Abstract
Background
Many hospitalized patients are not administered prescribed doses of pharmacologic venous thromboembolism prophylaxis.
Methods and Results
In this cluster‐randomized controlled trial, all adult non–intensive care units (10 medical, 6 surgical) in 1 academic hospital were randomized to either a real‐time, electronic alert–triggered, patient‐centered education bundle intervention or nurse feedback intervention to evaluate their effectiveness for reducing nonadministration of venous thromboembolism prophylaxis. Primary outcome was the proportion of nonadministered doses of prescribed pharmacologic prophylaxis. Secondary outcomes were proportions of nonadministered doses stratified by nonadministration reasons (patient refusal, other). To test our primary hypothesis that both interventions would reduce nonadministration, we compared outcomes pre‐ versus postintervention within each cohort. Secondary hypotheses were tested comparing the effectiveness between cohorts. Of 11 098 patient visits, overall dose nonadministration declined significantly after the interventions (13.4% versus 9.2%; odds ratio [OR], 0.64 [95% CI, 0.57–0.71]). Nonadministration decreased significantly (P<0.001) in both arms: patient‐centered education bundle, 12.2% versus 7.4% (OR, 0.56 [95% CI, 0.48–0.66]), and nurse feedback, 14.7% versus 11.2% (OR, 0.72 [95% CI, 0.62–0.84]). Patient refusal decreased significantly in both arms: patient‐centered education bundle, 7.3% versus 3.7% (OR, 0.46 [95% CI, 0.37–0.58]), and nurse feedback, 9.5% versus 7.1% (OR, 0.71 [95% CI, 0.59–0.86]). No differential effect occurred on medical versus surgical units. The patient‐centered education bundle was significantly more effective in reducing all nonadministered (P=0.03) and refused doses (P=0.003) compared with nurse feedback (OR, 1.28 [95% CI, 1.0–1.61]; P=0.03 for interaction).
Conclusions
Information technology strategies like the alert‐triggered, targeted patient‐centered education bundle, and nurse‐focused audit and feedback can improve venous thromboembolism prophylaxis administration.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03367364.
Keywords: deep vein thrombosis, patient‐centered care, prophylaxis, pulmonary embolism, randomized trial, venous thromboembolism
Subject Categories: Quality and Outcomes, Health Services, Risk Factors, Thrombosis
Nonstandard Abbreviations and Acronyms
- PCORI
Patient‐Centered Outcomes Research Institute
Clinical Perspective.
What Is New?
This trial shows that each intervention, (1) a real‐time electronic, alert‐triggered, patient‐centered education bundle and (2) nurse performance feedback, independently and successfully reduced missed doses of prescribed venous thromboembolism (VTE) prophylaxis among hospitalized patients.
What Are the Clinical Implications?
Hospital‐associated VTE is a largely preventable condition when hospitalized patients are prescribed and receive appropriate VTE prophylaxis; however, many doses of prescribed prophylaxis are not administered to hospitalized patients.
These interventions demonstrate that missed doses of VTE prophylaxis in hospitalized patients can be significantly reduced by engaging patients and nurses with timely, informative, actionable information about the importance of VTE prophylaxis.
Hospitalization is a primary risk factor for venous thromboembolism (VTE), 1 and deep vein thrombosis and pulmonary embolism are leading causes of preventable harm among patients in the hospital. 2 , 3 As a result, national organizations focused on health care quality improvement, including the Agency for Healthcare Research and Quality and The Joint Commission, measure and report VTE prevention practices. 4 , 5 Efforts to improve prescription of VTE prophylaxis for hospitalized patients have achieved great success. 6 , 7 , 8 , 9 However, evidence shows that many doses of prescribed pharmacologic VTE prophylaxis are not administered and most often attributed to patient refusal or poor patient–nurse communication. 10 , 11 , 12 , 13 , 14 Missing even 1 dose of a VTE prophylaxis medication has been associated with developing VTE events. 15 , 16 VTE prophylaxis medications are some of the most frequently nonadministered medications for hospitalized patients. 17 , 18
Reasons for nonadministration of prescribed pharmacologic VTE prophylaxis have been attributed to nurse attitudes, knowledge, and beliefs on the need for prophylaxis, 14 and patients refusing doses. Moreover, recent American Society of Hematology guidelines do not address nonadministration of prescribed doses, 19 , 20 which is a missed opportunity to encourage best practices to prevent VTE. Previous interventions have targeted nonadministration practices and significantly improved administration of prescribed prophylaxis doses, including comprehensive VTE‐specific nurse education 21 and widespread patient education. 11 , 22 Most recently, we showed that a real‐time, patient‐centered, education bundle (bundle) implemented on 4 adult non–intensive care units can reduce nonadministered doses by >40% in hospitalized patients. 23 This bundle intervention was part of an extramurally funded research project and had substantial human resources that are not sustainable long term or generalizable to a wide range of hospitals.
The current study is part of a dissemination effort. The goal was to scale use of the bundle to other adult non–intensive care units and transition implementation from the research team to the nurses for integration in their unit's routine medication administration process without additional support of a research nurse, as was done in the original trial. This approach has already been shown to be effective at a community hospital. 24 We aimed to test the effectiveness of unit staff nurses implementing the bundle triggered by a real‐time electronic alert. 23 While planning our intervention, individualized nurse performance feedback and coaching (nurse feedback) was suggested as an alternative approach to reduce nonadministration of prescribed in‐hospital pharmacologic VTE prophylaxis. We decided to implement both strategies simultaneously as part of a rigorously performed cluster‐randomized trial. Our primary a priori hypothesis was that each intervention would improve medication administration. Because patient refusals account for the majority of nonadministered doses, 10 and the bundle has significantly decreased refusals in prior studies, 23 , 24 our secondary a priori hypotheses were that the alert‐triggered patient‐ education bundle would be more effective than nurse feedback in reducing overall nonadministered doses for any reason and in reducing patient refusal.
METHODS
Data are available from the Johns Hopkins Medicine Institutional Data Trust for researchers who meet the criteria for access to confidential data. The data set in question contains protected health information, which by nature of the study cannot be deidentified. For the purpose of this study, identifiable information included patient identifiers, nurse identifiers, dates of hospitalization, dates/times of medication administration, and hospital location, which are all required components for the analysis that would potentially enable individuals to identify specific patients and nurses. To request access to the data, please reach out to Dr. Daniel E. Ford, MPH, Vice Dean for Clinical Investigation at dford@jhmi.edu.
Study Design and Setting
ENACT (Patient Education Bundle Versus Nurses Feedback and Coaching to Prevent Missed Doses of Venous Thromboembolism Prophylaxis) was a cluster‐randomized controlled trial involving all 16 adult non–intensive care medical (n=10) and surgical (n=6) units at The Johns Hopkins Hospital, an urban academic tertiary care hospital. The Johns Hopkins Medicine Institutional Review Board approved the trial and provided a waiver of consent. The trial was registered on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT03367364).
