Abstract
Background
The emergence of PCSK9i (proprotein convertase subtilisin kexin type 9 inhibitor) and icosapent ethyl (IPE) has expanded the role of lipid‐lowering therapies beyond statins. Despite recommendations by clinical practice guidelines, their national eligibility and use rates remain unclear.
Methods and Results
In the National Health and Nutrition Examination Survey data from 2017 to 2020, we assessed eligibility and the use of statins, PCSK9i, and IPE among US adults according to American College of Cardiology/American Heart Association guideline recommendations. Eligibility for PCSK9i and IPE were determined in the following 2 scenarios: (1) assuming existing lipid‐lowering therapy as the maximum tolerated before assessing eligibility for novel therapies and (2) assessing eligibility after assuming initiation and maximal escalation of preexisting lipid‐lowering therapies and accounting for expected lipid improvements. Of 2729 sampled individuals, representing 149.3 million adults, 1376 had indications for statins, representing 65.8 million or 44.0% (95% CI, 40.9%–47.2%) of adults. Current statin use was 45% of those eligible and was low across demographic groups. A total of 9.7 and 11.6 million adults would benefit from PCSK9i and IPE, respectively, based on lipid profiles and existing therapies. Assuming maximal escalation of statins and addition of ezetimibe, 4.1% (95% CI, 2.8%–5.4%) of adults or 6.1 million would benefit from PCSK9i and 6.8% (95% CI, 5.4%–8.3%) or 10.2 million from IPE.
Conclusions
Six and 10 million individuals have clinical profiles whereby PCSK9i and IPE, respectively, would be expected to improve cardiovascular outcomes even after maximum escalation of statins and ezetimibe use, but remain undertreated with lipid‐lowering therapies. Optimal use of lipid‐targeted agents that include these novel agents is needed to improve population health outcomes.
Keywords: icosapent ethyl, lipid‐lowering therapy, NHANES, PCSK9 inhibitor
Subject Categories: Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- IPE
icosapent ethyl
- NHANES
National Health and Nutrition Examination Survey
- PCSK9i
proprotein convertase subtilisin kexin type 9 inhibitor
Clinical Perspective.
What Is New?
This contemporary, nationally representative US study using the National Health and Nutrition Examination Survey from 2017 to 2020 evaluated the eligibility and use of statins, PCSK9i (proprotein convertase subtilisin kexin type 9 inhibitors), and icosapent ethyl (IPE).
Fewer than half of individuals with guideline indications for statin therapy were receiving these agents, and there was negligible use of PCSK9i and IPE.
Of US individuals, 6 million have clinical profiles for whom the use of PCSK9i and 10 million have clinical profiles for whom the use of IPE would be expected to improve cardiovascular outcomes, even after maximal escalation of statin intensities and the use of ezetimibe.
What Are the Clinical Implications?
Focused efforts to increase the use of novel lipid‐targeted therapies are needed to achieve their public health benefits.
Statins have remained a mainstay of prevention and treatment for atherosclerotic cardiovascular disease (ASCVD). 1 , 2 The emergence of novel lipid‐targeted therapies, such as PCSK9i (proprotein convertase subtilisin kexin type 9 inhibitors) and icosapent ethyl (IPE), has expanded therapeutic options to improve cardiovascular outcomes in patients with hypercholesterolemia. The 2018 lipid management guidelines from the American College of Cardiology and American Heart Association have recommended the use of adjunctive nonstatin agents such as PCSK9i for cardiovascular risk reduction in patients with established or high‐risk ASCVD. 1 These recommendations, in addition to the updated statement recommending IPE use by the National Lipid Association, 3 are based on evidence from large clinical trials that demonstrated improvement of cardiovascular outcomes in high‐risk patients receiving PCSK9i 4 , 5 , 6 and IPE 7 , 8 , 9 as adjunct therapies to statins.
Despite approval by the US Food and Drug Administration and recommendation by clinical practice guidelines, the proportion of the US population that has compelling indications for these novel agents has largely remained unknown. This is a particularly important knowledge gap given the early evidence of limited uptake of PCSK9i in clinical practice 10 and the emergence of IPE as an additional risk‐lowering therapy in patients with elevated triglyceride levels. In the absence of high‐quality data on the real‐world use of these medications, an unbiased assessment of the eligibility and use of PCSK9i and IPE is necessary to inform strategies for their broader clinical use and to maximize their public health benefits.
In this contemporary, national study of the US population from 2017 to 2020, we assessed the eligibility and use of PCSK9i and IPE across indications by clinical practice guidelines. We also assessed the estimated number of individuals who would benefit from PCSK9i and IPE after maximal initiation and escalation of their baseline lipid‐lowering therapies by applying known distributions of lipid profile changes derived from clinical trials that evaluated these agents.
METHODS
Data Source
Data from the National Health and Nutrition Examination Survey (NHANES) from 2017 to March 2020 were used for this study. All data and materials are publicly available online from the Centers for Disease Control and Prevention and can be accessed at https://wwwn.cdc.gov/nchs/nhanes/. The NHANES is a nationally representative database of standardized cross‐sectional surveys designed to assess the health and nutritional status of noninstitutionalized civilians across the United States. These surveys collect a wide range of demographic, socioeconomic, dietary, and other health‐related data such as vital signs, medications, and laboratory results for the monitoring of disease prevalence and trends in risk behaviors. 11 The NHANES employs a complex, multistage, stratified, probability‐based sampling design to select a nationally representative population. 12 In this study, we used data collected between 2017 and March 2020, which is the latest publicly available survey cycle. Notably, the operation of the NHANES survey from 2019 to 2020 was suspended in March 2020 because of the COVID‐19 pandemic, and data from 2019 through March 2020 were combined with the 2017 to 2018 cycle to form a representative sample for national estimates for this period. 13
Study Population and Exposure Groups
In this study, we included all adults ≥40 years of age who participated in the laboratory component of the survey. 14 We applied national guideline indications for statin, PCSK9i, and IPE to define their respective eligible populations. We used the 2018 American College of Cardiology/American Heart Association lipid management guideline to define eligibility for statins, including (1) patients with ASCVD; (2) patients with primary hypercholesterolemia, defined as low‐density lipoprotein cholesterol (LDL‐C) ≥190 mg/dL; (3) patients 40 to 75 years of age with diabetes and LDL‐C 70 to 189 mg/dL; and (4) patients 40 to 75 years of age with LDL‐C 70 to 189 mg/dL and an estimated 10‐year ASCVD risk of ≥7.5%. 15 We used the pooled cohort risk equations to estimate 10‐year ASCVD risk in those without ASCVD. 16 , 17
Eligibility for PCSK9i was defined as clinical ASCVD and LDL‐C ≥70 mg/dL on preexisting statin and ezetimibe therapies per the 2018 American College of Cardiology/American Heart Association lipid management guideline. 1 Although the American College of Cardiology/American Heart Association guideline also recommends the use of PCSK9i for primary ASCVD prevention in individuals with primary severe hypercholesterolemia, this recommendation requires knowledge of heterozygous familial hypercholesterolemia status and was classified as “weak” 1 ; therefore, we did not factor these criteria into eligibility for PSCK9i in this study. For IPE, we used the guideline outlined by the National Lipid Association in 2019, which defines the eligible population as individuals ≥45 years of age with clinical ASCVD or individuals ≥50 years of age with diabetes and at least 1 additional cardiovascular risk factor, including hypertension, current cigarette smoking, low high‐density lipoprotein cholesterol (HDL‐C), elevated high‐sensitivity C‐reactive protein, advanced age, kidney dysfunction, or the presence of micro‐ or macroalbuminuria, who are already taking statins and have a residual triglyceride level of 135 to 499 mg/dL. 3 Of note, all individuals eligible for novel agents were required to be on moderate‐high intensity statin therapy, which we define in the next section.
