Abstract
Background
Heart failure with reduced ejection fraction (HFrEF) is a chronic disease with substantial mortality. Management of HFrEF has seen significant breakthrough after the launch of neprilysin inhibitor. The PARADIGM‐HF (Prospective Comparison of ARNI with ACEI to Determine Impacton Global Mortality and Morbidity in Heart Failure) trial showed that sacubitril/valsartan significantly reduces HFrEF mortality and the heart failure hospitalization rate. However, in patients with advanced kidney disease, who have the highest prevalence of heart failure, the efficacy and safety of sacubitril/valsartan remains uncertain. We aim to study the efficiency of sacubitril/valsartan in patients with end‐stage kidney disease.
Methods and Results
Heart function was screened by echocardiogram among all patients with end‐stage kidney disease in 2 hospitals. Patients with HFrEF received either sacubitril/valsartan or conventional treatment. Fifteen echocardiographic parameters were compared before and after treatment. After 1‐year sacubitril/valsartan treatment, parameters of systolic (left ventricular ejection fraction 31.3% to 45.1%, P<0.0001; left ventricular end‐systolic volume 95.7 to 70.1 mL, P=0.006; left ventricular internal diameter at end‐systole phase 47.2 to 40.1 mm, P=0.005), and diastolic (E/A ratio 1.3 to 0.8, P=0.009; E/Med e' ratio 25.3 to 18.8, P=0.010) function improved in patients with HFrEF and end‐stage kidney disease. These parameters were unchanged in the conventional treatment group. Serum potassium did not increase in the sacubitril/valsartan group.
Conclusions
Sacubitril/valsartan improves left ventricular systolic and diastolic function in patients with HFrEF and end‐stage kidney disease.
Keywords: ARNI, end‐stage kidney disease (ESKD), heart failure with reduced ejection fraction (HFrEF), sacubitril/valsartan
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- ARNI
angiotensin receptor II blocker‐neprilysin inhibitor
- HFrEF
heart failure with reduced ejection fraction
Clinical Perspective.
What Is New?
Clinical trials showed sacubitril/valsartan has benefit effect in heart failure with reduced ejection fraction, but these trials excluded patients with an estimated glomerular filtration rate of <30 mL/min. The treatment of heart failure in patients with end‐stage kidney disease therefore is largely unknown.
The authors used 15 echocardiogram parameters to investigate sacubitril/valsartan therapeutic effect in patients with end‐stage kidney disease and heart failure with reduced ejection fraction; they found that 1‐year of treatment with sacubitril/valsartan significantly improves systolic and diastolic heart function in these patients.
Sacubitril/valsartan does not increase hyperkalemia or hypotension risk in patients with end‐stage kidney disease.
What Are the Clinical Implications?
Sacubitril/valsartan improves left ventricular systolic and diastolic function in patients with end‐stage kidney disease.
This result fulfills the unmet need of heart failure treatment in advanced chronic kidney disease and opens a window of opportunity for patients with end‐stage kidney disease who have heart failure with reduced ejection fraction.
Congestive heart failure affects 40 million people worldwide, 1 accounting for 2% of the global adult population. 2 The diagnosis is usually based on clinical symptoms, including shortness of breath, leg swelling, excessive tiredness, and pulmonary congestion. 2 , 3 , 4 Reduced cardiac output is generally accompanied by structural and functional abnormality of the heart, which disrupts its ejection of blood in systolic phase or refilling during the diastolic phase. 5 , 6 , 7 Based on whether the ability of the left ventricle to contract or to relax is affected, heart failure is divided into 2 types: heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction. 8 Patients with HFrEF, defined as ejection fraction <40%, 9 carry a very high morbidity and mortality rate. 10 , 11 , 12 Large‐scale epidemiology studies showed the survival rates of patients with HFrEF are even worse than those of common cancers. 13 , 14 , 15
Patients with heart failure are traditionally treated with oxygen supply, salt/water restriction, hypertension control, and medications including nitrates, diuretics, β‐blockers, mineralocorticoid receptor antagonists, angiotensin‐converting enzyme inhibitors (ACEi), and angiotensin II receptor blocker (ARB). 5 , 6 , 7 , 16 , 17 Novel therapies have emerged from an improved understanding of the pathophysiology of heart failure and cardiac remodeling. Among these new drugs, angiotensin receptor/neprilysin inhibitor 18 , 19 and sodium‐glucose cotransporter inhibitors 20 , 21 are now considered the “game changers.” The pathophysiology of heart failure involves a maladaptive response during which the renin‐angiotensin‐aldosterone system is activated. The natriuretic peptide system is simultaneously activated to work antagonistically to the renin‐angiotensin‐aldosterone system. Neprilysin inhibitor prevents the breakdown of natriuretic peptides and therefore prolongs the favorable effects of these natriuretic peptides. 22 , 23 , 24 , 25 , 26
Patients with chronic kidney disease (CKD) have a very high prevalence of heart failure. 27 , 28 These 2 diseases share common risk factors such as hypertension, diabetes, smoking, and older age. 29 , 30 , 31 Patients with advanced kidney disease also have higher left ventricular (LV) preload caused by fluid retention, arterial stiffness because of vessel calcification, and high output shunting through dialysis vascular access. Load‐independent factors including electrolyte imbalance, neurohormonal activation, anemia, ischemia, and FGF‐23 activation 32 , 33 , 34 , 35 , 36 also accelerate the progression of heart failure. 31 Although the angiotensin receptor II blocker‐neprilysin inhibitor (ARNI) combination has a fundamental role in HFrEF 18 treatment, these trials excluded patients with advanced CKD. Therefore, the efficacy and safety of sacubitril/valsartan in patients with advanced CKD remain unknown. 25 In the current study, we investigated the effects of sacubitril/valsartan on LV function in patients with concomitant HFrEF and ESKD.
