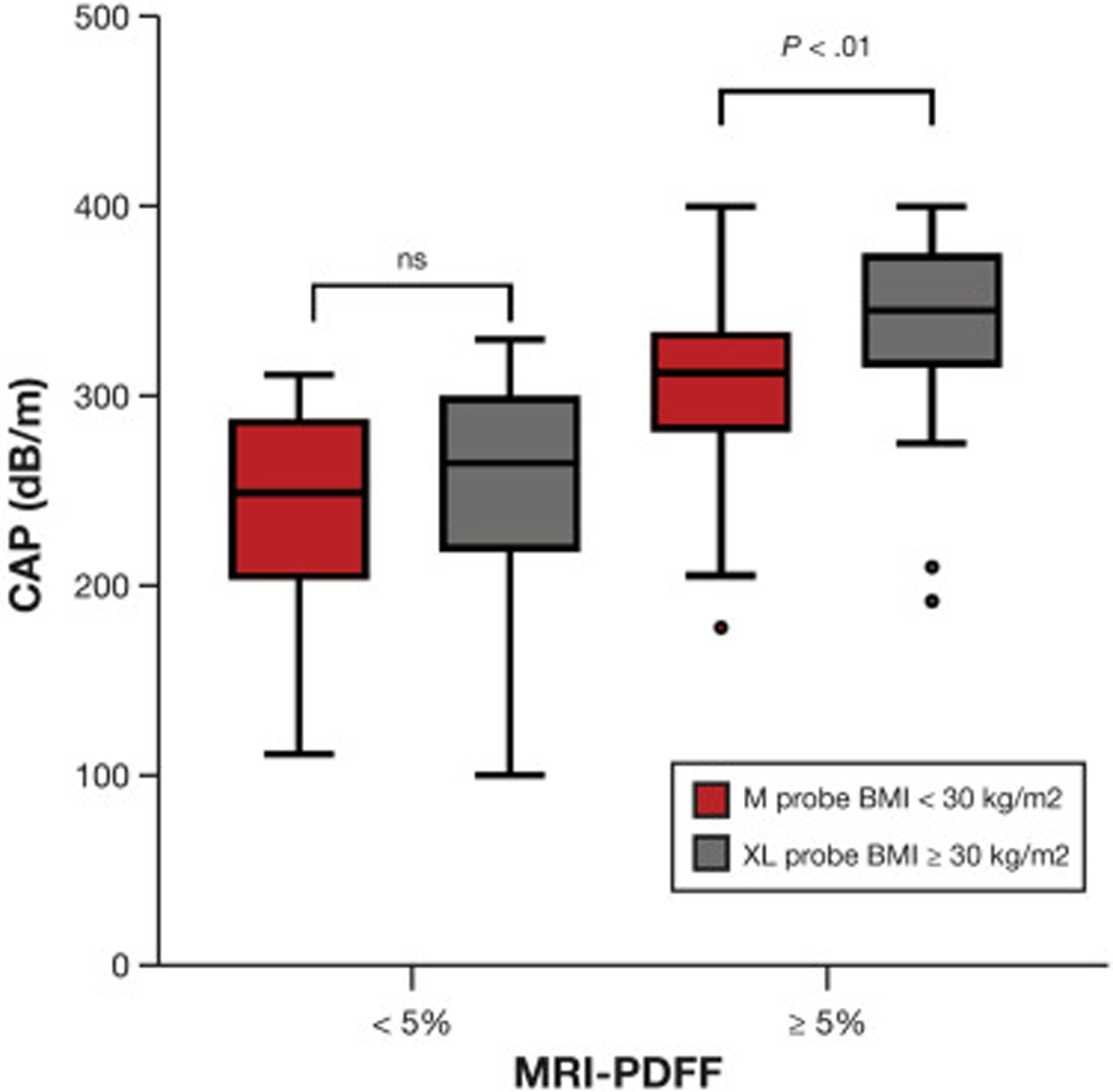
CAP measurements when the M probe is used in participants with BMI <30 kg/m2 and the XL probe in those with BMI ≥30 kg/m2. The horizontal lines in the boxes represent median values, the boxes present the IQRs, and the whiskers represent 1.5 times the IQR.