Defining Eligibilities for PCSK9i and IPE
Using the aforementioned eligibility criteria, we simulated eligibility populations for PCSK9i and IPE using the following 2 approaches: (1) assuming existing lipid‐lowering therapy as the maximum tolerated before determining eligibility for novel therapies and (2) assessing eligibility after assuming initiation and maximal escalation of preexisting lipid‐lowering therapies (ie, statin and ezetimibe) and accounting for their predicted lipid‐lowering effects using distributions of lipid profile changes extrapolated from previously published data. These assessments were designed to account for the underuse of existing agents in many patients with indications for their use and the recommendation that baseline lipid‐lowering therapy be maximized before the use of novel agents.
In modeling the second approach for simulating eligibility for PCSK9i, we assumed a stepwise escalation of lipid‐lowering therapy for those who were not already optimized on therapy, first with the addition or escalation of statin therapy then with the addition of ezetimibe and then accounted for the predicted LDL‐C lowering effects. In determining the simulated eligibility for IPE, we assumed maximal escalation of statin therapy and accounted for the predicted triglyceride lowering and HDL‐C increasing effects. These simulations were based on previously published distributions of lipid changes with varying statin intensities and ezetimibe and assumed full toleration of the maximum dose of each agent without adverse effects. Of note, because the NHANES surveys collect medication names but not the dosages, we were unable to determine the precise intensities of statin therapies. Therefore, we created statin intensity categories with rosuvastatin and atorvastatin as “moderate‐high” intensity statins and all other statins, including simvastatin, lovastatin, pravastatin, fluvastatin, and pitavastatin, as “low‐moderate” intensity statins. 1
Study Covariates
Demographic characteristics included age, sex, race and ethnicity, and health insurance status. Race and ethnicity were categorized according to standardized definitions in the NHANES and included non‐Hispanic White, non‐Hispanic Black, Mexican American, other Hispanic, non‐Hispanic Asian, and individuals of other races (including multiracial but not otherwise specified). Health insurance status was determined on a self‐reported basis and included private, employer, or government‐based coverage.
We applied standard strategies to identify medical comorbidities. For identification of individuals with clinical ASCVD, we included patients who self‐reported a history of coronary heart disease, myocardial infarction, angina pectoris, or stroke. Coronary or arterial revascularizations, peripheral artery disease, and transient ischemic attacks were not assessed in the NHANES. Diabetes was defined by a self‐reported history of diabetes, use of glucose‐lowering medications, hemoglobin A1C ≥6.5%, or fasting glucose ≥126 mg/dL. Hypertension was defined by a self‐reported history of hypertension, presence of antihypertensive medications, or mean systolic blood pressure ≥130 mm Hg and/or mean diastolic blood pressure ≥80 mm Hg on consecutive measurements. Chronic kidney disease was defined by a self‐reported history of kidney dysfunction or dialysis within the past year or a calculated glomerular filtration rate <60 mL/min per 1.73 m2 by the Modification of Diet in Renal Disease formula. Dyslipidemia was defined by a self‐reported history of high cholesterol, triglycerides ≥150 mg/dL, LDL‐C ≥130 mg/dL, HDL‐C ≤60 mg/dL, or total cholesterol ≥200 mg/dL. Smoking was defined by self‐reported current or recent cigarette use. Lastly, heart failure was identified based on self‐report.
Study Outcomes
The study outcomes included eligibility and the use of statins, PCSK9i, and IPE; and simulated eligibility for PCSK9i and IPE as defined previously. Eligibilities were assessed in the overall population and across demographic and comorbidity subgroups. For PCSK9i and IPE, these were specifically assessed for the overall population as well as for subgroups of patients with ASCVD for PCSK9i and with ASCVD and diabetes for IPE. Use of each agent was determined through standardized in‐person interviews. Individuals taking any of the following medications were identified as statin users: rosuvastatin, atorvastatin, simvastatin, lovastatin, pravastatin, fluvastatin, and pitavastatin. Individuals taking either evolocumab or alirocumab were considered PCSK9i users. Lastly, IPE users were defined by users of the agent.
