Abstract
Background
Diagnosis of congenital long‐QT syndrome (LQTS) is complicated by phenotypic ambiguity, with a frequent normal‐to‐borderline resting QT interval. A 3‐step algorithm based on exercise response of the corrected QT interval (QTc) was previously developed to diagnose patients with LQTS and predict subtype. This study evaluated the 3‐step algorithm in a population that is more representative of the general population with LQTS with milder phenotypes and establishes sex‐specific cutoffs beyond the resting QTc.
Methods and Results
We identified 208 LQTS likely pathogenic or pathogenic KCNQ1 or KCNH2 variant carriers in the Canadian NLQTS (National Long‐QT Syndrome) Registry and 215 unaffected controls from the HiRO (Hearts in Rhythm Organization) Registry. Exercise treadmill tests were analyzed across the 5 stages of the Bruce protocol. The predictive value of exercise ECG characteristics was analyzed using receiver operating characteristic curve analysis to identify optimal cutoff values. A total of 78% of male carriers and 74% of female carriers had a resting QTc value in the normal‐to‐borderline range. The 4‐minute recovery QTc demonstrated the best predictive value for carrier status in both sexes, with better LQTS ascertainment in female patients (area under the curve, 0.90 versus 0.82), with greater sensitivity and specificity. The optimal cutoff value for the 4‐minute recovery period was 440 milliseconds for male patients and 450 milliseconds for female patients. The 1‐minute recovery QTc had the best predictive value in female patients for differentiating LQTS1 versus LQTS2 (area under the curve, 0.82), and the peak exercise QTc had a marginally better predictive value in male patients for subtype with (area under the curve, 0.71). The optimal cutoff value for the 1‐minute recovery period was 435 milliseconds for male patients and 455 milliseconds for femal patients.
Conclusions
The 3‐step QT exercise algorithm is a valid tool for the diagnosis of LQTS in a general population with more frequent ambiguity in phenotype. The algorithm is a simple and reliable method for the identification and prediction of the 2 major genotypes of LQTS.
Keywords: diagnosis, exercise treadmill test, genetics, long‐QT syndrome, LQTS, sex, stress test
Subject Categories: Electrophysiology
Nonstandard Abbreviations and Acronyms
- FDR
first‐degree relative
- HiRO
Hearts in Rhythm Organization
- LP
likely pathogenic
- LQTS
long‐QT syndrome
- NLQTS
National Long‐QT Syndrome
- QTc
corrected QT interval
Clinical Perspective.
What Is New?
The addition of sex‐specific cutoffs in a previously developed 3‐step algorithm aids in accurately diagnosing long‐QT syndrome and predicting the 2 most common genotypes.
What Are the Clinical Implications?
The 3‐step screening algorithm is a simple and valid tool in the diagnosis of long‐QT syndrome in a population with frequent phenotypic ambiguity.
A sex‐specific approach to the interpretation of exercise testing should be used when screening for long‐QT syndrome.
Congenital long‐QT syndrome (LQTS) is an inherited heart rhythm condition characterized by QT prolongation on the surface ECG with failure of adequate QT shortening during exercise and recovery in LQTS1/2. 1 LQTS is associated with life‐threatening cardiac events, including torsade de pointes and sudden cardiac arrest in the young. 2 , 3 LQTS can be classified into distinct genetic subtypes, with LQTS1 to LQTS3 collectively comprising >90% of cases. 3 Variants in KCNQ1 (LQTS1) account for most cases associated with known LQTS genes at 35% to 50%, whereas variants in KCNH2 (LQTS2) account for 25% to 45% and variants in SCN5A (LQTS3) account for 5% to 10%. 3 , 4 , 5 Although 17 genes have been reported to cause LQTS, a recent appraisal suggests that only 6 genes (KCNQ1, KCNH2, SCN5A, and CALM1 to CALM3) definitely cause LQTS. 6 Genotype‐specific triggers are known to precipitate arrhythmias: physical exercise in LQTS1, emotional stress, loud noise, or startle in LQTS2, whereas adverse events most frequently occur at rest or during sleep in LQTS3. 7
Significant resting QT prolongation leads to a clear diagnosis of LQTS 3 ; however, given variable expressivity in LQTS, 30% to 50% of LQTS variant carriers may have QT intervals in the normal to borderline range, and are considered “silent carriers” 8 or “concealed LQTS.” 9 Thus, patients with a “normal” or borderline phenotype are commonly encountered, and remain a challenge for diagnosis. 8 Previous studies have developed a 3‐step screening algorithm based on exercise response of the QT interval to predict genotype from exercise testing in probands, 10 which was subsequently validated in first‐degree relatives (FDRs). 11 The algorithm stratifies patients from their resting corrected QT interval (QTc) and 4‐minute recovery QTc, to predict carrier status, and then further stratifies by 1‐minute recovery QTc to predict genotype. Both cohorts were composed of cases of LQTS with a severe phenotype that are less representative of the general population with LQTS, where there is often a milder phenotype and diagnostic ambiguity.
Sex differences in LQTS are well established. 12 , 13 Disease penetrance is reportedly higher in females than in males, with females composing 57% to 70% of LQTS cases. 2 , 14 , 15 Sex‐specific cutoffs for determining a prolonged resting QTc are 470 milliseconds for men and 480 milliseconds for women, 16 serving as step 1 in the screening algorithm. However, the second and third steps do not account for sex‐specific changes. We hypothesized that sex‐specific cutoff values would improve the algorithm's accuracy.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Subjects were enrolled in the Canadian NLQTS (National Long‐QT Syndrome) Registry, within the HiRO (Hearts in Rhythm Organization; www.heartsinrhythm.ca) Registry. The NLQTS Registry recruits LQTS probands and their family members from 21 Canadian adult and pediatric recruiting centers, which has been previously described. 17 During clinical evaluation, patients underwent standard diagnostic testing for LQTS, including baseline ECG, personal and family medical history, standardized treadmill testing, and genetic testing. Probands underwent LQTS‐based genetic testing (involving a minimum gene set of KCNQ1, KCNH2, and SCN5A), whereas their family members underwent variant‐specific genetic testing if the proband was identified to have a likely pathogenic (LP) or pathogenic LQTS variant, according to the American College of Medical Genetics criteria. 18 Variants were reviewed during follow‐up visits to ensure appropriate classification. 19 Ethical approval for this study was obtained from the University of British Columbia Providence Health Care Research Ethics Board. All participants provided written informed consent.
