Abstract
Background
Venous thromboembolism (VTE) contributes significantly to COVID‐19 morbidity and mortality. The urokinase receptor system is involved in the regulation of coagulation. Levels of soluble urokinase plasminogen activator receptor (suPAR) reflect hyperinflammation and are strongly predictive of outcomes in COVID‐19. Whether suPAR levels identify patients with COVID‐19 at risk for VTE is unclear.
Methods and Results
We leveraged a multinational observational study of patients hospitalized for COVID‐19 with suPAR and D‐dimer levels measured on admission. In 1960 patients (mean age, 58 years; 57% men; 20% Black race), we assessed the association between suPAR and incident VTE (defined as pulmonary embolism or deep vein thrombosis) using logistic regression and Fine‐Gray modeling, accounting for the competing risk of death. VTE occurred in 163 (8%) patients and was associated with higher suPAR and D‐dimer levels. There was a positive association between suPAR and D‐dimer (β=7.34; P=0.002). Adjusted for clinical covariables, including D‐dimer, the odds of VTE were 168% higher comparing the third with first suPAR tertiles (adjusted odds ratio, 2.68 [95% CI, 1.51–4.75]; P<0.001). Findings were consistent when stratified by D‐dimer levels and in survival analysis accounting for death as a competing risk. On the basis of predicted probabilities from random forest, a decision tree found the combined D‐dimer <1 mg/L and suPAR <11 ng/mL cutoffs, identifying 41% of patients with only 3.6% VTE probability.
Conclusions
Higher suPAR was associated with incident VTE independently of D‐dimer in patients hospitalized for COVID‐19. Combining suPAR and D‐dimer identified patients at low VTE risk.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04818866.
Keywords: COVID‐19, soluble urokinase plasminogen activator receptor, thromboembolism
Subject Categories: Biomarkers, Clinical Studies, Vascular Biology, Thrombosis, Embolism
Nonstandard Abbreviations and Acronyms
- ISIC
International Study of Inflammation in COVID‐19
- suPAR
soluble urokinase plasminogen activator receptor
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
Clinical Perspective.
What Is New?
This is the first study to evaluate the association between soluble urokinase plasminogen activator receptor (suPAR) and venous thromboembolism (VTE) in the setting of acute viral illness, such as COVID‐19.
There was a significant association between suPAR and risk for VTE in COVID‐19 that was independent of plasma D‐dimer level.
The subgroup of patients with suPAR <11 ng/mL and D‐dimer <1 mg/L had only a 3.6% probability of incident VTE and accounted for 41% of patients hospitalized for COVID‐19.
What Are the Clinical Implications?
Characterizing the association between suPAR and VTE risk furthers our understanding of the prognostic role of suPAR, a biomarker with established clinical utility in guiding immunotherapy treatment in COVID‐19.
The combined D‐dimer and suPAR diagnostic cutoffs could refine the risk stratification for VTE among patients hospitalized with COVID‐19.
Venous thromboembolism (VTE) is a highly prevalent COVID‐19 complication, estimated to occur in as many as 26% of patients hospitalized for the disease. 1 Pulmonary embolism remained prevalent despite certain immunomodulators effective at stalling the progression of respiratory illness. 2 It was the direct cause of death in close to one third of patients with COVID‐19 despite prophylaxis with anticoagulants. 3 Such excessive thromboembolic risk is thought to be attributable to not only critical illness but immunothrombosis, a condition where viral infection induces immune dysregulation and significant vascular inflammation, ultimately resulting in microangiopathy and macroangiopathy. 4 , 5 , 6 D‐dimer is the most commonly used biomarker for VTE but has been shown to be less reliable in COVID‐19. 7 Combining it with markers of immune dysregulation could refine the risk stratification of patients with COVID‐19.
The urokinase plasminogen activator (uPA)/urokinase plasminogen activator receptor (uPAR) system is abundant in various cell types, including vascular endothelial cells, and is known as a key regulator in the cross‐reactions between vascular inflammation, immunity, and coagulopathy. 8 , 9 The soluble uPAR (suPAR) is a cleavage product from the uPA/uPAR system, levels of which are thought to reflect the system's overall activity. 8 , 9 suPAR levels are 3‐ to 5‐fold higher in patients with COVID‐19, elevated earlier than other biomarkers in disease progression, and strongly associated with COVID‐19 complications, including death, respiratory failure requiring mechanical ventilation, and severe acute kidney injury. 10 , 11 suPAR may be an ideal biomarker to quantify hyperinflammation in COVID‐19. Recently, the European Commission authorized the drug anakinra, guided by suPAR levels, to target interleukin‐1 in COVID‐19. 2 , 12 As a long‐term inflammatory biomarker, suPAR levels measured in the nonacute setting have previously been shown to be associated with incident VTE. 13
We leveraged the ISIC (International Study of Inflammation in COVID‐19) to determine whether suPAR levels were associated with VTE risk in COVID‐19, independently of D‐dimer, and whether a role exists for suPAR in the risk stratification of patients with COVID‐19 for VTE.
METHODS
International Study of Inflammation in COVID‐19
ISIC is a multinational observational study primarily aiming to characterize the roles of inflammatory biomarkers in COVID‐19–related adverse outcomes. 14 Participants were enrolled at major health care institutions, including the University of Michigan, Ann Arbor, MI; Rush University, Chicago, IL; Copenhagen University Hospital Hvidovre, Copenhagen, Denmark; Attikon University Hospital, Athens, Greece; University of Thessaly, Thessaly, Greece; University Hospital of Düsseldorf, Düsseldorf, Germany; and Charité University Medicine, Berlin, Germany (Data S1). 10 , 14 , 15 , 16 , 17 Patients positive for SARS‐CoV‐2 in nasopharyngeal or oropharyngeal swab reverse transcription–polymerase chain reaction test who were hospitalized specifically for COVID‐19 and had a blood sample collected within 48 hours of hospitalization were included in this study. Clinical and laboratory data were obtained through a manual review of electronic medical records by at least 2 reviewers per site and entered into an online data repository hosted at the University of Michigan. Patient consent and institutional review board approvals were obtained at each clinical site, as per their institutional policies. Data from ISIC can be made available upon request through a collaborative process. Please contact penegonz@med.umich.edu for additional information.