Statistical Analysis
To account for the multistage sampling strategy of the NHANES, we used complex survey methods to calculate nationally representative estimates of eligibility and use for the US population. We used best practices in the selection of sample weights, choosing the sample weights corresponding to the laboratory subset as suggested by the National Center for Health Statistics. 18
In determining simulated PCSK9i and IPE eligibilities, the first model did not predicate PCSK9i and IPE eligibility on current statin therapy—this analysis essentially reflects the scenario where statin use is contraindicated or not tolerated in those who were not taking statins. Those who were already taking statins were still maximally escalated, if indicated. For the second model that focused on assuming possible initiation and maximal escalation of lipid‐lowering therapy, we created normal distributions of expected lipid changes using available trial data, including mean percentage changes and SDs. Distributions of lipid changes for low‐moderate and moderate‐high intensity statin categories were generated from pooled weighted means and SDs. For low‐moderate intensity statins, the distribution of LDL‐C reduction was generated using simvastatin 19 (mean reduction, 36%), and the distribution of triglyceride reduction was generated using simvastatin and pravastatin (mean reduction, 13%). 20 For moderate‐high intensity statins, the distribution of LDL‐C reduction was generated using atorvastatin and rosuvastatin 19 (mean reduction, 44%), and the distribution of triglyceride reduction was generated using atorvastatin (mean reduction, 21%). 20 The degree of lipid changes with escalation from low‐moderate to moderate‐high intensity statins was computed through the subtraction of the corresponding normal distributions. The distribution of LDL‐C reduction with ezetimibe when added onto preexisting statin therapy was obtained as well (mean reduction, 23%). 21 The effects of statins on HDL‐C lack obvious dose dependency; therefore, our distribution was generated using atorvastatin, simvastatin, and pravastatin (mean increase, 6%). 20 , 22
Simulated PCSK9i eligibility is based on cumulative LDL‐C reduction assuming stepwise escalation of therapy with statins and ezetimibe. For individuals not on any prior lipid‐lowering therapies, we assumed direct initiation of moderate‐high intensity statin therapy with the subsequent addition of ezetimibe. For individuals only on preexisting low‐moderate intensity statin therapy, we assumed escalation to moderate‐high statin therapy with the subsequent addition of ezetimibe. For individuals on preexisting low‐moderate intensity statin therapy and ezetimibe, we assumed escalation to moderate‐high statin therapy. For individuals on moderate‐high intensity statin therapy, we assumed the addition of ezetimibe alone. Lastly, for individuals on preexisting ezetimibe therapy without statin, we assumed escalation directly to moderate‐high intensity statin therapy. Simulated IPE eligibility accounted for the predicted triglyceride‐reducing and HDL‐C–increasing effects with statin therapy. For individuals not on prior statins, we assumed initiation of moderate‐high intensity statin therapy, and for those on prior low‐moderate intensity statin therapy, we assumed escalation to moderate‐high intensity statin therapy.
We employed bootstrapping methods—random sampling with replacement—across 1000 iterations using our distributions of lipid changes to generate postescalation lipid distributions for every sample individual. This simulation was designed to account for the inherent heterogeneity of lipid‐lowering effects with varying agents and, as such, incorporates a degree of stochasticity into our predictions. We generated postescalation LDL‐C distributions to simulate PCSK9i eligibility and postescalation triglyceride and HDL‐C distributions to simulate IPE eligibility. Probabilities of eligibility for novel agents for every sample individual were then computed using lipid‐level cutoffs and clinical profiles in accordance with guideline indications. These probabilities were then weighted using survey methods to create nationally representative population counts. A diagram of these methods is illustrated in Figure 1.
Figure 1. Methods for simulated PCSK9i and IPE eligibility assuming maximal escalation of lipid‐lowering therapy.
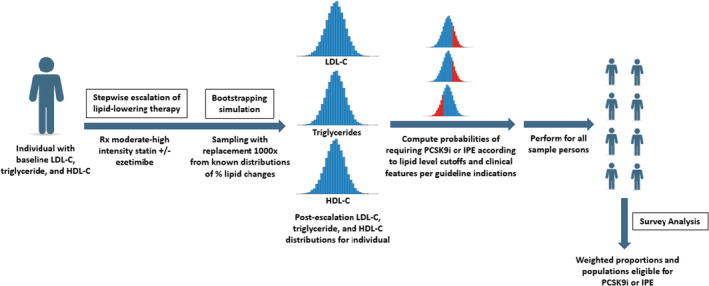
HDL‐C indicates high‐density lipoprotein cholesterol; IPE, icosapent ethyl; LDL‐C, low‐density lipoprotein cholesterol; PCSK9i, proprotein convertase subtilisin kexin type 9 inhibitor; and Rx, prescription.
We reported weighted proportions representative of the US population with likelihood‐based 95% CIs as well as counts at the sample and population levels. Categorical variables were presented as frequency and percentages, and continuous variables as means and SDs. The study uses public data with deidentified information; thus, this study represents nonhuman subject research outside the purview of an institutional review board. All data were analyzed using R software, version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Population Demographics and Characteristics
There were 15 560 participants in the NHANES from 2017 to 2020, of which 2729 were adults ≥40 years of age with participation in the laboratory component of the survey and LDL‐C measurements, representing 149.3 million US adults (Table S1). In this population, 32.9% (95% CI, 29.6%–36.2%) were ≥65 years of age, 52.5% (95% CI, 49.8%–55.1%) were women, 66.2% (95% CI, 62.1%–70.4%) were non‐Hispanic White participants, 10.7% (95% CI, 7.9%–13.6%) were non‐Hispanic Black participants, 5.7% (95% CI, 3.8%–7.6%) were non‐Hispanic Asian participants, 6.7% (95% CI, 5.0%–8.5%) were Mexican American participants, 6.8% (95% CI, 5.4%–8.2%) were other Hispanic participants, and 3.9% (95% CI, 2.6%–5.2%) were participants of other races, including multiracial individuals. Among these patients, 90.2% (95% CI, 87.5%–92.8%) reported having health insurance. In addition, 14.0% (95% CI, 11.3%–16.6%) of individuals had ASCVD, 43.3% (95% CI, 39.6%–47.1%) had hypertension, 48.1% (95% CI, 45.6%–50.7%) had dyslipidemia, 16.7% (95% CI, 14.5%–18.8%) had diabetes, 3.5% (95% CI, 2.4%–4.6%) reported a history of heart failure, and 15.3% (95% CI, 12.8%–17.9%) reported active smoking.
Statin Eligibility and Use
Of the 2729 sampled individuals, 1376 had indications for statins, representing 65.8 million or 44.0% (95% CI, 40.9%–47.2%) of US adults ≥40 years of age (Table). This statin‐eligible population also represented 66.2% (95% CI, 61.2%–71.2%) of individuals ≥65 years of age, 33.2% (95% CI, 29.4%–37.0%) of individuals 40 to 64 years of age, 35.3% (95% CI, 31.8%–38.7%) of women, and 53.7% (95% CI, 48.7%–58.8%) of men. Statin eligibility across race and ethnicity groups included 43.9% (95% CI, 39.7%–48.0%) of non‐Hispanic White individuals, 50.5% (95% CI, 45.5%–55.6%) of non‐Hispanic Black individuals, 39.4% (95% CI, 34.0%–44.8%) of non‐Hispanic Asian individuals, 38.7% (95% CI, 31.1%–46.3%) of Mexican American individuals, 42.8% (95% CI, 34.3%–51.4%) of other Hispanic individuals, and 47.6% (95% CI, 32.2%–62.9%) of individuals of other races. Key characteristics of this population are summarized in Table.
