Abstract
Background
Fine particulate (fine particles with aerodynamic diameters ≤2.5 μm [PM2.5]) exposure has been associated with a risk of cardiac arrhythmias in adults. However, the association between PM2.5 exposure and cardiac arrhythmias in adolescents remains unclear.
Methods and Results
To investigate the association and time course between PM2.5 exposure with cardiac arrhythmias in adolescents, we analyzed the data collected from 322 adolescents who participated in the PSCC (Penn State Child Cohort) follow‐up examination. We obtained individual‐level 24‐hour PM2.5 concentrations with a nephelometer. Concurrent with the PM2.5 measure, we obtained 24‐hour ECG data using a Holter monitor, from which cardiac arrhythmias, including premature atrial contractions and premature ventricular contractions (PVCs), were identified. PM2.5 concentration and numbers of premature atrial contractions/PVCs were summarized into 30‐minute‐based segments. Polynomial distributed lag models within a framework of a negative binomial model were used to assess the effect of PM2.5 concentration on numbers of premature atrial contractions and PVCs. PM2.5 exposure was associated with an acute increase in number of PVCs. Specifically, a 10 μg/m3 increase in PM2.5 concentration was associated with a 2% (95% CI, 0.4%–3.3%) increase in PVC counts 0.5 to 1.0, 1.0 to 1.5, and 1.5 to 2.0 hours after the exposure. Cumulatively, a 10 μg/m3 increment in PM2.5 was associated with a 5% (95% CI, 1%–10%) increase in PVC counts within 2 hours after exposure. PM2.5 concentration was not associated with premature atrial contraction.
Conclusions
PM2.5 exposure was associated with an acute increased number of ventricular arrhythmias in a population‐based sample of adolescents. The time course of the effect of PM2.5 on ventricular arrhythmia is within 2 hours after exposure.
Keywords: adolescents, air pollution, cardiac arrhythmia, premature ventricular contraction
Subject Categories: Arrhythmias, Electrophysiology, Epidemiology
Nonstandard Abbreviations and Acronyms
- PM2.5
fine particles with aerodynamic diameters ≤2.5 μm
- PSCC
Penn State Child Cohort
- SCD
sudden cardiac death
Clinical Perspective.
What Is New?
The current study is the first of its kind to show an acute association between air pollution of fine particles with aerodynamic diameters ≤2.5 μm and ventricular arrhythmia in a population‐based sample of adolescents.
The time course of the impact of air pollution on ventricular arrhythmia was within ≈2 hours after exposure.
What Are the Clinical Implications?
Because of the ubiquitous and involuntary nature of air pollution, air pollution control measures may be taken to reduce the risk of cardiac arrhythmia in adolescents.
Reducing cardiac arrhythmia risk during adolescence may further reduce cardiovascular disease burden in adults because of the importance of early prevention of cardiovascular disease in early life stages.
Sudden cardiac death (SCD) is a major public health threat. It is estimated that SCD accounts for ≈50% of cardiovascular‐related deaths and 15% to 20% of all‐cause mortality in Western countries. 1 , 2 , 3 Although relatively rare, SCD among otherwise healthy children and youths, including world‐class athletes, has a devastating impact on their families. In addition, considering the large number of life‐years lost, the public health burden related to SCD in youth is not trivial. As a result, causes for SCD among children and youth have been studied extensively. 4 , 5 , 6 Unlike older adults, among whom atherosclerotic coronary artery disease is the predominant cause of SCD, cardiac arrhythmia is one of the most prominent risk factors for SCD in youths. 4 For example, unexplained SCD (ie, SCD without apparent cause after comprehensive autopsy), which can be mostly attributed to cardiac arrhythmias, accounts for one‐third of SCD among children and young adults. 5 Therefore, it is of great public health importance to identify modifiable risk factors for cardiac arrhythmias among children and adolescents.
The occurrence of cardiac arrhythmias may be attributed to impaired cardiac autonomic balance, 7 , 8 systemic inflammation, 9 , 10 and oxidative stress. 11 Because ambient air pollution from fine particles with an aerodynamic diameter ≤2.5 μm (PM2.5) has been associated with these biological processes, 12 it is plausible that PM2.5 exposure may also relate to an increased risk of cardiac arrhythmias. In fact, the association between PM2.5 and cardiac arrhythmias has been observed in adult epidemiological studies based on mortality data, 13 , 14 arrhythmia episodes captured by implantable cardioverter defibrillator, 15 , 16 , 17 , 18 , 19 , 20 and ECG monitoring. 21 , 22 , 23 , 24 , 25 , 26 For example, long‐term PM2.5 exposure has been associated with increased mortality from arrhythmias, especially among smokers. 13 Moreover, acute exposure to PM2.5 has been associated with increased odds of atrial fibrillation recorded by implantable cardioverter defibrillator within hours of exposure. 16 In both the general adult population 22 , 23 , 26 and patients with cardiovascular disease, 21 , 25 exposure to ambient particles has also been associated with ventricular arrhythmias from ECG monitoring. Although these studies have provided convincing evidence to support that PM2.5 exposure is a risk factor for cardiac arrhythmias, nearly all of them were conducted in adults, including patients with preexisting cardiovascular diseases. Therefore, the findings cannot be generalized to low‐risk populations, such as adolescents who are free of underlying cardiovascular conditions. Given the paramount importance of arrhythmias in the cause of SCD among youths, there is an urgent need to understand whether PM2.5 exposure triggers cardiac arrhythmias in youth.
Therefore, we conducted this study to evaluate the association between ambient PM2.5 concentration and cardiac arrhythmias in a population‐based sample of adolescents. We hypothesize that elevated PM2.5 is associated with an acute increased number of cardiac arrhythmias within hours of exposure.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
In this study, we analyzed data collected from a population‐based sample of adolescents who completed the follow‐up examination of the PSCC (Penn State Child Cohort) study. Detailed descriptions of the PSCC baseline and follow‐up examinations have been published elsewhere. 27 , 28 , 29 , 30 Briefly, 700 children aged 6 to 12 years who were in 3 school districts in the Harrisburg metropolitan area were recruited for the baseline examination from 2002 to 2006. After an average of 7.4 years, 421 of them returned and completed the follow‐up examination from 2010 to 2013. No major differences in the demographic characteristics were observed between these participants and the 279 who did not return for the follow‐up examination. 28 , 30
During the follow‐up examination, the participants were given a personal PM2.5 monitor and connected with a Holter ECG to proceed with their daily routines. The PM2.5 monitor and holter ECG were collected from the participants after 24 hours of recording. All participants started the recording period at 8:00 am on day 1 and ended at 8:00 am on day 2.