Optimal Threshold of CAP for the Detection of Hepatic Steatosis (MRI-PDFF ≥5%) and MRI-PDFF ≥10% Depends on the Type of Probe
The diagnostic performance data of CAP using the M vs the XL probe for the detection of MRI-PDFF ≥5% and MIR-PDFF ≥10% are provided in Supplementary Figure 4 and Table 3. The optimal threshold for the detection of MRI-PDFF ≥ 5% was 294 dB/m using the M probe vs 307 dB/m using the XL probe. Likewise, the optimal threshold for the detection of MRI-PDFF ≥ 10% was 311 dB/m using the M probe vs 322 dB/m using the XL probe (Table 3).
Table 3.
Diagnostic Accuracy of CAP for the Detection of Hepatic Steatosis
| AUROC (95% CI) | Cutoff (dB/m) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| Detection of MRI-PDFF ≥5% | ||||||
| CAP XL probe | ||||||
| Threshold for 90% sensitivity | 0.836 (0.72–0.89) | 261.4 | 90 | 50.0 | 79.5 | 72.7 |
| Optimal threshold | 294 | 75.0 | 78.1 | 87.9 | 59.5 | |
| Threshold for 90% specificity | 316 | 58.8 | 90 | 90.9 | 50.0 | |
| CAP XL probe | ||||||
| Threshold for 90% sensitivity | 0.858 (0.73–0.90) | 281.4 | 90 | 65.6 | 84.9 | 77.8 |
| Optimal threshold | 307 | 73.5 | 75.0 | 86.2 | 57.1 | |
| Threshold for 90% specificity | 323.8 | 61.8 | 90 | 91.3 | 51.9 | |
| Detection of MRI-PDFF ≥10% | ||||||
| CAP M probe | ||||||
| Threshold for 90% sensitivity | 0.886 (0.80–0.94) | 293.6 | 90 | 70.8 | 73.3 | 69.2 |
| Optimal threshold | 311 | 79.2 | 84.6 | 82.6 | 81.5 | |
| Threshold for 90% specificity | 326.8 | 58.3 | 90 | 82.4 | 69.7 | |
| CAP XL probe | ||||||
| Threshold for 90% sensitivity | 0.93 (0.86–0.97) | 314.2 | 90 | 82.7 | 83.0 | 91.5 |
| Optimal threshold | 322 | 83.3 | 86.5 | 85.1 | 84.9 | |
| Threshold for 90% specificity | 323.8 | 83.3 | 90 | 87.0 | 85.2 |
AUROC, area under the receiver-operating characteristic curve; CAP, controlled attenuation parameter; CI, confidence of interval; MRI-PDFF, magnetic resonance imaging–proton density fat fraction; NPV, negative predictive value; PPV, positive predictive value.
Sensitivity Analyses of the Performance of CAP Using the M Probe vs the XL Probe for the Detection of Hepatic Steatosis
We have previously demonstrated that the diagnostic accuracy of CAP is more reliable when IQR of CAP is below 30 dB/m. When CAP measurements were stratified by IQR of CAP, the direct comparison of the AUROC of CAP for the detection of hepatic steatosis (MRI-PDFF ≥5%) showed that the CAP with IQR of CAP below 30 dB/m was significantly more accurate than the CAP with IQR of CAP above 30 dB/m with an AUROC of 0.97 (95% CI, 0.80–1.00) vs 0.82 (95% CI, 0.71–0.90) (P = .0129) when using the XL probe (Figure 2A) but not when using the M probe (Figure 2B, Table 3). In addition, no significant differences of the diagnostic accuracy of CAP using the XL probe or M probe were observed when stratified by the IQR of CAP below vs above 40 dB/m (Supplementary Table 2). Finally, CAP measurements using the M or XL probe were not correlated with liver stiffness assessed by magnetic resonance elastography (Supplementary Figure 5).