Study Design
Individuals were eligible for this study if they met the following inclusion criteria: (1) aged ≥18 years, (2) primary reason for hospitalization was COVID‐19, and (3) had available suPAR measurement from a blood sample collected on hospital admission. Individuals who tested positive for SARS‐CoV‐2 but were hospitalized for non–COVID‐19 reasons were excluded. All patients were followed up from hospital admission, which defines the baseline of this study, until hospital discharge or death. Clinical data were obtained from electronic medical records using established data mining tools, entered into a secure web‐based repository Research Electronic Data Capture, and reviewed by at least 2 independent reviewers per clinical site for accuracy.
Baseline Variables
Baseline variables in this study included age; sex; race; body mass index (BMI); anticoagulation therapy before hospital admission; comorbidities, including diabetes, hypertension, congestive heart failure, atrial fibrillation or flutter, stroke, peripheral artery disease, chronic obstructive pulmonary disease, and malignancy; and laboratory values, including creatinine, D‐dimer, and suPAR. We computed the estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation. 18
suPAR and D‐Dimer Measurements
Plasma suPAR levels were measured using a commercially available double monoclonal antibody sandwich assay (suPARnostic, ViroGates, Denmark). The assay's lower detection limit was 100 pg/mL. The intra‐assay and interassay variations were 2.8% and 9.2%, respectively. D‐dimer levels were measured by medical laboratories at each site (see details about D‐dimer assays and reference ranges in Table S1).
Outcomes
The outcome of interest was VTE, defined as a clinical diagnosis of deep vein thrombosis, pulmonary embolus, or both. Clinical imaging studies were used for the diagnoses of these conditions, which included computed tomography angiography scans of the lungs, ventilation/perfusion scans of the lungs, and lower extremity Doppler ultrasounds. We ascertained the outcome via a chart review of clinical notes and imaging study reports.
Statistical Analysis
We summarized baseline characteristics using descriptive statistics. Outcomes were described using total numbers and percentages. Continuous variables with skewed distributions (ie, suPAR and D‐dimer) were log transformed (base 2) to achieve approximate normal distribution. The independent association between suPAR and D‐dimer was assessed using multivariable linear regression.
We used multivariable logistic regression to assess the association between baseline suPAR tertile (0–5.2, 5.3–8.7, and 8.8–62.7 ng/mL) and VTE, adjusting for baseline covariables, including age, sex, Black race, BMI, anticoagulation therapy before hospital admission, history of diabetes, hypertension, congestive heart failure, and malignancy, and kidney function at hospital admission. In a separate model, we further adjusted for D‐dimer. The effect modification by D‐dimer was assessed by including an interaction term between suPAR and D‐dimer as a categorical variable (within or not within the reference range) in regression models.
We additionally assessed the cumulative incidence of VTE within 30 days of hospitalization by suPAR tertile using Kaplan‐Meier cumulative incidence curves. The difference in cumulative incidence by suPAR tertile was tested using a log‐rank test. The association between baseline suPAR and time to 30‐day VTE was examined using Cox proportional hazards models adjusted for the aforementioned covariates. Follow‐up time began at hospital admission until outcome, hospital discharge, or death within 30 days. Death was accounted for as a competing risk in Fine‐Gray models used to calculate the subdistribution hazard ratio (HR).
We performed the following sensitivity analyses: (1) subgroup analysis among patients in whom blood samples for laboratory testing were collected within 24 hours of hospital admission, (2) subgroup analysis among those enrolled at the University of Michigan alone, and (3) multivariable models adjusting for D‐dimer categorized on the basis of the respective assays' upper limits of normal (ULNs) at each clinical site (<ULN, 1–4× ULN, 4–6× ULN, and >6× ULN).
Sensitivity, specificity, and positive and negative predictive values for VTE were reported, stratified by quartiles of suPAR and D‐dimer. Risk discrimination for VTE was assessed with receiver operating characteristic curves of models including suPAR, D‐dimer, and the combination of both. Differences in the areas under the curve were examined using the DeLong test. 19
We identified the combination of suPAR and D‐dimer cutoffs that yielded the lowest probability of incident VTE by estimating the failure probability for each patient based on random forest and building a decision tree accordingly that estimated a regression relationship by binary recursive partitioning in a conditional inference framework. 20
A complete‐case analysis was performed. A 2‐sided P value of 0.05 was used as the threshold for statistical significance. Statistical analyses were performed using R (R Core Team, 2014). 21
RESULTS
Cohort Characteristics
A total of 1960 adults met this study's inclusion criteria. Among these patients, the mean age was 58±17 years, 1114 (57%) were men, and 385 (20%) were Black (Table 1). The median plasma D‐dimer was 1.0 (interquartile range, 0.6–2.6) mg/L. The median plasma suPAR was 6.7 (interquartile range, 4.5–10.1) ng/mL. VTE occurred in 163 (8%) patients, of whom 65, 88, and 10 had incident deep vein thrombosis, pulmonary embolus, and both, respectively. There were more men (75% versus 55%), more Black individuals (30% versus 20%), higher median D‐dimer (2.1 versus 1.0 mg/mL), and higher median suPAR (9.7 versus 6.5 ng/mL) among those who had VTE compared with those who did not.
Table 1.