The study protocol was approved by the Pennsylvania State University College of Medicine Institutional Review Board. Written informed consents were obtained from the participants and their parents/legal guardians if participants were aged <18 years.
Personal PM 2.5 Exposure
In the PSCC follow‐up examination, we fitted a personal PM2.5 DataRam (pDR, model 1200; Thermo Scientific, Boston, MA) in combination with a PM2.5 size‐selection cyclone (KTL SCC1.062; BGI, Inc., Winchester, NH), an air pump (BGI Inc.), and a battery pack in a backpack. A similar device has been used in our previous study to measure individual‐level acute exposure to PM2.5. 22 The entire PM2.5 monitoring system was cleaned and calibrated before it was dispatched to each participant. The participants were instructed to bring the monitor with them when performing outdoor activities and kept the monitor in the same room when they were indoors for 24 hours.
The real‐time PM2.5 concentration was originally recorded continuously at 1‐minute intervals by the pDR. In the statistical analysis phase, the recording was averaged for 30‐minute segments at the top and bottom of the whole hours (eg, 8:01–8:30 and 8:31–9:00 am). The segment‐specific PM2.5 concentrations were treated as repeated time‐varying measurements in the analysis, such that each participant contributed up to 48 data points during the 24‐hour period.
ECG and Cardiac Arrhythmias
Concurrent with the 24‐hour PM2.5 monitoring, a high‐fidelity (1000 Hz sampling rate) 12‐lead Holter (Mortara H12+; Mortara Instrument, Inc., Milwaukee, WI) was used to record the ECG data. The ECG recordings were subjected to automatic analyses by using the HScribe System software (version 4.21; Mortara Instrument, Inc.), which identifies various forms of cardiac arrhythmias. After which an experienced technician visually inspected the entire recording to remove recording artifacts, verified software‐identified arrhythmic heartbeats, and identified additional arrhythmias in the ECG data. Because our study population is a population‐based sample of adolescents, none of them presented with malignant cardiac arrhythmias (eg, atrial fibrillation or ventricular tachycardia) or reported having structural heart diseases. Therefore, we chose to focus on the most prevalent forms of arrhythmias in the general population, including premature atrial contractions 31 (PACs) and premature ventricular contractions 32 (PVCs). Specifically, a PAC is defined as a premature heartbeat with ≥25% reduction in the R‐R interval compared with the immediate prior R‐R interval; a PVC is a heartbeat characterized by an absence of P wave and a premature QRS complex with an abnormally large width and amplitude on ECG. The ECG characteristics of normal heartbeat, PAC, and PVC are presented in Figure S1. After labeling all arrhythmic heartbeats in the ECG recording, we calculated the numbers of PACs and PVCs every 30 minutes based on the top and bottom of the whole hour (eg, 8:01–8:30 and 8:31–9:00 am). As a result, each participant also contributed up to 48 repeated time‐varying PAC/PVC count data points.
Meteorological Covariates
In addition to the PM2.5 and ECG measurements, we obtained 24‐hour, real‐time, individual‐level temperature and relative humidity with a HOBO H8 logger (Onset Computer Corp., Bourne, MA) fixed to the backpack housing the PM2.5 monitoring system. Similar to the PM2.5 data, the logger recorded real‐time temperature and relative humidity on a 1‐minute basis and averaged into 30‐minute‐based segments corresponding to the time segments used in PM2.5 and arrhythmia data. Hence, these meteorological covariates were treated as repeated time‐varying measurements, such that each participant contributed up to 48 segments.
Other Covariates
In the PSCC follow‐up examination, a self‐administered demographic questionnaire was used to obtain participant age, race and ethnicity, and sex. As our study population consisted of 79.3% non‐Hispanic White participants, 30 race and ethnicity were treated as binary covariates (ie, non‐Hispanic White populations versus racial and ethnic minorities) in the analyses. In addition, each participant underwent a detailed physical examination, from which their body mass index was calculated as weight/height2 (kg/m2). Based on the body mass index, the age‐ and sex‐adjusted body mass index percentiles were calculated based on the algorithm provided in the 2000 US Centers for Disease Control and Prevention growth charts. 33
Statistical Analysis
Because of the limited availability of the PM2.5 monitoring system, PM2.5 data were available from 322 (76.5%) of the 421 adolescents. As a result, the effective sample size for the current report is 322. As shown in Table S1, there were no significant differences in demographic characteristics or arrhythmia measures between those with and without PM2.5 data.
As the independent variable, PM2.5 concentrations were continuously measured for 24 hours and partitioned into repeated time‐varying measurements. Therefore, a high‐level collinearity is expected between PM2.5 segments (ie, lag terms), especially when they are temporally close. Therefore, we chose polynomial distributed lag models 34 , 35 , 36 to reduce the potential collinearity of PM2.5 exposure metrics across the 30‐minute segments. We decided, a priori, to include second‐degree polynomials in all of our models. To compare the performance of models with different polynomial terms, we conducted sensitivity analyses by using distributed lag models that include first‐degree terms (ie, only include zero‐ and first‐degree terms) or third‐degree terms (ie, include zero‐, first‐, second‐, and third‐degree terms). Because the dependent variables (ie, PAC/PVC counts) were repeated count measurements, we nested the distributed lag model within the framework of a negative binomial model 37 with generalized estimating equation. 38 Because the outcome measurements were equally spaced (ie, 30 minutes apart), autoregressive order 1 was used to account for the potential autocorrelation in the arrhythmia counts.