Table 1.
Current Eligibility for Statins in Study Population Across Demographic and Comorbidity Profiles
| Overall, N | Eligible, n | Weighted percentage (95% CI) | Weighted population | |
|---|---|---|---|---|
| Overall | 2729 | 1376 | 44.0 (40.9–47.2) | 65.8M* |
| Age, y | ||||
| ≥65 | 990 | 655 | 66.2 (61.2–71.2) | 32.5M |
| 40–64 | 1739 | 721 | 33.2 (29.4–37.0) | 33.2M |
| Sex | ||||
| Female sex | 1384 | 574 | 35.3 (31.8–38.7) | 27.6M |
| Male sex | 1345 | 802 | 53.7 (48.7–58.8) | 38.2M |
| Race and ethnicity | ||||
| Non‐Hispanic White participants | 968 | 471 | 43.9 (39.7–48.0) | 43.4M |
| Non‐Hispanic Black participants | 698 | 407 | 50.5 (45.5–55.6) | 8.1M |
| Non‐Hispanic Asian participants | 339 | 143 | 39.4 (34.0–44.8) | 3.3M |
| Mexican American participants | 308 | 144 | 38.7 (31.1–46.3) | 3.9M |
| Other Hispanic participants | 297 | 157 | 42.8 (34.3–51.4) | 4.4M |
| Other race participants† | 119 | 54 | 47.6 (32.2–62.9) | 2.7M |
| Health insurance | ||||
| Yes | 2404 | 1230 | 44.7 (41.4–47.9) | 60.1M |
| No | 325 | 146 | 38.5 (28.1–48.9) | 5.6M |
| Comorbidities | ||||
| ASCVD | 435 | 435 | 100.0 | 20.8M |
| Hypertension | 1349 | 888 | 62.3 (58.2–66.3) | 40.3M |
| Dyslipidemia | 1304 | 803 | 54.3 (48.7–59.9) | 39.0M |
| Diabetes | 601 | 539 | 89.7 (85.8–93.5) | 22.3M |
| CKD | 396 | 261 | 65.1 (57.5–72.7) | 13.1M |
| Heart failure | 140 | 118 | 84.8 (76.9–92.8) | 4.4M |
| Smoking | 490 | 310 | 55.4 (49.2–61.7) | 12.7M |
Weighted percentages represent the proportion of individuals in each demographic or comorbidity subgroup who are eligible for statins. ASCVD indicates atherosclerotic cardiovascular disease; and CKD, chronic kidney disease.
M represents population in millions.
NHANES definitions of race and ethnicity included non‐Hispanic White, non‐Hispanic Black, non‐Hispanic Asian, Mexican American, other Hispanic, and individuals of other races (including multiracial but not otherwise specified).
Of the US population eligible for statins, 44.5% were active users of ≥1 statins (Figure 2), representing 29.6 million individuals. Individuals ≥65 years of age formed the group with the highest degree of statin indication at 66.2% as well as statin use at 50.9% of those eligible. Among all other demographic subgroups, proportions of statin use among those eligible were low throughout, with the lowest proportions of use in individuals 40 to 64 years of age (38.6%), non‐Hispanic Black individuals (37.0%), and other Hispanic individuals (35.0%). The distribution of statin usage by intensity category, regardless of indication, is shown in Table S2.
Figure 2. Use of statins among adults with indications for therapy by demographic subgroups.
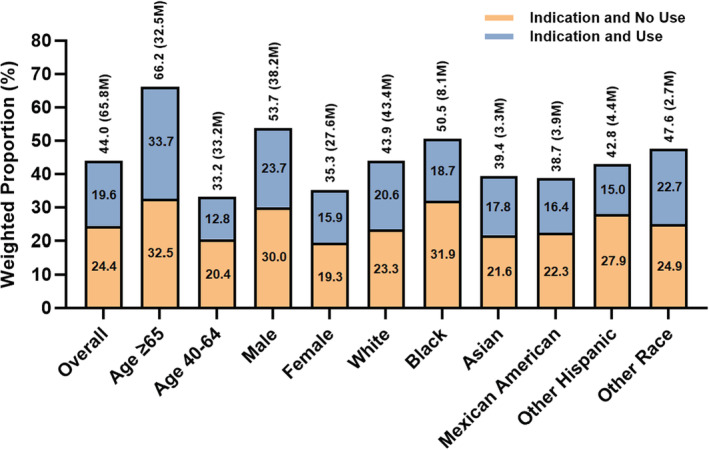
Bars represent weighted proportions (percentages) of individuals with indications for statins within each demographic subgroup. M represents population in millions.
PCSK9i and IPE Eligibility and Use
PCSK9i and IPE eligibility based on current clinical and lipid profiles is demonstrated in Table S3. In the first model, which did not predicate PCSK9i eligibility on current statin therapy, 6.5% (95% CI, 4.9%–8.1%) of US adults or 9.7 million were eligible for PCSK9i based on current lipid levels and clinical profiles, also representing 46.7% (95% CI, 40.6%–52.8%) of adults with ASCVD or 9.7 million individuals (Table S4, Figure 3A)–these weighted population counts are the same because all individuals eligible for PCSK9i must have ASCVD. In the second model, which simulated the possibility of initiation and maximal escalation of statin and ezetimibe therapies and accounted for the associated reductions in LDL‐C, we observed a lower number at 4.1% (95% CI, 2.8%–5.4%) of US adults or 6.1 million who would benefit from PCSK9i, also representing 29.3% (95% CI, 24.0%–34.6%) of adults with ASCVD or 6.1 million individuals (Table S4, Figure 3A).
Figure 3. Simulated eligibilities for PCSK9i and IPE in overall study population and across subgroups of guideline indications.