Figure 2.
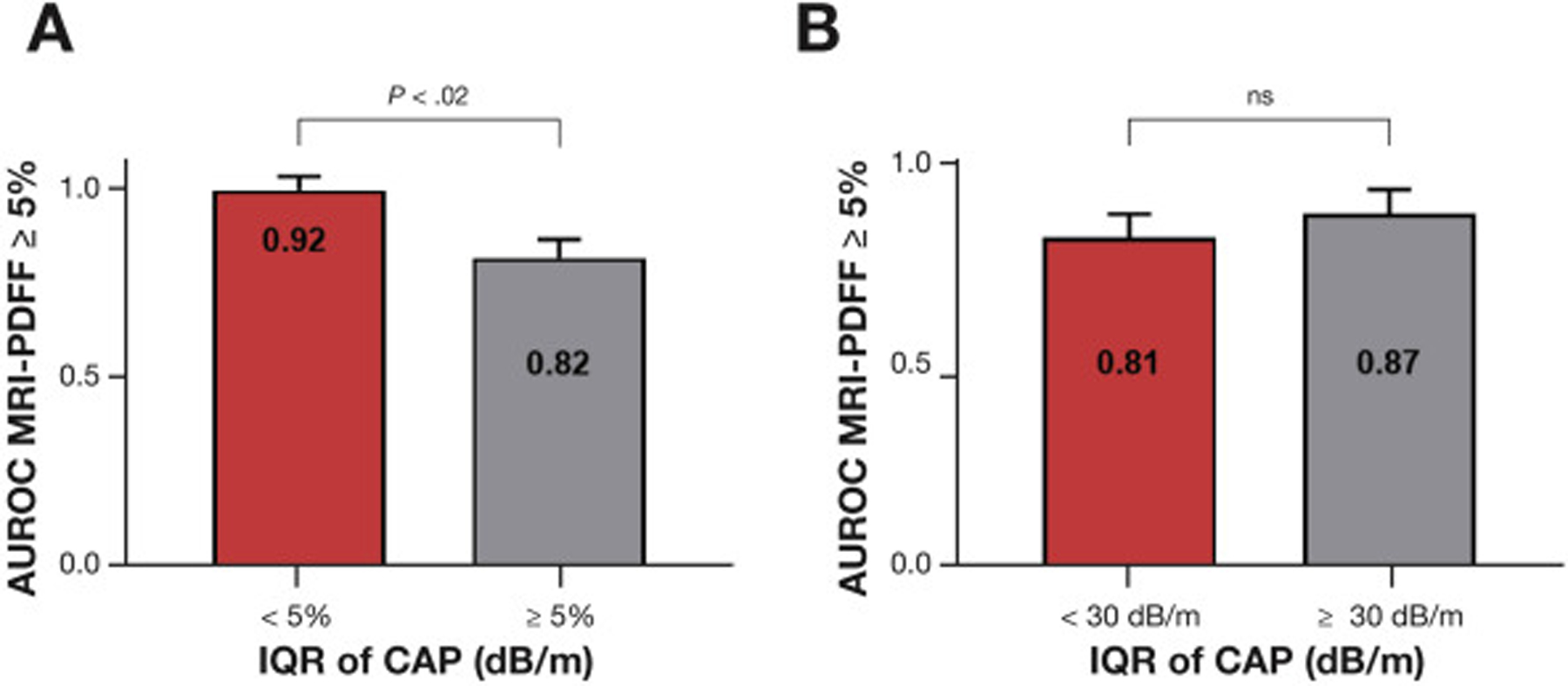
The diagnostic accuracy of CAP with only the XL probe increases when the IQR of CAP is <30 dB/m. The AUROC of CAP and the 95% CI for the detection of hepatic steatosis defined by MRI-PDFF ≥ 5% stratified by IQR of CAP using (A) the XL probe and (B) the M probe. P values were determined using the method by Hanley and McNeil.