Baseline Characteristics, Overall and by Incident VTE, in Patients Hospitalized for COVID‐19
| Characteristic | Overall (N=1960) | Incident VTE during hospitalization (N=163) | No VTE during hospitalization (N=1797) |
|---|---|---|---|
| Age, y | 58 (17) | 57 (15) | 58 (17) |
| Men | 1114 (57) | 122 (75) | 992 (55) |
| Body mass index, kg/m2 | 32 (9) | 31 (10) | 32 (9) |
| Black race | 385 (20) | 48 (30) | 337 (20) |
| Diabetes | 571 (29) | 51 (31) | 520 (29) |
| Hypertension | 954 (49) | 91 (56) | 863 (48) |
| Congestive heart failure | 179 (9) | 10 (6) | 169 (9) |
| Atrial fibrillation or flutter | 172 (9) | 7 (4) | 165 (9) |
| Stroke | 121 (6) | 9 (5) | 112 (9) |
| Chronic obstructive pulmonary disease | 170 (9) | 15 (9) | 155 (9) |
| Malignancy | 82 (4) | 14 (9) | 68 (4) |
| Before admission anticoagulation therapy | 177 (9) | 9 (6) | 168 (9) |
| Creatinine, mg/dL* | 1.0 (0.8–1.4) | 1.0 (0.9–1.5) | 1.0 (0.8–1.3) |
| D‐dimer, mg/L† | 1.0 (0.6–2.6) | 2.1 (1.1–10.4) | 1.0 (0.5–2.2) |
| suPAR, ng/mL | 6.7 (4.5–10.1) | 9.7 (6.2–13.8) | 6.5 (4.4–9.7) |
Data are given as mean (SD), number (percentage), or median (interquartile range). suPAR indicates soluble urokinase plasminogen activator receptor; and VTE, venous thromboembolism.
Serum creatinine normal range: 0.65 to 1.00 mg/dL (University of Michigan and Rush University Medical Center), 0.57 to 1.02 mg/dL for women and 0.68 to 1.19 mg/dL for men (Copenhagen University Hospital Hvidovre), 0.50 to 0.90 ng/mL (University of Thessaly), and 0.50 to 0.9 mg/dL for women and 0.70 to 1.20 mg/dL for men (Attikon University Hospital in Athens, University Hospital of Düsseldorf, and Charité University Medicine).
D‐dimer normal range: 0.000 to 0.500 mg/L (University of Michigan, Copenhagen University Hospital Hvidovre, Attikon University Hospital in Athens, University Hospital of Düsseldorf, and Charité University Medicine), 0.000 to 0.600 mg/L (Rush University Medical Center), and 0.000 to 0.255 mg/L (University of Thessaly).
Correlation Between Circulating suPAR and D‐Dimer Levels
Per each doubling of plasma suPAR, the D‐dimer level was 7.34 (β=7.34 [95% CI, 1.49–13.2]; P=0.002) times higher after controlling for age, sex, Black race, BMI, comorbidities, and estimated glomerular filtration rate (Table 2).
Table 2.
Independent Associations Between Baseline Variables and D‐Dimer
| Variable | Regression coefficient (95% CI) | P value |
|---|---|---|
| suPAR, per log (ng/mL)/log (2) higher | 7.34 (1.49 to 13.2) | 0.014 |
| Age, per 10 y older | −0.31 (−2.67 to 2.05) | 0.79 |
| Men vs women | 0.27 (−5.83 to 6.36) | 0.93 |
| Black vs White, Asian, American Indian and Pacific Islander | −1.03 (−8.25 to 6.20) | 0.78 |
| Body mass index, per 5 kg/m2 higher | −2.02 (−3.72 to −0.31) | 0.02 |
| History of diabetes vs none | 3.72 (−3.19 to 10.63) | 0.29 |
| History of hypertension vs none | 1.64 (−5.49 to 8.76) | 0.65 |
| History of congestive heart failure vs none | −0.79 (−10.6 to 9.03) | 0.87 |
| Estimated glomerular filtration rate, per 5 mL/min per 1.73 m2 higher | 0.28 (−0.32 to 0.88) | 0.36 |
Regression coefficients are interpreted as fold changes in D‐dimer level. suPAR indicates soluble urokinase plasminogen activator receptor.
Association Between suPAR and Incident VTE
In an unadjusted model, the odds of incident VTE were 72% (odds ratio [OR], 1.72 [95% CI, 0.96–3.09]; P=0.07; Figure 1) and 344% (OR, 4.44 [95% CI, 2.62–7.51]; P<0.001) higher for the second (5.3–8.7 ng/mL) and third (8.8–62.7 ng/mL) tertiles of suPAR compared with the first tertile (0–5.2 ng/mL). After adjusting for baseline age, sex, Black race, BMI, anticoagulation therapy, comorbidities, and kidney function, the third tertile of suPAR was associated with a 168% (adjusted OR, 2.68 [95% CI, 1.51–4.75]; P<0.001) higher odds of VTE when compared with the first tertile of suPAR. This association was mildly attenuated after additionally adjusting for D‐dimer level (adjusted OR, 1.98 [95% CI, 1.08–3.66]; P=0.028). The association was unchanged in patients with D‐dimer ≤ULN compared with those with D‐dimer >ULN (P‐interaction=0.21).
Figure 1. Odds ratios and 95% CIs for venous thromboembolism per soluble urokinase plasminogen activator receptor (suPAR) tertile in patients hospitalized with COVID‐19, with and without adjustments for baseline covariables and D‐dimer level.
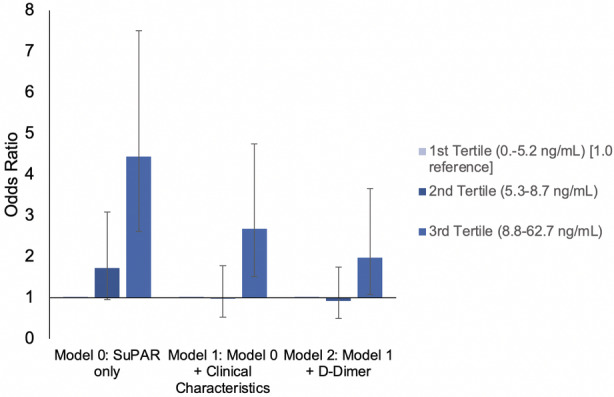
Baseline clinical characteristics included in model 1: age, sex, Black race, body mass index, history of diabetes, history of hypertension, history of congestive heart failure, history of atrial fibrillation or flutter, history of stroke, history of chronic obstructive pulmonary disease, history of malignancy, before admission anticoagulation therapy, and estimated glomerular filtration rate.