Taken together, we performed repeated‐measurement analyses using distributed lag models under a negative binomial model framework 22 to assess the acute association between PM2.5 exposure and arrhythmia counts. In these models, 1 lag period indicates a 30‐minute separation between the exposure and outcome. In other words, a lag‐0 effect indicates the instantaneous impact of PM2.5 on cardiac arrhythmias, and a lag‐1 effect indicates the impact of PM2.5 on the arrhythmia counts measured 30 minutes later, and so on. The cumulative effects of multiple lags of PM2.5 concentrations on arrhythmia counts may be considered as the sum of multiple individual‐lag effects. To determine the number of lag terms included in our final model, we decided, a priori, to start from the model with the smallest number of lags (ie, lag 0 only) and incrementally increase the number of lags included (ie, lag 0; lag 0, 1; lag 0, 1, 2; etc), until the largest cumulative effect was obtained, conditional on the significance of individual lag terms not changing (ie, such that including additional lag terms did not attenuate to null terms of the earlier lags). Moreover, because the ECG and air pollution data were obtained concurrently, arrhythmia data obtained during the first 6 hours of the recording period (ie, segments 0–12) would not have a corresponding PM2.5 concentration measured at 6.5 hours before them (ie, lag‐13 PM2.5). Consequently, the first 6 hours of data would be excluded from analysis in the model of lags 0 to 13. To preserve the validity of the statistical analyses, it is commonly accepted that ≥75% of the nominally available data (ie, 18 hours of the 24‐hour recording) need to be nonmissing in regression models. Therefore, the largest number of lag terms included in our model was 13 (ie, lags 0–12).
In the regression models, we adjusted for major meteorological and demographic covariates. The meteorological covariates, including temperature and relative humidity, were treated as time‐varying covariates, whereas demographics, including age, race, sex, and body mass index percentile, were treated as time‐invariant covariates. To ensure the results were not impacted by segments with an excessive number of arrhythmias, we conducted further sensitivity analyses by excluding segments with >60 (or >30) of PACs and PVCs or truncating extremely high levels of environmental exposure (eg, >95th percentile of PM2.5 concentration). All results were expressed as the rate ratios (RRs) in cardiac arrhythmia counts for a 10 μg/m3 increase in PM2.5. SAS 9.4 (SAS Institute, Inc., Cary, NC) was used to perform all analysis. A P≤0.05 was used to determine statistical significance.
RESULTS
Participant Characteristics
The demographic characteristics, arrhythmia counts, and environmental exposures with related variables in the study sample are summarized in Table 1. The study sample had a mean age of 16.95±2.25 years and consisted of 79.19% non‐Hispanic White participants, and 55.90% were boys. The average 30‐minute‐based PM2.5 concentration was 17.13±38.55 μg/m3. Overall, the mean±SD 30‐minute‐based PAC and PVC counts were 0.47±3.00 and 0.36±3.09, respectively. Among the 322 participants, 254 (78.88%) had at least 1 arrhythmic beat during the 24‐hour study period. Of these 254 adolescents, 102 (40.2%) had only PAC, 30 (11.8%) had only PVC, and 122 (48.0%) had both PAC and PVC. Although not significantly different in any of the demographic characteristics, the univariable analyses showed that adolescents who had arrhythmias, compared with those who were free of cardiac arrhythmias, were exposed to significantly higher levels of PM2.5 concentration, temperature, and relative humidity.
Table 1.
Demographic Characteristics of the Study Sample
| Overall, N=322 | Arrhythmia=yes, n=254 | Arrhythmia=no, n=68 | P value* | |
|---|---|---|---|---|
| Age, y | 16.95±2.25 | 16.83±2.27 | 17.11±2.22 | 0.27 |
| Male sex, n (%) | 180 (55.90) | 144 (56.69) | 38 (52.94) | 0.90 |
| Non‐Hispanic White participants, n (%) | 255 (79.19) | 201 (79.13) | 54 (79.41) | 0.96 |
| BMI percentile | 65.35±29.09 | 64.14±30.44 | 66.89±27.31 | 0.40 |
| PAC, counts | 0.47±3.00 | 0.55±3.26 | 0.00±0.00 | N/A |
| PVC, counts | 0.34±3.09 | 0.40±3.35 | 0.00±0.00 | N/A |
| PM2.5, μg/m3 | 17.13±38.55 | 21.54±49.10 | 16.34±36.17 | <0.01 |
| Temperature, °C | 22.24±3.56 | 22.34±3.57 | 21.72±3.53 | <0.01 |
| Relative humidity, % | 43.71±11.75 | 43.96±11.57 | 42.29±12.05 | <0.01 |
Data are presented as mean±SD for continuous variables and number (percentage) for binary variables. BMI indicates body mass index; N/A, not applicable; PAC, premature atrial contraction; PM2.5, fine particles with aerodynamic diameters ≤2.5 μm; and PVC, premature ventricular contraction.
The t tests and χ2 tests were used to obtain P values for continuous and categorical variables, respectively.
Distributions of PM 2.5 and Cardiac Arrhythmias
Detailed distribution of PM2.5 concentrations, related environmental variables, and cardiac arrhythmia counts are presented in Table 2. As shown in the table, there was a substantial amount of variation in the environmental exposure during the study period. Although the average PM2.5 concentration was relatively low, the concentration was extremely high in some of the segments. Specifically, 5% of the segments had a concentration >62.83 μg/m3, and ≈1% of them were >250 μg/m3. The time‐specific distribution of PM2.5 is shown in Figure 1, which depicts that PM2.5 concentrations increased in the morning (ie, at 6:30 am) and started to decrease late at night (ie, at 11:00 pm) into the early morning. Such a pattern is consistent with ordinary human time‐activity patterns. The figure further illustrates that the PM2.5 concentrations in each time segment were highly variable.
Table 2.
Distribution of the 30‐Minute‐Based Environmental Factors and Cardiac Arrhythmias
| Mean | SD | Median | IQR | Minimum | Maximum | Fifth percentile | 95th percentile | |
|---|---|---|---|---|---|---|---|---|
| Environmental factors | ||||||||
| PM2.5, μg/m3 | 17.13 | 38.55 | 5.90 | 12.90 | 0.00 | 3849.03 | 1.92 | 62.83 |
| Temperature, °C | 22.24 | 3.56 | 22.09 | 4.05 | 2.52 | 49.92 | 17.25 | 27.72 |
| Relative humidity, % | 43.71 | 11.75 | 43.63 | 15.58 | 15.58 | 100.00 | 24.57 | 62.43 |
| Cardiac arrhythmias | ||||||||
| PAC, counts | 0.47 | 3.00 | 2.00 | 1.00 | 0.00 | 127.00 | 0.00 | 7.00 |
| PVC, counts | 0.36 | 3.09 | 1.00 | 1.00 | 0.00 | 123.00 | 0.00 | 4.00 |
IQR indicates interquartile range; PAC, premature atrial contraction; PM2.5, fine particles with aerodynamic diameters ≤2.5 μm; and PVC, premature ventricular contraction.
Figure 1. Time‐specific distributions of PM2.5 concentrations during the study period.