Discussion
Main Findings
We conducted a prospective study including well-characterized American adults with NAFLD and without NAFLD who underwent CAP measurement using both the M and XL probes for the quantification of liver fat content and MRI-PDFF assessment as the gold standard. We report that CAP measurements using the M probe underestimate the hepatic fat content compared with CAP measurements using the XL probe for the same quantity of MRI-PDFF hepatic fat content, even when the probe is selected according to the BMI of the participant with NAFLD. The key novelty of this study is to prospectively provide head-to-head comparison between CAP assessment using the M and XL probes for the detection of the same hepatic fat content by an accurate and quantitative reference using MRI-PDFF in a Western population with and without NAFLD. These results have important implication in the clinical use of CAP, as they consequently impact the optimal thresholds for the detection of NAFLD. Finally, this study confirms that an IQR of CAP below 30 dB/m significantly improves the diagnostic accuracy of CAP measurement but only using the XL probe. Hence, these results have direct application in routine clinical practice, as it will help clinicians interpreting CAP measurements depending on the type of probe used (Figure 3).
Figure 3.
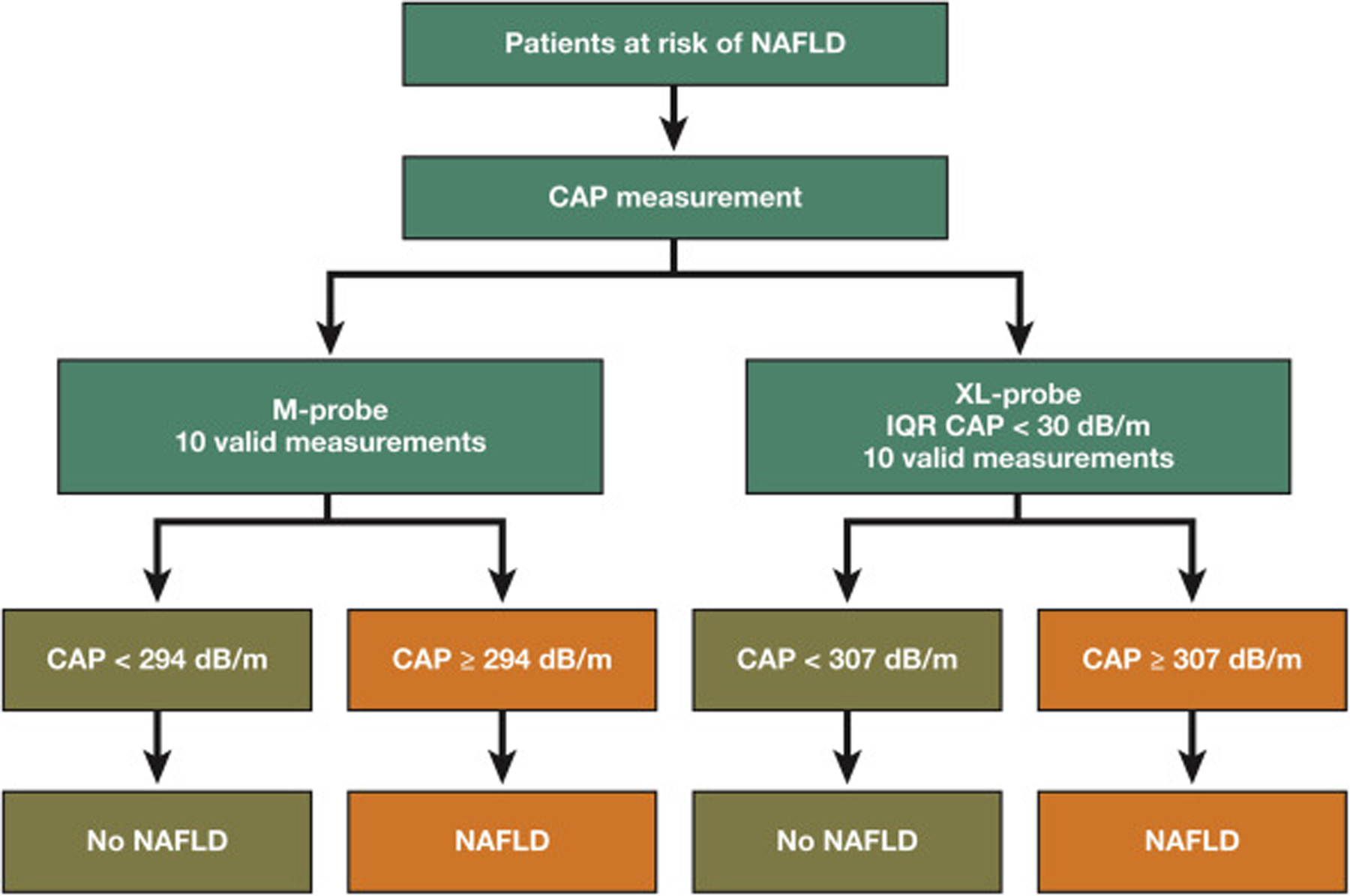
Probe specific optimal strategy for the screening of NAFLD using CAP measurement.
In Context With the Published Literature
This is the first prospective study to compare CAP measurements using both the M and XL probes for the detection of hepatic steatosis in a well-characterized cohort of American adults with and without NAFLD using advanced quantitative MRI-PDFF as the gold standard.
