Abstract
Background
Differences in death rate and cardiovascular disease (CVD) between Black and White patients with chronic kidney disease is attributed to sociocultural factors, comorbidities, genetics, and inflammation.
Methods and Results
We examined the interaction of race, plasma IL‐6 (interleukin‐6), and TMPRSS6 genotype as determinants of CVD and mortality in 3031 Chronic Renal Insufficiency Cohort study participants. The primary outcomes were all‐cause mortality and a composite of incident myocardial infarction, peripheral artery disease, stroke, and heart failure. During the median follow‐up of 10 years, Black patients with chronic kidney disease experienced a significantly higher mortality (34% versus 26%) and CVD composite (41% versus 28%) compared with White patients. After adjustment, TMPRSS6 genotype did not associate with the outcomes. The adjusted hazard ratio for mortality (4.11 [2.48–6.80], P<0.001) and CVD composite (2.52 [1.96–3.24], P<0.001) were higher for the highest versus lowest IL‐6 quintile. The adjusted hazards for death per 1‐quintile increase in IL‐6 in White and Black individuals were 1.53 (1.42–1.64) versus 1.29 (1.20–1.38) (P<0.001), respectively. For CVD composite they were 1.61 (1.50–1.74) versus 1.30 (1.22–1.39) (P<0.001), respectively. In Cox proportional hazard models that included IL‐6, there was no longer a racial disparity for death (1.01 [0.87–1.16], P=0.92), but significant unexplained mediation remained for CVD (1.24 [1.07–1.43]; P=0.004). Path models that included IL‐6, diabetes, and urine albumin to creatinine ratio were able to identify variables responsible for racial disparity in mortality and CVD.
Conclusions
Racial differences in mortality and CVD among patients with chronic kidney disease could be explained by good‐fitting path models that include selected mediator variables including diabetes and plasma IL‐6.
Keywords: anemia, cytokines, genetics, inflammation, mortality, progression of chronic kidney disease
Subject Categories: Inflammatory Heart Disease
Nonstandard Abbreviations and Acronyms
- CRIC
Chronic Renal Insufficiency Cohort
- MT‐2
matriptase‐2
- SBP
systolic blood pressure
- STAT5
signal transducer and activator of transcription 5
- TMPRSS6
transmembrane serine protease 6
- UACR
urine albumin to creatinine ratio
Clinical Perspective.
What Is New?
Path models examining the association of race with mortality and cardiovascular events in patients with chronic kidney disease show that a set of mediator variables that included plasma IL‐6 (interleukin‐6) could explain the racial disparity in mortality and cardiovascular events.
What Are the Clinical Implications?
Despite Black patients with chronic kidney disease having higher levels of IL‐6, the association of IL‐6 with mortality and cardiovascular events was stronger in White patients.
The less robust association of inflammation and outcomes for Black compared with White patients may explain in part the survival advantage among Black patients with chronic kidney disease.
Despite intensive cardiovascular risk reduction strategies through optimizing blood pressure, glycemic control, and lipid levels, many patients continue to experience cardiovascular disease (CVD), indicating the presence of residual risk factors. 1 Among the general population, the burden of CVD remains the primary driver of racial disparities in life expectancy. 2 Among individuals with chronic kidney disease (CKD), Black individuals have a survival advantage compared with White patients with CKD. 3 , 4 Several theories have been proposed to explain this paradox, including survival bias, genetics, and inflammation. 5 IL‐6 (interleukin‐6) is a pleiotropic cytokine that plays a pivotal role in mediating an integrated immune response. 6 Ziltivekimab, a fully human monoclonal antibody directed against the IL‐6 ligand is being explored as a potential treatment for CVD risk reduction. 7
A J‐shaped association between biomarkers of iron load with both cardiovascular and all‐cause mortality is reported. 8 The TMPRSS6 (transmembrane serine protease 6) gene encodes MT‐2 (matriptase‐2), which inhibits hepcidin transcription and facilitates iron absorption. 9 , 10 Mutations in TMPRSS6 can result in overexpression of hepcidin, leading to iron refractory anemia. 11 TMPRSS6 expression is downregulated by IL‐6 via STAT5 (signal transducer and activator of transcription 5) signaling. 12 Preliminary evidence suggests that TMPRSS6 gene polymorphism (rs855791) could modify the cellular responsiveness to IL‐6. 13
In this study, we examine the independent and combined association of IL‐6 and TMPRSS6 genotype on all‐cause mortality and CVD events among the Chronic Renal Insufficiency Cohort (CRIC) study participants. We further asses the interaction of race with IL‐6 and TMPRSS6 genotype as a determinant of racial differences in clinical outcomes in patients with CKD.
METHODS
We used clinical and genome‐wide association study data from the CRIC study. 1 , 14 Because of the ongoing nature of the study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the CRIC study (http://www.cristudy.org). The CRIC study includes 3939 individuals with CKD, with approximately half of all participants having diabetes. 1 The primary exclusion criteria were cirrhosis, class III or IV heart failure, HIV infection, cancer, active or recent immunosuppression, polycystic kidney disease, and pregnancy. The study complies with the Declaration of Helsinki and is approved by the institutional review board at each participating site. All participants provided written informed consent. The enrollment period was 2003 to 2007, and follow‐up was recorded through March 2013.
Reporting race and ethnicity in this study was mandated by the US National Institutes of Health, consistent with the Inclusion of Women, Minorities, and Children policy. Demographic characteristics and medical history were recorded at baseline and updated during follow‐up visits. Race and ethnicity was self‐reported and categorized by the investigators as Black, White, Hispanic/Latino, and Asian American/Pacific Islander/American Indian, based on the US Office of Management and Budget's Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. Estimated glomerular filtration rate (eGFR) was estimated using the Modification of Diet in Renal Disease equation. Albuminuria was assessed by urine albumin to creatinine ratio (UACR). Study participants were queried twice annually during study visits about possible CVD events and hospitalizations. When the International Classification of Diseases, Ninth Revision (ICD‐9) discharge codes indicated myocardial infraction, cerebrovascular accident, peripheral artery disease, or death, medical records were reviewed using event‐specific guidelines by 2 physicians. 1 , 15
A genome‐wide association study was performed using the Illumina HumanOmni 1‐Quad Array Platform in 3635 study participants, and 3527 samples passed quality control metrics. 14 IL‐6 was measured in baseline blood samples using high‐sensitivity ELISA (Quantikine HS; R&D Systems, Minneapolis, MN). 16
Statistical Analysis
We examined the data from CRIC participants who had nonmissing data for IL‐6, rs855791 genotype, outcomes, and relevant covariates. Missing UACR (89 cases) was imputed with multiple imputation using the fully conditional specification method in the SAS (Cary, NC) multiple imputation procedure, using the mean value from 25 imputed data sets with the following variables in the imputation model: pooled cohort equation 10‐year CVD risk estimate, baseline eGFR, IL‐6 quintile, rs855791 genotype, level of education, death during follow‐up, CVD composite outcome, and hemoglobin outcome. Multivariable analyses were adjusted for the first 3 principal components of population stratification, which were determined separately by race.