Eligible cases from September 2014 to May 2020 were included. Study subjects consisted of LQTS probands and FDRs with an LP/pathogenic variant. Patients with LQTS with a phenotypic diagnosis of long QT (based on Schwartz score 16 ) but without an LP/pathogenic variant were excluded. Patients with LQTS without treadmill test strips available were also excluded. The control group was composed of participants enrolled in the HiRO Registry who tested negative for a known LQTS familial variant; FDRs of gene‐negative probands of other inherited arrhythmia and cardiomyopathy conditions who had no phenotypic evidence of disease; and last, unaffected FDRs of unexplained cardiac arrest and sudden cardiac death probands where the proband did not have an LQTS phenotype. 17 Carriers in this study were restricted to variants in KCNQ1 and KCNH2, because of the limited number of other LQTS subtypes identified for analysis.
Data Extraction
All clinical information pertaining to the cardiac history of each patient was extracted from the NLQTS/HiRO Registry, including demographic information, medications, family history, pedigree, clinical cardiac history, and genetic data.
Treadmill Tests
As part of their clinical evaluation, patients underwent treadmill testing consisting of the standard Bruce protocol. The 12‐lead ECGs were taken during the standard Bruce protocol at 5 stages: supine at rest, after 30 seconds of standing, at peak exercise, 1 minute into recovery, and 4 minutes into recovery. Two expert cardiologists (H.‐C.H. and A.K.) independently reviewed treadmill tests in the registry for QT interval and heart rate across the 5 stages for each patient, blinded to the carrier status of the patient. The QTc was calculated using the Bazett formula, which has been demonstrated to accurately identify LQTS at high heart rates. 20 , 21 The QT interval was measured in leads II and V5 from the beginning of the QRS complex to the end of the T wave, determined using the maximum slope technique or tangent method. 22 , 23 The mean of 3 consecutive QT intervals was used. Of all available treadmill tests read for each patient, pre‐β and post‐β blockade were identified through review of medical history.
Genetic Variants
Patients with LP/pathogenic variants in KCNQ1 and KCNH2 were included. Genetic variant classifications were reviewed by an independent reviewer (L.Y.) before inclusion as an LP/pathogenic variant carrier 19 and were classified according to the American College of Medical Genetics criteria. 18 Classifications that were borderline between variants of unknown significance and LP were reviewed by a genetic counselor and second reviewer (B.D.). To ensure replicability of other classifications, the second reviewer independently classified an additional 10 variants. High agreement (>80%) among classifications was found between reviewers (L.Y. and B.D.), and discrepancies were resolved by consensus. The LP/pathogenic KCNQ1 and KCH2 variants included are listed in Table S1.
The 3‐Step Algorithm
The proposed algorithm 10 was applied, using genetic testing and variant carrier status as the gold standard for test performance. β‐Blocker naïve tests were selected for analysis when patients had multiple tests. All study participants were stratified as normal, borderline, or abnormal, according to their resting QTc based on previous literature. 16 In male patients, the normal QTc was defined as <440 milliseconds, borderline QTc as 440 to 470 milliseconds, and abnormal QTc as >470 milliseconds. In female patients, the normal QTc was defined as <450 milliseconds, borderline QTc as 450 to 480 milliseconds, and abnormal QTc as >480 milliseconds. Those with normal and borderline resting QTc proceeded to the second step of the algorithm, where they were stratified as normal or abnormal, according to the exercise ECG parameter with the best predictive value. Those identified as having a normal QTc, based on the exercise ECG parameter with the best predictive value and the corresponding optimal cutoff value, were subsequently classified as unaffected, whereas those who were identified to have abnormal QTc were classified as affected carriers. The affected carriers proceeded to the third step of the algorithm to determine LQTS subtype, differentiating LQTS1 from LQTS2, using receiver operating characteristic curve analysis for the highest area under the curve (AUC).
The utility of the initial 2 steps of the algorithm was compared by genotype (ie, patients with LQTS1 versus noncarriers, and patients with LQTS2 versus noncarriers). Further analysis was performed on the basis of previous reports that variants in S6 segment mutations surrounding p.A341 are associated with higher arrhythmic risk in patients with LQTS1, 24 whereas variants in the pore region are linked to worse outcomes in patients with LQTS2. 25 For KCNQ1, p.A341‐neighboring regions were defined as amino acid residues in the S6 segment, residues 328 to 348, 24 whereas for KCNH2, the pore region was defined as residues spanning 548 to 659. 26 , 27
Statistical Analysis
Data analysis was performed using R version 4.0.2. Discrete variables were compared using the χ2 test, and continuous variables were compared by linear regression for baseline characteristics and subsequent analyses. The regression models were adjusted for age, sex, and familial relatedness, where appropriate. P values for multiple comparisons were adjusted using the Bonferroni correction. The predictive value of exercise ECG characteristics was analyzed using receiver operating characteristic curve analysis. Optimal cutoff values for exercise ECG characteristics (supine, standing, peak exercise, and 1 and 4 minutes into recovery) were selected to attain a minimum sensitivity of 0.80 for carrier status and 0.75 for subtype, allowing for maximization of specificity given these sensitivity constraints. The DeLong test was used to compare AUCs. To ensure measurability of QTc in clinical practice, the optimal cutoff value was reported to the nearest 5 milliseconds. A sex‐specific analysis was decided a priori. The familywise type 1 error rate was 0.05.