The current study confirms the good diagnosis accuracy of CAP for the detection of hepatic steatosis using both probes.10,11 Previous studies have reported lower CAP values with the M probe compared with the XL probe.15,24 However, these studies were limited by the lack of consecutive CAP assessments using both M and XL probes in the same participants and for the same quantity of hepatic fat content. Recently, Chan et al14 assessed CAP measurements using both M and XL probe in an Asian cohort of patients with NAFLD using histological grade of steatosis as the reference. The authors showed a significant lower value of CAP using the M probe compared with the XL probe using histological grade of steatosis as the reference.14 In line with our findings, Chan et al have observed that CAP measurements using M probe were significantly lower compared with the XL probe in a higher grade of steatosis (S2 and S3) but not in a low grade of steatosis (S0 and S1). Contrary to this study that used the liver biopsy as reference, we used a highly accurate and quantitative method for the quantification of hepatic steatosis, which allowed direct comparison between 2 quantitative modalities as opposed to the comparison with histological subjective estimates of steatosis grade on an ordinal scale limited by sampling error. Finally, in the study performed by Chan et al, only a minority of patients with steatosis grade 0 (9.4% [which would equate with an MRI-PDFF of <5%]) were included. These differences are crucial, as differences in CAP with the M vs the XL probe are observed when the quantity of liver fat increased but not in lower hepatic fat content with PDFF below 5%. Therefore, our study is complimentary to this previous study and provides a more robust comparison of CAP using M vs the XL probe for detection of hepatic steatosis at a threshold of MRI-PDFF of 5% and 10%.
Studies have reported that although the M and XL probes may yield different liver stiffness values when applied to the same patient, the optimal thresholds for both probes are similar when they are used according to BMI12,25; our study demonstrates that this is not the case for CAP measurements, as CAP measurement remains significantly lower with the XL probe when the probe is selected based on the BMI.