The primary clinical outcomes were all‐cause mortality and a composite of congestive heart failure, myocardial infarction, peripheral artery disease, and stroke (CVD composite). Secondary outcomes were CKD progression, as measured by a 50% eGFR decline or reaching end‐stage renal disease, and a decline over time in level of hemoglobin to below‐normal levels (<11.7 for women or <12.4 for men). We examined incidence of these events during the 10‐year follow‐up period, as well as time‐to‐event using survival analysis methods. Death during follow‐up was binary (yes/no). In the original raw data, cardiovascular events had possible values of 0 (no event), 1 (event), 2 (died before any event), or 9 (withdrew before event). Based on the assumption that death could have been associated with having a relevant condition, those participants who died before an event were coded missing for this outcome, whereas those who withdrew without an event were coded as no event.
For continuous biological measures, we examined the distribution for nonnormality and outliers, and if present, we either transformed the variable using natural log or coded into quintiles, which could then be used as a continuous or categorical measure. For the single nucleotide polymorphism of interest in the TMPRSS6 gene (rs855791), the associations with baseline patient variables and outcomes were examined using χ2 or analysis of variance, based on an additive genetic model. Univariable associations of IL‐6 quintile with outcomes were assessed using χ2.
The multivariable model was tested for multicollinearity of relevant predictors (genotype, IL‐6, pooled cohort equation 10‐year CVD probability, 17 proteinuria, baseline eGFR) by examining the variance inflation factors as well as the collinearity diagnostic matrix. If 2 predictors loaded above 0.40 on the same component, or if the variance inflation factors were >2, this would indicate a problem. We also examined genotype by race and IL‐6 by race interactions in both univariable and multivariable analyses.
We used Cox proportional hazard models (Cox model) to examine the association of genotype and IL‐6 with time‐to‐event for death, CVD composite, CKD progression, and hemoglobin decline. This adjusted for the possibility of differences in time at risk of participants attributable to differences in start times or loss to follow‐up. In these models, genotype was coded as AA, AG, or GG, with GG as the reference group, and IL‐6 was coded in quintiles (with the lowest quintile as reference group). First, for each outcome we tested a model including genotype and IL‐6 quintile as the only covariates, and examined the cumulative hazard functions for genotype, adjusted for IL‐6, and for IL‐6 adjusted for genotype. For testing whether IL‐6 was associated with outcomes differently by the rs855791 genotype, we examined the genotype by IL‐6 interaction. We then tested these models for violations of the proportionality assumption, using the proportionality test option in the SAS PHREG procedure, which examines the effects of covariate by time interactions. When these tests were significant for either genotype or IL‐6, we included a predictor×time interaction in the model. These models were then adjusted for the pooled cohort equation 10‐year CVD probability estimate (which is calculated from age, sex, race, total cholesterol, high‐density lipoprotein, systolic blood pressure, smoking, diabetes, antihypertensive medication), 17 UACR, baseline eGFR, education, and population stratification. Finally, genotype by race and IL‐6 by race interactions were tested in models using genotype, IL‐6, race and their interactions, along with all the other covariates. If an interaction was significant, we tested Cox proportional hazard models using genotype and IL‐6 as covariates, stratified by race.
We examined the association of race with outcomes in univariable Cox models. We then adjusted for variables that might have acted as confounders or mediators of the race‐to‐outcome relationship, including age, sex, total cholesterol, high‐density lipoprotein, systolic blood pressure, smoking, diabetes, use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers or diuretics, baseline eGFR, baseline UACR, and IL‐6. If the association of race with outcome remains significant in the fully adjusted model, it suggests that either race has a direct (unmediated) association with the outcome, the mediating variables have not been measured, or there are complex relationships between mediators that are not accounted for in regression models. The presence of unmeasured confounders is also possible but less likely, given that there are few if any variables that could have a causal effect on race.
We used the SAS CALIS procedure to fit structural equation models showing possible causal paths from race through mediating variables to outcomes. Fit statistics included the adjusted goodness‐of‐fit index, standardized mean residual, and probability of close fit. SAS version 9.4 was used for all data analysis, with P<0.05 considered significant.
RESULTS
Study participant characteristics stratified by race are shown in Table 1. There were 3031 participants with genotypes and covariate data available whose data were included in the study. The median plasma IL‐6 was 1.9 pg/mL (interquartile range, 1.1–3.1 pg/mL). For the rs855791 single nucleotide polymorphism, 1569 (52%) were homozygous for the major allele (GG), 340 (11%) were homozygous for the minor allele (AA), and 1122 (37%) were heterozygous (AG). The prevalence of the G allele was more frequent among Black than White individuals. Baseline IL‐6 was significantly higher in Black compared with White individuals (2.1 [1.3–3.4 pg/mL] versus 1.6 pg/mL [1.0–2.8 pg/mL], P<0.001). During the median follow‐up of 10 years (interquartile range, 7–11 years), 903 (30%) died, 891 (34%) had cardiovascular events, progression of CKD was seen in 988 (33%), and hemoglobin decline over time was noted in 1249 (41%) of the study participants. At any given level of IL‐6, the incidence of the clinical outcomes was higher with the presence of the G allele (Figure 1A through 1D).
Table 1.