Within 30 days of hospitalization, there were 148 (7.6%) incident VTE events. The cumulative incidence of VTE within 30 days was 3.5%, 6.0%, and 12.6% for the first, second, and third tertiles of suPAR, respectively (log‐rank P<0.001; Figure 2). In survival analyses, the risk of VTE within 30 days was 3% lower (adjusted HR, 0.97 [95% CI, 0.48–1.58]; P=0.15) and 52% higher (adjusted HR, 1.52 [95% CI, 0.86–2.68]; P=0.32) for the second and third tertiles of suPAR, respectively, compared with the first tertile, adjusting for the aforementioned covariates, including D‐dimer (Table 3). The associations were similar in a competing risk analysis accounting for death within 30 days of hospitalization (n=149 [7.6%]) as a competing event.
Figure 2. Cumulative incidence of venous thromboembolism (VTE) within 30 days of hospitalization by soluble urokinase plasminogen activator receptor (suPAR) tertile.
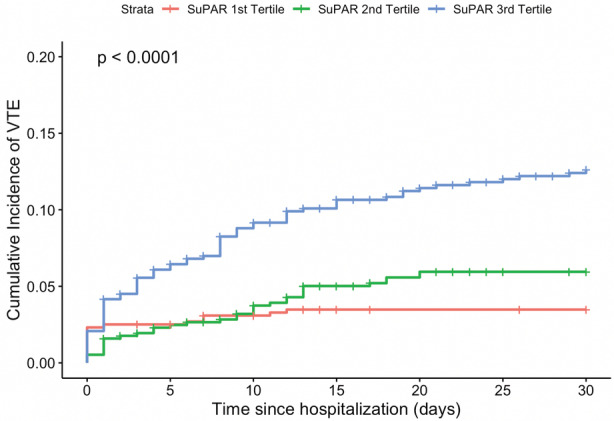
suPAR categories are defined as follows: first tertile, 0 to 5.2 ng/mL; second tertile, 5.3 to 8.7 ng/mL; and third tertile, 8.8 to 62.7 ng/mL
Table 3.
Cox Proportional Hazards and Competing Risk Regressions for the Association Between suPAR and Time to Incident VTE Within 30 Days of Hospitalization
| Variable | Cox model | Fine‐Gray model | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| suPAR | ||||
| First tertile (0–5.2 ng/mL) | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Second tertile (5.3–8.7 ng/mL) | 0.87 (0.48–1.58) | 0.64 | 0.88 (0.48–1.61) | 0.67 |
| Third tertile (8.8–62.7 ng/mL) | 1.52 (0.86–2.68) | 0.15 | 1.52 (0.84–2.74) | 0.17 |
| Age, per 10 y older | 0.93 (0.81–1.07) | 0.32 | 0.93 (0.81–1.07) | 0.32 |
| Men, vs women | 2.25 (1.45–3.47) | <0.001 | 2.24 (1.45–3.46) | 0.003 |
| Black, vs White, Asian, American Indian and Pacific Islander | 1.87 (1.23–2.86) | 0.004 | 1.86 (1.22–2.84) | 0.004 |
| Body mass index, per 5 kg/m2 higher | 1.06 (0.98–1.14) | 0.15 | 1.06 (0.99–1.13) | 0.12 |
| History of diabetes, vs none | 0.89 (0.58–1.37) | 0.60 | 0.90 (0.60–1.34) | 0.59 |
| History of hypertension, vs none | 0.88 (0.56–1.38) | 0.58 | 0.88 (0.55–1.41) | 0.60 |
| History of congestive heart failure, vs none | 0.57 (0.25–1.27) | 0.17 | 0.57 (0.25–1.28) | 0.17 |
| History of malignancy, vs none | 2.22 (1.13–4.36) | 0.021 | 2.21 (1.13–4.32) | 0.021 |
| Before admission anticoagulation therapy, vs none | 0.40 (0.16–1.01) | 0.053 | 0.41 (0.16–1.04) | 0.06 |
| Admission estimated glomerular filtration rate, per 5 mL/min per 1.73 m2 higher | 0.99 (0.95–1.02) | 0.39 | 0.99 (0.95–1.02) | 0.38 |
| D‐dimer, per fold higher compared with baseline | 1.44 (1.30–1.60) | <0.001 | 1.43 (0.70–1.30) | <0.001 |
HR indicates hazard ratio; suPAR, soluble urokinase plasminogen activator receptor; and VTE, venous thromboembolism.
Sensitivity Analyses
We found consistent results in the subgroup of patients in whom blood samples were obtained during the first 24 hours of hospital admission (Table S2), among those enrolled at the University of Michigan (Table S3), and in multivariable models adjusting for D‐dimer scaled to the assays' upper normal limits at respective clinical sites (Table S4).
Diagnostic Power and Risk Discrimination for Incident VTE Comparing suPAR and D‐Dimer
The sensitivity values for incident VTE at the 25th, 50th and 75th percentiles of suPAR levels were 0.91 (95% CI, 0.87–0.96), 0.70 (95% CI, 0.63–0.77), and 0.47 (95% CI, 0.40–0.55), respectively (Table 4). The sensitivity values for incident VTE at the 25th, 50th, and 75th D‐dimer percentiles were 0.92 (95% CI, 0.88–0.96), 0.78 (95% CI, 0.72–0.83), and 0.48 (95% CI, 0.41–0.55), respectively. The 95% sensitivity cutoff was 4.12 ng/mL for suPAR (negative predictive value, 0.98) and 0.50 mg/dL for D‐dimer (negative predictive value, 0.97). The C‐statistic values for incident VTE were 0.677 (95% CI, 0.630–0.724) and 0.713 (95% CI, 0.673–0.752) for suPAR and D‐dimer in their respective univariate logistic regression models. Including both suPAR and D‐dimer in one model improved the C‐statistic to 0.718 (95% CI, 0.673–0.764), although the increment compared with D‐dimer alone was not statistically significant (P=0.86).