Results
Baseline Characteristics
The study was composed of 208 LP/pathogenic variant‐positive patients with LQTS (age, 33±18 years), consisting of 87 probands and 121 FDRs, and 215 noncarriers (age, 36±19 years; Table 1) from 366 families. Most variants were in KCNQ1 (78%). There were 80 of 208 (38%) patients with LQTS who were symptomatic on initial referral at baseline. As expected, the mean QTc was higher in carriers (461±43 milliseconds) than noncarriers (407±33 milliseconds), although the extent of QTc prolongation in affected cases was modest, in keeping with a “general” population with LQTS. A density plot of resting QTc is shown in Figure S1. β Blockade was used in 60% of patients with LQTS. The QTc of carriers using other formulas is shown in Table S2.
Table 1.
Baseline Characteristics of LQTS LP/Pathogenic Carriers and Unaffected Noncarriers
| Characteristic | Carriers | Noncarriers | P value |
|---|---|---|---|
| Total No. | 208 | 215 | |
| Age at test, mean (SD), y | 33 (18) | 36 (19) | 0.05 |
| Male sex, n (%) | 87 (42) | 101 (47) | 0.33 |
| Race or ethnicity, n (%) | <0.001 | ||
| East Asian | 14 (7) | 25 (12) | |
| European ancestry | 143 (69) | 152 (71) | |
| Indigenous | 39 (18) | 4 (2) | |
| KCNQ1 variant, n (%) | 163 (78) | … | … |
| KCNH2 variant, n (%) | 45 (22) | … | … |
| Resting HR, mean (SD), bpm | 68 (13) | 73 (14) | <0.001* |
| Resting QTc, mean (SD), ms | 461 (43) | 407 (33) | <0.001* |
| β‐Blocker use, n (%) | 87 (40) | … | … |
| Symptomatic at baseline, n (%) | 80 (38) | … | … |
| Syncope | 39 | ||
| Chest pain | 19 | ||
| Cardiac arrest | 5 | ||
| Palpitations | 23 | ||
| Presyncope | 17 |
BPM indicates beats per minute; HR, heart rate; LP, likely pathogenic; LQTS, long‐QT syndrome; and QTc, corrected QT interval.
Adjusted for age, sex, and familial relatedness.
Resting QTc
The frequencies of resting QTc classifications, stratified by sex and carrier status, are shown in Figure 1. A total of 78% of male carriers and 74% of female carriers had a resting QTc value in the normal‐to‐borderline range. For male patients, carriers accounted for 100% of an overall population with an overtly abnormal resting QTc, 60% with borderline resting QTc prolongation, and 36% with a normal resting QTc. For female patients carriers accounted for 100% of female patients with an overtly abnormal resting QTc, 76% with borderline resting QTc prolongation, and 33% with a normal resting QTc. Those with an overtly abnormal resting QTc were considered carriers (n=51) and were streamlined to the final step of the algorithm. The receiver operating characteristic curve for resting QTc in male patients and female patients is shown in Figure 2. Male patients had an AUC of 0.82, whereas female patients had an AUC of 0.84. The resting QTc was highly specific but not sensitive. Given a cutoff value of 470 milliseconds, there was a sensitivity of 0.22 and a specificity of 0.99 for male patients. In female patients given a cutoff value of 480 milliseconds, there was a sensitivity of 0.27 and a specificity of 0.96.
Figure 1. Frequencies of resting corrected QT interval (QTc) classification by sex and carrier status.
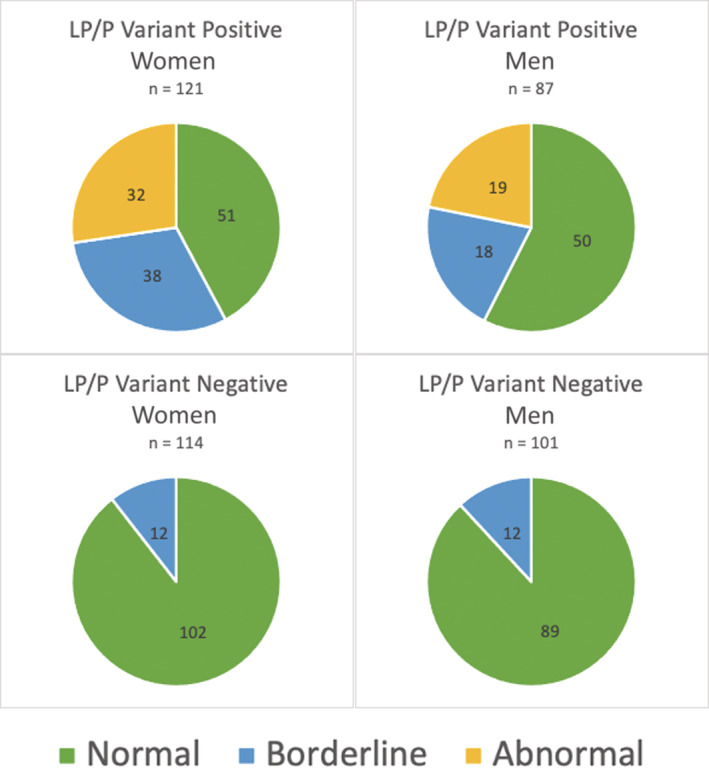
Male patients: normal QTc <440 milliseconds; borderline QTc as 440 to 470 milliseconds; abnormal QTc >470 milliseconds. Female patients: normal QTc <450 milliseconds; borderline QTc as 450 to 480 milliseconds; abnormal QTc >480 milliseconds. LP indicates likely pathogenic; and P, pathogenic.
Figure 2. Receiver operating characteristic curve for exercise treadmill test stages (standing, peak exercise, 1‐minute recovery, and 4‐minute recovery).