Finally, different quality criteria of CAP have been previously reported.15,26 Wong et al26 reported a higher diagnostic performance of CAP when the IQR of CAP was below 40 dB/m using only the M probe in a cohort of patients with various causes of chronic liver diseases with a mean BMI of 27.2 kg/m2. In our previous study, the IQR of CAP below 30 dB/m significantly increased the diagnostic accuracy of CAP using either the M or XL probe.15 In a recent study with CAP measurement using either the M or XL probe based on an automatic probe selection tool compared with histological grade of steatosis, Eddowes et al13 did not observe that IQR of CAP <30 dB/m or <40 dB/m improves the diagnosis performance of CAP. However, in this study, we confirm that the diagnostic accuracy of CAP for the detection of hepatic steatosis is significantly more reliable when the IQR of CAP is below 30 dB/m, but only using the XL probe and not using the M probe, in an independent cohort with a mean BMI of 30.6 kg/m2, which represents the usual population screened for NAFLD. As Eddowes et al did not perform head-to-head comparison of CAP measurement with both the M and XL probes, this important difference could not have been observed. Moreover, as an IQR of CAP <30 dB/m significantly improves the diagnostic accuracy of CAP only with the XL probe, the pooled analysis of CAP measurements with either the M or XL probe performed by Eddowes et al may have underestimated the impact of the IQR of CAP as quality criteria using the XL probe. Hence, the type of probe used should be considered when interpreting quality criteria of CAP measurement, and an IQR of CAP below 30 dB/m should be considered as a quality indicator that significantly improves the diagnostic performance of CAP using the XL probe for the detection of hepatic steatosis in NAFLD.
Strengths and Limitations
There are several notable strengths of this study including the prospective design that included well-characterized participants with and without NAFLD systematically screened by a standardized liver assessment to exclude for any other cause of chronic liver disease including excessive alcohol consumption before inclusion in the study. In addition, the cohort was recruited by experienced investigators at a dedicated research center specialized for both clinical and radiologic research in NAFLD. All participants underwent consecutive CAP assessment using both the M and XL probe and advanced MRI-PDFF for the quantification of hepatic fat content, with majority of the assessment performed on the same day.
However, we acknowledge the following limitations of this study. The cross-sectional design of the study did not allow for comparison of CAP monitoring using the M vs the XL probe for monitoring longitudinal changes in hepatic fat content. In addition, the study was conducted in a highly specialized tertiary center using an MRI technique that may not be available in other centers, and the generalizability of these findings in other clinical settings is unknown. Moreover, the liver biopsy was not performed in this study, as the study was designed to compare CAP measurements with the M vs XL probe, which comprise quantitative modalities; therefore, a quantitative biomarker of hepatic fat content should be used as a gold standard. We used the most accurate noninvasive quantitative modality that has emerged as a novel standardized biomarker for assessing hepatic steatosis.27 We acknowledge that the higher prevalence NAFLD may impact the interpretation of the result, as differences between CAP with the M and XL probes are observed especially when liver fat content increases above 5%. However, the proportion of participants with NAFLD in this cohort may represent the context of use in the setting of the screening for NAFLD using CAP in a high-risk population such type 2 diabetes patients with a similar prevalence of approximately 60%–70% of NAFLD in this population.28
Implications for Clinical Care and Future Research
Using a prospective study, we demonstrated that CAP measurements using the M probe yielded a lower value compared with the XL probe for the same hepatic fat content in individuals with NAFLD, especially when hepatic fat content increases. These important findings suggest that probe-specific thresholds should be used when interpreting CAP measurement. The use of these new thresholds will help to further assess the clinical utility of CAP for the detection of hepatic steatosis and its cost-effectiveness compared with other modalities to develop optimal strategies for the screening of NAFLD.
Supplementary Material
What You Need to Know.
Background
The controlled attenuation parameter (CAP) is measured by FibroScan and involves use of the M or XL probe to identify patients with nonalcoholic fatty liver disease (NAFLD).
Findings
Measurements of CAP using the M probe significantly underestimated hepatic fat content, compared with measurements made by the XL probe, when magnetic resonance imaging–proton density fat fraction was used as the standard, independent of body mass index. CAP interquartile ranges below 30 dB/m significantly increase the diagnostic accuracy of CAP measurements but only for the XL probe.