Table 4.
Sensitivity and Specificity Parameters for Incident VTE by Different suPAR and D‐Dimer Centiles
| Marker | Cutoff | Centile | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) |
|---|---|---|---|---|---|---|
| suPAR, ng/mL | 4.12 | 20 | 0.95 (0.91–0.98) | 0.21 (0.19–0.23) | 0.10 (0.09–0.10) | 0.98 (0.96–0.99) |
| 4.54 | 25 | 0.91 (0.87–0.96) | 0.26 (0.24–0.28) | 0.10 (0.10–0.11) | 0.97 (0.96–0.98) | |
| 6.70 | 50 | 0.70 (0.63–0.77) | 0.52 (0.50–0.54) | 0.12 (0.11–0.13) | 0.95 (0.94–0.96) | |
| 10.1 | 75 | 0.47 (0.40–0.55) | 0.75 (0.75–0.79) | 0.16 (0.13–0.18) | 0.94 (0.93–0.95) | |
| D‐dimer, mg/L | 0.50 | 20 | 0.95 (0.92–0.98) | 0.21 (0.20–0.24) | 0.12 (0.11–0.12) | 0.97 (0.96–0.99) |
| 0.56 | 25 | 0.92 (0.88–0.96) | 0.26 (0.25–0.29) | 0.12 (0.12–0.13) | 0.97 (0.95–0.98) | |
| 1.03 | 50 | 0.78 (0.72–0.83) | 0.53 (0.50–0.56) | 0.15 (0.14–0.16) | 0.96 (0.94–0.97) | |
| 2.40 | 75 | 0.48 (0.41–0.55) | 0.77 (0.75–0.79) | 0.19 (0.16–0.21) | 0.93 (0.92–0.94) |
suPAR indicates soluble urokinase plasminogen activator receptor; and VTE, venous thromboembolism.
suPAR and D‐Dimer Cutoff Combination to Identify Patients With the Lowest Probability of VTE
A decision tree based on predicted probabilities from random forest identified a subgroup of patients with suPAR <11 ng/mL and D‐dimer <1.0 mg/L who had a 3.6% probability of incident VTE during a COVID‐19 hospitalization (Figure 3). This subgroup represented 41% of the total study population. No other suPAR and D‐dimer cutoff combinations yielded a <5% probability of the outcome.
Figure 3. A decision tree based on predicted probabilities from random forest exploring soluble urokinase plasminogen activator receptor (suPAR) and D‐dimer cutoff combinations for the stratification of patients by probability of venous thromboembolism (VTE).
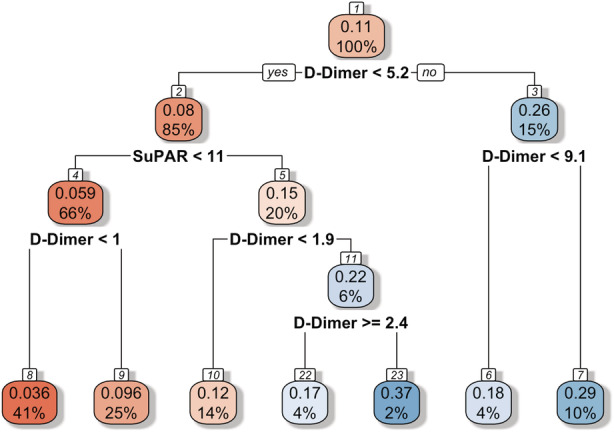
The subgroup of patients with suPAR <11 ng/mL and D‐dimer <1.0 mg/L had a 3.6% probability of incident VTE during a COVID‐19 hospitalization. This subgroup represented 41% of the total study population. No other suPAR and D‐dimer cutoff combinations yielded a <5% probability of the outcome. The red color indicates lower probabilities, and the blue color indicates higher probabilities. Units for suPAR and D‐dimer as shown in the figure are ng/mL and mg/L, respectively.
DISCUSSION
In this multinational observational cohort study of 1960 adults hospitalized for COVID‐19, we found significantly more incident VTE events among individuals with higher compared with lower suPAR levels on hospital admission. The significant association between suPAR and VTE was independent of established clinical thromboembolic markers, such as D‐dimer, suggesting that suPAR may account for the excessive thromboembolic risk in COVID‐19, possibly secondary to immunothrombosis. Using a plasma suPAR level of 4.12 ng/mL as a cutoff, we were able to identify patients at risk for VTE with 95% sensitivity and a negative predictive value of 0.98. We also illustrated the use of suPAR and D‐dimer cutoff combinations to identify patient subgroups with a low probability of VTE, which may aid in the risk stratification of patients with COVID‐19.