Participant Baseline Variables, Stratified by Race
| Patient variable | All, n=3031 | Black, n=1467 | White, n=1564 | P value |
|---|---|---|---|---|
| Age, y | 58±11 | 58±11 | 59±11 | 0.003 |
| Female sex | 1375 (45%) | 750 (51%) | 625 (40%) | <0.001 |
| BMI, kg/m2 | 32±8 | 33±8 | 31±7 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 45.1±15 | 43.8±15 | 46.3±15 | <0.001 |
| UACR, μg/mg, median [IQR] | 40 [8–342] | 78 [12–523] | 24 [6–211] | <0.001 |
| Hemoglobin, g/dL | 12.7±1.7 | 12.2±1.7 | 13.2±1.6 | <0.001 |
| Baseline IL‐6, pg/mL, median [IQR] | 1.9 [1.1–3.1] | 2.1 [1.3–3.4] | 1.6 [1.0–2.8] | <0.001 |
| TMPRSS6 rs855791 genotype | ||||
| GG | 1569 (52%) | 1070 (73%) | 499 (32%) | <0.001 |
| GA | 1122 (37%) | 366 (25%) | 756 (48%) | |
| AA | 340 (11%) | 31 (2%) | 309 (20%) | |
| Cholesterol, mg/dL | 182.8±44.0 | 185.9±46.0 | 179.9±41.9 | <0.001 |
| Current smoker | 427 (14%) | 279 (19%) | 148 (9%) | <0.001 |
| College graduate | 1003 (33%) | 250 (17%) | 753 (48%) | <0.001 |
| Hypertension | 2599 (86%) | 1364 (93%) | 1235 (79%) | <0.001 |
| Diabetes | 1383 (46%) | 756 (52%) | 627 (40%) | <0.001 |
| History of CVD | 1055 (35%) | 565 (39%) | 490 (31%) | <0.001 |
| ACEi/ARB | 2087 (69%) | 1044 (71%) | 1043 (67%) | 0.009 |
| Aspirin | 1347 (45%) | 627 (43%) | 720 (46%) | 0.08 |
| Diuretic | 1804 (60%) | 1013 (69%) | 791 (51%) | <0.001 |
| Outcomes | ||||
| Death | 903 (30%) | 492 (34%) | 411 (26%) | <0.001 |
| CVD composite | 891 (34%) | 507 (41%) | 384 (28%) | <0.001 |
| CKD progression | 988 (33%) | 612 (42%) | 376 (24%) | <0.001 |
| Hemoglobin decline | 1249 (41%) | 783 (53%) | 466 (30%) | <0.001 |
P values are based on χ2 for categorical variables or the Kruskal‐Wallis test for continuous variables. ACEi/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; IL‐6, interleukin‐6; IQR, interquartile range; TMPRSS6, transmembrane serine protease 6 gene; and UACR, urine albumin to creatinine ratio.
Figure 1. Association of IL‐6 and rs855791 genotype with outcomes.
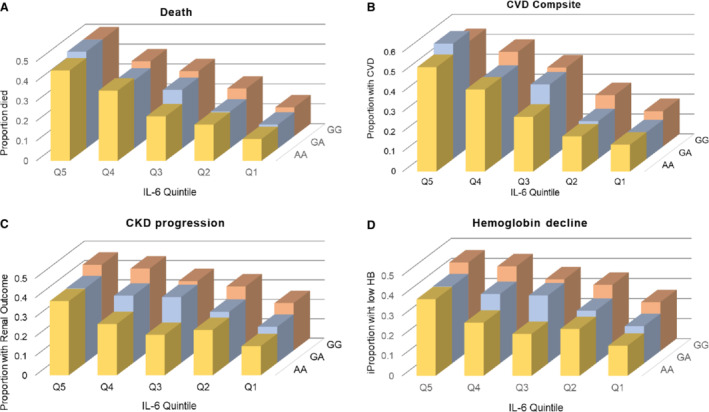
This shows the combined associations of IL‐6 quintile (Q) and genotype on each of the 4 outcomes: (A) mortality, (B) CVD composite, (C) CKD progression, and (D) hemoglobin (HB) decline. CKD indicates chronic kidney disease; CVD, cardiovascular disease; and IL‐6, interleukin‐6.
Death and CVD Outcome
Baseline plasma IL‐6 level was significantly and monotonically associated with an increase in mortality and cardiovascular composite outcome (Table S1). Cumulative hazard for death and CVD composite during the 10‐year follow‐up, stratified by IL‐6, is shown in Figure S1A and S1B. A stepwise increase in the hazard of death and CVD composite was seen with higher quartiles of IL‐6 quintile. The adjusted hazard ratio (aHR) for death (4.11 [2.48–6.80], P<0.001) and CVD composite (2.52 [1.96–3.24], P<0.001) were significantly higher for the top IL‐6 quintile compared with the reference group (Table 2). In the Cox model adjusted for age, race, sex, genotype, and population stratification, the aHR for mortality for each increasing quintile of IL‐6 was 1.40 (95% CI, 1.33–1.47; P<0.001), indicating that for each 1‐quintile increase in the level of IL‐6, the hazard for death increased by 40%. In the model for the CVD composite outcome, the aHR for continuous IL‐6 quintile was 1.43 (95% CI, 1.36–1.51; P<0.001), indicating that for each 1‐quintile increase in IL‐6, the hazard for a CVD composite outcome increased by 43%.
Table 2.