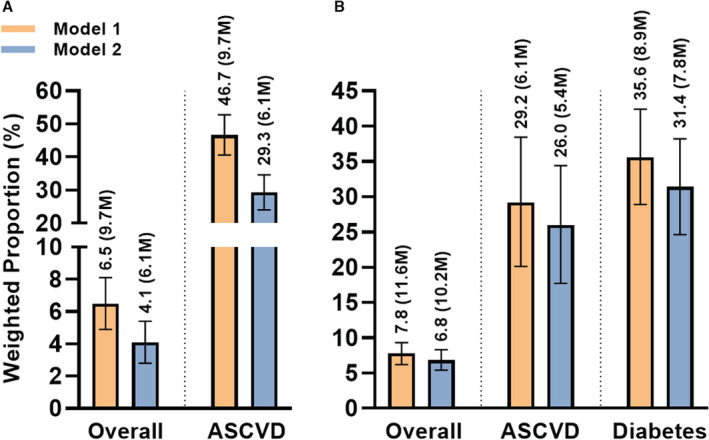
Simulated eligibilities for (A) PCSK9i and (B) IPE. Model 1: assumes existing lipid‐lowering therapy as the maximum tolerated. Model 2: assumes initiation and maximal escalation of preexisting lipid‐lowering therapies and accounting for expected lipid profile changes. Bars represent weighted proportions (percentages) with 95% CIs of eligible individuals for each drug class. ASCVD indicates atherosclerotic cardiovascular disease; IPE, icosapent ethyl; and PCSK9i, proprotein convertase subtilisin kexin type 9 inhibitor. M represents population in millions.
In determining the eligibility for IPE, the first model suggested an eligibility of 7.8% (95% CI, 6.2%–9.3%) of US adults or 11.6 million individuals, which includes 29.2% (95% CI, 20.1%–38.4%) of adults with ASCVD or 6.1 million individuals, and 35.6% (95% CI, 28.9%–42.4%) of adults with diabetes or 8.9 million individuals (Table S4, Figure 3B). After simulating the possibility of initiation and maximal escalation of statin therapy and accounting for associated reductions in triglycerides and increases in HDL‐C, 6.8% (95% CI, 5.4%–8.3%) of US adults or 10.2 million would benefit from IPE, which includes 26.0% (95% CI, 17.7%–34.4%) of adults with ASCVD or 5.4 million individuals, and 31.4% (95% CI, 24.6%–38.2%) of adults with diabetes or 7.8 million individuals (Table S4, Figure 3B). Stratification of PCSK9i and IPE eligibility by key demographics is shown in Figure S1. Original and simulated mean postescalation LDL‐C and triglyceride levels are shown in Figure 4 and Figure S2.
Figure 4. Distributions of original and simulated mean LDL‐C and triglyceride levels after maximal escalation of lipid‐lowering therapy in the overall study population.
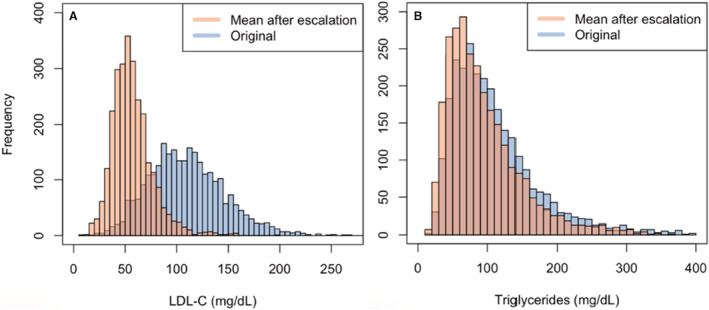
A, Original and mean postescalation LDL‐C assuming initiation and maximal escalation of statin and ezetimibe. B, Original and mean postescalation triglycerides assuming initiation and maximal escalation of statins. Postescalation LDL‐C and triglycerides are presented as mean values as a result of the bootstrapping method. LDL‐C indicates low‐density lipoprotein cholesterol.
Current use of PCSK9i and IPE in the United States was nearly negligible. There were only 5 individuals at the sample level who were PCSK9i users, representing 253 528 or 0.17% (95% CI, 0.01%–0.33%) of US adults. Similarly, there were only 2 individuals at the sample level who were IPE users, representing 233 064 or 0.16% (95% CI, 0.00%–0.43%) of US adults (Table S2).
DISCUSSION
In this nationally representative US study, fewer than half of individuals who had guideline indications for lipid lowering with statins were receiving these agents, with particular underuse among Black adults and younger adults 40 to 64 years of age. Furthermore, based on the lipid profiles of US adults and their existing therapies, 9.7 million or 6.5% of those >40 years of age would merit consideration for PCSK9i. In addition, 11.6 million or 7.8% of those in this age group would benefit from reduced risk of cardiovascular adverse events with the initiation of IPE. However, a substantial proportion of individuals who may be candidates for PCSK9i and IPE are on suboptimal therapy with statins and/or ezetimibe, and if optimally managed on these agents, 6.1 and 10.2 million US adults >40 years of age are expected to be candidates for PCSK9i and IPE, respectively.
Our findings underscore the degree to which standard lipid‐lowering therapies are underused, but also highlight the potential population that would benefit from PCSK9i and IPE in the United States. Prior studies have demonstrated the suboptimal use of standard lipid‐lowering therapies, including statins and ezetimibe, in individuals with ASCVD. 21 , 23 , 24 , 25 , 26 , 27 Moreover, a recent study using the GOULD (Getting to an Improved Understanding of Low‐Density Lipoprotein Cholesterol and Dyslipidemia Management) clinical registry demonstrated only modest lipid‐lowering therapy intensification during a 2‐year period in patients with ASCVD, with particularly low rates of intensification in non‐White individuals. 23 The impact of continued suboptimal use of standard lipid‐lowering therapies on public health can be substantial. Estimates of PCSK9i eligibility range from modest to large; several studies estimate PCSK9i eligibility at around 14% of those with ASCVD after simulating lipid‐lowering therapy intensification. 21 , 28 , 29 Other studies estimate that up to one‐half of patients with ASCVD could be eligible for PCSK9i therapy. 30 , 31
In our study, we also found a substantial number of individuals who would be eligible for IPE therapy. Although the literature on IPE is limited in comparison with that of PCSK9i, studies have suggested a broad degree of eligibility. Prior studies using national data showed that a significant proportion of US adults had residual hypertriglyceridemia from 2007 to 2014 despite statin therapy, suggesting that nearly 5 million adults were potentially eligibility for IPE. 32 , 33 In addition, a large study from the Veterans Affairs reported an eligibility of 14.5% among adults with ASCVD and 17% among adults with diabetes. 34 A study using the international CLARIFY (Prospective Observational Longitudinal Registry of Patients with Stable Coronary Artery Disease) registry suggested a similar eligibility of 15% in individuals with coronary artery disease, and another study conducted using the CANHEART (Cardiovascular Health in Ambulatory Care Research Team) cohort suggested that 25% of individuals with ASCVD could be eligible for novel therapy. Of note, these studies were based on the original REDUCE‐IT (Reduction of Cardiovascular Events with Icosapent Ethyl‐Intervention) inclusion and exclusion criteria and excluded those with LDL‐C outside the range of 41 to 100 mg/dL. 7 Another study evaluating IPE eligibility in patients with a history of coronary artery bypass graft reported 22% eligibility based on the original REDUCE‐IT criteria as well as higher eligibility rates of 26% and 34% in subcohorts based on criteria outlined by the US Food and Drug Administration and Health Canada, which did not exclude individuals on the basis of LDL‐C or age. 37 Our eligibility criteria were based on the 2019 National Lipid Association statement supporting the use of IPE, which did not restrict eligibility based on LDL‐C 3 ; this may explain why our predicted eligibility was higher than what has been previously reported.