VTE is highly prevalent among those with COVID‐19 and can contribute significantly to morbidity and mortality. 22 , 23 This has led to increasingly widespread use of prophylactic and therapeutic anticoagulation. Pulmonary embolism, however, remained the direct cause of mortality in up to one third of patients who died from COVID‐19. VTE may be unnoticed throughout the course of the disease; extensive thrombi, including those in pulmonary and glomerular microcirculations, were identified during autopsies of patients with COVID‐19. 3 , 24 Even in the absence of critical illness, and with therapy targeting certain inflammatory pathways effective at controlling the progression of severe respiratory disease, thromboembolic events are common. 2 , 5 , 25 Aside from critical illness, the disproportionately high thromboembolic risk in COVID‐19 was attributed in part to immunothrombosis, thromboembolism secondary to a distinct proinflammatory milieu caused by immune dysregulation and host defense against the SARS‐CoV‐2 virus (viral infection‐induced significant immune dysregulation), and local vascular inflammation (ie, endotheliitis), thereby triggering extensive microangiopathy and macroangiopathy. 4 Unfortunately, conventional clinical imaging studies are incapable of discerning this type of thromboembolism from that arising from nonimmune causes. Traditional VTE markers (eg, D‐dimer and fibrinogen) have been less reliable in predicting incident VTE events in COVID‐19. Although D‐dimer levels are higher in the presence of VTE, they do not always correlate with the risk of developing new VTE in COVID‐19, making D‐dimer less helpful in clinical decision making. This finding may be explained by the direct release of D‐dimer from the inflammation‐injured lungs. 26 , 27 Whether suPAR can be released from the lungs in a similar manner in COVID‐19 has yet to be investigated. We note that only a few of the novel VTE markers being investigated in non–COVID‐19 settings have been found to be independently associated with incident VTE in COVID‐19 (eg, plasma P‐selectin, an activated platelet and endothelial membrane protein, presumably a mediator of immunothrombosis, via facilitating leukocyte rolling). 28 , 29 , 30
The uPAR is a glycosylphosphatidylinositol‐anchored 3‐domain membrane protein bound to various cells, such as myeloid and endothelial cells, which is likely a key regulator of cross‐linking vascular inflammation, immunity, and coagulation. 9 In the presence of inflammatory stimuli, uPAR is cleaved and released into circulation, becoming suPAR, enhancing local vascular inflammation, and exerting immune effects distally, possibly via what is known as a “danger‐associated molecular pattern.” Mounting evidence has pointed to suPAR being a key immune component in certain renal and cardiovascular conditions (eg, hypertension‐attributed chronic kidney disease). 31 , 32 , 33 , 34 , 35 , 36 suPAR can be elevated by 3‐ to 5‐fold in patients with COVID‐19, and often earlier than other inflammatory biomarkers, including D‐dimer, with higher suPAR levels portending detrimental complications, such as severe acute kidney injury and respiratory failure requiring mechanical ventilation. 10 , 11 , 37 An association between suPAR and thromboembolism independent of D‐dimer, a downstream, direct biomarker of thromboembolism, suggests that suPAR and the uPA/uPAR system may reflect upstream immunothrombotic activity and explain the excessive thromboembolic risk in COVID‐19. 6 , 8 , 9 However, in survival analyses with and without accounting for the competing risk of death, despite a trend toward higher risk for 30‐day VTE with higher suPAR, consistent with results from multivariate logistic regression, the association was not statistically significant, which may be explained by insufficient power and delays in clinical diagnosis among at‐risk individuals. Of note, previous studies have reported higher D‐dimer in those with obesity and COVID‐19, presumably because of hyperinflammation. 38 Interestingly, we observed a small but statistically significant negative association between BMI and D‐dimer after accounting for suPAR. Such finding suggests suPAR, as an early biomarker of hyperinflammation in COVID‐19, may explain the association between obesity and elevated D‐dimer.
There have been ongoing efforts to integrate suPAR testing, which is now commercially available and affordable, into clinical practice; the European Commission recently authorized the drug, anakinra, to target interleukin‐1 in COVID‐19 using suPAR levels for guidance. 2 In light of these developments, our findings provide additional data to improve the understanding of suPAR's role in clinical settings. For example, with suPAR levels >4.12 ng/mL, we identified with a 95% sensitivity patients at risk for developing VTE. Combining suPAR and D‐dimer cutoffs, we also identified patient subgroups with a low probability of VTE.
To our knowledge, only one published observational study has explored the association between suPAR and VTE, which was in a general European population in the 1990s. 13 No published study to date has demonstrated an independent association between suPAR and thromboembolism in the setting of acute viral illness, such as COVID‐19. The strength of this study lies in its large sample size and detailed ascertainment of clinical and laboratory variables in all participants. This study also has limitations. First, we are unable to ascribe causality with an observational study design. The possibility of residual confounding cannot be excluded. However, we have adjusted for most known thromboembolic risk factors in our analyses and performed a competing risk analysis. Second, there was possibly an underascertainment of outcomes, given assessment for VTE was not systematic and was performed on the basis of clinical suspicion. However, the incidence of VTE in this study population was comparable to that of others. 1 Third, blood samples were collected for laboratory testing within the first 48 hours of hospitalization. Laboratory values later during this period may have been affected by inpatient COVID‐19 treatments. However, a sensitivity analysis including blood samples obtained within the first 24 hours of hospitalization showed consistent findings. Fourth, different D‐dimer assays were used at different clinical sites, which may lead to inconsistent accuracy in measurements. Nonetheless, subgroup analysis within the University of Michigan cohort produced consistent findings, and multivariable analyses adjusting for D‐dimer rescaled to respective centers' ULNs did not change results.
In conclusion, in a multinational observational cohort of adults hospitalized with COVID‐19, we found suPAR levels measured on admission to be associated with incident VTE independently of D‐dimer levels, a well‐established clinical marker of coagulopathy. This finding implies the possible roles of suPAR in COVID‐19–related immunothrombosis. suPAR may be used in conjunction with D‐dimer in clinical practice for improved risk stratification of patients with COVID‐19 for VTE.
APPENDIX
ISIC (International Study of Inflammation in COVID‐19) Group Investigators
University of Michigan in Ann Arbor, MI: Salim S. Hayek*, Pennelope Blakely, Christopher Launius, Hanna Berlin, Kingsley Amadi, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Elizabeth Anderson, Danny Perry, Abbas Bitar, Rayan Kaakati, Lili Zhao, Peiyao Zhao, Erinleigh Michaud, Yiyuan Huang, Toniemarie Catalan, Ibrahim Khaleel.
Rush University in Chicago, IL: Jochen Reiser*, Beata Samelko, Alexander Hlepas, Xuexiang Wang, Priya Patel.