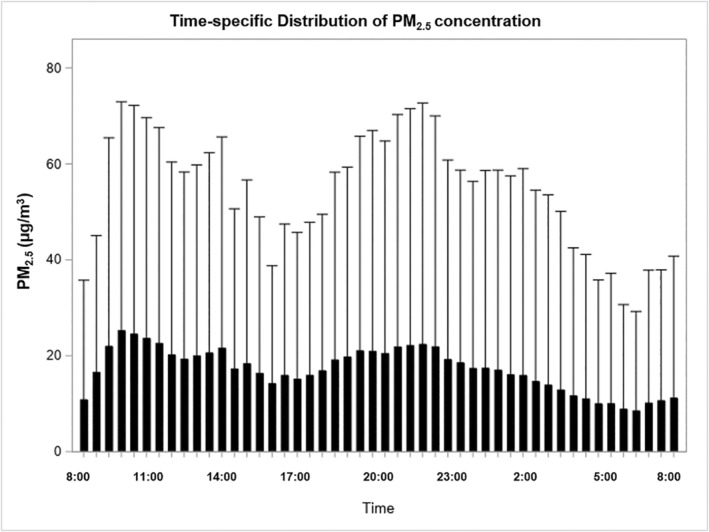
PM2.5 indicates fine particles with aerodynamic diameters ≤2.5 μm.
As expected in otherwise healthy adolescents, the numbers of PACs and PVCs in 30‐minute‐based segments were small. Of the segments, >50% had ≤2 PACs and ≤1 PVC, respectively. We further illustrate the time‐specific distributions of PAC and PVC counts in Figure 2, which indicate a large amount of variation in the arrhythmia counts in each of the segment. Overall, the arrhythmia counts were relatively high in the early morning (ie, at 4:00–4:30 am) and around noon (ie, 11:00–11:30 am), but low in the afternoon (ie, 4:30–5:00 pm).
Figure 2. Time‐specific distributions of PAC and PVC counts during the study period.
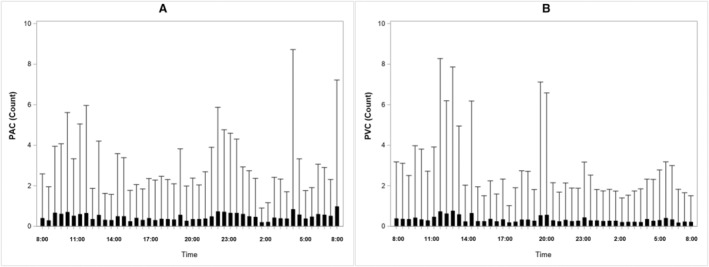
A, Time‐specific distributions of PAC counts. B, Time‐specific distributions of PVC counts. PAC indicates premature atrial contraction; and PVC, premature ventricular contraction.
Associations Between PM2.5 and Cardiac Arrhythmias
Individual lag effects and cumulative effects in the association between PM2.5 and arrhythmias from all models are summarized in Table S2. The cumulative effect of PM2.5 on PVC was significant in all models except the lag‐0 model. Also, the cumulative effects gradually increased as the number of lag terms increases. However, we observed a pattern that the significance of the closer lag terms changed when the further lag terms were included in the model. For example, lags 1, 2, and 3 were significant in the model of lags 0 to 3, whereas they were no longer significant in the model of lags 0 to 12. This phenomenon may be attributed to the following 2 potential reasons: (1) the large number of lag terms were competing for the limited amount of variance in the PVC count that may be explained by PM2.5 air pollution, and (2) the PVC count was, indeed, impacted by the PM2.5 exposure that occurred 3 to 6 hours (ie, lags 6–12) before the ECG measure instead of within 2 hours (ie, lags 0–3). Because there is little prior knowledge of the actual time course of this association, we followed the aformentioned model selection strategy, that is, adding additional lag term (eg, lag 5) into the model should not attenute the assocation between eariler terms (eg, lags 0–4) and cardiac arrhythmia to null. Therefore, the model of lags 0 to 3 was selected as our final model.
The multivariable‐adjusted RRs and the corresponding 95% CIs from the final model are presented in Table 3. As shown in the table, every 10 μg/m3 increase in PM2.5 concentration was associated with a 5% increase in PVC count within 2 hours after exposure. Given that the mean PVC count was 0.36, the effect size was approximately equivalent to an additional 0.02 PVCs every 30 minutes. In contrast, increased PM2.5 was not significantly associated with PAC counts.
Table 3.
RRs (95% CI) and P Values in Associations Between 10 μg/m3 Increments of PM2.5 Concentrations and Cardiac Arrhythmia Counts*
| PAC | PVC | |||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Lag 0, instantaneous | 0.995 (0.989, 1.002) | 0.17 | 0.998 (0.983, 1.013) | 0.76 |
| Lag 1, 0.5–1.0 h prior | 0.998 (0.990, 1.003) | 0.25 | 1.015 (1.004, 1.027) | <0.01 |
| Lag 2, 1.0–1.5 h prior | 1.000 (0.995, 1.006) | 0.88 | 1.022 (1.011, 1.033) | <0.01 |
| Lag 3, 1.5–2.0 h prior | 1.003 (0.998, 1.009) | 0.24 | 1.016 (1.002, 1.029) | 0.02 |
| Cumulative, within 2.0 h | 0.989 (0.970, 1.009) | 0.27 | 1.051 (1.002, 1.102) | 0.04 |
All models were adjusted for age, race, sex, body mass index percentile, temperature, and relative humidity. P values for zero‐, first‐, and second‐degree polynomial terms were 0.76, <0.01, and <0.01, respectively. PAC indicates premature atrial contraction; PM2.5, fine particles with aerodynamic diameters ≤2.5 μm; PVC, premature ventricular contraction; and RR, rate ratio.
RRs were calculated by exponentiating the regression coefficients from distributed lag‐negative binomial models. An RR of 1.05 indicates a 5% increase in arrhythmia counts/30 min in association with a 10 μg/m3 increase in PM2.5 concentration.
The individual lag effects further showed that a 10 μg/m3 increase in PM2.5 in lags 1, 2, and 3 were each significantly associated with an ≈2% increase in PVC counts, but lag 0 was not significantly related to PVC count. This suggests that the most significant impact of PM2.5 on PVC occurred between 0.5 and 2.0 hours after exposure. Because the second‐degree polynomial term was statistically significant (P<0.01), the individual lag effects exhibited nonlinearity and followed a quadratic form. That is, the effect sizes gradually increased from lag 0 to lag 2 and slightly decreased in lag 3. Similar to the cumulative effects, PM2.5 was not associated with PAC counts in any of the lags.