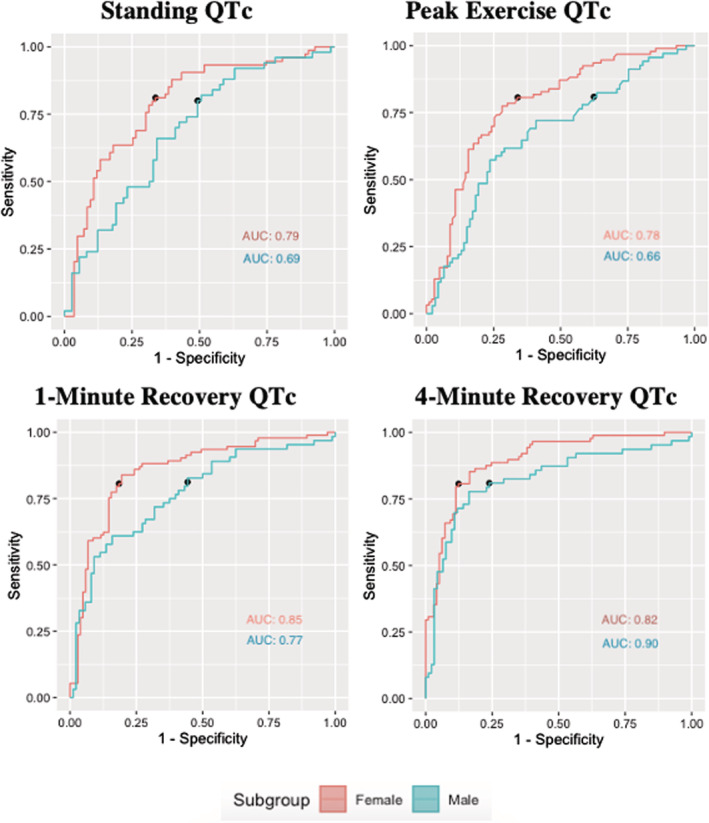
See Table S2 for the raw data of this figure. Adjusted for age and familial relatedness. AUC indicates area under the curve; and QTc, corrected QT interval.
QTc Changes With Exercise
The 5 treadmill test stages in carriers and noncarriers were compared and are shown in Table 2. There were significant differences in QTc at all 5 stages of the Bruce protocol in carriers versus noncarriers (P<0.001). Given that 60% of patients with LQTS were on β blockers, heart rate was significantly different in the peak exercise, 1‐minute recovery, and 4‐minute recovery stages (P<0.001), as well as at the standing stage (P=0.004), but borderline at rest (P=0.06). The QTc remained longer in carriers than noncarriers across all stages of the exercise test. The normative data for QTc values in treadmill testing stages in noncarriers at 95th and 98th percentiles are shown in Table S3. The QTc and heart rate during treadmill testing stages in prepubescent (age, <14 years) versus postpubescent (age, ≥14 years) patients with LQTS are included in Table S4, and in symptomatic versus asymptomatic patients with LQTS in Table S5.
Table 2.
QTc and HR at Various Stages of Treadmill Testing in Carriers and Noncarriers
| Variable | Carriers (N=208) | Noncarriers (N=215) | P value* |
|---|---|---|---|
| Resting QTc, ms | 461 (44) | 407 (33) | <0.001 |
| Resting HR, bpm | 71 (13) | 73 (14) | 0.06 |
| Standing QTc, ms | 478 (52) | 430 (51) | <0.001 |
| Standing HR, bpm | 82 (16) | 88 (22) | 0.004 |
| Peak exercise QTc, ms | 480 (76) | 431 (61) | <0.001 |
| Peak exercise HR, bpm | 155 (25) | 171 (21) | <0.001 |
| Min 1 recovery QTc, ms | 474 (65) | 409 (43) | <0.001 |
| Min 1 recovery HR, bpm | 123 (24) | 130 (22) | <0.001 |
| Min 4 recovery QTc, ms | 488 (65) | 427 (35) | <0.001 |
| Min 4 recovery HR, bpm | 92 (20) | 97 (14) | <0.001 |
Data are given as mean (SD). BPM indicates beats per minute; HR, heart rate; and QTc, corrected QT interval.
Adjusted for age, sex, familial relatedness, and multiple comparisons.
Patients in step 1 of the algorithm who were identified to have a normal‐to‐borderline resting QTc (n=372) were analyzed for the predictive value of exercise ECG characteristics by receiver operating characteristic curve analysis. The predictive utilities of standing QTc, peak exercise QTc, and 1 and 4 minutes into recovery QTc are summarized in Figure 3 and Table S6. Recovery AUCs were numerically greater than standing or peak AUCs. The 4‐minute recovery QTc had the greatest predictive value for both male and female patients.
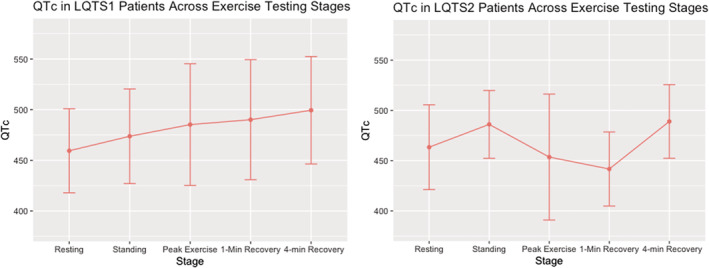
Figure 3. Corrected QT interval (QTc) values during exercise and recovery in long‐QT syndrome (LQTS) 1 and LQTS2.
Sex‐Specific QTc Changes With Exercise
For step 2 of the algorithm, using the 4‐minute recovery QTc, the AUCs in males and females were 0.82 and 0.90, respectively. Male patients had an optimal cutoff value of 440 milliseconds and a specificity of 0.76, whereas female patients had an optimal cutoff value of 450 milliseconds and a specificity of 0.88. For male patients the positive and negative predictive values were 0.67 and 0.86, respectively. For female patients the positive and negative predictive values were 0.79 and 0.84, respectively. AUC was higher in female than in male patients across all stages by a mean difference of 0.10±0.02, but this was only statistically greater for the 1‐minute recovery QTc (P=0.002). Additional sensitivity values corresponding with 0.80, 0.90, 0.95, and 0.99 specificity are shown in Table S7. In step 2, all values (100%) >480 milliseconds 16 represented patients with LQTS; however, 62% of carriers in step 2 had a 4‐minute recovery QTc <480 milliseconds.
LQTS Subtypes and QTc Changes With Exercise
Patients with a probable LQTS diagnosis based on either an abnormal supine QTc (n=53) or a normal‐to‐borderline supine QTc with an abnormal 4‐minute recovery QTc (n=130) were included in further analysis for LQTS subtype (n=183). Figure 4 shows the QTc at various stages of treadmill testing, stratified by genotype. There were no significant differences (P=not significant) in QTc between LQTS1 and LQTS2 at rest and 4 minutes into recovery, whereas there were significant differences in standing (P=0.04), peak exercise (P<0.001), and 1‐minute recovery (P<0.001) QTc.
