Abstract
The cross-bactericidal and cross-protective activities of a monoclonal antibody (MAb) named Me-7, which is directed against an antigenically highly conserved epitope on the meningococcal NspA protein, were studied. This MAb efficiently killed in vitro, in the presence of rabbit or human serum, 13 of 14 meningococcal strains tested, including 9 of 9, 2 of 3, and 2 of 2 strains of serotypes B, A, and C, respectively. MAb Me-7 also significantly reduced by more than 75% the levels of bacteremia recorded for mice challenged with 10 of 11 meningococcal strains tested. Analysis of the predicted amino acid sequence of the NspA protein from the meningococcal strain MCH88 (A:4:P1.10), which was not killed by MAb Me-7, indicated the presence of an additional glutamine residue at position 73, compared to the three other NspA sequences. The data presented in this study suggest that antibodies directed against this highly conserved outer membrane protein could protect against meningococcal infections.
Neisseria meningitidis, the etiologic agent of meningococcal meningitis and meningococcemia, is still an important cause of mortality and morbidity throughout the world (12, 19). However, there is presently no vaccine available against serogroup B meningococci, which are responsible for between 30 and 70% of the meningococcal infections in industrialized countries (4–6). Since this capsular polysaccharide is poorly immunogenic in humans, the emphasis for the development of a serogroup B vaccine has therefore been directed toward the identification of protective surface antigens (5, 6, 20). Ideally, such an antigen would be a conserved protein, exposed at the surface of the meningococcus, that would elicit the production of bactericidal antibodies. Such bactericidal antibodies have been strongly correlated with human immunity and protection (7–9).
We have recently reported the identification of a surface-exposed meningococcal outer membrane (OM) protein which was designated NspA for neisserial surface protein A (15). Immunization of mice with recombinant NspA protein purified from transformed Escherichia coli protected against lethal meningococcal infections. In the present study, the cross-reactive bactericidal and protective activities of a monoclonal antibody (MAb) directed against the NspA protein were studied by using a panel of 14 serologically distinct meningococcal strains, including isolates of serogroups A, B, and C, which cause most of the diseases. In addition, to evaluate the molecular conservation of the NspA protein and to possibly localize the epitope recognized by this cross-reactive MAb, two additional nspA genes were cloned and sequenced from two serogroup A strains of N. meningitidis. These new sequences were compared to the original one and found to be nearly identical. Our results confirm that the NspA protein is highly conserved and suggest that antibodies directed against this particular protein could protect against infection by all strains of meningococci.
Generation of NspA-specific MAb Me-7.
To generate additional MAbs directed against the NspA protein, a BALB/c mouse (Charles River Laboratories, Montréal, Québec, Canada) was immunized with a meningococcal OM fraction enriched in NspA protein. Meningococcal OM from strain 608B (B:2a:P1.2:L3) was first obtained by lithium chloride extraction as described previously (11). The membrane extract was then solubilized for 30 min at room temperature by using a solution of 10% (wt/vol) Triton X-100 (Sigma Chemical Co., St. Louis, Mo.) in 50 mM Tris buffer (pH 8.0). After ultracentrifugation at 100,000 × g for 1 h, the supernatant was dialyzed overnight at 4°C with a solution of 0.1% (wt/vol) Triton X-100 in 50 mM Tris-HCl buffer (pH 8.0). The dialyzed supernatant was filtered and then applied to a cation-exchanger Macro-Prep High S column (Bio-Rad Laboratories, Mississauga, Ontario, Canada) and eluted with an increasing NaCl salt gradient. This procedure generated a meningococcal membrane fraction enriched in NspA protein. The mouse was injected subcutaneously three times at 3-week intervals with 50 μg of the NspA-enriched meningococcal OM proteins mixed with 20 μg of QuilA adjuvant (Cedarlane Laboratories, Hornby, Ontario, Canada). Three days before the fusion procedure, this mouse received a final intravenous injection of 5 μg of NspA-enriched meningococcal OM proteins. After the fusion procedure (11), one hybridoma was selected and subcloned twice by limiting dilution and the class, subclass, and light-chain specificity of the MAb were determined to be immunoglobulin G2a(κ). This MAb, designated Me-7, was shown to react with different meningococcal OM protein preparations by immunoblot (data not shown). This MAb reacted with two protein bands of approximately 22 and 18 kDa which were previously shown to correspond to the NspA protein (15).