University of Copenhagen at Hvidovre Hospital, Denmark: Jesper Eugen‐Olsen*, Izzet Altintas, Jens Tingleff, Marius Stauning, Morten Baltzer Houlind, Mette B. Lindstrøm, Ove Andersen, Hejdi Gamst‐Jensen, Line Jee Hartmann Rasmussen, Christian Rasmussen, Jan O. Nehlin, Thomas Kallemose, Imran Parvaiz.
Attikon University Hospital in Athens, Greece: Evangelos J. Giamarellos‐Bourboulis*, Maria‐Evangelia Adami, Nicky Solomonidi, Maria Tsilika, Maria Saridaki, Vasileios Lekakis.
University Hospital Dusseldorf, Germany: Sven Loosen*, Tom Luedde, Verena Keitel.
University of Thessaly, Greece: Athanasios Chalkias*, Ioannis Pantazopoulos, Eleni Laou, Anargyros Skoulakis.
Charité University Medicine Berlin, Germany: Frank Tacke*, Pinkus Tober‐Lau, Raphael Mohr, Florian Kurth, Leif Erik Sander, Christoph Jochum.
University Hospital of Cologne, Germany: Philipp Koehler.
*Site Principal Investigator
Sources of Funding
Dr Vasbinder is supported by a National Heart, Lung, and Blood Institute–funded postdoctoral fellowship (T32HL007853). Dr Hayek is funded by National Heart, Lung, and Blood Institute 1R01HL153384‐01, the National Institute of Diabetes and Digestive and Kidney Diseases 1R01DK12801201A1 and U01‐DK119083‐03S1, and the Frankel Cardiovascular Center COVID‐19: Impact Research Ignitor (U‐M G024231) award. Dr Giamarellos‐Bourboulis is supported by the Hellenic Institute for the Study of Sepsis. Dr Tacke is supported through intramural funds from Charité Universitaetsmedizin Berlin and the Berlin Institute of Health.
Disclosures
Drs Hayek and Reiser are members of the scientific advisory board of Walden Biosciences. Dr Eugen‐Olsen is a cofounder, shareholder, and chief scientific officer of Virogates, and named inventor on patents related to soluble urokinase plasminogen activator receptor. Other authors have no disclosures.
Supporting information
Tables S1–S4
Acknowledgments
The authors acknowledge the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation, management, and distribution services in support of the research reported in this publication. The authors are grateful to the services of the Microbiome Core, supported by U2CDK110768, especially Chris Blair; the Michigan Clinical Research Unit, including Wrenn Woodard and Dexter Hobdy; and the University of Michigan Medical School Central Biorepository for providing biospecimen storage, management, and distribution services in support of the research reported in the publication.
ISIC (International Study of Inflammation in COVID‐19) Group investigators are listed in the Appendix at the end of the article.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.025198
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Salim S. Hayek, Email: shayek@med.umich.edu.
the ISIC (International Study of Inflammation in COVID‐19) Group:
Salim S. Hayek, Pennelope Blakely, Christopher Launius, Hanna Berlin, Kingsley Amadi, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’Hayer, Chelsea Meloche, Rafey Feroze, Kishan J. Padalia, Elizabeth Anderson, Danny Perry, Abbas Bitar, Rayan Kaakati, Lili Zhao, Peiyao Zhao, Erinleigh Michaud, Yiyuan Huang, Toniemarie Catalan, Ibrahim Khaleel, Jochen Reiser, Beata Samelko, Alexander Hlepas, Xuexiang Wang, Priya Patel, Jesper Eugen‐Olsen, Izzet Altintas, Jens Tingleff, Marius Stauning, Morten Baltzer Houlind, Mette B. Lindstrøm, Ove Andersen, Hejdi Gamst‐Jensen, Line Jee Hartmann Rasmussen, Christian Rasmussen, Jan O. Nehlin, Thomas Kallemose, Imran Parvaiz, Evangelos J. Giamarellos‐Bourboulis, Maria‐Evangelia Adami, Nicky Solomonidi, Maria Tsilika, Maria Saridaki, Vasileios Lekakis, Sven Loosen, Tom Luedde, Verena Keitel, Athanasios Chalkias, Ioannis Pantazopoulos, Eleni Laou, Anargyros Skoulakis, Frank Tacke, Pinkus Tober‐Lau, Raphael Mohr, Florian Kurth, Leif Erik Sander, Christoph Jochum, and Philipp Koehler
References
- 1. Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AWS, Di Nisio M. Venous thromboembolism in patients with COVID‐19: systematic review and meta‐analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, et al. Early treatment of COVID‐19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double‐blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loo J, Spittle DA, Newnham M. COVID‐19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412–420. doi: 10.1136/thoraxjnl-2020-216243 [DOI] [PubMed] [Google Scholar]
- 5. Levi M, Coppens M. Vascular mechanisms and manifestations of COVID‐19. Lancet Respir Med. 2021;9:551–553. doi: 10.1016/S2213-2600(21)00221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elberts SJ, Bateman R, Koutsoubis A, London KS, White JL, Fields JM. The impact of COVID‐19 on the sensitivity of D‐dimer for pulmonary embolism. Acad Emerg Med. 2021;28:1142–1149. doi: 10.1111/acem.14348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Alonzo D, De Fenza M, Pavone V. COVID‐19 and pneumonia: a role for the uPA/uPAR system. Drug Discov Today. 2020;25:1528–1534. doi: 10.1016/j.drudis.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Rosso M, Margheri F, Serratì S, Chillà A, Laurenzana A, Fibbi G. The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation. Curr Pharm Des. 2011;17:1924–1943. doi: 10.2174/138161211796718189 [DOI] [PubMed] [Google Scholar]
- 10. Azam TU, Shadid HR, Blakely P, O'Hayer P, Berlin H, Pan M, Zhao P, Zhao L, Pennathur S, Pop‐Busui R, et al. Soluble urokinase receptor (SuPAR) in COVID‐19‐related AKI. J Am Soc Nephrol. 2020;31:2725–2735. doi: 10.1681/ASN.2020060829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rovina N, Akinosoglou K, Eugen‐Olsen J, Hayek S, Reiser J, Giamarellos‐Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID‐19 pneumonia. Crit Care. 2020;24:187. doi: 10.1186/s13054-020-02897-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Medicines Agency . EMA recommends approval for use of Kineret in adults with COVID‐19. European Medicines Agency Offficial Website, Date Published: 12/16/2021. 2021. Available at: https://www.ema.europa.eu/en/news/ema‐recommends‐approval‐use‐kineret‐adults‐covid‐19. Accessed June 12, 2022.