Randomization and Blinding
We performed a stratified block randomization to balance the distribution of medicine (n=10) and surgery (n=6) units and of baseline dose administration performance (high versus low performers) in each intervention arm. We leveraged unit‐specific data on VTE prophylaxis nonadministration practices of nurses from the electronic health record (EHR) system, and in a research team meeting rank ordered units by performance. After group consensus on the block assignment, an actual physical coin toss (heads or tails) within each block assigned units to either the bundle or the nurse feedback intervention. Eight units (3 surgical, 5 medical) were assigned to each arm. Our biostatisticians (J.W., G.Y.) were blinded to unit intervention assignments.
Interventions
Patient‐Centered Education Bundle
The bundle was developed and validated in the original study and the details published. 23 , 24 , 25 , 26 Briefly, the bundle used the same educational approach, which included a 1‐on‐1 personalized discussion with the patient, supplemented by a 2‐page paper handout (available in 13 languages), and a 10‐minute video (bit.ly/bloodclots). 23 In the original study, a Patient‐Centered Outcomes Research Institute (PCORI)‐funded research nurse educator implemented the bundle, asking the patient their preferred mode of education, spending a median of 10 minutes on the patient intervention. 23 In the current study, the nursing teams providing routine clinical care on the units replaced that funded nurse educator and implemented the bundle. The bundle process following the alert involved engaging the bedside nurse who documented the nonadministered dose to determine the cause and intervening with education targeting the nurse, the patient, or both. To prepare units for implementation, we provided standardized educational materials (posters, badge cards, and slides) and trained nurses to deliver the education in real time, offering them scripted talking points when discussing VTE prevention with patients. Because the intent of dissemination was independent implementation of the bundle during routine medication administration, fidelity to the intervention and patient preferences to which education they preferred were not tracked.
Real‐Time Alert
At The Johns Hopkins Hospital, every patient is VTE risk assessed on admission using a computerized clinical decision support tool in the EHR, which has been in place for over a decade. 12 , 27 , 28 Prescription of appropriate VTE prophylaxis has improved dramatically via numerous quality improvement interventions and is well over 90% in many patient populations. 7 , 29 , 30 When the bedside nurse documented a nonadministered dose in the EHR, it automatically generated an alert in real‐time to a pager and triggered the bundle intervention. Each unit could choose which team members would hold the pager (eg, nurse manager, charge nurse, nurse educator) and respond in a timely manner to the alert.
Nurse Feedback
The research team provided unit nurse managers with a monthly performance scorecard describing VTE prophylaxis administration practices for each individual nurse (number of doses prescribed, number of doses administered, and number of doses refused) (Figure S1). The unit nurse managers were exclusively responsible for conducting the nurse feedback intervention and used their preferred style. Although the research team recommended options to provide feedback (eg, 1‐on‐1 coaching, sharing data via email, posting blinded/unblended results), all decisions and actions were left to the nurse manager for each participating unit. We did not collect information on approaches used. When performing our analysis, we used an intention‐to‐treat concept, aggregating missed doses for all patients in this cohort, regardless of what, if any, nurse feedback was provided.
Data Collection and Variables
Data on patient characteristics (age, sex, race), length of stay, and number of prescribed and administered prophylaxis doses were extracted directly from the EHR, achieving 100% automated data capture. The EHR system required documentation of all administered and nonadministered prescribed prophylaxis doses and the reason when not administered. We categorized reasons for nonadministration into patient refusal and other reasons, as documented by the nurse. We included patient visits (a hospitalization) with ≥1 dose of VTE prophylaxis prescribed. Baseline data included the period July 1, 2017 to December 31, 2017. We introduced the interventions to units in January 2018 and excluded these data from our analysis (washout period). We collected postintervention data from February 1, 2018 through April 30, 2018.
Outcomes
The primary outcome was the proportion of all prescribed pharmacologic VTE prophylaxis doses not administered. Secondary outcomes were the proportions of nonadministered doses from patient refusal or for other reasons.
Statistical Analysis
We performed a power calculation using historical data of prescribed VTE doses to determine whether we would have sufficient power for our planned analyses. Individual dose was the unit of analysis. Patient demographic and clinical characteristics for the baseline and postintervention periods are described in aggregate by intervention arm. The primary analysis was a pre‐ versus postintervention comparison to determine if both cohorts improved administration of prophylaxis, treating individual floors as their own historical control to account for differences by floor. We conducted 2‐sample t tests with equal variance to compare mean (SD) for age, and χ2 tests to compare proportions for sex and race. Nonparametric Wilcoxon rank sum tests were used to compare the number of prescribed doses per patient visit and length of stay; these were reported as median (interquartile range) and mean (SD). Our secondary analyses compared each intervention to determine which (if either) was more effective in reducing nonadministered doses, comparing overall reasons for missed doses, patient refusal, and other reasons not related with patient refusal.
For our primary and secondary hypotheses, mixed‐effects logistic regression models and Poisson regression models with random intercepts for unit and nurse were used to account for the nurse‐unit correlation when comparing VTE prophylaxis nonadministration by intervention arm and time. We included indicator variables for arm (nurse feedback versus patient bundle), time periods (pre‐ versus postintervention) and interaction term between arm and time periods as predictors. For the subgroup analysis by hospital unit, we included indicator variables for arm, pre‐ versus postintervention periods, and hospital unit (surgery versus medicine), as well as all the interaction terms among indicator variables for arm, time, and hospital unit as predictors. We used a multiple outputation approach to account for multiple VTE doses per patient across nurses and/or units. 31 This approach randomly selects 1 VTE prophylaxis dose per patient and repeats the procedure 1000 times to bootstrap the conditional odds ratio (OR) and proportions with corresponding 95% CI and P values for these comparisons, reducing hierarchical structure to the nurse‐unit level. 31 All comparisons were specified a priori and performed using a 0.05 α level for statistical significance. Statistical analyses were performed by a blinded team of biostatisticians using Stata version 14.1 MP–Parallel Edition (StataCorp, College Station, TX).
RESULTS
Of 12 958 patient visits prescribed pharmacologic VTE prophylaxis, 1860 were excluded (multiple floors n=495, doses in multiple periods n=1365), resulting in 11 098 visits from 9657 unique patients included in our analysis (Figure 1). Of 11 098 patient visits, 6158 were in the bundle arm and 4940 in the nurse feedback arm. A significant difference was observed in the racial distribution of patients between the bundle and nurse feedback arms in the preintervention period (White: 55.9% versus 49.3%, Black: 35.5% versus 42.3%, and other races: 8.6% versus 8.4%; P<0.001), but no significant differences were observed within unit type and between periods (Table 1). Median length of stay and median number of prescribed VTE doses per patient visit were significantly higher on nurse feedback units in the preintervention period. The proportion of nonadministered doses for all reasons for the patient education bundle was 12.2% (95% CI, 8.5–17.6) preintervention and 7.4% (95% CI, 5.1–10.8) postintervention, and for nurse feedback was 14.7% (95% CI, 10.3–21.2) preintervention and 11.2% (95% CI, 7.7–16.4) postintervention (Table 2).