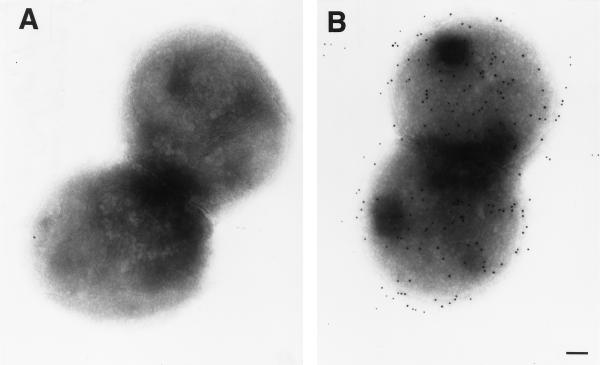
To determine whether the NspA protein was not only present in the meningococcal OM but also exposed at the surface of the bacteria, immunogold electron microscopy was used (17). The photograph presented in Fig. 1B clearly demonstrated that MAb Me-7 recognized the NspA protein on intact meningococci and that this protein was evenly distributed at the surface of the cells. Control MAb P2-4 (16), which is directed against Haemophilus influenzae porin, did not react with the meningococci (Fig. 2A).
FIG. 1.
Evaluation of the attachment of the NspA-specific MAb Me-7 to intact meningococci. Electron microphotograph of whole cells of meningococcal strain 608B probed with MAb P2-4 (A) or Me-7 (B), followed by gold-labeled goat anti-mouse immunoglobulin G (bar = 10 nm).
FIG. 2.
Comparison of the predicted amino acid sequence of the NspA proteins from the serogroup B strain 608B (B:2a:P1.3:L3) and three serogroup A strains MCH88 (A:4:P1.10), Z4063 (A:4:P1.7), and Z2491 (A:4,21:P1.7b,13a:L9). The NspA sequence from the strain Z2491 was produced by the N. meningitidis Sequencing Group at the Sanger Centre. Differences are indicated by one-letter codes and identities by a period. A 19-amino-acid-residue leader peptide is underlined.
Distribution of the nspA gene and corresponding NspA protein in N. meningitidis.
To determine whether the nspA gene was present in the genome of meningococcal strains in general, DNA dot hybridizations were performed by using the previously cloned nspA gene from serogroup B strain 608B (15) as a digoxigenin (DIG)-labeled DNA probe. The nspA probe was labeled by random priming with the DIG DNA Labeling and Detection Kit (Roche Diagnostics, Laval, Québec, Canada) according to the manufacturer’s instructions with these oligonucleotide primers: NC-01 (5′-ATG AAA AAA GCA CTT GCC ACA CTG-3′) and NC-18 (5′-TCA GAA TTT GAC GCG CAC GCC G-3′). This probe reacted with all 71 meningococcal isolates tested, even though these strains belong to many different serogroups. Of these 71 strains, 19 were serogroup A, 23 were serogroup B, 13 were serogroup C, 6 were serogroup W-135, 2 each were serogroup Y and Z, 1 each was serogroup 29E and X, and four were nontypeable strains. All of these strains were obtained from the following sources: Caribbean Epidemiology Centre (Port of Spain, Trinidad and Tobago), Children’s Hospital of Eastern Ontario (Ottawa, Ontario, Canada), Laboratory Centre for Disease Control (Ottawa, Ontario, Canada), Laboratoire de Santé Publique du Québec (Montréal, Québec, Canada), Department of Saskatchewan Health (Regina, Saskatchewan, Canada), Max-Planck-Institut für molekulare Genetik (Berlin, Germany), Victoria General Hospital (Halifax, Nova Scotia, Canada), and our own strain collection. Similarly, dot immunoblots with the NspA-specific MAb Me-7 performed on the same 71 meningococcal strains showed reactivity with all of these strains. However, MAb Me-7 barely recognized the meningococcal strain MCH88, a serogroup A strain (A:4:P1.10). This strain and a serogroup B strain, identified as CHEO22 (B:15:P1.−), were not recognized by the previously described NspA-specific MAb Me-1 (15). Immunoblotting experiments indicated that MAb Me-7 reacted strongly with the NspA protein produced by the meningococcal strain CHEO22 but failed to react with strain MCH88.