Figure 4. Revised algorithm.
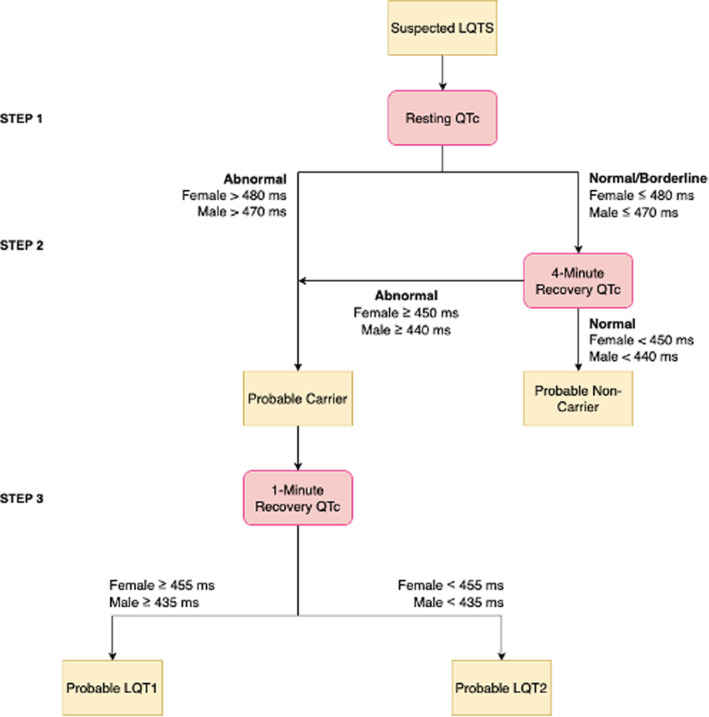
LQTS indicates long‐QT syndrome; and QTc, corrected QT interval.
Peak exercise and 1‐minute recovery QTc in male patients had comparable performance (AUC, 0.71 versus 0.70), whereas the 1‐minute recovery QTc clearly had the greatest utility in female patients (AUC, 0.82; Table 3). The 1‐minute recovery QTc can be reasonably applied for both sexes as the final step of the algorithm to differentiate subtype; the optimal cutoff value was 435 milliseconds for male patients and 455 milliseconds for female patients. The sex‐stratified cutoff values were not statistically better than the published cutoff values (Table S8).
Table 3.
Prediction of Subtype (LQTS1 vs LQTS2)
| Variable | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male patients (N=78) | Female patients (N=111) | P value | |||||||||||
| AUC | Cutoff, ms | Specificity | Sensitivity | PPV | NPV | AUC | Cutoff, ms | Specificity | Sensitivity | PPV | NPV | ||
| Peak exercise QTc | 0.71 | 435 | 0.53 | 0.80 | 0.46 | 0.74 | 0.72 | 460 | 0.63 | 0.76 | 0.65 | 0.80 | 0.82 |
| 1‐Min recovery QTc | 0.70 | 435 | 0.44 | 0.76 | 0.80 | 0.39 | 0.82 | 455 | 0.91 | 0.75 | 0.95 | 0.49 | 0.15 |
Total N=189. Modeling adjusted for familial relatedness, and P values corrected for multiple comparisons. AUC indicates area under the curve; LQTS, long‐QT syndrome; NPV, negative predictive value; PPV, positive predictive value; and QTc, corrected QT interval.
The utility of the algorithm by genotype was compared using the newly established sex‐stratified cutoff values (Table 4). There were no statistically significant differences seen between LQTS1 versus LQTS2 in male and female patients.
Table 4.
Utility of Steps 1 and 2 in LQTS1 vs LQTS2
| Variable | LQTS1 (n=163) | LQTS2 (n=45) | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff, ms | Specificity | Sensitivity | PPV | NPV | AUC | Cutoff, ms | Specificity | Sensitivity | PPV | NPV | ||
| Resting | |||||||||||||
| Male patients | 0.80 | 470 | 0.99 | 0.17 | 1 | 0.65 | 0.88 | 470 | 0.99 | 0.43 | 1 | 0.89 | 0.08 |
| Female patients | 0.87 | 480 | 0.99 | 0.27 | 1 | 0.62 | 0.81 | 480 | 0.99 | 0.29 | 1 | 0.87 | 0.32 |
| 4‐Min recovery | |||||||||||||
| Male patients | 0.83 | 442 | 0.79 | 0.76 | 0.62 | 0.87 | 0.82 | 442 | 0.80 | 0.76 | 0.47 | 0.94 | 0.87 |
| Female patients | 0.90 | 451 | 0.82 | 0.88 | 0.75 | 0.88 | 0.87 | 451 | 0.76 | 0.86 | 0.24 | 0.97 | 0.36 |
Modeling adjusted for age and familial relatedness, and P values corrected for multiple comparisons. P values compare male patients vs female patients. AUC indicates area under the curve; LQTS, long‐QT syndrome; NPV, negative predictive value; and PPV, positive predictive value.
Variant Site
There were a total of 163 carriers with KCNQ1 variants and 45 carriers with KCNH2 variants. Within KCNQ1, there were 11 (7%) patients with variants in the p.A341‐neighboring region, compared with 152 (93%). No statistical differences were seen in the p.A341‐neighboring and non–p.341‐neighboring variant sites at any stage of treadmill testing (P=not significant; Table S9). Within KCNH2, there were 11 (24%) patients with variants in the pore region, compared with 34 (75%) in a nonpore region. There were no differences seen in pore versus nonpore regions across the exercise test stages (P=not significant; Table S10).
β Blockade
The heart rates of carriers were significantly different comparing before and on β blockade at all stages of exercise testing (P<0.001; Table S11). The QTc was lower after blockade than before blockade at rest and standing stages (P=0.001 and P=0.01, respectively), but there were no statistically significant differences before and after β blockade in carriers in the subsequent stages (P=not significant).