Implications for patient care
The type of probe should be considered when interpreting CAP data from patients with NAFLD. Different thresholds should be applied depending of the type of probe used for the detection of hepatic fat content ≥5% and ≥10%.
Funding
This work was supported by Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association, and National Institutes of Health grant K23-DK090303 (to Rohit Loomba). Rohit Loomba is supported in part by the American Gastroenterological Association Foundation–Sucampo–ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. The project described and Rohit Loomba were partially supported by National Institute of Environmental Health Sciences grant 5P42ES010337 and National Center for Advancing Translational Sciences grant 5UL1TR001442. Claude B. Sirlin and Rohit Loomba also received support from the National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK106419. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper:
- AUROC
area under the receiver-operating characteristic curve
- BMI
body mass index
- CAP
controlled attenuation parameter
- CI
confidence interval
- IQR
interquartile range
- MRI
magnetic resonance imaging
- MRI-PDFF
magnetic resonance imaging–proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- UCSD
University of California, San Diego
Footnotes
Publisher's Disclaimer: This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e95. Learning Objective–Upon completion of this activity, successful learners will be able to manage a patient with nonalcoholic fatty liver disease including noninvasive tests for the stage of the disease and treatment.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2019.11.060.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017–1044. [DOI] [PubMed] [Google Scholar]
- 3.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–1906. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015; 61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idilman IS, Aniktar H, Idilman R, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013;267:767–775. [DOI] [PubMed] [Google Scholar]
- 8.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasso M, Miette V, Sandrin L, et al. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol 2012; 36:13–20. [DOI] [PubMed] [Google Scholar]
- 10.de Ledinghen V, Hiriart JB, Vergniol J, et al. Controlled attenuation parameter (CAP) with the XL probe of the Fibroscan(R): a comparative study with the M probe and liver biopsy. Dig Dis Sci 2017;62:2569–2577. [DOI] [PubMed] [Google Scholar]
- 11.Chan WK, Nik Mustapha NR, Wong GL, et al. Controlled attenuation parameter using the FibroScan(R) XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United Eur Gastroenterol J 2017; 5:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong VW, Irles M, Wong GL, et al. Unified interpretation of liver stiffness measurement by M and XL probes in non-alcoholic fatty liver disease. Gut 2019;68:2057–2064. [DOI] [PubMed] [Google Scholar]
- 13.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019; 156:1717–1730. [DOI] [PubMed] [Google Scholar]
- 14.Chan WK, Nik Mustapha NR, Mahadeva S, et al. Can the same controlled attenuation parameter cut-offs be used for M and XL probes for diagnosing hepatic steatosis? J Gastroenterol Hepatol 2018;33:1787–1794. [DOI] [PubMed] [Google Scholar]
- 15.Caussy C, Alquiraish MH, Nguyen P, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018;67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caussy C, Tripathi A, Humphrey G, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun 2019;10:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caussy C, Bhargava M, Villesen IF, et al. Collagen formation assessed by N-terminal propeptide of type 3 procollagen is a heritable trait and is associated with liver fibrosis assessed by magnetic resonance elastography. Hepatology 2019; 70:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caussy C, Ajmera VH, Puri P, et al. Serum metabolites detect the presence of advanced fibrosis in derivation and validation cohorts of patients with non-alcoholic fatty liver disease. Gut 2019;68:1884–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caussy C, Soni M, Cui J, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest 2017;127:2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeder SB. Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology 2013; 58:1877–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36. [DOI] [PubMed] [Google Scholar]
- 24.Vuppalanchi R, Siddiqui MS, Van Natta ML, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology 2018; 67:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger A, Shili S, Zuberbuhler F, et al. Liver stiffness measurement with FibroScan: use the right probe in the right conditions! Clin Transl Gastroenterol 2019;10:e00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong VW, Petta S, Hiriart JB, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol 2017;67:577–584. [DOI] [PubMed] [Google Scholar]
- 27.Caussy C, Reeder SB, Sirlin CB, et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018;68:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48:835–847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