Evaluation of the biological activity of MAb Me-7.
The biological activity of MAb Me-7 was evaluated by three different methods, an in vitro bactericidal assay and two in vivo murine models of infection: the bacteremia and the mortality models. The bactericidal activity of MAb Me-7 was tested in vitro as described previously (15) with the following modifications. Fifty microliters of selected dilutions in Hanks balanced salt solution (Gibco BRL, Gaithersburg, Md.) containing 0.15 mM CaCl2, 0.5 mM MgCl2, and 1% (wt/vol) casein hydrolysate plus either purified MAbs, heat-inactivated ascitic fluids containing MAb Me-7, or a negative control MAb P2-4 which is specific for H. influenzae porin (16) was added into appropriate wells of a sterile flat-bottom 96-well plate (Gibco BRL). Then, 30 μl of an overnight meningococcal culture adjusted to 8 × 103 CFU/ml was added to each well, and the plate was shaken at 200 rpm for 15 min at 37°C under an 8% CO2 atmosphere. As the source of complement for this assay, 20 μl of freshly thawed baby rabbit (Pel-Freez, Brown Deer, Wis.) or human serum was dispensed into appropriate wells. The human serum was collected from a healthy adult volunteer with a low reactivity against meningococcal strains. Duplicate bacterium-antibody mixtures were also incubated with heat-inactivated serum. The plate was shaken at 200 rpm for 1 h at 37°C with 8% CO2. The content of each well was mixed before 10 μl was plated onto chocolate agar. The chocolate agar plates were incubated overnight at 37°C with 8% CO2, and the numbers of CFU were quantified. The bactericidal titer of MAb Me-7 was determined to be the last dilution of antibodies which still reduced the numbers of CFU by at least 50% compared to the numbers of CFU in control wells containing an unrelated MAb and the appropriate amount of complement. The Mann-Whitney U test for nonparametric analysis was used to compare the numbers of CFU recorded in control mice to the values obtained for mice injected with MAb Me-7.
The mouse bacteremia model was described previously with the following modifications (2). Groups of four or five female CFW mice (8 to 10 weeks old; Charles River) were injected intraperitoneally 18 h before bacterial challenge with 400 μl of ascitic fluid or 50 μg of purified MAb Me-7 or MAb P2-4. To increase their virulence, each N. meningitidis strain was passaged twice in mice before the bacterial challenge. After the second passage, the meningococci were incubated on the chocolate agar plates for 15 to 18 h at 37°C with 5% CO2, and the bacteria were suspended and adjusted to 1,000 to 5,000 CFU/ml in heart infusion broth (Difco Laboratories, Detroit, Mich.) supplemented with 2 mg of iron dextran (Sigma) per ml. The appropriate bacterial quantities were determined from preliminary challenge experiments for each meningococcal strain. Mice were injected intraperitoneally with 1 ml of the adjusted meningococcal suspension. After 5 h, a blood sample was harvested by cardiac puncture from each mouse, and 10 μl of undiluted and diluted blood was plated onto chocolate agar plates. The plates were incubated overnight at 37°C with 5% CO2 and the numbers of CFU were counted. The percentage of bacteremia was calculated relative to the control mice that received MAb P2-4 as follows: [(mean CFU for control mice − mean CFU for Me-7-injected mice)/mean CFU for control mice] × 100.