Multivariable Cox Proportional Hazard Models for Outcomes
| IL‐6 quintile | HR (95% CI) adjusted for IL‐6 | P value | HR (95% CI) adjusted for all covariates* | P value |
|---|---|---|---|---|
| Death | ||||
| Q1 | Reference | Reference | ||
| Q2 | 1.71 (1.29–2.26) | <0.001 | 1.34 (0.99–1.83) | 0.06 |
| Q3 | 2.59 (1.99–3.37) | <0.001 | 2.03 (1.42–2.91) | <0.001 |
| Q4 | 3.38 (2.61–4.37) | <0.001 | 2.54 (1.65–3.92) | <0.001 |
| Q5 | 4.82 (3.76–6.18) | <0.001 | 4.11 (2.48–6.80) | <0.001 |
| CVD composite | ||||
| Q1 | Reference | Reference | ||
| Q2 | 1.47 (1.11–1.94) | 0.006 | 1.02 (0.77–1.34) | 0.92 |
| Q3 | 2.79 (2.17–3.59) | <0.001 | 1.64 (1.26–2.12) | <0.001 |
| Q4 | 3.68 (2.87–4.72) | <0.001 | 1.84 (1.42–2.38) | <0.001 |
| Q5 | 4.93 (3.87–6.29) | <0.0001 | 2.52 (1.96–3.24) | <0.001 |
| CKD progression | ||||
| Q1 | Reference | Reference | ||
| Q2 | 1.54 (1.23–1.93) | <0.001 | 1.05 (0.83–1.32) | 0.69 |
| Q3 | 1.96 (1.57–2.44) | <0.001 | 1.07 (0.85–1.34) | 0.57 |
| Q4 | 2.43 (1.96–3.02) | <0.001 | 1.06 (0.84–1.33) | 0.63 |
| Q5 | 3.06 (2.47–3.79) | <0.001 | 1.42 (1.14–1.78) | 0.002 |
| Hemoglobin decline | ||||
| Q1 | Reference | Reference | ||
| Q2 | 1.54 (1.25–1.90) | <0.001 | 1.30 (1.03–1.64) | 0.026 |
| Q3 | 2.06 (1.68–2.53) | <0.001 | 1.71 (1.30–2.26) | <0.001 |
| Q4 | 2.63 (2.16–3.22) | <0.001 | 2.19 (1.56–3.07) | <0.001 |
| Q5 | 3.25 (2.67–3.95) | <0.001 | 2.95 (1.99–4.38) | <0.001 |
CKD indicates chronic kidney disease; CVD, cardiovascular disease; HR, hazard ratio; IL‐6, interleukin‐6; and Q, quintile.
Adjusted for pooled cohort equation 10‐year CVD risk, education, urine albumin to creatinine ratio, estimated glomerular filtration rate, population stratification, and if necessary, for nonproportionality.
In univariable analysis, the rs855791 genotype was significantly associated with both outcomes with increase in incidence as the number of G alleles increased (Table S2). The cumulative hazard for death and CVD composite stratified by rs865781 genotype is shown in Figure S2A and S2B. In fully adjusted Cox models, the rs855791 genotype was not associated with death or CVD composite (Table S3). We did not find any significant IL‐6 by genotype interactions, indicating that the association of IL‐6 with outcomes did not vary by rs855791 genotype. Variance inflation factors were all <1.3, indicating no problem with multicollinearity.
CKD Progression and Hemoglobin Decline
Baseline IL‐6 level was significantly associated with CKD progression and hemoglobin decline (Table S1). The cumulative hazards for CKD progression and hemoglobin decline during follow‐up for the quintiles of IL‐6 are shown in Figure S1C and S1D. In Cox models, IL‐6 was significantly associated with both outcomes, exhibiting progressive increase in hazard with higher IL‐6 quintiles (Table 2). The aHR for CKD progression (1.42 [1.14–1.78], P=0.002) and hemoglobin decline (2.95 [1.99–4.38], P<0.001) were higher for the top IL‐6 quintile compared with the reference group.
Both CKD progression and hemoglobin decline were most likely for participants with the GG genotype and least likely for those with the AA genotype (Figure S2C and S2D). After adjusting for demographics, pooled cohort equation risk estimates, and education, the only outcome for which genotype remained significantly associated with was risk for CKD progression (Table S3). Compared with the GG genotype, both AG (aHR, 0.85 [0.74–0.97], P=0.02) and AA (aHR, 0.69 [0.54–0.87], P=0.002) genotypes were associated with reduced risk for CKD progression.
Association of Race With Outcomes
The incidence of death (34% versus 26%), CVD composite (41% versus 28%), CKD progression (42% versus 24%), and hemoglobin drop (53% versus 30%) were more frequent in Black compared with White patients with CKD (all P<0.001). In general, the IL‐6 association with outcomes was stronger for White than for Black individuals (Figure 2). There were no significant interactions for genotype with race, but the race×IL‐6 interaction was significant for death, CVD composite, and hemoglobin decline. This was true for death, CVD composite, and the hemoglobin decline, but not for CKD progression. The aHR for the highest versus lowest IL‐6 quintile was higher among White versus Black individuals for death (3.09 [2.15–4.45] versus 2.01 [1.40–2.90]; P=0.02), CVD composite (3.14 [2.18–4.52] versus 2.00 [1.39–2.89], P=0.01), and hemoglobin decline (2.13 [1.55–2.93] versus 1.59 [1.22–2.08], P=0.015) (Table 3).
Figure 2. Adjusted hazard ratio (aHR) for outcomes by IL‐6 quintile, stratified by race.
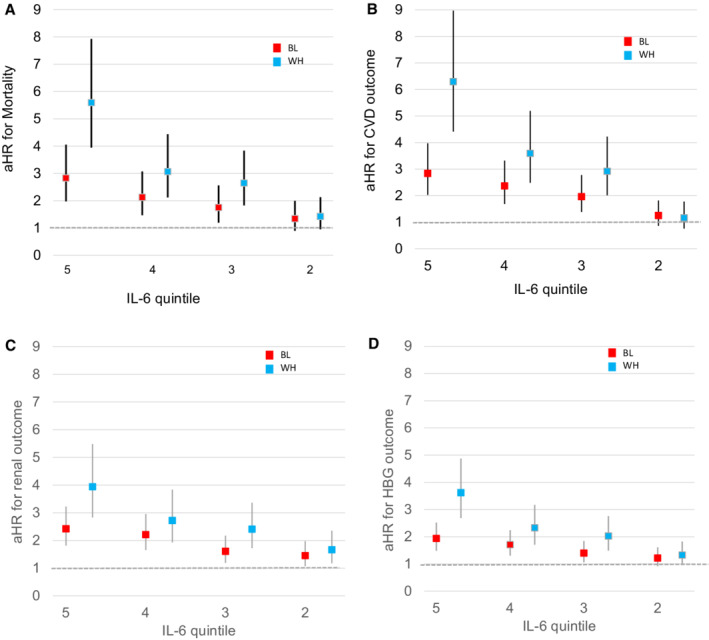
Adjusted for sex, age, and population stratification. aHRs with 95% CIs. The numbers 5, 4, 3, and 2 refer to the IL‐6 quintile. Quintile 1 is the reference group. A, The aHR per 1‐quintile increase in IL‐6 for death in White and Black participants, respectively, were 1.53 (1.42–1.64) vs 1.29 (1.20–1.38) (P<0.001 for interaction). B, The aHR for CVD composite for White and Black participants, respectively, were 1.61 (1.50–1.74) vs 1.30 (1.22–1.39) (P<0.001 for interaction). C, The aHR for progression of CKD for White and Black participants, respectively, were 1.38 (1.28–1.48) vs 1.23 (1/16–1.31) (P=0.02 for interaction). D, The aHR for hemoglobin decline were, respectively, 1.37 (1/28–1.46), and 1.18 (1.12–1.24), (P<0.001 for interaction). BL indicates Black patients; CKD, chronic kidney disease; CVD, cardiovascular disease; HGB, hemoglobin; IL‐6, interleukin‐6; and WH, White patients.