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram of hospital units receiving the patient‐centered education bundle vs nurse feedback interventions.
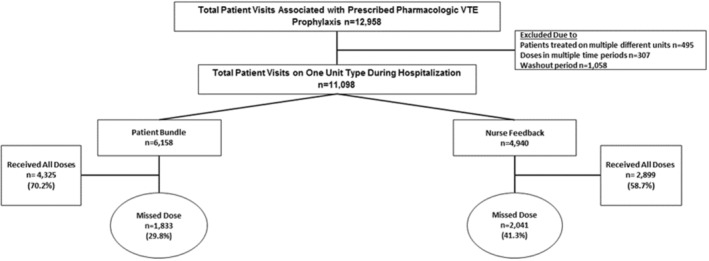
Patient visits reflect patients with prescribed pharmacologic venous thromboembolism (VTE) prophylaxis doses during 1 hospital encounter. In the patient‐centered education bundle arm, a nonadministered prophylaxis dose triggers an alert leading to the delivery of the patient‐centered education bundle intervention. The patient‐centered education bundle is only delivered once to patients. Nurse leaders in the nurse feedback arm received monthly scorecards detailing VTE prophylaxis administration practices by individual nurse.
Table 1.
Demographic and Clinical Characteristics for Pre‐ and Postintervention Visits by Patient Bundle and Nurse Feedback Arms
| Characteristics | Patient education bundle | P value | Nurse feedback | P value | P value comparing pre patient bundle and pre nurse feedback | ||
|---|---|---|---|---|---|---|---|
| Period | Period | ||||||
| Pre, n=4025 | Post, n=2133 | Pre, n=3342 | Post, n=1598 | ||||
| Unique patients | 3497 | 1926 | 2811 | 1423 | |||
| Unique nurses | 529 | 446 | 476 | 421 | |||
| Mean age, y (SD)* | 56.1 (16.8) | 56.1 (16.1) | 0.97 | 56.1 (16.2) | 56.7 (16.2) | 0.17 | 0.99 |
| Sex, n (%)† | |||||||
| Men | 2107 (52.3) | 1126 (52.8) | 0.74 | 1754 (52.5) | 864 (54.1) | 0.30 | 0.91 |
| Women | 1918 (47.7) | 1007 (47.2) | 1588 (47.5) | 734 (45.9) | |||
| Race, n (%)† | |||||||
| Black | 1428 (35.5) | 755 (35.4) | 0.79 | 1414 (42.3) | 724 (45.3) | 0.14 | <0.001 |
| White | 2251 (55.9) | 1212 (56.8) | 1646 (49.3) | 744 (46.6) | |||
| Other | 346 (8.6) | 166 (7.8) | 282 (8.4) | 130 (8.1) | |||
| Unit type, n (%)† | |||||||
| Medicine units | 1569 (39.0) | 839 (39.3) | 0.98 | 1849 (55.3) | 882 (55.2) | 0.93 | <0.001 |
| Surgery units | 2456 (61.0) | 1394 (60.7) | 1493 (44.7) | 716 (44.8) | |||
| No. of prescribed doses per patient visit (Q1–Q3) | |||||||
| Median (IQR) | 6.0 (3.0–12.0) | 6.0 (3.0–11.0) | 0.45 | 7.0 (3.0–13.0) | 6.0 (3.0–1.0) | 0.001 | <0.001 |
| Mean (SD)‡ | 9.22 (11.3) | 8.81 (10.1) | 10.44 (12.4) | 8.88 (9.7) | |||
| Length of stay, d (Q1–Q3) | |||||||
| Median (IQR) | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 0.68 | 5.0 (3.0–9.0) | 5.0 (3.0–8.0) | 0.64 | <0.001 |
| Mean (SD)‡ | 6.49 (9.6) | 6.52 (8.94) | 7.41 (8.4) | 7.22 (7.9) | |||
Patient education bundle refers to the Patient‐Centered Education Bundle. IQR indicates interquartile range; and Q, quartile.
P values calculated using 2‐sample t tests with equal variances.
P values calculated using χ2 tests.
P values calculated using Wilcoxon rank sum tests.
Table 2.
Proportion of Doses Nonadministered for All Reasons, Refused, and Other Reasons: Comparisons Between Overall Pre‐ and Postintervention by Patient Bundle and Nurse Feedback Arms* , †
| Period | Overall | Patient education bundle | Nurse feedback | OR nurse feedback/patient education bundle (95% CI) | P value* |
|---|---|---|---|---|---|
| Any nonadministered dose | |||||
| Preintervention, % (95% CI) | 13.4 (10.3–17.5) | 12.2 (8.5–17.6) | 14.7 (10.3–21.2) | 1.24 (0.68–2.26) | 0.49 |
| Postintervention, % (95% CI) | 9.2 (7.0–12.1) | 7.4 (5.1–10.8) | 11.2 (7.7–16.4) | 1.59 (0.86–2.94) | 0.14 |
| OR, post/pre, (95% CI) | 0.64 (0.57–0.71) | 0.56 (0.48–0.66) | 0.72 (0.62–0.84) | ||
| P value* | <0.001 | <0.001 | <0.001 | ||
| Refused dose | |||||
| Preintervention, % (95% CI) | 8.3 (5.6–12.2) | 7.3 (4.3–12.4) | 9.5 (5.6–16.1) | 1.35 (0.59–3.06) | 0.48 |
| Postintervention, % (95% CI) | 5.2 (3.5–7.8) | 3.7 (2.1–6.4) | 7.1 (4.2–12.2) | 2.07 (0.89–4.83) | 0.09 |
| OR, post/pre (95% CI) | 0.59 (0.51–0.68) | 0.46 (0.37–0.58) | 0.71 (0.59–0.86) | ||
| P value* | <0.001 | <0.001 | <0.001 | ||
| Other reasons for nonadministered doses (not patient refused) | |||||
| Preintervention, % (95% CI) | 3.6 (3.1–4.2) | 3.4 (2.8–4.3) | 3.8 (3.0–4.7) | 1.09 (0.80–1.50) | 0.57 |
| Postintervention, % (95% CI) | 2.9 (2.4–3.5) | 2.8 (2.2–3.6) | 3.0 (2.3–3.9) | 1.08 (0.75–1.56) | 0.68 |
| OR, post/pre (95% CI) | 0.79 (0.68–0.93) | 0.80 (0.64–0.99) | 0.79 (0.63–0.99) | ||
| P value* | 0.004 | 0.04 | 0.04 | ||
Patient education bundle refers to the Patient‐Centered Education Bundle. OR indicates odds ratio.
P values for the ORs were calculated using multiple outputation of the mixed‐effects logistic regression models.
Exponentiated 2‐way interactions were performed including pre vs post time period, and nurse feedback vs patient bundle: OR, 1.28 (95% CI, 1.02–1.61); P=0.03 for any nonadministered dose; OR, 1.53 (95% CI, 1.16–2.05); P=0.003 for patient refused dose; OR, 0.99 (95% CI, 0.72–1.35); P=0.93 for other reasons for nonadministered doses (excluding patient refused).