Despite their broad eligibility and known benefits in mitigating cardiovascular risk as demonstrated by large randomized controlled trials, uptake of novel agents into clinical practice remains limited. An analysis using PCORnet (National Patient‐Centered Clinical Research Network) from 2015 to 2017 reported PCSK9i prescriptions in <1% of individuals with dyslipidemia and coronary artery disease, with virtually no increase in PCSK9i prescription over time for those with dyslipidemia and modest increases for those with ASCVD. 10 Similarly, an analysis derived from claims‐based data demonstrated <1% of PCSK9i prescriptions in those with ASCVD. 38 We could not identify any studies that evaluated current patterns of IPE use in the United States, which possibly indicates a lack of awareness and delayed uptake of IPE into modern clinical practice. Altogether, these findings suggest that suboptimal treatment with standard lipid‐lowering therapies contribute, in large part, to the limited eligibility for these novel agents, and more aggressive efforts should be made by providers to escalate lipid‐lowering therapies to expand eligibility for novel agents.
There are multiple potential reasons for our observed findings. Our results suggest that statin underuse may be partly driven by racial and ethnic disparities. Despite the higher burden of ASCVD and cardiovascular risk factors in Black individuals, 39 which is also reflected by a higher degree of statin eligibility in comparison with other races, we observed that Black and Hispanic individuals in our cohort had 1 of the highest percentages of statin underuse among those eligible for therapy. Multiple studies have highlighted the racial disparities in prescribing patterns of lipid‐lowering therapy, with Black individuals and certain ethnic minorities being less likely than White individuals to receive guideline‐appropriate statin therapy. 40 , 41 Statin underuse in these individuals may also be driven in part by misinformation and lack of trust; Black individuals were less likely to believe that statins are effective or safe and were less likely to trust their providers compared with their White counterparts. 40 In addition, fear of adverse effects was the most commonly cited reason for declining or discontinuing statins according to a study using the PALM (Patient and Provider Assessment of Lipid Management) registry. 42 The same study found that more than half of patients who were eligible but not prescribed statins reported never being offered the agent. 42 Greater proactivity and targeted efforts among providers are needed, especially toward these vulnerable populations, to increase the use of standard lipid‐lowering therapies and thus optimize eligibility for novel agents.
Cost of therapy may also represent a major barrier for patients interested in novel agents and may also partly explain the low use rates. At the introductory list price of ≈$14 000 annually, PCSK9i were deemed cost‐ineffective at the commonly accepted threshold of $100 000 per quality‐adjusted life‐year. 43 , 44 These simulations estimated that prices would need to drop >60% to reach the cost‐effectiveness threshold. Fortunately, with the 2018 price reduction of PCSK9i from ≈$14 000 to $5850 annually, we may begin to observe an accelerated uptake of PCSK9i into modern clinical practice. In fact, there was a modest increase in patients initiated on PCSK9i in the year after this price reduction. 38 Moreover, an updated cost‐effectiveness analysis of alirocumab from ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab) demonstrated that the agent met the quality‐adjusted life‐year threshold with especially good value among patients with baseline LDL‐C ≥100 mg/dL. 45 Similarly, an analysis of evolocumab, also at the reduced price point, showed that the agent met the quality‐adjusted life‐year threshold for a range of simulated cardiovascular event rates. 46 On the other hand, cost‐effectiveness analyses focusing on IPE have consistently demonstrated cost‐effectiveness well below various quality‐adjusted life‐year thresholds. 47 , 48 , 49 As such, IPE is considered to be an inexpensive yet high‐value therapy for reducing cardiovascular risk in certain high‐risk populations. Nevertheless, our study suggests that there is still room for further optimization with affordable standards of lipid‐lowering therapy before consideration of these novel agents.
There are limitations in this study that warrant consideration. First, although the NHANES collects data from in‐person interviews and questionnaires administered by trained professionals, many components remain self‐reported, which can potentially lead to inaccuracies in the reporting of nuanced medical information. As such, we attempted to supplement the data obtained from questionnaires wherever possible using objective measurements such as vital signs and laboratory results. Second, the NHANES does not include information on coronary or arterial revascularizations, peripheral artery disease, or transient ischemic attacks and thus could have resulted in underestimation of eligible populations for the novel agents. Third, the cross‐sectional nature of the NHANES data does not capture changes in clinical and lipid profiles over time and thus may impact our determination of eligibility. Fourth, nationally representative population counts are strictly estimates based on sample‐level data and respective weighting factors. Fifth, PCSK9i use in particular may be underestimated as it is a monthly or semi‐monthly injection and may not be reported as a typical prescription medication. Sixth, IPE received expanded US Food and Drug Administration approval for these indications (clinical ASCVD or diabetes with an additional cardiovascular risk factor) partway through our NHANES study period in 2019. 48 However, IPE had already received US Food and Drug Administration approval for adults with hypertriglyceridemia with years of data supporting its safety and efficacy, and hypertriglyceridemia is known to be independently associated with increased ASCVD risk, cardiovascular mortality, and all‐cause mortality. 48 Lastly, our NHANES data set represents clinical practice from 2017 to 2020, and thus our results may underestimate current use patterns if there has been an increased uptake of novel agents in clinical practice.
CONCLUSIONS
In this nationally representative US study, 6 and 10 million individuals have clinical profiles that would merit PCSK9i and IPE, respectively, for improving cardiovascular outcomes but many remain undertreated with lipid‐lowering therapies. A focus on optimal lipid‐targeted therapies that includes these novel agents is necessary to improve public health.