Table 3.
Interactions of IL‐6 and Genotype With Race for All Outcomes
| Outcome | Interaction | Significant multivariable effects | Interaction P * | Interpretation | aHR by race |
|---|---|---|---|---|---|
| Death | IL‐6×race | Q5 vs Q1 | 0.02 | Smaller effect in Black participants | WH 3.09 (2.15–4.45), BL 2.01 (1.40–2.90) |
| CVD composite | IL‐6×race | Q5 vs Q1 | 0.01 | Smaller effect in Black participants | WH 3.14 (2.18–4.52), BL 2.00 (1.39–2.89) |
| CKD progression | IL‐6×race | None | |||
| Hemoglobin decline | IL‐6×race | Q5 vs Q1 | 0.015 | Smaller effect in Black participants | WH 2.13 (1.55–2.93), BL 1.59 (1.22–2.08) |
| Death | Genotype×race | None | |||
| CVD composite | Genotype×race | None | |||
| CKD progression | Genotype×race | None | |||
| Hemoglobin decline | Genotype×race | None |
Hemoglobin decline over time is measured using a binary indicator for having a below‐normal final hemoglobin value (<11.7 g/dL for women or <12.4 g/dL for men). CKD progression is the composite of either a 50% or larger drop in estimated glomerular filtration rate from baseline or reaching end‐stage renal disease. CVD composite is composite of myocardial infarction, stroke, peripheral artery disease, or congestive heart failure. aHR indicates adjusted hazard ratio; BL, Black; CKD, chronic kidney disease; CVD, cardiovascular disease; IL‐6, interleukin‐6; Q, quintile; and WH, White.
P is for the interaction in a Cox proportional hazards model adjusted for IL‐6 or genotype, race, urine albumin to creatinine ratio, estimated glomerular filtration rate, education, pooled cohort equation 10‐year CVD risk, population stratification.
In a sensitivity analysis, after adjusting for genotype, age, sex, and population stratification predicting mortality, the interaction of race×IL‐6 remained significant when IL‐6 quintile is coded as continuous. The association of IL‐6 with mortality was stronger in White than Black individuals (aHR, 1.53 [1.42–1.64] in White versus aHR, 1.29 [1.20–1.38] in Black participants; P<0.001). In other words, the hazard for mortality increases by 53% for each 1‐quintile increase in IL‐6 in White versus an increase by 29% per 1‐quintile increase of IL‐6 in Black individuals. Using the same model to predict time to the composite CVD outcome, the race×IL‐6 interaction also remained significant, with the IL‐6 quintile coded as continuous. The aHR for CVD is 1.30 [1.22–1.39] in Black versus 1.61 [1.50–1.74] in White individuals (P<0.001). For each 1‐quintile increase in IL‐6, the hazard for CVD composite increased by 30% versus 61% in Black versus White individuals.
In the Cox models for outcomes by race, Black participants had significantly higher hazard ratios for all 4 outcomes than White participants in unadjusted models (Table 4). When covariates were included, there was no longer a significant association of race with mortality (1.01 [0.87–1.16], P=0.92). However, the association of race with CVD composite remained significant after adjustment (1.25 [1.08–1.45]; P=0.002). To better understand the relationship between the variables and these outcomes in the context of race, we performed path analysis. 18 We found that a good‐fitting path model showed that the association of race with mortality could be explained by the mediator variables that included diabetes, eGFR, albuminuria, and plasma IL‐6 (Figure 3A). A more complex model was needed to explain how mediator variables led to the association of race to the composite CVD outcome (Figure 3B). To summarize, the primary association of race was with UACR, which in turn associates with systolic blood pressure, eGFR, and diabetes in predicting CVD composite outcome. Race also associates with IL‐6, which directly influences CVD. IL‐6 also strongly influences diabetes, which in turn impacts CVD.
Table 4.
Hazard Ratios for Black Versus White Race From Cox Proportional Hazard Models
| Unadjusted model | Adjusted model* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Death | 1.34 (1.17–1.52) | <0.001 | 1.01 (0.87–1.16) | 0.92 |
| CVD composite | 1.65 (1.44–1.88) | <0.001 | 1.25 (1.08–1.45) | 0.002 |
| CKD progression | 2.14 (1.88–2.43) | <0.001 | 1.56 (1.36–1.79) | <0.001 |
| Hemoglobin decline | 1.86 (1.66–2.09) | <0.001 | 1.44 (1.27–1.64) | <0.001 |
CKD progression is the composite of either a 50% or larger decrease in estimated glomerular filtration rate from baseline or reaching end‐stage renal disease. CVD composite is the composite of myocardial infarction, stroke, peripheral artery disease, or congestive heart failure. Hemoglobin decline is the hemoglobin decline over time measured using a binary indicator for having a below‐normal final hemoglobin value (<11.7 g/dL for women or <12.4 g/dL for men). CKD indicates chronic kidney disease; CVD, cardiovascular disease; and HR, hazard ratio.
Adjusted for age, sex, education, total cholesterol, high‐density lipoprotein, systolic blood pressure, smoking, diabetes, angiotensin‐converting enzyme/angiotensin receptor blocker, diuretic, estimated glomerular filtration rate, urine albumin to creatinine ratio, and interleukin‐6.