Sensitivity Analyses
To compare the results from distributed lag models with different polynomial terms, we replicated the analyses by using first‐degree polynomial and third‐degree polynomial models. As summarized in Table 4, the RRs for lags 0 to 3 cumulative effects from both models were 1.05, which is identical to the second‐degree polynomial model. However, the lag‐1 PM2.5 concentration was no longer significantly related to PVC counts in the first‐degree model. On the other hand, the third‐degree polynomial term was not significant (P=0.17) in the third‐degree polynomial model, although including it did not substantially change the estimates. Taken together, the second‐degree polynomial model was the best‐fitted model for our data. Nevertheless, all models showed a consistent positive association between PM2.5 and PVC counts.
Table 4.
RRs (95% CI) and P Values in Associations Between 10 μg/m3 Increments of PM2.5 Concentrations and PVC Counts in First‐Degree and Third‐Degree Polynomial Distributed Lag Models
| First‐degree model* | Third‐degree model† | |||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Lag 0, instantaneous | 1.003 (0.997, 1.016) | 0.68 | 0.994 (0.981, 1.008) | 0.42 |
| Lag 1, 0.5–1.0 h prior | 1.009 (0.997, 1.022) | 0.13 | 1.025 (1.008, 1.042) | <0.01 |
| Lag 2, 1.0–1.5 h prior | 1.016 (1.004, 1.028) | <0.01 | 1.013 (1.003, 1.023) | 0.01 |
| Lag 3, 1.5–2.0 h prior | 1.023 (1.004, 1.035) | 0.02 | 1.018 (1.001, 1.035) | 0.04 |
| Cumulative, within 2.0 h | 1.052 (1.003, 1.103) | 0.04 | 1.050 (1.002, 1.101) | 0.04 |
All models were adjusted for age, race, sex, body mass index percentile, temperature, and relative humidity. PM2.5 indicates fine particles with aerodynamic diameters ≤2.5 μm; PVC, premature ventricular contraction; and RR, rate ratio.
In the first‐degree model, P values for zero‐ and first‐degree polynomials were 0.68 and <0.01, respectively.
In the third‐degree model, P values for zero‐, first‐, second‐, and third‐degree polynomials were 0.42, <0.01, 0.04, and 0.17, respectively.
To eliminate the potential impact of segments with extremely high PVC counts, we further performed the regression analysis by excluding the 30‐minute‐based segments with >60 (ie, 2 PVC/min) or >30 (ie, 1 PVC/min) PVCs. As suggested in Table 5, the results did not change from our estimates obtained from the primary analysis as presented in Table 3. Therefore, the significant association between PM2.5 exposure and PVC cannot be attributed to the few segments with extremely high arrhythmia counts.
Table 5.
RRs (95% CI) and P Values in Associations Between 10 μg/m3 Increments of PM2.5 Concentrations and PVC Counts After Excluding Segments With Large Numbers of PVCs
| Excluding segments with >60 PVCs | Excluding segments with >30 PVCs | |||
|---|---|---|---|---|
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Lag 0, instantaneous | 0.992 (0.997, 1.007) | 0.30 | 0.991 (0.972, 1.011) | 0.38 |
| Lag 1, 0.5–1.0 h prior | 1.016 (1.006, 1.027) | 0.13 | 1.016 (1.005, 1.027) | <0.01 |
| Lag 2, 1.0–1.5 h prior | 1.024 (1.014, 1.034) | <0.01 | 1.025 (1.015, 1.035) | <0.01 |
| Lag 3, 1.5–2.0 h prior | 1.013 (1.002, 1.025) | 0.03 | 1.017 (1.005, 1.030) | <0.01 |
| Cumulative, within 2.0 h | 1.046 (1.002, 1.009) | 0.04 | 1.050 (1.000, 1.103) | 0.05 |
All models were adjusted for age, race, sex, body mass index percentile, temperature, and relative humidity. PM2.5 indicates fine particles with aerodynamic diameters ≤2.5 μm; PVC, premature ventricular contraction; and RR, rate ratio.
Given the extremely high maximum PM2.5 concentration (ie, 3849.0 μg/m3) and temperature (49.9 °C) in our data, we conducted further sensitivity analyses by truncating these 2 variables to their respective 95th percentiles (62.8 μg/m3 and 27.7 °C). As summarized in Table S3, every 10 μg/m3 increase in PM2.5 was associated with a 6.2% (95% CI, 0.1%–14.6%) increase in the PVC count in the model of lags 0 to 3. The association remained with the further adjustment of key lifestyle factors, including physical activity and self‐reported cigarette smoking or exposure to secondhand smoking.
DISCUSSION
The present study found that higher ambient PM2.5 concentration is associated with an acute increase in ventricular arrhythmias in a population‐based sample of adolescents. Specifically, our results revealed that elevated PM2.5 concentration triggers the onset of PVCs within 2 hours after exposure. Such a significant effect was supported by multiple sensitivity analyses. In addition, the most significant impact of PM2.5 on ventricular arrhythmias occurred between 0.5 and 2.0 hours after exposure, and the strongest effect occurred at 1.0 hour after exposure. To the best of our knowledge, this is the first study that reports the association between PM2.5 air pollution and cardiac arrhythmias among otherwise healthy adolescents.
PM2.5 air pollution has been established as a risk factor for cardiovascular morbidity and mortality. 12 Although the association between air pollution, especially gaseous pollutants, and cardiac arrhythmias is not conclusive, 39 it is generally accepted that cardiac arrhythmias induced by PM2.5 is 1 of the major pathogenesis pathways linking air pollution and cardiovascular diseases. For example, it has been shown that short‐term PM2.5 from traffic is associated with hospital admissions for arrhythmia in elderly individuals. 40 In a study conducted in 176 patients with implantable cardioverter defibrillators, Link et al 16 also found that 2‐hour average PM2.5 concentration was significantly associated with an increased odds of atrial fibrillation. In addition to acute exposure, short‐term 23 and long‐term 26 PM2.5 exposure have also been associated with higher odds of having arrhythmias in population‐based adults. The consistent association between air pollution and arrhythmias from these observational studies have been further replicated in experimental studies in both human 41 , 42 and animal models. 43 , 44 Our current study results are consistent with these previous findings that PM2.5 is related to an increased risk of cardiac arrhythmias. However, as cardiac arrhythmias are less prevalent in adolescents, it is expected that the strength of their association with air pollution would be weaker than that in adults. Indeed, we found a 5% increase in PVC counts within 2 hours in adolescents and a 10% increase in PVC counts within 1 hour in adults. 22 However, it should be noted that the number of arrhythmic events that occur in the early morning (eg, 4:00–6:00 am) are relatively high, whereas the PM2.5 concentration is low in the early morning. Such a misalignment may attenuate the association between PM2.5 and cardiac arrhythmias toward null. Therefore, our estimation with regard to the strength of the association was conservative.