VTE Prophylaxis Medication Administration
Overall, the odds of nonadministration of a pharmacologic VTE prophylaxis dose significantly decreased from the pre‐ to the postintervention period (OR, 0.64 [95% CI, 0.57–0.71]). Both the nurse feedback (OR, 0.72 [95% CI, 0.62–0.84]) and the bundle (OR, 0.56 [95% CI, 0.48–0.66]) interventions significantly decreased the odds of nonadministration after implementation. Our analysis of intervention effect for reducing nonadministration found the bundle to be more effective (exponentiated 2‐way interaction term [ratio of the nurse feedback OR versus the bundle] 1.28 [95% CI, 1.02–1.61]; P=0.03; Table 2).
Nonadministered Doses Stratified by Reason
Patient refusal was the most common reason for dose nonadministration (Table 2). Refused doses for the patient education bundle were 7.3% (95% CI, 4.3–12.4) preintervention and 3.7% (95% CI, 2.1–6.4) postintervention, and for nurse feedback were 9.5% (95% CI, 5.6–16.1) preintervention and 7.1% (95% CI, 4.2–12.2) postintervention (Table 2). The overall odds of dose refusal significantly decreased after the intervention (OR, 0.59 [95% CI, 0.51–0.68]; P<0.001). Both interventions significantly decreased the odds of dose refusal (P<0.001). The bundle had a larger decrease in odds of refused doses compared with nurse feedback (OR, 0.46 versus OR, 0.71) and was significantly more effective (2‐way interaction term [ratio of the nurse feedback OR versus the bundle OR] 1.53, [95% CI, 1.16–2.05]; P=0.003; Table 2). There was a smaller, yet still statistically significant decrease in nonadministration for other reasons, and the bundle and nurse feedback were equally effective (OR, 0.80 and OR, 0.79), and the ratio between the 2 odds ratios is not statistically different from 1 (exponentiated 2‐way interaction term 0.99 [95% CI, 0.72–1.35]; P=0.93).
Stratified Analysis by Unit Type (Surgery Versus Medicine)
Overall, both unit types (surgery and medicine) had significantly lower odds of nonadministered doses for patient refusal and other reasons postintervention (P<0.001; Table 3). On the medicine units, the bundle led to a statistically significant improvement in the odds of refusal compared with nurse feedback (OR 0.49 [95% CI, 0.38–0.64] versus OR, 0.79 [95% CI, 0.63–0.97]; P=0.008). There were no significant differential effects for any other comparisons.
Table 3.
Subgroup Analysis by Hospital Unit (Surgery Versus Medicine) on the Proportion of Nonadministered Venous Thromboembolism Prophylaxis Medication Doses by Patient Bundle and Nurse Feedback Arms* , † , ‡
| Surgery | Medicine | |||||||
|---|---|---|---|---|---|---|---|---|
| Period | Patient education bundle | Nurse feedback | OR nurse feedback/patient bundle (95% CI) | P value* | Patient education bundle | Nurse feedback | OR nurse feedback/patient education bundle (95% CI) | P value* |
| Any nonadministered dose | ||||||||
| Preintervention, % (95% CI) | 6.3% (4.6%–8.6%) | 10.0% (7.4%–13.7%) | 1.68 (1.00–2.84) | 0.05 | 18.6% (14.5%–23.7%) | 18.9% (14.8%–24.0%) | 1.03 (0.68–1.55) | 0.89 |
| Postintervention (95% CI) | 3.3% (2.3%–4.7%) | 6.7% (4.6%–9.5%) | 2.11 (1.18–3.79) | 0.01 | 12.2% (9.2%–16.1%) | 15.2% (11.7%–19.9%) | 1.31 (0.84–2.06) | 0.24 |
| OR post/pre (95% CI) | 0.50 (0.39–0.65) | 0.63 (0.49–0.81) | 0.60 (0.49–0.74) | 0.77 (0.63–0.93) | ||||
| P value* | <0.001 | <0.001 | <0.001 | 0.01 | ||||
| Patient refused dose | ||||||||
| Preintervention (95% CI) | 2.7% (1.8%–4.2%) | 5.5% (3.6%–8.5%) | 2.13 (1.07–4.22) | 0.03 | 13.5% (9.7%–18.8%) | 13.8% (10.0%–19.1%) | 1.04 (0.62–1.75) | 0.89 |
| Postintervention (95% CI) | 1.0% (0.6%–1.8%) | 3.2% (1.9%–5.2%) | 3.12 (1.39–6.99) | 0.01 | 7.3% (5.0%–10.6%) | 11.2% (7.9%–15.9%) | 1.64 (0.93–2.89) | 0.09 |
| OR post/pre (95% CI) | 0.37 (0.23–0.59) | 0.55 (0.39–0.77) | 0.49 (0.38–0.64) | 0.79 (0.63–0.97) | ||||
| P value* | <0.001 | <0.001 | <0.001 | 0.02 | ||||
| Other reason for nonadministered dose (excluding patient refused) | ||||||||
| Preintervention (95% CI) | 3.0% (2.4%–3.9%) | 3.5% (2.7%–4.7%) | 1.18 (0.80–1.74) | 0.41 | 3.8% (3.0%–4.9%) | 4.0% (3.1%–5.1%) | 1.04 (0.72–1.49) | 0.84 |
| Postintervention (95% CI) | 1.9% (1.4%–2.6%) | 2.8% (1.9%–4.2%) | 1.50 (0.90–2.50) | 0.12 | 4.0% (3.0%–5.4%) | 3.2% (2.4%–4.2%) | 0.77 (0.51–1.17) | 0.22 |
| OR post/pre (95% CI) | 0.62 (0.46–0.83) | 0.79 (0.55–1.13) | 1.05 (0.78–1.43) | 0.78 (0.59–1.05) | ||||
| P value* | 0.002 | 0.19 | 0.74 | 0.10 | ||||
Patient education bundle refers to the Patient‐Centered Education Bundle. OR indicates odds ratio.
P values for the odds ratios were calculated using multiple outputation of the mixed‐effects logistics regression models.
Exponentiates 2‐way interactions were performed including pre vs post time period, and nurse feedback vs patient bundle units for surgery: OR, 1.26 (95% CI, 0.87–1.81); P=0.22 for any nonadministered dose; OR, 1.47 (95% CI, 0.83–2.60); P=0.19 for patient refused dose; OR, 1.27 (95% CI, 0.79–2.06); P=0.33 for other reasons for nonadministered doses (excluding patient refused). Medicine: OR, 1.28 (95% CI, 0.96–1.70); P=0.09 for any nonadministered dose; OR, 1.58 (95% CI, 1.13–2.20); P=0.01 for patient refused dose; OR, 0.74 (95% CI, 0.49–1.13); P=0.16 for other reasons for nonadministered doses (excluding patient refused).