Figure 3. Good‐fitting path model showing the association of race with mortality (A) and path model showing association of race with CVD events through mediating variables (B).
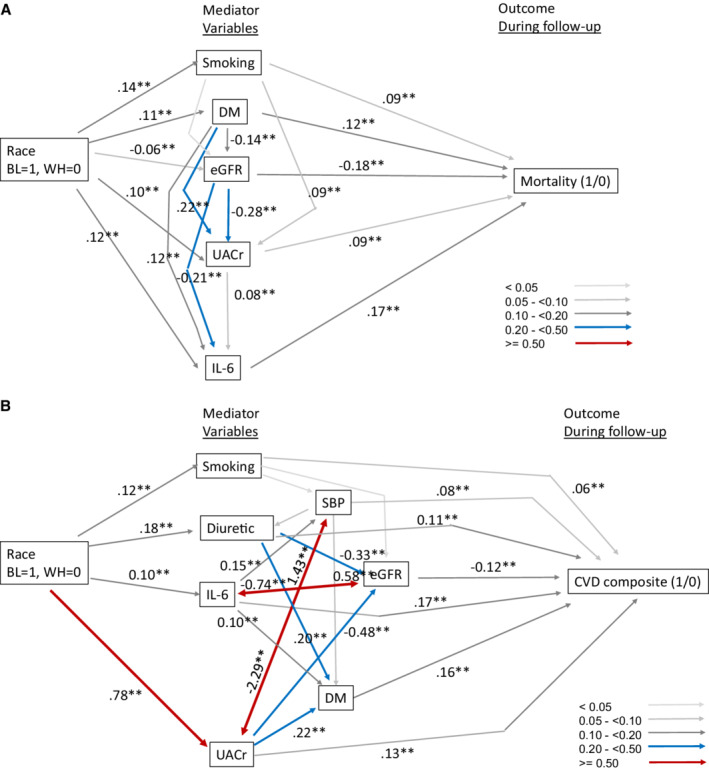
Path coefficients are standardized. 2‐headed arrows indicate bi‐directional paths. Fit statistics: A) Adjusted Goodness of Fit Index;=0.99, Standard mortality ratio =0.01, P(close fit)=0.85. B) AGFI 0.993, SRMR 0.014, probability of close fit=1.00. BL indicates Black; CVD, cardiovascular disease; DM, diabetes; eGFR, estimated glomerular filtration rate; IL‐6, interleukin‐6; SBP, systolic blood pressure; UACr, urine albumin to creatinine ratio; and WH, White.
DISCUSSION
Despite a higher prevalence of cardiovascular risk factors, lower socioeconomic status, and barriers to health care, Black individuals with CKD do not experience excess mortality or CVD compared with White individuals. 3 , 19 , 20 Although plasma IL‐6 level was higher in Black individuals in this study, the association of IL‐6 with mortality and CVD composite was stronger in White patients with CKD. Fully adjusted Cox models that included IL‐6 were able to entirely explain the racial disparity in mortality, but not the difference in CVD outcome. This indicates either the presence of residual unmeasured factors that are not included in the model, or complex relationships among mediator variables that are not accounted for by simple regression models. We show 2 separate plausible path models that explain the links underlying the observed association of race with mortality and race with CVD composite.
Reduced renal function is a harbinger for adverse CVD outcomes. 21 , 22 The cause for the increased risk of CVD in CKD could be because of shared risk factors. 1 , 23 Shlipak et al reported that among the novel risk factors, only C reactive protein and IL‐6 were associated with cardiovascular mortality. 23 In the present study, during the 10‐year follow‐up, the cumulative hazard for CVD composite increased by 43% per quintile increase in IL‐6. Evidence from laboratory investigations and epidemiological studies associate IL‐6 with atherosclerosis, left ventricular hypertrophy, heart failure, and cardiovascular death. 24 , 25 , 26 , 27 A large collaborative meta‐analysis showed that IL‐6 signaling is causally relevant to coronary heart disease. 28 Among individuals presenting with unstable angina, early intervention benefited only those with elevated IL‐6, further affirming the clinical significance of measuring IL‐6. 27 IL‐6 inhibition is emerging as a potential method for cardiovascular protection in patients with CKD. Hence, findings from this study are highly relevant for risk stratification. 7 , 13
Among the US general population, Black individuals are 30% more likely to die from heart disease than White individuals. 2 , 29 However, analysis of pooled data from the Multi‐Ethnic Study of Atherosclerosis, Family Heart Study, and Diabetes Heart Study reported that coronary artery calcification was reduced in Black individuals compared with White individuals. 30 In adult participants of the Third National Health and Nutrition Examination Survey, younger Black individuals in early stages of CKD had higher all‐cause and cardiovascular mortality. 31 However, Black patients with end‐stage renal disease are reported to have lower prevalence of CVD compared with White individuals. 32 In a prospective, observational cohort study of 271 102 patients with incident dialysis, Black, Asian, and Hispanic individuals had lower rates of nonfatal and fatal myocardial infarction compared with White individuals. 33 It is speculated that the cause for CKD‐related racial disparities could be because of survival bias, sociocultural factors, and underlying biological variation. 5 Differential association of inflammation with outcomes has been reported in patients with dialysis, with Black patients exhibiting survival advantage over White patients, especially in the presence of excess inflammation. 34 Polymorphisms in the IL‐6 promoter region as well as polymorphisms in the IL‐6R gene are associated with inflammation and increased disease risk. 28 , 35 , 36 IL‐6 polymorphism −174G/C is associated with CVD and mortality in healthy European individuals and in White patients with dialysis. 37 , 38 We did not find any significant interaction between race and TMPRSS6 genotype in determining the outcomes, but a significant interaction was noted for IL‐6 and race. In general, the association of IL‐6 with all outcomes was stronger in White compared with Black patients with CKD. Findings from the MESA (Multi‐Ethnic Study of Atherosclerosis) showed that persons of a lower socioeconomic position have greater inflammatory burden than those of high socioeconomic position as a result of the cumulative effects of multiple behavioral, psychosocial, and metabolic characteristics. 39 The less robust association of inflammation and outcomes for Black patients compared with White patients may be because of the potential impact of racial inequalities on a range of biological systems over time. 40 , 41 , 42
A pooled analysis of community‐based studies noted that the presence of CKD is an independent risk factor for both all‐cause mortality and CVD in Black individuals, but only for all‐cause mortality in White individuals. 43 Mehrotra et al reported a higher all‐cause and cardiovascular mortality for younger Black individuals with early‐stage CKD. 31 Weiner et al attributed the interaction between race and CKD to residual confounding, related to greater severity of hypertension and/or diabetes in Black individuals. 43 In our study, we performed Cox models to test for interaction of race with outcomes, and found that that the association of race with mortality is fully explained by the variables included in the model. The association of race with CVD composite was not fully explained by the model, indicating the existence of other unmeasured mediators for CVD, or a more complex interplay between mediators that is not captured by simple regression models.