Discussion
Our study shows that a sex‐specific approach to interpretation of exercise testing should be used when assessing for LQTS and adds additional insight into the 2 most common genotypic causes.
QTc Changes With Exercise
The QTc of carriers remained longer at all stages of exercise testing compared with noncarriers, despite the inclusion of milder LQTS phenotypes. The 4‐minute recovery had the best predictive utility for carrier status in both sexes. Sy et al 11 had previously identified 445 milliseconds as the optimal cutoff value for both sexes at the 4‐minute recovery stage, and the Schwartz score was revised 16 to include the 4‐minute recovery QTc as 1 criterion point, but with a higher specificity and cutoff value of 480 milliseconds. Given the normal‐to‐borderline phenotype that is prevalent in the general population with LQTS, there may be reduced utility in increasing specificity with a higher cutoff value to identify cases, and this further underscores the importance of genetic testing as the gold standard for diagnosis. Schwartz and Crotti suggested that the algorithm has the greatest utility in probands at initial presentation, where LQTS‐based genetic testing is not yet offered or available. 16 Our current study includes both LQTS probands and FDR carriers, and the algorithm shows value for both populations. However, because of the inclusion of an LQTS patient population representing a broad and not just a high‐risk phenotype, the predictive utility of the algorithm was somewhat lower overall. The resting QTc was insensitive for identifying LQTS in the current population. The phenotypic ambiguity (normal‐to‐borderline resting QTc) was substantially greater in our cohort than the 52% previously reported by Sy et al. 11 This further compounds the challenge of detecting LQTS in the general population when the majority have frank overlap with normal and borderline cases. Population screening would thus determine that the vast majority of these individuals are unaffected. Exercise testing would enhance latent LQTS detection, but choosing specific cutoffs will fail to identify a substantial proportion of the low‐risk carriers whose QTs cannot be provoked to prolong into the overtly abnormal range. The algorithm performed similarly in patients with LQTS without an overtly prolonged resting QTc, demonstrated in Table S12.
Sex‐Specific QTc Changes With Exercise
Although the AUC remained numerically higher in female than male patients there was a modest difference in predictive utility by sex. We also found that the stages of the algorithm provided good discrimination even when used in a population with more ambiguity, as the 4‐minute recovery QTc had the highest predictive value for both male and female patients and the 1‐minute recovery QTc had the highest predictive value for LQTS subtype in female patients but not male patients. As expected, optimal cutoff values were higher in female than in male patients across all stages.
Sex‐Specific Considerations in LQTS Subtypes
Although the 1‐minute recovery QTc had the best predictive value for subtype differentiation in our female patients with LQTS, there was more uncertainty in male patients where peak exercise and 1‐minute recovery QTc values yielded similar AUC values in predicting subtype. Ultimately, we selected the 1‐minute recovery QTc for male patients for consistency, ease of measurement, and the negligible differences when compared with the peak exercise QTc. In female patients the 1‐minute recovery QTc had a clear best predictive value of subtype. For patients with LQTS1, there was an evident increase in QTc from rest to peak exercise, and in patients with LQTS2, the QTc normalized at peak exercise. The differential QT response by genotype after exercise was described by Chattha et al in 2010, 28 highlighting the 1‐minute recovery period where patients with LQTS1 had a significantly longer 1‐minute recovery QTc compared with their counterparts with LQTS2.
Horner et al reported findings consistent with Wong et al and Sy et al, while noting several discrepancies; they reported that patients with LQTS1 had an unchanged or marginally increased QTc from rest to peak exercise, whereas patients with LQTS2 had a decreased QTc from rest to peak exercise. 15 They also found no major differences in QTc in the recovery when adjusted for sex and age of presentation. Notably, their cohort with LQTS had a mean age of 25±14 years, which is substantially younger than our cohort, as well as the previous cohorts by Sy et al. As well, the shortening of the QT in male patients and lengthening of the QT in female patients only occurs after puberty, 12 and the inclusion of more pediatric and prepubertal patients in the cohort of Horner et al may have lessened the effects of sex hormones and contributed to the discrepancy in findings between cohorts.
Variant Sites
Our present study did not find differences in QTc between KCNQ1 p.A341‐neighboring and other variants at any treadmill testing stage. Recently, in a multicenter cohort of >1300 patients with LQTS, Schwartz et al 24 identified missense p.A341V and the neighboring mutations to be of greatest risk. We suspect that differences may not have been evident given the limited number of participants with these high‐risk variants available for analysis. However, the number was proportional to the cohort of Schwartz et al, where 7% of patients had a missense p.A346‐neighboring variant. Sex differences in variant sites have been previously highlighted 29 ; however, given the limited number of participants with the variants of interest, a sex‐specific analysis was not performed.
Clinical Application of the Algorithm
The 3‐step screening algorithm is a feasible and simple method for diagnosing LQTS. There is further utility in identifying LQTS1 and LQTS2 where the patient preference is not to undergo genetic testing, genetic testing is not immediately accessible, where cost is a limitation, or while awaiting results. The algorithm would allow for the detection of LQTS2 as early as possible, allowing for the immediate administration of β blockers in the morning and in the evening. 30 There is also additional utility where the patient may have a normal QTc, and genetic testing identifies variants of unknown significance in KCNQ1 or KCNH2, but the 4‐minute recovery QTc would help to inform whether the variant of unknown significance is more likely pathogenic or benign. Ultimately, the algorithm was not designed to direct genetic testing, but rather inform clinical evidence of LQTS, and aid in the uncommon environment where genetic testing is unavailable or difficult to access. Genetic testing remains a key tool in the diagnosis of LQTS, as even patients in whom the algorithm showed best performance, it was still far from perfect.