Exponentiated 3‐way interactions were performed to calculate the ratio of ratios between ORs that examine whether the differences in ORs are different, including pre vs post time period, and nurse feedback vs patient bundle units, and surgery vs medicine with no significant differences observed: OR, 0.94 for any nonadministered dose; OR, 0.80 for patient refused dose; OR, 0.09 for other reasons for nonadministered doses (excluding patient refused).
Time Trend Analysis
Figure 2 shows an overall graph of the conditional probability of nonadministered doses by month, including unit type strata for surgery (Figure 2B) and medicine (Figure 2C) units. These analyses demonstrate improvements in the postimplementation period. Similar improvements were observed for both unit types.
Figure 2. Time series analysis for all units (A) of the patient‐centered education bundle and nurse feedback arms stratified by surgery (B) and medicine (C) hospital units.
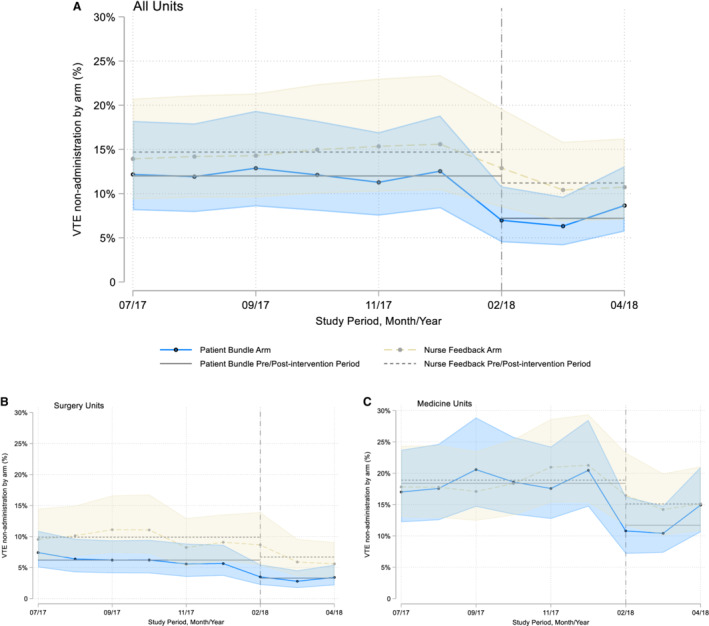
This trend analysis reflects the monthly data for nonadministered doses of venous thromboembolism (VTE) prophylaxis in the preintervention (July 1, 2017 to December 31, 2017) and postintervention (February 1, 2018 to April 30, 2018) periods, comparing both intervention arms. Data for January 2018 were excluded (washout period). Our findings show that no changes are evident before the postimplementation period.
DISCUSSION
In this cluster‐randomized controlled trial, we found significant reductions in nonadministered doses of pharmacologic VTE prophylaxis among hospitalized patients following implementation of both the patient‐centered education bundle and the nurse feedback intervention. The trial implemented 2 unique strategies: a real‐time EHR alert prompting delivery of patient‐centered education materials, or a monthly scorecard reporting the dose administration percentages for unit nurses relative to their peers. Although both interventions improved administration practices, the bundle showed a superior effect, evident in a 44% reduction in the odds of nonadministration and a 54% reduction in patient refusal of pharmacologic prophylaxis. This finding is important, because we sought to determine if frontline nurses could implement the bundle and effectively reduce nonadministered doses as part of their routine work, even without the interventions being done by a research‐funded nurse. These data replicate a similar, yet smaller, scale intervention at a community hospital. 24 When stratified by unit type, both interventions showed a statistically significant improvement in the odds of both nonadministered and refused doses, confirming that the intervention was beneficial for both surgery and medicine patients, and the effect size was similar in this larger scale implementation, which grew the intervention from 4 to 16 floors at our hospital.
To our knowledge, this is the first study to implement multiple quality improvement interventions in a randomized fashion, targeting this key point of failure in VTE prevention, administration of VTE prophylaxis doses. In addition, we harnessed health information technology to automate the intervention and empowered nurses and nurse leaders to use evidence‐based medicine in daily practice. The monthly scorecard in this trial was the only such clinical performance feedback provided to nurses on participating units during the study period. This trial expands on our previous evaluation of the impact of the bundle, in which we achieved significant improvements in nonadministered doses of VTE prophylaxis. 23 , 24
In the current trial, we demonstrated that the bundle delivered after an EHR real‐time alert can be scaled for real‐world use, independent of a dedicated research team or extra financial resources. Our approach used an implementation science framework to translate evidence into routine practice. 32 We developed and studied the bundle first and showed its effectiveness. 23 , 25 Then, nursing staff took ownership and integrated it into routine, daily practice, and finally, we independently measured whether their efforts were making significant improvements in delivering prescribed prophylaxis doses. This approach has achieved sustained success in previous quality improvement research. 33 , 34 Evidence from quality improvement research can contribute to effective and efficient patient‐centered care. 35 Synthesis from such evidence has informed health care policy and formulated guidelines. 36 However, translating evidence for VTE prophylaxis into practice can be an unpredictable and slow process. 32 , 37 Some areas of uncertainty may relate to structural and cultural influences or the health care professionals involved in crystallizing these findings into practice. 14
Studies consistently show that audit and feedback changes behaviors and improves professional practice. 30 , 38 , 39 Yet, there are different degrees to which feedback has affected sustained change. 6 , 40 Shojania and colleagues reviewed a variety of quality improvement strategies aimed at provider adherence to care practices for diabetes management and alluded to the greater chance of success when using multiple targeted strategies over 1 strategy alone. 41 Strategies that address multiple layers of defect‐free VTE prophylaxis have also been suggested. 42 Our current study highlights the advantage of a multifaceted approach aimed at numerous critical steps to optimal VTE prevention, namely nurse administration and patient acceptance of prophylaxis. In addition, we implemented the interventions as part of an overarching strategy at our institution, which already has a computerized clinical decision support tool that stratifies patients by major risk categories and has improved prescription of risk‐appropriate VTE prophylaxis. 7 , 8 , 28 , 43 Our targeted alert approach is supported by previous research that recommends reducing unneeded electronic alert interventions to mitigate alert fatigue. 44 , 45
This dissemination study relied on nurses' endorsement and welcoming of the intervention into routine clinical practice. The easily adaptable nature of our intervention was an added benefit and required minimal deviation from nurses' workflow, which already includes dedicated time for patient education. The vast majority of nurses had already been exposed to targeted VTE prophylaxis knowledge through a prior study of required online VTE education module. 21 The nurse education module improved dose administration, and therefore, our baseline may have been better than hospitals naive to any nurse education intervention. The results of this study are predicated on first providing education to nurses about the importance of delivering all prescribed prophylaxis doses for VTE prevention, which is a critical first step before implementing the patient‐centered education bundle or the nurse feedback intervention.