Our study is unique in its study population and should not be considered as a simple replication of previous studies. As summarized previously, prior reports, including our own, were based on data collected from adults, including patients with preexisting cardiovascular diseases and those with implanted pacemakers. There is clear evidence that adults, especially elderly individuals with underlying cardiopulmonary diseases, are particularly vulnerable to the adverse effects of air pollution. 12 Hence, it is not surprising that particulate air pollution may associated with an increased risk of cardiac arrhythmia in adults who have high cardiometabolic burdens. On the contrary, our current study was performed in a sample of population‐based adolescents who were, on average, aged 17 years and free of any major cardiovascular conditions. With a relatively mature circulatory system and low cardiometabolic burden, our sample of adolescents may be considered as a low‐risk group with respect to their vulnerability to the adverse impact of air pollution. In addition, the arrhythmic burden in our study population was low (ie, <1 PVC/h). Therefore, it is extremely alarming that such a significant adverse impact of air pollution on PVC was observed in these otherwise healthy adolescents. This is the first time that the arrhythmogenesis effect of PM2.5 has been reported in such a low‐risk population. When considering the level of PM2.5 exposure in our study, our findings are even more concerning as the increased risk of PVC was observed at a PM2.5 concentration (ie, 17 μg/m3) well under the primary (ie, health‐based) standard of 35 μg/m3 established by the US Environmental Protection Agency. 45 These novel findings suggests that the adverse impact of air pollution on cardiac arrhythmias may be even larger in regions with high levels of PM2.5 (eg, highly populated inner cities).
PACs and PVCs are the most common forms of cardiac arrhythmias. Although highly prevalent and generally considered benign, 31 large numbers of PACs are responsible for the initiation of atrial fibrillation and increased risks of stroke, pacemaker implantation, and mortality. 46 , 47 , 48 , 49 Similarly, although isolated PVC is common and usually not harmful, 32 excessive amounts and severe forms of PVCs (eg, ventricular tachycardia) are independent risk factors for major cardiovascular events, including acute myocardial infarction, stroke, and SCD. 50 , 51 , 52 Because cardiac arrhythmias, especially ventricular tachycardia, is a predominate cause of SCD in otherwise healthy adolescents and young adults, 4 our finding that PM2.5 is related to ventricular arrhythmias suggests that PM2.5 may contribute to the risk of SCD among youth. It should be noted that increased PM2.5 was not related to PACs, which is consistent with previous studies conducted in other general population samples. 22 , 23 , 26 Only Riediker et al 24 found that PM2.5 exposure was associated with an increased number of both ventricular and supraventricular arrhythmias in a study conducted in young male highway patrol troopers. Highway patrol troopers are usually exposed to high levels of particulate matter air pollution from traffic for an extended period of time. Therefore, their study results may not reflect the association between air pollution and cardiac arrhythmias in a typical youth population.
Multiple causal mechanisms for the association between PM2.5 and cardiac arrhythmias have been proposed. 53 , 54 As summarized in these 2 comprehensive reviews, particulate matter‐induced systematic inflammation responses, oxidative stress, autonomic imbalance, direct effects of particulate matter in circulation, and particulate matter‐promoted cardiac structural remodeling may be responsible for the proarrhythmic effects of PM2.5.
The present study has some noteworthy strengths. First, we collected individual‐level PM2.5 data with a portable nephelometer for 24 hours. The personal monitor enabled us to adequately measure the air quality from the microenvironment around the participants, whereas geostatistical‐based estimations generated based on regional‐level data may not be able to capture the variations in PM2.5 concentrations in small geographical areas. Second, we divided the entire 24‐hour recordings into 30‐minute‐based segments and treated them as time‐varying repeated measurements. This approach substantially increased our statistical power. Because air pollution usually explains a small proportion of the variance in ECG outcomes, especially among healthy populations, the repeated‐measurement data structures played a critical role in allowing us to detect the small yet significant effect of PM2.5 on PVC. Third, we nested a polynomial distributed lag model within a framework of a negative binomial model to assess the association between PM2.5 concentrations and arrhythmia counts. With this model, the autocorrelation in both independent and dependent variables are accounted for simultaneously and consequently minimized the biases in the regression estimates. We further conducted sensitivity analyses by including different polynomial terms and excluding segments with high PVC counts. The consistent results from different models suggests that the observed association was highly robust.
Some of the limitations of the present study should not be ignored. First, because of the limited availability of PM2.5 monitors, we were not able to obtain air quality data from 23.5% of the PSCC. However, there was no significant difference between adolescents with and without PM2.5 data. Second, although the participants were instructed to bring the air pollution monitor with them when performing outdoor activities, it is possible that some of them kept the monitor indoors. Third, the PACs were identified solely based on the ratio of 2 consecutive R‐R intervals. In the meantime, we largely relied on the ECG processing software to identify PVCs. However, the software limited our ability to further stratify the PVC according to its origin or specify the prematurity of PVCs. Therefore, there is a chance for misclassification. However, this misclassification bias is systematic because only 1 research technician was involved in the ECG processing. Fourth, our model selection approach is data driven. As discussed briefly previously, the cumulative effects of PM2.5 on PVCs were statistically significant in all of our models except the lag‐0 model. Because there was little prior knowledge regarding the time course of the impact of PM2.5 on cardiac arrhythmias, we decided, a priori, to select our final model based on the strengths of the cumulative effect and the significance of the individual lag terms. As a result, we chose the model of lags 0 to 3 as our final model, which suggested an acute impact of PM2.5 on arrhythmias within 2 hours after exposure. In fact, such an acute effect is supported by some of the previous studies. Lastly, other variables, in particular cigarette smoking, may be an important confounding factor. However, the vast majority of our study participants were younger than the legal smoking age, and their self‐reported smoking histories are likely to be underestimated. Nonetheless, the acute impact of PM2.5 on PVC remained significant after adjusting for self‐reported exposure to cigarette smoking.