By using the in vitro bactericidal assay and the mouse bacteremia model, the biological activity of MAb Me-7 was tested against 14 serologically different strains of N. meningitidis, including nine serogroup B, three serogroup A, and two serogroup C strains (Table 1). During preliminary assays, it was shown that after 1 h of incubation at 37°C, the loss in viability for most of the meningococcal strains was less than 20% when human serum was used as a complement source at a final concentration of 25%. For that reason, the bactericidal activity of MAb Me-7 was always evaluated by using control wells containing both an unrelated MAb and the appropriate amount of complement. Of this panel of meningococcal strains, strains MCH88 and CHEO22 were shown to be highly sensitive to both sources of complement. For that reason, the concentration of sera used in the in vitro bactericidal assay had to be reduced to 10% in order to determine the bactericidal titer of Me-7 against these two meningococcal strains. A higher sensitivity to serum for certain meningococcal strains was reported previously (1).
TABLE 1.
Evaluation of the bactericidal titer and passive protection conferred by MAb Me-7 against serologically distinct meningococcal strains
| Meningococcal strain (serological classification) | Bactericidal titera
|
Level of bacteremia (CFU ± SD/ml of blood)b 5 h after challenge in:
|
% Reduction of bacteremia levels by MAb Me-7c | ||
|---|---|---|---|---|---|
| Rabbit serum | Human serum | Control mice injected with MAb P2-4 | Mice injected with MAb Me-7 | ||
| 4B (B:15:P1.−:L3) | 300 | 160 | 18,890 ± 8,872 | 4,688 ± 3,241 | 75 |
| 27B (B:2b:P1.2,5) | 10,000 | 600 | 46,800 ± 51,470 | 6,338 ± 7,494 | 86 |
| 44B (B:4:P1.12) | 950 | 120 | 46,710 ± 16,930 | 250 ± 370 | 99 |
| 58B (B:−:P1.10:L2) | 540 | 100 | 78,840 ± 51,060 | 10,160 ± 7,149 | 87 |
| 164B (B:15:P1.7,1.6) | 9,000 | 1,800 | 75,500 ± 82,390 | 12 ± 35 | 99 |
| 180B (B:4:P1.2,5:L1/3) | <10 | 40 | 57,760 ± 32,150 | 11,140 ± 8,893 | 81 |
| 187B (B:14:P1.−) | 2,250 | 1,800 | 28,730 ± 18,120 | 21 ± 43 | 99 |
| 608B (B:2a:P1.2:L3) | 4,500 | 1,500 | 19,230 ± 12,020 | 350 ± 558 | 98 |
| CHEO22 (B:15:P1.−)d | 200 | 100 | NDe | ND | ND |
| MCH88 (A:4:P1.10)d | <10 | <10 | 176,400 ± 90,490 | 164,500 ± 100,800NS | 7 |
| Z4734 (A:4:21:P1.16) | 2,500 | 1,400 | ND | ND | ND |
| Z4063 (A:4:P1.7) | 10,000 | 2,000 | 203,300 ± 98,520 | 7,188 ± 6,451 | 96 |
| 3C (C) | 2,500 | 900 | ND | ND | ND |
| 41C (C:2b:P1.2,5) | 8,000 | 3,000 | 83,210 ± 65,570 | 10,850 ± 8,062 | 87 |
The bactericidal titer of MAb Me-7 was determined to be the last dilution of antibodies which still reduced the numbers of CFU by at least 50% compared to the numbers of CFU in control wells containing an unrelated MAb and the appropriate amount of complement.
The control mice were injected intraperitoneally 18 h before the meningococcal challenge with either 400 μl of ascitic fluid or 50 μg of purified MAb Me-7 or control MAb P2-4. The level of bacteremia induced by each meningococcal strain was calculated from at least two separate experiments as the arithmetic mean CFU per ml of blood ± the standard deviation. Each value represents a mean of data obtained from at least eight animals. The Mann-Whitney U test for nonparametric analysis was used to compare the numbers of CFU recorded in control mice to the values obtained for mice injected with MAb Me-7. P < 0.05 for all Me-7 values except for the one marked “NS” (not significant). The bacterial inocula were adjusted to approximately 1,000 CFU for strains 4B, 44B, 58B, and 187B; to 2,500 CFU for strains 27B, 164B, and Z4063; and to 5,000 CFU for strains 608B, 180B, 41C, and MCH88.