We performed path analysis to assess the fit of structural equation models to the data. These models illustrate potential mechanisms through which a set of mediator variables may connect race to outcome via multiple pathways. We show here that the association of race with mortality could be explained by a set of variables that included IL‐6. On the other hand, elucidating the association of race with CVD composite outcome required a more complex path model that showed that mediators of the association of race and CVD outcome are albuminuria and IL‐6.
Limitations
The purpose of this study was to identify the variables driving racial disparity in clinical outcomes in patients with CKD. IL‐6 was chosen as a marker of inflammation because it is believed to be a central regulator of the inflammatory process. The circulating level and actions of IL‐6 are altered by IL‐6 polymorphisms, which was not addressed in this study. Path models are based on correlations and cannot prove causation. 44 Social factors were not measured as part of this study, although they are likely to be important exogenous variables that affect the associations of race with other variables used in this study.
CONCLUSIONS
Our findings demonstrate that plasma levels of IL‐6 strongly associate with mortality and cardiovascular events among patients with CKD. Despite Black individuals having higher levels of IL‐6, the association of IL‐6 with mortality and cardiovascular events were stronger in White patients with CKD. Path models linking race to mortality and CVD show how mediator variables that included plasma IL‐6 could explain the racial disparity in mortality and cardiovascular events. In fully adjusted Cox models, rs855791 genotype was not associated with death or CVD composite. Thus, racial disparity in mortality and CVD in patients with CKD is because of a complex interplay of biological, social, and cultural factors.
APPENDIX
CRIC Study Investigators
Lawrence J. Appel, MD, MPH; Debbie Cohen, MD; Harold I. Feldman, MD, MSCE; James P. Lash, MD; Robert G. Nelson, MD, PhD, MS; Mahboob Rahman, MD; Panduranga S. Rao, MD; Vallabh O. Shah, PhD, MS; and Mark L. Unruh, MD, MS.
Sources of Funding
Funding for the CRIC study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health/National Center for Advancing Translational Sciences UL1TR000003, Johns Hopkins University UL1 TR‐000424, University of Maryland GCRC M01 RR‐16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and National Institutes of Health Roadmap for Medical Research, Michigan Institute for Clinical and Health Research UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Awards UL1RR029879, Tulane Centers of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente National Institutes of Health/National Center for Research Resources UCSF‐CTSI UL1 RR‐024131, Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque, NM R01DK119199. The study is supported by a grant from Corvidia Therapeutics. D. Raj is supported by funding from the National Institutes of Health through 1 R01DK125256, R01 DK073665‐01A1, 1U01DK099924‐01, and 1U01DK099914‐01. He has received research funding from Relypsa and serves in the advisory board for Novo Nordisc and Corvidia Therapeutics.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S2
See Editorial by Nicholas et al.
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Richard L. Amdur, Email: ramdur@mfa.gwu.edu.
the CRIC Study Investigators:
Mark L. Unruh, Vallabh O. Shah, Panduranga S. Rao, Mahboob Rahman, Robert G. Nelson, James P. Lash, Harold I. Feldman, Debbie Cohen, and Lawrence J. Appel
References
- 1. Amdur RL, Feldman HI, Dominic EA, Anderson AH, Beddhu S, Rahman M, Wolf M, Reilly M, Ojo A, Townsend RR, et al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis. 2019;73:344–353. doi: 10.1053/j.ajkd.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/cir.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 3. Newsome BB, McClellan WM, Coffey CS, Allison JJ, Kiefe CI, Warnock DG. Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol. 2006;1:993–999. doi: 10.2215/cjn.01251005 [DOI] [PubMed] [Google Scholar]
- 4. Kovesdy CP, Quarles LD, Lott EH, Lu JL, Ma JZ, Molnar MZ, Kalantar‐Zadeh K. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62:228–235. doi: 10.1053/j.ajkd.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powe NR. Reverse race and ethnic disparities in survival increase with severity of chronic kidney disease: what does this mean? Clin J Am Soc Nephrol. 2006;1:905–906. doi: 10.2215/cjn.02660706 [DOI] [PubMed] [Google Scholar]
- 6. Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997 [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj D, et al. IL‐6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double‐blind, randomised, placebo‐controlled, phase 2 trial. Lancet. 2021;397:2060–2069. doi: 10.1016/S0140-6736(21)00520-1 [DOI] [PubMed] [Google Scholar]
- 8. Grammer TB, Scharnagl H, Dressel A, Kleber ME, Silbernagel G, Pilz S, Tomaschitz A, Koenig W, Mueller‐Myhsok B, Marz W, et al. Iron metabolism, hepcidin, and mortality (the Ludwigshafen Risk and Cardiovascular Health Study). Clin Chem. 2019;65:849–861. doi: 10.1373/clinchem.2018.297242 [DOI] [PubMed] [Google Scholar]
- 9. Nai A, Pagani A, Silvestri L, Campostrini N, Corbella M, Girelli D, Traglia M, Toniolo D, Camaschella C. TMPRSS6 rs855791 modulates hepcidin transcription in vitro and serum hepcidin levels in normal individuals. Blood. 2011;118:4459–4462. doi: 10.1182/blood-2011-06-364034 [DOI] [PubMed] [Google Scholar]