Sources of Funding
This study is supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (1K23HL153775) awarded to Dr Khera. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
Dr Bhatt discloses the following relationships: advisory boards of Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; boards of directors of Boston VA Research Institute, DRS.LINQ (stock options), Society of Cardiovascular Patient Care, and TobeSoft; inaugural chair at American Heart Association Quality Oversight Committee; data monitoring committees of Acesion Pharma, Assistance Publique‐Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY‐DM trial, funded by Concept Medical), Novartis, Population Health Research Institute, and Rutgers University (for the National Institutes of Health–funded MINT trial); honoraria from American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol‐Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor, Associate Editor), K2P (co‐chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), and Wiley (steering committee). Dr Bhatt also reports the following: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry steering committee (chair), VA CART Research and Publications Committee (chair); research funding from Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, and 89Bio; royalties from Elsevier (Editor, Braunwald's Heart Disease); site coinvestigator at Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, and Svelte; trustee at American College of Cardiology; and unfunded research at FlowCo and Takeda. Dr Nasir has received research support from the Katz Academy of Translational Research and is an advisory board member for Amgen, Novartis, and Novo Nordisk. Dr Khera also receives support from the Doris Duke Charitable Foundation (under award, 2022060) and from Bristol‐Myers Squibb, through Yale. He is a coinventor of U.S. Pending Patent Application No. 63/177,117, “Methods for neighborhood phenomapping for clinical trials”, and U.S. Provisional Patent Application No. 63/346,610, “Articles and methods for format independent detection of hidden cardiovascular disease from printed electrocardiographic images using deep learning”. He is a founder of Evidence2Health, a precision health platform to improve evidence‐based cardiovascular care. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S2
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, De Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:E1082–E1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orringer CE, Jacobson TA, Maki KC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin‐treated patients with elevated triglycerides and high or very‐high ASCVD risk. J Clin Lipidol. 2019;13:860–872. doi: 10.1016/j.jacl.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 4. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 5. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. doi: 10.1093/eurheartj/ehv028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with Icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 8. Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg PG, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, et al. REDUCE‐IT USA: results from the 3146 patients randomized in the United States. Circulation. 2020;141:367–375. doi: 10.1161/CIRCULATIONAHA.119.044440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma S, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Dhingra NK, Ketchum SB, Juliano RA, Jiao L, et al. Icosapent ethyl reduces ischemic events in patients with a history of previous coronary artery bypass grafting: REDUCE‐IT CABG. Circulation. 2021;144:1845–1855. doi: 10.1161/CIRCULATIONAHA.121.056290 [DOI] [PubMed] [Google Scholar]
- 10. Chamberlain AM, Gong Y, Shaw KMA, Bian J, Song WL, Linton MF, Fonseca V, Price‐Haywood E, Guhl E, King JB, et al. PCSK9 inhibitor use in the real world: data from the National Patient‐Centered Research Network. J Am Heart Assoc. 2019;8:e011246. doi: 10.1161/JAHA.118.011246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS) . National Health and Nutrition Examination Survey Data. Available at: http://www.cdc.gov/nchs/nhanes/. Accessed November 28, 2021.
- 12. Nargesi AA, Jeyashanmugaraja GP, Desai N, Lipska K, Krumholz H, Khera R. Contemporary national patterns of eligibility and use of novel cardioprotective antihyperglycemic agents in type 2 diabetes mellitus. J Am Heart Assoc. 2021;10:e021084. doi: 10.1161/JAHA.121.021084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS) . National Health and Nutrition Examination Survey Data. NHANES 2017‐March 2020 Pre‐pandemic. Available at: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?cycle=2017‐2020. Accessed November 28, 2021.
- 14. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 16. Jaeger B. PooledCohort: predict 10‐year risk for atherosclerotic cardiovascular disease. R package version 0.0.1. 2021. Available at: https://cran.r‐project.org/package=PooledCohort. Accessed November 28, 2021.
- 17. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS) . National Health and Nutrition Examination Survey Data. Module 3: weighting. Available at: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx. Accessed November 28, 2021.
- 19. Karlson BW, Wiklund O, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Variability of low‐density lipoprotein cholesterol response with different doses of atorvastatin, rosuvastatin, and simvastatin: results from VOYAGER. Eur Heart J Cardiovasc Pharmacother. 2016;2:212–217. doi: 10.1093/ehjcvp/pvw006 [DOI] [PubMed] [Google Scholar]
- 20. Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol. 1998;81:582–587. doi: 10.1016/s0002-9149(97)00965-x [DOI] [PubMed] [Google Scholar]
- 21. Cannon CP, Khan I, Klimchak AC, Reynolds MR, Sanchez RJ, Sasiela WJ. Simulation of lipid‐lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:959–966. doi: 10.1001/jamacardio.2017.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McTaggart F, Jones P. Effects of statins on high‐density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008;22:321–338. doi: 10.1007/s10557-008-6113-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cannon CP, De Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Gao Q, Palagashvilli T, Alam S, Mues KE, Bhatt DL, et al. Use of lipid‐lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6:1060–1068. doi: 10.1001/jamacardio.2021.1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cannon CP, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Yazdi D, Elliott‐Davey M, Mues KE, Bhatt DL, Kosiborod MN. Getting to an ImprOved understanding of low‐density lipoprotein‐cholesterol and dyslipidemia management (GOULD): methods and baseline data of a registry of high cardiovascular risk patients in the United States: GOULD registry methods and baseline data. Am Heart J. 2020;219:70–77. doi: 10.1016/j.ahj.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 25. Colantonio LD, Rosenson RS, Deng L, Monda KL, Dai Y, Farkouh ME, Safford MM, Philip K, Mues KE, Muntner P. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc. 2019;8:e010376. doi: 10.1161/JAHA.118.010376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salami JA, Warraich H, Valero‐Elizondo J, Spatz ES, Desai NR, Rana JS, Virani SS, Blankstein R, Khera A, Blaha MJ, et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the Medical Expenditure Panel Survey. JAMA Cardiol. 2017;2:56–65. doi: 10.1001/jamacardio.2016.4700 [DOI] [PubMed] [Google Scholar]