The percentage of bacteremia was calculated relative to the control mice receiving MAb P2-4 as follows: [(mean CFU for control mice − mean CFU for Me-7-injected mice)/mean CFU for control mice] × 100.
The concentration of serum used in the in vitro bactericidal assay was reduced to 10%.
ND, not done.
As shown in Table 1, the source of complement used did influence the outcome of the in vitro bactericidal assay. Higher bactericidal titers (between 300 and 10,000) were obtained when baby rabbit serum was used compared to the results recorded with human serum (between 40 and 3,000). Such differences when rabbit serum was replaced with nonimmune human serum were previously reported (10, 13, 23). In all cases, heat inactivation for 30 min at 56°C of either source of complement abolished the bactericidal activity of MAb Me-7. The NspA-specific MAb Me-7 efficiently killed 13 of 14 meningococcal strains, including nine serogroup B, two serogroup A, and two serogroup C strains (Table 1). This result clearly demonstrated that the bactericidal activity of MAb Me-7 was not restricted to serogroup B strains but was also directed at serologically and genetically distant meningococcal strains. No bactericidal activity of MAb Me-7 was recorded against the serogroup A strain MCH88 (A:4:P1.10). However, with 10% rabbit or human serum as complement in the in vitro bactericidal assay, this strain was efficiently killed by a mouse hyperimmune serum obtained after immunization with meningococcal OM preparation (data not shown).
Also presented in Table 1 are the passive protection data recorded for MAb Me-7 by using the bacteremia model. A good correlation was obtained between the in vitro bactericidal assay and the mouse bacteremia model. Indeed, with the exception of strain MCH88, MAb Me-7 significantly reduced by more than 75% the levels of bacteremia (P < 0.05) recorded for mice challenged with all of the meningococcal strains tested compared to the levels obtained in the corresponding control groups that received MAb P2-4. These results indicated that, upon passive immunization of mice, the cross-reactive bactericidal MAb Me-7 considerably reduced the levels of bacteremia induced by serologically distinct meningococcal strains.
A mouse mortality model which was described previously (3, 15) was also used to evaluate the ability of MAb Me-7 to passively protect mice against meningococcal lethal challenge. In this model, the meningococci were suspended in heart infusion broth containing 4% mucin (Sigma) and 1.6% hemoglobin (Oxoid, Ltd., Nepean, Ontario, Canada), two substances that were previously shown to enhance the infectivity of different microorganisms (3). It was observed that, compared to the previous model, the levels of bacteremia increased over time until death occurred. It was also observed that only a limited number of meningococcal strains could repeatedly induce a lethal infection in mice when that particular model was used. In this assay, 400 μl of ascitic fluid containing the MAb or 400 to 600 μg of purified MAb was injected intraperitoneally 18 h before the mice were challenged with approximately 1,000 CFU of N. meningitidis 608B. MAb Me-7 increased the survival rate of the mice from 25% observed for the control group to at least 70% (Table 2). Lower amounts of MAb Me-7 were not tested. Also shown in Table 2 is the protection conferred by MAb Me-7 against the heterologous meningococcal strain 164B (B:15:P1.7,1.6). In this case, the administration of MAb Me-7 18 h prior to the challenge protected 100% of the mice (12 of 12).
TABLE 2.
Passive protection of BALB/c mice conferred by MAb Me-7 against infection with N. meningitidis 608B or 164Ba
| Challenge strain (serological classification) | Group | No. of living mice at 72 h after the bacterial challenge/total no. | % Survival |
|---|---|---|---|
| 608B (2a:P1.2:L3) | 400 μl of ascitic fluid containing MAb Me-7 | 7/10 | 70 |
| 600 μg of MAb Me-7 | 12/15 | 80 | |
| 400 μg of MAb Me-7 | 8/10 | 80 | |
| 400 μl of ascitic fluid containing P2-4 or PBS | 4/15 | 26 | |
| 164B (B:15:P1.7,1.6) | 400 μl of ascitic fluid containing MAb Me-7 | 12/12 | 100 |
| 400 μl of PBS | 3/12 | 25 |
The MAbs or phosphate-buffered saline (PBS) was administered intraperitoneally 18 h before challenge. The mice were then challenged with 1 ml of a meningococcal suspension containing 4% mucin and 1.6% hemoglobin. The meningococcal strains 608B and 164B were adjusted to 1,500 and 1,000 CFU/ml, respectively, and 1 ml of the bacterial suspension was injected intraperitoneally into each mouse.