A key element highlighted by previous quality improvement efforts to optimize VTE prophylaxis administration is to target interventions to appropriate clinical groups. 38 , 46 , 47 , 48 Although surgical attendings did not benefit from VTE prophylaxis prescription feedback, 38 surgical residents' significantly improved prophylaxis prescribing practices following performance feedback. 30 Although audit and feedback can be useful tools, implementation may be influenced by structural or institutional capacities and differential buy‐in that can effect intervention success. 46 , 47 , 48 In the current trial, our findings show that providing targeted feedback to nurses can be successful. Although feedback improved practice, it was not as effective as the bundle. Perhaps adding feedback in a multipronged approach alongside other interventions would show added benefit.
Our study has several limitations. First, our trial was in a single tertiary care center with robust health information technology and a modifiable EHR system, limiting the generalizability of our findings to other settings. However, delivery of the bundle can be adapted based on workflows in different settings. At hospitals without an EHR system, the approach potentially can be implemented without the triggered alert if the bedside nurse uses the same educational bundle at the time of dose administration refusal. The materials are freely available for widespread use (bit.ly/bloodclots), and were created with patient stakeholder input. 23 Second, differences in patient demographics and length of stay between the arms in the preintervention period may have influenced our findings. We mitigated this through our randomization approach and analytic models, which provided a fair distribution of patients by arm and accounted for any residual bias. Moreover, each unit served as its own historical control for the pre‐ and postintervention comparisons, also limiting bias in our analysis. Third, nurses and/or patients moving between units may have contaminated the data. However, at the time of this study, nurses at our hospital were predominantly assigned to specific units; thus, moving between units was likely not a problem. We also limited contamination by excluding patients who moved between units. Finally, we did not control intervention implementation, and consciously decided not to jeopardize buy‐in by overburdening frontline nurses and nurse leaders with vast amounts of added data collection (ie, which patients got which bundle elements, how exactly nurses received individual or group feedback). Although we would have liked to know exactly which patients were intervened upon and the frequency and methods that nurse leaders used to deliver feedback, these data are not available. A benefit of this intention to treat approach was the demonstrated real‐world applicability for what originally began as funded clinical research.
CONCLUSIONS
Our study provides clear evidence that supports the use of information technology strategies combined with targeted patient‐centered education to bolster best practices of VTE prophylaxis medication administration. Future research and quality improvement efforts should target strategies that leverage information technology solutions to scale and translate evidence into practice to improve a wider variety of clinical practices.
Sources of Funding
The PCORI Award (DI‐1603‐34596) funded evaluation of the implementation of the real‐time alert‐triggered patient education bundle. The PCORI had no role in the design or conduct of this trial, nor the decision to approve publication of the finished article. The statements in this work are solely the responsibility of the authors and do not necessarily represent the views of the PCORI, its Board of Governors, or the Methodology Committee.
Disclosures
B.D. Lau, D.L. Shaffer, Drs Owodunni, Webster, Aboagye, Farrow, Pronovost, Streiff, and Haut were/are supported by contracts from the PCORI titled Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient‐Centered Care Via Health Information Technology (CE‐12‐11–4489) and/or Preventing Venous Thromboembolism (VTE): Engaging Patients to Reduce Preventable Harm From Missed/Refused Doses of VTE Prophylaxis (DI‐1603‐34596). B.D. Lau, V. Kia, D.l. Shaffer, and Drs Haut, Streiff, and Owodunni are supported by a grant from the Agency for Healthcare Research and Quality (R18HS025341) titled Disseminating an Evidence‐Based Venous Thromboembolism Prevention Bundle. Dr Haut, B.D. Lau, and V. Kia receive research grant support for a project titled The Pathogenesis of Post‐Traumatic Pulmonary Embolism: A Prospective Multicenter Investigation by the CLOTT Study Group (CLOTT) from the Department of Defense/Army Medical Research Acquisition Activity. B.D. Lau, and Drs Streiff and Haut are supported by a grant from the Agency for Healthcare Research and Quality (1R01HS024547) titled Individualized Performance Feedback on Venous Thromboembolism Prevention Practice, and a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (R21HL129028) titled “Analysis of the Impact of Missed Doses of Venous Thromboembolism Prophylaxis.” Dr Haut is supported by a contract from PCORI, A Randomized Pragmatic Trial Comparing the Complications and Safety of Blood Clot Prevention Medicines Used in Orthopedic Trauma Patients (PCS‐1511‐32745). Dr Haut has received grant support from the Henry M. Jackson Foundation for the Advancement of Military Medicine and the Department of Defense. B.D. Lau was supported by the Institute for Excellence in Education Berkheimer Faculty Education Scholar Grant and a contract (AD‐1306‐03980) from PCORI titled “Patient‐Centered Approaches to Collect Sexual Orientation/Gender Identity Information in the Emergency Department.” Dr Pronovost reports consultancy fees from the Association for Professionals in Infection Control and Epidemiology, honoraria from various hospitals and the Leigh Bureau (Somerville, NJ), and royalties from his book, Safe Patients Smart Hospitals, and received grant or contract support from the Agency for Healthcare Research and Quality, National Institutes of Health, Robert Wood Johnson Foundation, PCORI, and The Commonwealth Fund. Dr Streiff has received research funding from Boehringer‐Ingelheim, Janssen, NovoNordisk, Roche, and Sanofi; consulted for Janssen and Portola; and has given expert witness testimony in various medical malpractice cases. Dr Haut receives royalties from Lippincott, Williams, & Wilkins for a book titles Avoiding Common ICU Errors. Dr Haut was a paid speaker for the Vizient Hospital Improvement Innovation Network VTE Prevention Acceleration Network. The remaining authors have no disclosures to report.
Supporting information
Figure S1
Acknowledgments
The authors express their appreciation to the patients who informed the development of the patient education bundle, and to nurse leadership, nurse managers, and bedside nurses for their participation in this study. In particular the authors thank D. Baker, Senior Vice President of Nursing for the Johns Hopkins Health System; M. Morris, Director of Nursing, Departments of Surgery and Physical Medicine and Rehabilitation; R. Langlotz, Director of Nursing, and T. Nelson, Assistant Director of Nursing, Department of Medicine. The authors are grateful to Dr Fayzullin for his assistance in obtaining medication administration data from our electronic medication administration record. The authors thank their key stakeholder organizations: the National Blood Clot Alliance, the North American Thrombosis Forum, and the Johns Hopkins Hospital Patient and Family Advisory Council.