- 27. Rosenson RS, Kent ST, Brown TM, Farkouh ME, Levitan EB, Yun H, Sharma P, Safford MM, Kilgore M, Muntner P, et al. Underutilization of high‐intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65:270–277. doi: 10.1016/j.jacc.2014.09.088 [DOI] [PubMed] [Google Scholar]
- 28. Gencer B, Koskinas KC, Räber L, Karagiannis A, Nanchen D, Auer R, Carballo D, Carballo S, Klingenberg R, Heg D, et al. Eligibility for PCSK9 inhibitors according to American College of Cardiology (ACC) and European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines after acute coronary syndromes. J Am Heart Assoc. 2017;6:e006537. doi: 10.1161/JAHA.117.006537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koskinas KC, Gencer B, Nanchen D, Branca M, Carballo D, Klingenberg R, Blum MR, Carballo S, Muller O, Matter CM, et al. Eligibility for PCSK9 inhibitors based on the 2019 ESC/EAS and 2018 ACC/AHA guidelines. Eur J Prev Cardiol. 2021;28:59–65. doi: 10.1177/2047487320940102 [DOI] [PubMed] [Google Scholar]
- 30. Ko DT, Khan AM, Kotrri G, Austin PC, Wijeysundera HC, Koh M, Chu A, Jackevicius CA, Lawler PR, Tu JV. Eligibility, clinical outcomes, and budget impact of PCSK9 inhibitor adoption: the CANHEART PCSK9 study. J Am Heart Assoc. 2018;7:e010007. doi: 10.1161/JAHA.118.010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarak B, Savu A, Kaul P, McAlister FA, Welsh RC, Yan AT, Goodman SG. Lipid testing, lipid‐modifying therapy, and PCSK9 (proprotein convertase subtilisin‐Kexin type 9) inhibitor eligibility in 27 979 patients with incident acute coronary syndrome. Circ Cardiovasc Qual Outcomes. 2021;14:e006646. doi: 10.1161/CIRCOUTCOMES.120.006646 [DOI] [PubMed] [Google Scholar]
- 32. Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Prevalence of US adults with triglycerides ≥ 150 mg/dl: NHANES 2007–2014. Cardiol Ther. 2020;9:207–213. doi: 10.1007/s40119-020-00170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan W, Philip S, Toth PP, Granowitz C, Wong ND. Prevalence of United States adults with triglycerides ≥ 135 mg/dL: NHANES 2007–2014. Cardiol J. 2019;26:604–606. doi: 10.5603/CJ.2019.0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jia X, Akeroyd JM, Nasir K, Nambi V, Ballantyne CM, Petersen LA, Virani SS. Eligibility and cost for icosapent ethyl based on the reduce‐it trial: insight from the Veterans Affairs Healthcare System. Circulation. 2019;139:1341–1343. doi: 10.1161/CIRCULATIONAHA.118.038691 [DOI] [PubMed] [Google Scholar]
- 35. Lawler PR, Kotrri G, Koh M, Goodman SG, Farkouh ME, Lee DS, Austin PC, Udell JA, Ko DT. Real‐world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41:86–94. doi: 10.1093/eurheartj/ehz767 [DOI] [PubMed] [Google Scholar]
- 36. Picard F, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG. Generalizability of the REDUCE‐IT trial in patients with stable coronary artery disease. J Am Coll Cardiol. 2019;73:1362–1364. doi: 10.1016/j.jacc.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 37. Kosmopoulos A, Verma S, Meglis G, Bhatt DL, Verma R, Mazer CD, Voisine P. Generalizability of reduction of cardiovascular events with Icosapent ethyl‐intervention trial in patients with a history of coronary artery bypass graft surgery. Curr Opin Cardiol. 2021;36:172–178. doi: 10.1097/HCO.0000000000000800 [DOI] [PubMed] [Google Scholar]
- 38. Dayoub EJ, Eberly LA, Nathan AS, Khatana SAM, Adusumalli S, Navar AM, Giri J, Groeneveld PW. Adoption of pcsk9 inhibitors among patients with atherosclerotic disease. J Am Heart Assoc. 2021;10:e019331. doi: 10.1161/JAHA.120.019331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 40. Nanna MG, Navar AM, Zakroysky P, Xiang Q, Goldberg AC, Robinson J, Roger VL, Virani SS, Wilson PWF, Elassal J, et al. Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: insights from the patient and Provider Assessment of Lipid Management Registry. JAMA Cardiol. 2018;3:739–748. doi: 10.1001/jamacardio.2018.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kalra DK. Bridging the racial disparity gap in lipid‐lowering therapy. J Am Heart Assoc. 2021;10:e019533. doi: 10.1161/JAHA.120.019533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, Virani SS, Louie MJ, Lee LV, Peterson ED, et al. Patient‐reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. J Am Heart Assoc. 2019;8:e011765. doi: 10.1161/JAHA.118.011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins‐Domingo K. Cost‐effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–753. doi: 10.1001/jama.2016.11004 [DOI] [PubMed] [Google Scholar]
- 44. Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nasir K. Updated cost‐effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2017;2:1369–1374. doi: 10.1001/jama.2016.11004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhatt DL, Briggs AH, Reed SD, Annemans L, Szarek M, Bittner VA, Diaz R, Goodman SG, Harrington RA, Higuchi K, et al. Cost‐effectiveness of alirocumab in patients with acute coronary syndromes: the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2020;75:2297–2308. doi: 10.1016/j.jacc.2020.03.029 [DOI] [PubMed] [Google Scholar]
- 46. Fonarow GC, Van Hout B, Villa G, Arellano J, Lindgren P. Updated cost‐effectiveness analysis of evolocumab in patients with very high‐risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:691–695. doi: 10.1001/jamacardio.2019.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weintraub WS, Bhatt DL, Zhang Z, Dolman S, Boden WE, Bress AP, King JB, Bellows BK, Tajeu GS, Derington CG, et al. Cost‐effectiveness of Icosapent ethyl for high‐risk patients with hypertriglyceridemia despite statin treatment. JAMA Netw Open. 2022;5:e2148172. doi: 10.1001/jamanetworkopen.2021.48172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boden WE, Baum S, Toth PP, Fazio S, Bhatt DL. Impact of expanded FDA indication for icosapent ethyl on enhanced cardiovascular residual risk reduction. Future Cardiol. 2021;17:155–174. doi: 10.2217/fca-2020-0106 [DOI] [PubMed] [Google Scholar]
- 49. Ollendorf D, McQueen RB, Campbell J, Herron‐Smith S, Fazioli K, Synnott P, Zaim R, Chapman R, Adair E, Quinlan TA, et al. Additive therapies for cardiovascular disease: effectiveness and value. Institute for Clinical and Economic Review; 2019. Available at: https://icer.org/wp‐content/uploads/2020/10/ICER_CVD_Final_Evidence_Report_101719_.pdf. Accessed March 3, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S2