The clinical utility of the algorithm can be best illustrated with the following example. A 23‐year‐old woman on anti‐anxiety medication presents with a borderline QTc of 468 milliseconds, which remains unchanged off medication. She has no family history suggestive of LQTS. It is nearly certain that she is unaffected; however, proceeding to step 2 of the algorithm confirms that she is an unaffected noncarrier, given her 4‐minute recovery QTc is within the normal range at 438 milliseconds. On the other hand, an asymptomatic 21‐year‐old woman who is an FDR of an LQTS proband presented with a borderline QTc of 461 milliseconds. She also proceeded to step 2 of the algorithm, where her 4‐minute recovery QTc was prolonged at 478 milliseconds. This patient was asked to avoid QT‐prolonging medications and initiate β blockers, while pending genetic testing, which eventually revealed a pathogenic KCNQ1 variant (see Figure S2 for stress test strips).
Directions for Future Research
There remains a gap in knowledge surrounding the application of the algorithm in uncommon LQTS subtypes, races and ethnicities other than of European descent, and gene‐negative patients with LQTS with a diagnosis based on the Schwartz score. Future large‐scale assessment to capture more diverse genotypes and races and ethnicities would be helpful to further explore sex differences in stratification. The use of the treadmill testing and the algorithm should be examined in the pediatric population specifically.
Limitations
Carriers were restricted to KCNQ1 and KCNH2 variants, limiting the generalizability of the results of this study to other LQTS subtypes. Furthermore, capturing a more diverse ethnic population would strengthen the generalizability of the findings of this study, which has previously been highlighted. 31 , 32 Our control group included FDRs who were not evaluated in the context of familial LQTS and therefore did not have negative genetic tests for LQTS‐related variants. Therefore, there remains a small chance that they are affected; however, the exclusion of these FDRs would have hampered the number of controls eligible, and an analysis including only LQTS variant‐negative FDRs yielded comparable results (data not shown). The algorithm targets patients with LQTS at initial diagnosis, and β blockade would follow. Treatment was subsequently recommended on the basis of clinical presentation, severity of phenotype, and family context. Patients on β blockade at the time of their treadmill test were not excluded, but like the cohort of Sy et al, a subanalysis of β‐blocker naïve patients demonstrated similar performance (Table S13). The measurement of the QTc value at peak exercise remains a challenge, regardless of experience, as it is often difficult to identify the return to baseline of the T wave. Similarly, the cutoff values between male and female patients were within a few milliseconds, which has practical challenges given that accurate identification and calculation remains a challenge, even among cardiologists. 33
Conclusions
The current study demonstrates that sex‐specific cutoff values should be applied to accurately diagnose LQTS and related prediction of the 2 major genotypes in postpubertal patients. The screening algorithm is a valid and simple method for identifying LQTS in probands and FDR carriers in whom phenotypic ambiguity is common.
Sources of Funding
Dr Krahn receives support from the Sauder Family and Heart and Stroke Foundation Chair in Cardiology (Vancouver, BC, Canada), the Paul Brunes Chair in Heart Rhythm Disorders (Vancouver, BC, Canada), and the Paul Albrechtson Foundation (Winnipeg, MB, Canada). The study is supported by the Canadian Institutes of Health Research (HiRO [Hearts in Rhythm Organization] Registry, Dr Krahn, Principal Investigator, RN380020‐406814; and NLQTS [National Long‐QT Syndrome] Registry, Dr Krahn, Principal Investigator, MOP‐142218). Dr Cadrin‐Tourigny receives support from the Philippa and Marvin Carsley Chair. Dr Tadros receives support from the Canada Research Chairs program.
Disclosures
The authors had full access to the data and take full responsibility for their integrity. All authors have read and agreed to the article as written. The authors have no conflicts of interest to declare.
Supporting information
Table S1–S13
Figures S1–S2
Acknowledgments
We are deeply appreciative of the work of the HiRO (Hearts in Rhythm Organization) study coordinators across Canada and of our patients and their families who participate in research to advance our understanding and care of long‐QT syndrome and other inherited arrhythmia and cardiomyopathy conditions.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.025108
For Sources of Funding and Disclosures, see page 11.
References
- 1. Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantù F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene‐specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381 [DOI] [PubMed] [Google Scholar]
- 2. Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, et al. Age‐ and sex‐related differences in clinical manifestations in patients with congenital long‐QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237 [DOI] [PubMed] [Google Scholar]
- 3. Schwartz PJ, Crotti L, Insolia R. Long‐QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–877. doi: 10.1161/CIRCEP.111.962019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilde AAM, Amin AS, Postema PG. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart. 2022;108:332–338. doi: 10.1136/heartjnl-2020-318259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 6. Adler A, Novelli V, Amin AS, Abiusi E, Care M, Nannenberg EA, Feilotter H, Amenta S, Mazza D, Bikker H, et al. An international, multicentered, evidence‐based reappraisal of genes reported to cause congenital long QT syndrome. Circulation. 2020;141:418–428. doi: 10.1161/CIRCULATIONAHA.119.043132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, et al. Genotype‐phenotype correlation in the long‐QT syndrome: gene‐specific triggers for life‐threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89 [DOI] [PubMed] [Google Scholar]
- 8. Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long‐QT syndrome: clinical impact. Circulation. 1999;99:529–533. doi: 10.1161/01.cir.99.4.529 [DOI] [PubMed] [Google Scholar]