CONCLUSIONS
In summary, our study results suggest that PM2.5 air pollution is associated with an acute increase in the numbers of ventricular arrhythmias among otherwise healthy adolescents. Importantly, such an adverse health effect of PM2.5 on cardiac arrhythmia was observed in this low‐risk population in an environment with PM2.5 concentrations well below the US Environmental Protection Agency–mandated, health‐based air quality standards. In addition, we identified that the time course of the effect of PM2.5 on arrhythmia was ≈2 hours, with the most significant impacts occurring between 0.5 and 2.0 hours. Although pending confirmation, such an acute impact of particulate air pollution on ventricular arrhythmias during adolescence may increase the risk of SCD during early adulthood.
Sources of Funding
This work was supported by National Institutes of Health Grant Numbers R01 HL63772, R01 HL97165, and R21 HL 87858 and Penn State Clinical and Translational Science Institute Grant Number UL TR000127.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026370
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–1906. doi: 10.1161/CIRCRESAHA.116.304521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katritsis DG, Gersh BJ, Camm AJ. A clinical perspective on sudden cardiac death. Arrhythmia Electrophysiol Rev. 2016;5:177–182. doi: 10.15420/aer.2016:11:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, Sanders P. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ. 2019;28:6–14. doi: 10.1016/j.hlc.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 4. Ackerman M, Atkins DL, Triedman JK. Sudden cardiac death in the young. Circulation. 2016;133:1006–1026. doi: 10.1161/CIRCULATIONAHA.115.020254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam L, Davis AM, Thompson T, Connell V, Wallace J, et al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. doi: 10.1056/NEJMoa1510687 [DOI] [PubMed] [Google Scholar]
- 6. Kaltman JR, Thompson PD, Lantos J, Berul CI, Botkin J, Cohen JT, Cook NR, Corrado D, Drezner J, Frick KD, et al. Screening for sudden cardiac death in the young: report from a National Heart, Lung, and Blood Institute Working Group. Circulation. 2011;123:1911–1918. doi: 10.1161/CIRCULATIONAHA.110.017228 [DOI] [PubMed] [Google Scholar]
- 7. Herring N, Kalla M, Paterson DJ. The autonomic nervous system and cardiac arrhythmias: current concepts and emerging therapies. Nat Rev Cardiol. 2019;16:707–726. doi: 10.1038/s41569-019-0221-2 [DOI] [PubMed] [Google Scholar]
- 8. Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549 [DOI] [PubMed] [Google Scholar]
- 9. Lazzerini PE, Capecchi PL, Laghi‐Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208 [DOI] [PubMed] [Google Scholar]
- 10. Vonderlin N, Siebermair J, Kaya E, Köhler M, Rassaf T, Wakili R. Critical inflammatory mechanisms underlying arrhythmias. Herz. 2019;44:121–129. doi: 10.1007/s00059-019-4788-5 [DOI] [PubMed] [Google Scholar]
- 11. Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative‐stress‐induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8 [DOI] [PubMed] [Google Scholar]
- 13. Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long‐term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F [DOI] [PubMed] [Google Scholar]
- 14. Ueda K, Nitta H, Ono M. Effects of fine particulate matter on daily mortality for specific heart diseases in Japan. Circ J. 2009;73:1248–1254. doi: 10.1253/circj.cj-08-1149 [DOI] [PubMed] [Google Scholar]
- 15. Dockery DW, Luttmann‐Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, Koutrakis P, Schwartz JD, Verrier RL. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113:670–674. doi: 10.1289/ehp.7767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Link MS, Luttmann‐Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol. 2013;62:816. PMID: 23770178–825. doi: 10.1016/j.jacc.2013.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ljungman PL, Berglind N, Holmgren C, Gadler F, Edvardsson N, Pershagen G, Rosenqvist M, Sjögren B, Bellander T. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–2901. doi: 10.1093/eurheartj/ehn463 [DOI] [PubMed] [Google Scholar]
- 18. Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005 [DOI] [PubMed] [Google Scholar]
- 19. Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann‐Gibson H, Catalano PJ, Speizer FE, Gold DR, Dockery DW. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006;114:120–123. doi: 10.1289/ehp.8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vedal S, Rich K, Brauer M, White R, Petkau J. Air pollution and cardiac arrhythmias in patients with implantable cardioverter defibrillators. Inhal Toxicol. 2004;16:353–362. doi: 10.1080/08958370490439506 [DOI] [PubMed] [Google Scholar]
- 21. Berger A, Zareba W, Schneider A, Rückerl R, Ibald‐Mulli A, Cyrys J, Wichmann HE, Peters A. Runs of ventricular and supraventricular tachycardia triggered by air pollution in patients with coronary heart disease. J Occup Environ Med. 2006;48:1149–1158. doi: 10.1097/01.jom.0000245921.15916.03 [DOI] [PubMed] [Google Scholar]
- 22. He F, Shaffer ML, Rodriguez‐Colon S, Yanosky JD, Bixler E, Cascio WE, Liao D. Acute effects of fine particulate air pollution on cardiac arrhythmia: the APACR study. Environ Health Perspect. 2011;119:927–932. doi: 10.1289/ehp.1002640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liao D, Whitsel EA, Duan Y, Lin HM, Quibrera PM, Smith R, Peuquet DJ, Prineas RJ, Zhang ZM, Anderson G. Ambient particulate air pollution and ectopy—the environmental epidemiology of arrhythmogenesis in Women's Health Initiative study, 1999–2004. J Toxicol Environ Health A. 2009;72:30–38. doi: 10.1080/15287390802445483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, Williams RW, Devlin RB. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169:934–940. doi: 10.1164/rccm.200310-1463OC [DOI] [PubMed] [Google Scholar]