Molecular conservation of the nspA gene and corresponding NspA protein in N. meningitidis.
The nspA gene from strain MCH88 (A:4:P1.10), which was isolated in Montreal in 1984, was cloned and sequenced in order to better understand the observed lack of protection of MAb Me-7 against that particular serogroup A meningococcal strain. The DNA sequence of the nspA of another serogroup A strain, designated Z4063 (A:4:P1.7), which was isolated during an epidemic in China in 1979 and was generously provided by M. Atchman (Max-Planck-Institut für molekulare Genetik), was also determined. Meningococcal genomic DNA was isolated as previously described by Marmur (14). In each case, a 2.75-kb ClaI fragment, which was shown by Southern blotting (21) to contain the nspA gene, was cloned in the ClaI site of the low-copy-number plasmid pWKS30 (22) and sequenced as previously described (15). The sequences were analyzed and compared by using the program GeneWorks (Intelligenetics, Inc., Mountain View, Calif.). The nspA sequence from strain Z2491 was produced by the N. meningitidis Sequencing Group at the Sanger Centre and can be obtained from their website (16a).
The nucleotide and deduced amino acid sequences were found to be highly conserved (Fig. 2). Indeed, at the nucleotide level these four nspA genes show differences in only 17 of 525 bp, which makes them 97% identical (data not shown). Similarly, at the amino acid level these proteins differ at only 8 of 174 residues, making them 95% identical (Fig. 2). An insertion at position 73 of a glutamine residue was identified for the predicted polypeptide from strain MCH88 which was not present in the other three predicted polypeptides. The insertion of a glutamine at position 73 is not an isolated case, since we also identified such an insertion in one of the two N. gonorrhoeae nspA genes that were recently described (18). NH2-terminal amino acid analysis of the 22-kDa protein band present in the OM preparation of strain 608B indicated the presence of a 19-amino-acid-residue leader peptide which is cleaved in the mature meningococcal protein (15). This leader peptide is highly conserved among the four NspA predicted polypeptides (Fig. 2).
From the sequence comparison presented in Fig. 2, it is possible to speculate as to the probable location of the epitope(s) recognized by the two NspA-specific MAbs Me-1 (15) and Me-7. Indeed, these epitopes must be located in an area of the NspA protein where the meningococcal strains 608B, Z2491, and Z4063 are similar but where strain MCH88 differs from them. There are only three such areas throughout the entire NspA protein sequence. The first one can immediately be dismissed since it is at position 7 of the protein which is located in the signal sequence region (15) and is not present in the mature protein. The two other regions are located at positions 73 to 74 and 115 to 116 of the NspA protein (Fig. 2). Indeed, the epitopes recognized by MAb Me-1 and Me-7 might be located in either portion of the protein. Alternatively, the differences in amino acid residues in either of these areas might have an impact on the tertiary structure of the protein and thus restrict the efficient binding of the MAbs. Interestingly, these regions were determined to be hydrophilic and could possibly constitute exposed loops at the surface of intact meningococcal protein (15). Although this analysis is mainly speculative, it does give indications as to which important areas of the NspA protein should be investigated further.
In conclusion, we believe that the present report confirms the importance of the NspA protein as a potential vaccine candidate. Indeed, this protein is present in all strains tested, and it is highly conserved at the molecular level, is exposed at the surface of the bacterium, and induces the production of cross-reactive bactericidal and cross-protective antibodies.
Nucleotide sequence accession number.
The N. meningitidis nspA genes from strains 608B, MCH88, and Z4063 have been submitted to the GenBank database under accession no. U52066, U52067, and U52068.