- 9. Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;43:657–662. doi: 10.1136/bjsm.2008.054734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long‐QT syndrome. Circ Arrhythm Electrophysiol. 2010;3:120–125. doi: 10.1161/CIRCEP.109.907865 [DOI] [PubMed] [Google Scholar]
- 11. Sy RW, van der Werf C, Chattha IS, Chockalingam P, Adler A, Healey JS, Perrin M, Gollob MH, Skanes AC, Yee R, et al. Derivation and validation of a simple exercise‐based algorithm for prediction of genetic testing in relatives of LQTS probands. Circulation. 2011;124:2187–2194. doi: 10.1161/CIRCULATIONAHA.111.028258 [DOI] [PubMed] [Google Scholar]
- 12. Asatryan B, Yee L, Ben‐Haim Y, Dobner S, Servatius H, Roten L, Tanner H, Crotti L, Skinner JR, Remme CA, et al. Sex‐related differences in cardiac channelopathies: implications for clinical practice. Circulation. 2021;143:739–752. doi: 10.1161/CIRCULATIONAHA.120.048250 [DOI] [PubMed] [Google Scholar]
- 13. Linde C, Bongiorni MG, Birgersdotter‐Green U, Curtis AB, Deisenhofer I, Furokawa T, Gillis AM, Haugaa KH, Lip GYH, van Gelder I, et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018;20:1565. doi: 10.1093/europace/euy067 [DOI] [PubMed] [Google Scholar]
- 14. Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, et al. Risk stratification in the long‐QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147 [DOI] [PubMed] [Google Scholar]
- 15. Horner JM, Horner MM, Ackerman MJ. The diagnostic utility of recovery phase QTc during treadmill exercise stress testing in the evaluation of long QT syndrome. Heart Rhythm. 2011;8:1698–1704. doi: 10.1016/j.hrthm.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 16. Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long‐QT syndrome. Circulation. 2011;124:2181–2184. doi: 10.1161/CIRCULATIONAHA.111.062182 [DOI] [PubMed] [Google Scholar]
- 17. Davies B, Roberts JD, Tadros R, Green MS, Healey JS, Simpson CS, Sanatani S, Steinberg C, MacIntyre C, Angaran P, et al. The hearts in rhythm organization: a Canadian National Cardiogenetics Network. CJC Open. 2020;2:652–662. doi: 10.1016/j.cjco.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies B, Bartels K, Hathaway J, Xu F, Roberts JD, Tadros R, Green MS, Healey JS, Simpson CS, Sanatani S, et al. Variant re‐interpretation in survivors of cardiac arrest with preserved ejection fraction (CASPER registry) by clinicians and clinical commercial laboratories. Circ Genom Precis Med. 2021;14:e003235. doi: 10.1161/CIRCGEN.120.003235 [DOI] [PubMed] [Google Scholar]
- 20. Stramba‐Badiale M, Karnad DR, Goulene KM, Panicker GK, Dagradi F, Spazzolini C, Kothari S, Lokhandwala YY, Schwartz PJ. For neonatal ECG screening there is no reason to relinquish old Bazett's correction. Eur Heart J. 2018;39:2888–2895. doi: 10.1093/eurheartj/ehy284 [DOI] [PubMed] [Google Scholar]
- 21. Phan DQ, Silka MJ, Lan YT, Chang RK. Comparison of formulas for calculation of the corrected QT interval in infants and young children. J Pediatr. 2015;166:e961–e962. doi: 10.1016/j.jpeds.2014.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McLaughlin NB, Campbell RW, Murray A. Accuracy of four automatic QT measurement techniques in cardiac patients and healthy subjects. Heart. 1996;76:422–426. doi: 10.1136/hrt.76.5.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vink AS, Neumann B, Lieve KVV, Sinner MF, Hofman N, el Kadi S, Schoenmaker MHA, Slaghekke HMJ, de Jong JSSG, Clur SAB, et al. Determination and interpretation of the QT interval. Circulation. 2018;138:2345–2358. doi: 10.1161/CIRCULATIONAHA.118.033943 [DOI] [PubMed] [Google Scholar]
- 24. Schwartz PJ, Moreno C, Kotta MC, Pedrazzini M, Crotti L, Dagradi F, Castelletti S, Haugaa KH, Denjoy I, Shkolnikova MA, et al. Mutation location and IKs regulation in the arrhythmic risk of long QT syndrome type 1: the importance of the KCNQ1 S6 region. Eur Heart J. 2021;42:4743–4755. doi: 10.1093/eurheartj/ehab582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moss AJ, Zareba W, Kaufman ES, Gartman E, Peterson DR, Benhorin J, Towbin JA, Keating MT, Priori SG, Schwartz PJ, et al. Increased risk of arrhythmic events in long‐QT syndrome with mutations in the pore region of the human ether‐a‐go‐go‐related gene potassium channel. Circulation. 2002;105:794–799. doi: 10.1161/hc0702.105124 [DOI] [PubMed] [Google Scholar]
- 26. Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, Zareba W, et al. Mutation and gender‐specific risk in type 2 long QT syndrome: implications for risk stratification for life‐threatening cardiac events in patients with long QT syndrome. Heart Rhythm. 2011;8:1537–1543. doi: 10.1016/j.hrthm.2011.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lahrouchi N, Tadros R, Crotti L, Mizusawa Y, Postema PG, Beekman L, Walsh R, Hasegawa K, Barc J, Ernsting M, et al. Transethnic genome‐wide association study provides insights in the genetic architecture and heritability of long QT syndrome. Circulation. 2020;142:324–338. doi: 10.1161/CIRCULATIONAHA.120.045956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chattha IS, Sy RW, Yee R, Gula LJ, Skanes AC, Klein GJ, Bennett MT, Krahn AD. Utility of the recovery electrocardiogram after exercise: a novel indicator for the diagnosis and genotyping of long QT syndrome? Heart Rhythm. 2010;7:906–911. doi: 10.1016/j.hrthm.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 29. Salama G, Bett GC. Sex differences in the mechanisms underlying long QT syndrome. Am J Physiol Heart Circ Physiol. 2014;307:H640–H648. doi: 10.1152/ajpheart.00864.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34:3109–3116. doi: 10.1093/eurheartj/eht089 [DOI] [PubMed] [Google Scholar]
- 31. Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78:1479–1487. doi: 10.4065/78.12.1479 [DOI] [PubMed] [Google Scholar]
- 32. Crotti L, Spazzolini C, Schwartz PJ, Shimizu W, Denjoy I, Schulze‐Bahr E, Zaklyazminskaya EV, Swan H, Ackerman MJ, Moss AJ, et al. The common long‐QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation‐specific risk stratification. Circulation. 2007;116:2366–2375. doi: 10.1161/CIRCULATIONAHA.107.726950 [DOI] [PubMed] [Google Scholar]
- 33. Viskin S, Rosovski U, Sands AJ, Chen E, Kistler PM, Kalman JM, Rodriguez Chavez L, Iturralde Torres P, Cruz F FES, Centurión OA, et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–574. doi: 10.1016/j.hrthm.2005.02.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S13
Figures S1–S2