- 10. Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase‐2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K, et al. Mutations in TMPRSS6 cause iron‐refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meynard D, Sun CC, Wu Q, Chen W, Chen S, Nelson CN, Waters MJ, Babitt JL, Lin HY. Inflammation regulates TMPRSS6 expression via STAT5. PLoS One. 2013;8:e82127. doi: 10.1371/journal.pone.0082127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pergola PE, Devalaraja M, Fishbane S, Chonchol M, Mathur VS, Smith MT, Lo L, Herzog K, Kakkar R, Davidson MH. Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: results from a phase 1/2 multicenter, randomized, double‐blind, placebo‐controlled trial. J Am Soc Nephrol. 2021;32:211–222. doi: 10.1681/ASN.2020050595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parsa A, Kanetsky PA, Xiao R, Gupta J, Mitra N, Limou S, Xie D, Xu H, Anderson AH, Ojo A, et al. Genome‐wide association of CKD progression: the Chronic Renal Insufficiency Cohort study. J Am Soc Nephrol. 2017;28:923–934. doi: 10.1681/asn.2015101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bansal N, Zelnick LR, Soliman EZ, Anderson A, Christenson R, DeFilippi C, Deo R, Feldman HI, He J, Ky B, et al. Change in cardiac biomarkers and risk of incident heart failure and atrial fibrillation in CKD: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2021;77:907–919. doi: 10.1053/j.ajkd.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/cjn.03500412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/cir.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Streiner DL. Finding our way: an introduction to path analysis. Can J Psychiatry. 2005;50:115–122. doi: 10.1177/070674370505000207 [DOI] [PubMed] [Google Scholar]
- 19. Lash JP, Ricardo AC, Roy J, Deo R, Fischer M, Flack J, He J, Keane M, Lora C, Ojo A, et al. Race/ethnicity and cardiovascular outcomes in adults with CKD: findings from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic CRIC studies. Am J Kidney Dis. 2016;68:545–553. doi: 10.1053/j.ajkd.2016.03.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Norris KC, Shlipak MG. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP). Clin J Am Soc Nephrol. 2011;6:1858–1865. doi: 10.2215/cjn.00500111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x [DOI] [PubMed] [Google Scholar]
- 22. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007 [DOI] [PubMed] [Google Scholar]
- 23. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman‐Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737 [DOI] [PubMed] [Google Scholar]
- 24. Schieffer B, Selle T, Hilfiker A, Hilfiker‐Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, et al. Impact of interleukin‐6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.Cir.0000148135.08582.97 [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin‐6 signalling pathway and incidence rates of atherosclerotic events and all‐cause mortality: analyses from the Canakinumab Anti‐Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39:3499–3507. doi: 10.1093/eurheartj/ehy310 [DOI] [PubMed] [Google Scholar]
- 26. Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin‐6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin‐6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–398. doi: 10.1016/s0735-1097(97)00494-4 [DOI] [PubMed] [Google Scholar]
- 27. Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–2113. doi: 10.1001/jama.286.17.2107 [DOI] [PubMed] [Google Scholar]
- 28. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, et al. Interleukin‐6 receptor pathways in coronary heart disease: a collaborative meta‐analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/s0140-6736(11)61931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/cir.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 30. Wagenknecht LE, Divers J, Bertoni AG, Langefeld CD, Carr JJ, Bowden DW, Elbein SC, Shea S, Lewis CE, Freedman BI. Correlates of coronary artery calcified plaque in blacks and whites with type 2 diabetes. Ann Epidemiol. 2011;21:34–41. doi: 10.1016/j.annepidem.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19:1403–1410. doi: 10.1681/asn.2007070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Volkova N, McClellan W, Soucie JM, Schoolwerth A. Racial disparities in the prevalence of cardiovascular disease among incident end‐stage renal disease patients. Nephrol Dial Transplant. 2006;21:2202–2209. doi: 10.1093/ndt/gfl078 [DOI] [PubMed] [Google Scholar]
- 33. Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ. Racial and ethnic differences in incident myocardial infarction in end‐stage renal disease patients: the USRDS. Kidney Int. 2006;69:1691–1698. doi: 10.1038/sj.ki.5000346 [DOI] [PubMed] [Google Scholar]
- 34. Crews DC, Sozio SM, Liu Y, Coresh J, Powe NR. Inflammation and the paradox of racial differences in dialysis survival. J Am Soc Nephrol. 2011;22:2279–2286. doi: 10.1681/asn.2011030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, Pfister R, et al. The interleukin‐6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/s0140-6736(12)60110-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith AJ, Zheng D, Palmen J, Pang DX, Woo P, Humphries SE. Effects of genetic variation on chromatin structure and the transcriptional machinery: analysis of the IL6 gene locus. Genes Immun. 2012;13:583–586. doi: 10.1038/gene.2012.32 [DOI] [PubMed] [Google Scholar]
- 37. Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The interleukin‐6 ‐174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J. 2001;22:2243–2252. doi: 10.1053/euhj.2001.2678 [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Berthier‐Schaad Y, Fallin MD, Fink NE, Tracy RP, Klag MJ, Smith MW, Coresh J. IL‐6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol. 2006;17:863–870. doi: 10.1681/asn.2005050465 [DOI] [PubMed] [Google Scholar]
- 39. Ranjit N, Diez‐Roux AV, Shea S, Cushman M, Ni H, Seeman T. Socioeconomic position, race/ethnicity, and inflammation in the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2007;116:2383–2390. doi: 10.1161/circulationaha.107.706226 [DOI] [PubMed] [Google Scholar]
- 40. Assari S. Blacks' diminished health returns of educational attainment: Health and Retirement Study. J Med Res Innov. 2020;4:e000212. doi: 10.32892/jmri.212 [DOI] [PubMed] [Google Scholar]
- 41. Assari S, Cobb S, Saqib M, Bazargan M. Diminished returns of educational attainment on heart disease among black Americans. Open Cardiovasc Med J. 2020;14:5–12. doi: 10.2174/1874192402014010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geronimus AT, Hicken M, Keene D, Bound J. "Weathering" and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/ajph.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all‐cause mortality: a pooled analysis of community‐based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2 [DOI] [PubMed] [Google Scholar]
- 44. Petraitis PS, Dunham AE, Niewiarowski PH. Inferring multiple causality: the limitations of path analysis. Funct Ecol. 1996;10:421–431. doi: 10.2307/2389934 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S2