Acknowledgments
We thank Edith Gagnon and Michèle Lussier for their excellent technical assistance and for their contributions to the in vitro bactericidal assay and animal models of infection. We also gratefully acknowledge Mario Jacques for performing the electron microscopy.
This research was financially supported by a grant from Biochem Pharma, Inc.
REFERENCES
- 1.Ala’Aldeen D A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. doi: 10.1016/0264-410x(95)00136-o. [DOI] [PubMed] [Google Scholar]
- 2.Ashton F E, Ryan J A, Michon F, Jennings H J. Protective efficacy of mouse serum to the N-propionyl derivative of meningococcal group B polysaccharide. Microb Pathog. 1989;6:455–458. doi: 10.1016/0882-4010(89)90087-9. [DOI] [PubMed] [Google Scholar]
- 3.Brodeur B R, Larose Y, Tsang P, Hamel J, Ashton F, Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985;50:510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz Romero J, Outschoorn I M. Current status of meningococcal group B vaccine candidates: capsular or noncapsular? Clin Microbiol Rev. 1994;7:559–575. doi: 10.1128/cmr.7.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frasch C E. Vaccines for prevention of meningococcal disease. Clin Microbiol Rev. 1989;2(Suppl.):S134–S138. doi: 10.1128/cmr.2.suppl.s134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frasch C E. Meningococcal vaccines: past, present and future. In: Cartwright K, editor. Meningococcal disease. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 245–283. [Google Scholar]
- 7.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotschlich E C, Goldschneider I, Artenstein M S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiss J M, Goroff D K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983;130:2882–2885. [PubMed] [Google Scholar]
- 11.Hamel J, Brodeur B R, Larose Y, Tsang P S, Belmaaza A, Montplaisir S. A monoclonal antibody directed against a serotype-specific, outer-membrane protein of Haemophilus influenzae type b. J Med Microbiol. 1987;23:163–170. doi: 10.1099/00222615-23-2-163. [DOI] [PubMed] [Google Scholar]
- 12.Hart C A, Rogers T R. Meningococcal disease. J Med Microbiol. 1993;39:3–25. doi: 10.1099/00222615-39-1-3. [DOI] [PubMed] [Google Scholar]
- 13.Mandrell R E, Azmi F H, Granoff D M. Complement-mediated bactericidal activity of human antibodies to poly α2→8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 14.Marmur J. A procedure for the isolation of ribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–216. [Google Scholar]
- 15.Martin D, Cadieux N, Hamel J, Brodeur B R. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med. 1997;185:1173–1183. doi: 10.1084/jem.185.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin D, Munson R, Jr, Grass S, Chong P, Hamel J, Zobrist G, Klein M, Brodeur B R. Mapping of B-cell epitopes on the outer membrane P2 porin protein of Haemophilus influenzae by using recombinant proteins and synthetic peptides. Infect Immun. 1991;59:1457–1464. doi: 10.1128/iai.59.4.1457-1464.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Neisseria meningitidis Sequencing Group. 26 August 1998, posting date. [Online.] http://www.Sanger.ac.uk/Projects/N_meningitidis/. [26 February 1999, last date accessed.]
- 17.Paradis S-E, Dubreuil D, Rioux S, Gottschalk M, Jacques M. High-molecular-mass lipopolysaccharides are involved in Actinobacillus pleuropneumoniae adherence to porcine respiratory tract cells. Infect Immun. 1994;62:3311–3319. doi: 10.1128/iai.62.8.3311-3319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plante M, Cadieux N, Rioux C R, Hamel J, Brodeur B R, Martin D. Antigenic and molecular conservation of the gonococcal NspA protein. Infect Immun. 1999;67:2855–2861. doi: 10.1128/iai.67.6.2855-2861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollard A J, Faust S N, Levin M. Meningitidis and meningococcal septicaemia. J R Coll Physicians Lond. 1998;32:319–328. [PMC free article] [PubMed] [Google Scholar]
- 20.Poolman J T. Development of a meningococcal vaccine. Infect Agents Dis. 1995;4:13–28. [PubMed] [Google Scholar]
- 21.Southern E M. Detection of sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 23.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]