Drs Haut and Owodunni, J. Wang, D. Shaffer, D. Hobson, P.S. Kraus, Drs Farrow, Florecki, and Webster, J. Aboagye, V. Popoola, Drs Pronovost and Streiff, and B. Lau all contributed to the study design. Drs Haut and Owodunni, J. Wang, Dr Yenokyan, J. Canner, and B. Lau all contributed to analysis and interpretation of results, and M.V. Kia contributed to interpretation of the results. Drs Haut and Owodunni, J. Wang, C. Holzmueller, and B. Lau all contributed to the writing the first draft of the article. D. Shaffer, D. Hobson, P.S. Kraus, Dr Farrow, J. Canner, Dr Florecki, Dr Webster, C. Holzmueller, J. Aboagye, V. Popoola, M.V. Kia, Dr Pronovost, and Dr Streiff all provided critical revisions to the article. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Data access, responsibility, and analysis: B. Lau had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J. Wang, and Dr Yenokyan from the Department of Biostatistics in the Johns Hopkins Bloomberg School of Public Health conducted the data analysis.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/atvbaha.108.162545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haut ER, Lau BD. Prevention of venous thromboembolism: brief update review. Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Agency for Healthcare Research and Quality; 2013:Chapter 28. [Google Scholar]
- 3. US Department of Health and Human Services . The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 4. Lau BD, Streiff MB, Pronovost PJ, Haut ER. Venous thromboembolism quality measures fail to accurately measure quality. Circulation. 2018;137:1278–1284. doi: 10.1161/CIRCULATIONAHA.116.026897 [DOI] [PubMed] [Google Scholar]
- 5. Streiff MB, Haut ER. The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063. doi: 10.1001/jama.301.10.1063 [DOI] [PubMed] [Google Scholar]
- 6. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23:187–195. doi: 10.1136/bmjqs-2012-001782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeidan AM, Streiff MB, Lau BD, Ahmed SR, Kraus PS, Hobson DB, Carolan H, Lambrianidi C, Horn PB, Shermock KM, et al. Impact of a venous thromboembolism prophylaxis "smart order set": improved compliance, fewer events. Am J Hematol. 2013;88:545–549. doi: 10.1002/ajh.23450 [DOI] [PubMed] [Google Scholar]
- 8. Haut ER, Lau BD, Kraenzlin FS, Hobson DB, Kraus PS, Carolan HT, Haider AH, Holzmueller CG, Efron DT, Pronovost PJ, et al. Improved prophylaxis and decreased preventable harm with a mandatory computerized clinical decision support tool for venous thromboembolism (VTE) prophylaxis in trauma patients. Arch Surg. 2012;147:901–907. doi: 10.1001/archsurg.2012.2024 [DOI] [PubMed] [Google Scholar]
- 9. Borab ZM, Lanni MA, Tecce MG, Pannucci CJ, Fischer JP. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta‐analysis. JAMA Surg. 2017;152:638–645. doi: 10.1001/jamasurg.2017.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shermock KM, Lau BD, Haut ER, Hobson DB, Ganetsky VS, Kraus PS, Efird LE, Lehmann CU, Pinto BL, Ross PA, et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8:e66311. doi: 10.1371/journal.pone.0066311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fanikos J, Stevens LA, Labreche M, Piazza G, Catapane E, Novack L, Goldhaber SZ. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123:536–541. doi: 10.1016/j.amjmed.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 12. Lau BD, Streiff MB, Kraus PS, Hobson DB, Shaffer DL, Aboagye JK, Pronovost PJ, Haut ER. Missed doses of venous thromboembolism (VTE) prophylaxis at community hospitals: cause for alarm. J Gen Intern Med. 2018;33:19–20. doi: 10.1007/s11606-017-4203-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haut ER, Lau BD, Kraus PS, Hobson DB, Maheshwari B, Pronovost PJ, Streiff MB. Preventability of hospital‐acquired venous thromboembolism. JAMA Surg. 2015;150:912–915. doi: 10.1001/jamasurg.2015.1340 [DOI] [PubMed] [Google Scholar]
- 14. Elder S, Hobson DB, Rand CS, Streiff MB, Haut ER, Efird LE, Kraus PS, Lehmann CU, Shermock KM. Hidden barriers to delivery of pharmacological venous thromboembolism prophylaxis: the role of nursing beliefs and practices. J Patient Saf. 2016;12:63–68. doi: 10.1097/PTS.0000000000000086 [DOI] [PubMed] [Google Scholar]
- 15. Louis SG, Sato M, Geraci T, Anderson R, Cho SD, Van PY, Barton JS, Riha GM, Underwood S, Differding J, et al. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA Surg. 2014;149:365–370. doi: 10.1001/jamasurg.2013.3963 [DOI] [PubMed] [Google Scholar]
- 16. Khorfan R, Kreutzer L, Love R, Schlick CJR, Chia M, Bilimoria KY, Yang AD. Association between missed doses of chemoprophylaxis and VTE incidence in a statewide colectomy cohort. Ann Surg. 2021;273:e151–e152. doi: 10.1097/sla.0000000000004349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Popoola VO, Lau BD, Tan E, Shaffer DL, Kraus PS, Farrow NE, Hobson DB, Aboagye JK, Streiff MB, Haut ER. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75:392–397. doi: 10.2146/ajhp161057 [DOI] [PubMed] [Google Scholar]
- 18. Popoola VO, Tavakoli F, Lau BD, Lankiewicz M, Ross P, Kraus P, Shaffer D, Hobson DB, Aboagye JK, Farrow NA, et al. Exploring the impact of route of administration on medication acceptance in hospitalized patients: implications for venous thromboembolism prevention. Thromb Res. 2017;160:109–113. doi: 10.1016/j.thromres.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 19. Owodunni OP, Lau BD, Streiff MB, Kraus PS, Hobson DB, Shaffer DL, Webster KLW, Kia MV, Holzmueller CG, Haut ER. What the 2018 ASH venous thromboembolism guidelines omitted: nonadministration of pharmacologic prophylaxis in hospitalized patients. Blood Adv. 2019;3:596–598. doi: 10.1182/bloodadvances.2018030510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer‐Westendorf J, Spencer FA, Rezende SM, Zakai NA, Bauer KA, Dentali F, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lau BD, Shaffer DL, Hobson DB, Yenokyan G, Wang J, Sugar EA, Canner JK, Bongiovanni D, Kraus PS, Popoola VO, et al. Effectiveness of two distinct web‐based education tools for bedside nurses on medication administration practice for venous thromboembolism prevention: a randomized clinical trial. PLoS One. 2017;12:e0181664. doi: 10.1371/journal.pone.0181664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baillie CA, Guevara JP, Boston RC, Hecht TE. A unit‐based intervention aimed at improving patient adherence to pharmacological thromboprophylaxis. BMJ Qual Saf. 2015;24:654–660. doi: 10.1136/bmjqs-2015-003992 [DOI] [PubMed] [Google Scholar]