- 25. Sarnat SE, Suh HH, Coull BA, Schwartz J, Stone PH, Gold DR. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occup Environ Med. 2006;63:700–706. doi: 10.1136/oem.2006.027292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Kang J, Hong YS, Chang Y, Ryu S, Park J, Cho J, Guallar E, Shin HC, Zhao D. Long‐term particulate matter exposure and incidence of arrhythmias: a cohort study. J Am Heart Assoc. 2020;9:e016885. doi: 10.1161/JAHA.120.016885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela‐Bueno A, Fedok F, Vlasic V, Graff G. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–736. doi: 10.1093/sleep/32.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bixler EO, Fernandez‐Mendoza J, Liao D, Calhoun S, Rodriguez‐Colon SM, Gaines J, He F, Vgontzas AN. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47:1402–1409. doi: 10.1183/13993003.01771-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He F, Bixler EO, Berg A, Imamura Kawasawa Y, Vgontzas AN, Fernandez‐Mendoza J, Yanosky J, Liao D. Habitual sleep variability, not sleep duration, is associated with caloric intake in adolescents. Sleep Med. 2015;16:856–861. doi: 10.1016/j.sleep.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He F, Dong H, Fernandez‐Mendoza J, Bixler EO, Liao J, Liao D. Racial/ethnic disparity in habitual sleep is modified by caloric intake in adolescents. Sleep Med. 2020;76:65–71. doi: 10.1016/j.sleep.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conen D, Adam M, Roche F, Barthelemy JC, Felber Dietrich D, Imboden M, Künzli N, von Eckardstein A, Regenass S, Hornemann T, et al. Premature atrial contractions in the general population: frequency and risk factors. Circulation. 2012;126:2302–2308. doi: 10.1161/CIRCULATIONAHA.112.112300 [DOI] [PubMed] [Google Scholar]
- 32. Marcus GM. Evaluation and management of premature ventricular complexes. Circulation. 2020;141:1404–1418. doi: 10.1161/CIRCULATIONAHA.119.042434 [DOI] [PubMed] [Google Scholar]
- 33. Kuczmarski RJ, Ogden CL, Guo SS, Grummer‐Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC growth charts for the United States: methods and development. Vital Health Stat. 2000;11:1–190. PMID: 12043359 [PubMed] [Google Scholar]
- 34. Almon S. The distributed lag between capital appropriations and expenditures. Econometrica. 1965;33:178–196. doi: 10.2307/1911894 [DOI] [Google Scholar]
- 35. Pope CA III, Schwartz J. Time series for the analysis of pulmonary health data. Am J Respir Crit Care Med. 1996;154:S229–S233. doi: 10.1164/ajrccm/154.6_Pt_2.S229 [DOI] [PubMed] [Google Scholar]
- 36. Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11:320–326. doi: 10.1097/00001648-200005000-00016 [DOI] [PubMed] [Google Scholar]
- 37. Hilbe JM. Negative Binomial Regression. Cambridge University Press; 2007. [Google Scholar]
- 38. Hardin J, Hilbe JM. Generalized Estimating Equations. Chapman and Hall/CRC; 2003. [Google Scholar]
- 39. Watkins A, Danilewitz M, Kusha M, Massé S, Urch B, Quadros K, Spears D, Farid T, Nanthakumar K. Air pollution and arrhythmic risk: the smog is yet to clear. Can J Cardiol. 2013;29:734–741. doi: 10.1016/j.cjca.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 40. Halonen JI, Lanki T, Yli‐Tuomi T, Tiittanen P, Kulmala M, Pekkanen J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology. 2009;20:143–153. doi: 10.1097/EDE.0b013e31818c7237 [DOI] [PubMed] [Google Scholar]
- 41. Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl. 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403 [DOI] [PubMed] [Google Scholar]
- 42. Gong H Jr, Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, Cascio WE, Devlin RB. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal Toxicol. 2008;20:533–545. doi: 10.1080/08958370801911340 [DOI] [PubMed] [Google Scholar]
- 43. Nadziejko C, Fang K, Narciso S, Zhong M, Su WC, Gordon T, Nádas A, Chen LC. Effect of particulate and gaseous pollutants on spontaneous arrhythmias in aged rats. Inhal Toxicol. 2004;16:373–380. doi: 10.1080/08958370490439533 [DOI] [PubMed] [Google Scholar]
- 44. Wellenius GA, Saldiva PH, Batalha JR, Krishna Murthy GG, Coull BA, Verrier RL, Godleski JJ. Electrocardiographic changes during exposure to residual oil fly ash (ROFA) particles in a rat model of myocardial infarction. Toxicol Sci. 2002;66:327–335. doi: 10.1093/toxsci/66.2.327 [DOI] [PubMed] [Google Scholar]
- 45. U.S. Environmental Protection Agency . Review of the national ambient air quality standards to particulate matter. Fed Regist. 2020;85:82684–82748. [Google Scholar]
- 46. Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982 [DOI] [PubMed] [Google Scholar]
- 47. Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF, Lau CP, Tse HF, Siu CW. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389 [DOI] [PubMed] [Google Scholar]
- 48. Himmelreich JCL, Lucassen WAM, Heugen M, Bossuyt PMM, Tan HL, Harskamp RE, van Etten‐Jamaludin FS, van Weert HCPM. Frequent premature atrial contractions are associated with atrial fibrillation, brain ischaemia, and mortality: a systematic review and meta‐analysis. Europace. 2019;21:698–707. doi: 10.1093/europace/euy276 [DOI] [PubMed] [Google Scholar]
- 49. Lin CY, Lin YJ, Chen YY, Chang SL, Lo LW, Chao TF, Chung FP, Hu YF, Chong E, Cheng HM, et al. Prognostic significance of premature atrial complexes burden in prediction of long‐term outcome. J Am Heart Assoc. 2015;4:e002192. doi: 10.1161/JAHA.115.002192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheriyath P, He F, Peters I, Li X, Alagona P Jr, Wu C, Pu M, Cascio WE, Liao D. Relation of atrial and/or ventricular premature complexes on a two‐minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011;107:151–155. doi: 10.1016/j.amjcard.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 51. Ofoma U, He F, Shaffer ML, Naccarelli GV, Liao D. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc. 2012;1:e002519. doi: 10.1161/JAHA.112.002519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Frederiksen BS, Davanlou M, Hansen JF. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age > or =55 years. Am J Cardiol. 2006;97:1351–1357. doi: 10.1016/j.amjcard.2005.11.067 [DOI] [PubMed] [Google Scholar]
- 53. Shahrbaf MA, Akbarzadeh MA, Tabary M, Khaheshi I. Air pollution and cardiac arrhythmias: a comprehensive review. Curr Probl Cardiol. 2021;46:100649. doi: 10.1016/j.cpcardiol.2020.100649 [DOI] [PubMed] [Google Scholar]
- 54. Zhang S, Liu W, Wei Z, Zhang H. Air pollution and cardiac arrhythmias: from epidemiological and clinical evidences to cellular electrophysiological mechanisms. Front Cardiovasc Med. 2021;8:736151. doi: 10.3389/fcvm.2021.736151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