- 13. Engström G, Zöller B, Svensson PJ, Melander O, Persson M. Soluble urokinase plasminogen activator receptor and incidence of venous thromboembolism. Thromb Haemost. 2016;115:657–662. doi: 10.1160/TH15-06-0511 [DOI] [PubMed] [Google Scholar]
- 14. Pan M, Vasbinder A, Anderson E, Catalan T, Shadid HR, Berlin H, Padalia K, O'Hayer P, Meloche C, Azam TU, et al. Angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and outcomes in patients hospitalized for COVID‐19. J Am Heart Assoc. 2021;10:e023535. doi: 10.1161/JAHA.121.023535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azam TU, Berlin H, Anderson E, Pan M, Shadid HR, Padalia K, O'Hayer P, Meloche C, Feroze R, Michaud E, et al. Differences in inflammation, treatment, and outcomes between black and non‐black patients hospitalized for COVID‐19: a prospective cohort study. Am J Med. 2022;135:360–368. doi: 10.1016/j.amjmed.2021.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayek SS, Roderburg C, Blakely P, Launius C, Eugen‐Olsen J, Tacke F, Ktena S, Keitel V, Luedde M, Giamarellos‐Bourboulis EJ, et al. Circulating osteopontin levels and outcomes in patients hospitalized for COVID‐19. J Clin Med. 2021;10:3907. doi: 10.3390/jcm10173907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vasbinder A, Anderson E, Shadid H, Berlin H, Pan M, Azam TU, Khaleel I, Padalia K, Meloche C, O'Hayer P, et al. Inflammation, hyperglycemia, and adverse outcomes in individuals with diabetes mellitus hospitalized for COVID‐19. Diabetes Care. 2022;45:692–700. doi: 10.2337/dc21-2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 20. Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15:651–674. doi: 10.1198/106186006x133933 [DOI] [Google Scholar]
- 21. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available at: https://www.R‐project.org/
- 22. Tan BK, Mainbourg S, Friggeri A, Bertoletti L, Douplat M, Dargaud Y, Grange C, Lobbes H, Provencher S, Lega JC. Arterial and venous thromboembolism in COVID‐19: a study‐level meta‐analysis. Thorax. 2021;76:970–979. doi: 10.1136/thoraxjnl-2020-215383 [DOI] [PubMed] [Google Scholar]
- 23. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunt BJ, Levi M. Re The source of elevated plasma D‐dimer levels in COVID‐19 infection. Br J Haematol. 2020;190:e133–e134. doi: 10.1111/bjh.16907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vincent JL, Levi M, Hunt BJ. Prevention and management of thrombosis in hospitalised patients with COVID‐19 pneumonia. Lancet Respir Med. 2022;10:214–220. doi: 10.1016/S2213-2600(21)00455-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fenyves BG, Mehta A, Kays KR, Beakes C, Margolin J, Goldberg MB, Hacohen N, Filbin MR. Plasma P‐selectin is an early marker of thromboembolism in COVID‐19. Am J Hematol. 2021;96:E468–E471. doi: 10.1002/ajh.26372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mulder MMG, Brandts L, Brüggemann RAG, Koelmann M, Streng AS, Olie RH, Gietema HA, Spronk HMH, van der Horst ICC, Sels JEM, et al. Serial markers of coagulation and inflammation and the occurrence of clinical pulmonary thromboembolism in mechanically ventilated patients with SARS‐CoV‐2 infection; the prospective Maastricht intensive care COVID cohort. Thromb J. 2021;19:35. doi: 10.1186/s12959-021-00286-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van de Sande D, van Genderen ME, Rosman B, Diether M, Endeman H, van den Akker JPC, Ludwig M, Huiskens J, Gommers D, van Bommel J. Predicting thromboembolic complications in COVID‐19 ICU patients using machine learning. J Clin Transl Res. 2020;6:179–186. doi: 10.18053/jctres.06.202005.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo S, Coresh J, Tin A, Rebholz CM, Chen TK, Hayek SS, Tracy M, Lipkowitz MS, Appel LJ, Levey AS, et al. Soluble urokinase‐type plasminogen activator receptor in black Americans with CKD. Clin J Am Soc Nephrol. 2018;13:1013–1021. doi: 10.2215/CJN.13631217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eapen DJ, Manocha P, Ghasemzadeh N, Patel RS, Al Kassem H, Hammadah M, Veledar E, Le NA, Pielak T, Thorball CW, et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3:e001118. doi: 10.1161/JAHA.114.001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, Samelko B, Lee H, Dande RR, Lee HW, et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23:945–953. doi: 10.1038/nm.4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodges GW, Bang CN, Wachtell K, Eugen‐Olsen J, Jeppesen JL. suPAR: a new biomarker for cardiovascular disease? Can J Cardiol. 2015;31:1293–1302. doi: 10.1016/j.cjca.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 36. Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, Wadhwani S, Cao Y, Peev V, Zloza A, et al. Bone marrow‐derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23:100–106. doi: 10.1038/nm.4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayek SS, Leaf DE, Samman Tahhan A, Raad M, Sharma S, Waikar SS, Sever S, Camacho A, Wang X, Dande RR, et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:416–426. doi: 10.1056/NEJMoa1911481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, Small‐Saunders J, Rajagopalan KN, Greendyk R, Chae SR, et al. Body mass index and risk for intubation or death in SARS‐CoV‐2 infection: a retrospective cohort study. Ann Intern Med. 2020;173:782–790. doi: 10.7326/M20-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