- 23. Haut ER, Aboagye JK, Shaffer DL, Wang J, Hobson DB, Yenokyan G, Sugar EA, Kraus PS, Farrow NE, Canner JK, et al. Effect of real‐time patient‐centered education bundle on administration of venous thromboembolism prevention in hospitalized patients. JAMA Netw Open. 2018;1:e184741. doi: 10.1001/jamanetworkopen.2018.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Owodunni OP, Lau BD, Shaffer DL, McQuigg D, Samuel D, Kantsiper M, Harris JE, Hobson DB, Kraus PS, Webster KL, et al. Disseminating a patient‐centered education bundle to reduce missed doses of pharmacologic venous thromboembolism (VTE) prophylaxis to a community hospital. J Patient Saf Risk Manag. 2021;26:22–28. doi: 10.1177/2516043520969324 [DOI] [Google Scholar]
- 25. Popoola VO, Lau BD, Shihab HM, Farrow NE, Shaffer DL, Hobson DB, Kulik SV, Zaruba PD, Shermock KM, Kraus PS, et al. Patient preferences for receiving education on venous thromboembolism prevention—a survey of stakeholder organizations. PLoS One. 2016;11:e0152084. doi: 10.1371/journal.pone.0152084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owodunni OP, Haut ER, Shaffer DL, Hobson DB, Wang J, Yenokyan G, Kraus PS, Aboagye JK, Florecki KL, Webster KLW, et al. Using electronic health record system triggers to target delivery of a patient‐centered intervention to improve venous thromboembolism prevention for hospitalized patients: is there a differential effect by race? PLoS One. 2020;15:e0227339. doi: 10.1371/journal.pone.0227339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Streiff MB, Carolan H, Hobson DB, Kraus PS, Holzmueller C, Demski R, Lau B, Biscup‐Horn P, Pronovost PJ, Haut ER. Lessons from the Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935. doi: 10.1136/bmj.e3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Streiff MB, Lau BD, Hobson DB, Kraus PS, Shermock KM, Shaffer DL, Popoola VO, Aboagye JK, Farrow NA, Horn PJ, et al. The Johns Hopkins Venous Thromboembolism Collaborative: multidisciplinary team approach to achieve perfect prophylaxis. J Hosp Med. 2016;11:S8–S14. doi: 10.1002/jhm.2657 [DOI] [PubMed] [Google Scholar]
- 29. Lau BD, Arnaoutakis GJ, Streiff MB, Howley IW, Poruk KE, Beaulieu R, Ellison TA, Van Arendonk KJ, Kraus PS, Hobson DB, et al. Individualized performance feedback to surgical residents improves appropriate venous thromboembolism prophylaxis prescription and reduces potentially preventable VTE: a prospective cohort study. Ann Surg. 2016;264:1181–1187. doi: 10.1097/SLA.0000000000001512 [DOI] [PubMed] [Google Scholar]
- 30. Lau BD, Streiff MB, Hobson DB, Kraus PS, Shaffer DL, Popoola VO, Farrow NE, Efron DT, Haut ER. Beneficial "halo effects" of surgical resident performance feedback. J Surg Res. 2016;205:179–185. doi: 10.1016/j.jss.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 31. Follmann D, Proschan M, Leifer E. Multiple outputation: inference for complex clustered data by averaging analyses from independent data. Biometrics. 2003;59:420–429. doi: 10.1111/1541-0420.00049 [DOI] [PubMed] [Google Scholar]
- 32. Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. doi: 10.1136/bmj.a1714 [DOI] [PubMed] [Google Scholar]
- 33. Shadman KA, Ralston SL, Garber MD, Eickhoff J, Mussman GM, Walley SC, Rice‐Conboy E, Coller RJ. Sustainability in the AAP bronchiolitis quality improvement project. J Hosp Med. 2017;12:905–910. doi: 10.12788/jhm.2830 [DOI] [PubMed] [Google Scholar]
- 34. Pronovost PJ, Goeschel CA, Colantuoni E, Watson S, Lubomski LH, Berenholtz SM, Thompson DA, Sinopoli DJ, Cosgrove S, Sexton JB, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309. doi: 10.1136/bmj.c309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan E, Laupacis A, Pronovost PJ, Guyatt GH, Needham DM. How to use an article about quality improvement. JAMA. 2010;304:2279. doi: 10.1001/jama.2010.1692 [DOI] [PubMed] [Google Scholar]
- 36. Carr SM, Lhussier M, Forster N, Geddes L, Deane K, Pennington M, Visram S, White M, Michie S, Donaldson C, et al. An evidence synthesis of qualitative and quantitative research on component intervention techniques, effectiveness, cost‐effectiveness, equity and acceptability of different versions of health‐related lifestyle advisor role in improving health. Health Technol Assess. 2011;15:iii–iv. doi: 10.3310/hta15090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duff J, Walker K, Omari A. Translating venous thromboembolism (VTE) prevention evidence into practice: a multidisciplinary evidence implementation project. Worldviews Evid Based Nurs. 2011;8:30–39. doi: 10.1111/j.1741-6787.2010.00209.x [DOI] [PubMed] [Google Scholar]
- 38. Lau BD, Streiff MB, Pronovost PJ, Haider AH, Efron DT, Haut ER. Attending physician performance measure scores and resident physicians' ordering practices. JAMA Surg. 2015;150:813–814. doi: 10.1001/jamasurg.2015.0891 [DOI] [PubMed] [Google Scholar]
- 39. Michtalik HJ, Carolan HT, Haut ER, Lau BD, Streiff MB, Finkelstein J, Pronovost PJ, Durkin N, Brotman DJ. Use of provider‐level dashboards and pay‐for‐performance in venous thromboembolism prophylaxis. J Hosp Med. 2015;10:172–178. doi: 10.1002/jhm.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process. J Gen Intern Med. 2007;22:1762–1770. doi: 10.1007/s11606-007-0369-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shojania KG, Ranji SR, Shaw LK, Charo LN, Lai JC, Rushakoff RJ, McDonald KM, Owens DK. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Agency for Healthcare Research and Quality; 2004. [PubMed] [Google Scholar]
- 42. Kemble JV. PH changes on the surface of burns. Br J Plast Surg. 1975;28:181–184. doi: 10.1016/0007-1226(75)90126-5 [DOI] [PubMed] [Google Scholar]
- 43. Lau BD, Haider AH, Streiff MB, Lehmann CU, Kraus PS, Hobson DB, Kraenzlin FS, Zeidan AM, Pronovost PJ, Haut ER. Eliminating health care disparities with mandatory clinical decision support: the venous thromboembolism (VTE) example. Med Care. 2015;53:18–24. doi: 10.1097/MLR.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians' decisions to override computerized drug alerts in primary care. Arch Intern Med. 2003;163:2625–2631. doi: 10.1001/archinte.163.21.2625 [DOI] [PubMed] [Google Scholar]
- 45. Kucher N, Koo S, Quiroz R, Cooper JM, Paterno MD, Soukonnikov B, Goldhaber SZ. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–977. doi: 10.1056/NEJMoa041533 [DOI] [PubMed] [Google Scholar]
- 46. Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, O'Brien MA, Johansen M, Grimshaw J, Oxman AD. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6:CD000259. doi: 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayes RP, Ballard DJ. Review: feedback about practice patterns for measurable improvements in quality of care—a challenge for PROs under the Health Care Quality Improvement Program. Clin Perform Qual Health Care. 1995;3:15–22. [PubMed] [Google Scholar]
- 48. Jamtvedt G, Young JM, Kristoffersen DT, Thomson O'Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2003;7:CD000259. doi: 10.1002/14651858.CD000259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