METHODS
The data that support the findings of this study are available from the corresponding author upon request.
Study Design
This is a case–control study. In 2019, heart failure was screened among regular stable dialysis patients in Taipei Veterans General Hospital and Cheng Hsin General Hospital dialysis centers by echocardiogram. In those patients with HFrEF, some had taken sacubitril/valsartan thereafter, where others received conventional treatment. The decision of whether to take sacubitril/valsartan was decided after discussion between patient and their nephrologist and/or cardiologist. The starting dose of sacubitril/valsartan is 24 mg/26 mg twice daily and up‐titration by doubling of dose every 2 to 4 weeks until a target dose of 97 mg/103 mg twice daily is reached. All patients with HFrEF were requested to receive follow‐up echocardiogram 12 months later. For those who took sacubitril/valsartan, an echocardiogram was performed 12 months after the maximal tolerated dosage.
The inclusion criteria were age >18 years, ESKD on regular renal replacement therapy for at least 6 months, and left ventricular ejection fraction (LVEF) ≤40% at baseline echocardiography. Exclusion criteria were recent acute coronary syndrome and inadequate dialysis. Because coronary angioplasty and/or bypass surgery increase cardiomyocyte perfusion and function, patients who received percutaneous transluminal coronary angioplasty or coronary artery bypass graft in the follow‐up period were also excluded from analysis. The institutional review board reviewed and approved the study design (IRB No 2021–12‐007C). All enrolled patients provided written informed consent.
Data Collection
Demographic data, medical history, and medications were obtained from medical records. Cardiac structure and function were assessed by 2‐dimensional echocardiography (Philips EPIQ CVx) the next day after hemodialysis treatment in order to avoid potential biases caused by fluid overload or postdialysis myocardial stunning. Fifteen echo cardiac parameters were recorded, including aortic root diameter, left atrial dimension, left atrial volume index, left ventricular internal diameter at end‐diastole phase, left ventricular internal diameter at end‐systole phase, interventricular septum thickness in diastole phase, left ventricular posterior wall thickness in diastole phase, biplane Simpson determined left ventricular end‐diastolic volume (LVEDV), left ventricular end‐systolic volume (LVESV), and Doppler‐derived E/A ratio across the mitral valve, tissue Doppler‐derived lateral E/e' ratio, medial E/e' ratio, and peak tricuspid regurgitation velocity (peak TR). All measurements were performed according to the updated American Society of Echocardiography and the European Association of Cardiovascular Imaging criteria. LV mass index was calculated by LV mass divided by body surface area, where LV mass (g)=0.8[1.04[([left ventricular end‐diastolic diameter (LVEDD)+interventricular septum thickness in diastole phase+posterior wall thickness in diastole]^3 – LVEDD^3)]]+0.6, where RWT=2* posterior wall thickness in diastole/LVEDD. 37 The echocardiogram results were cross‐checked by 2 independent cardiologists who were blinded to the patient's clinical condition and grouping. Blood pressures were recorded 3 times a week in patients undergoing hemodialysis and monthly in patients undergoing peritoneal dialysis. Serum potassium and other blood biochemistry data were checked in all individuals monthly.
Statistical Analysis
Statistical analyses were performed using SPSS version 25.0 and GraphPad Prism. Descriptive results of continuous variables are presented as means±SD,and categorical variables are reported as percentages and numbers. For quantitative data, independent t test was used to compare characteristics of the 2 groups and baseline and after 1‐year treatment. Paired t test was used to compare self‐matching data before and after treatment in each group. Categorical variables were analyzed by χ2 and Fisher exact test. All tests were 2‐tailed, and a P<0.05 was considered statistically significant. The Holm‐Bonferroni method was used to adjust probability (P) values because of the increased risk of a type I error when making multiple tests.
RESULTS
Baseline Characteristics and Heart Function in Study Subjects
There were 805 regular patients with ESKD who received the echocardiogram screening, and 61 (7.5%) had an LVEF <40% (Figure 1). Among the 61 enrolled patients with HFrEF, 9 did not have the 1‐year follow‐up echocardiograph because of transplant/emigration, and another 3 patients who received coronary angioplasty or coronary artery bypass graft surgery were also excluded from analysis. Finally, 49 patients with HFrEF with a mean dialysis vintage of 5 years were analyzed. Thirty‐one patients were on hemodialysis and the other 18 patients were on peritoneal dialysis. Among these patients, 26 patients received sacubitril/valsartan, starting from 50 mg (sacubitril 24 mg+valsartan 26 mg) twice daily and titrating every 2 to 4 weeks to 200 mg (sacubitril 97 mg+valsartan 103 mg) twice daily. The other 23 patients received conventional treatment and maintained their regular medications. All patients in the sacubitril/valsartan group maintained ARNI treatment throughout the study period, but not all of them reached maximal dose, mainly because of hypotension. The final dose of sacubitril/valsartan in the study group is listed in Table 1. The 2 groups were comparable in demographics, cause of ESKD, and comorbid diseases. Sixty‐one percent of patients in the conventional treatment group received ACEi or ARB. The sacubitril/valsartan group had a lower β‐blocker utilization rate (61.54% versus 95.65%, P=0.055) but still lower blood pressure (136±19 mm Hg versus 147±13 mm Hg, P=0.054). Detailed demographics and laboratory data of the 2 groups are illustrated in Table 2. A baseline echocardiogram showed these patients had a mean LVEF of 33% and LVESV of 89 mL, which indicates they had moderate to severe systolic heart failure. The mean mitral E/A ratio was 1.35, average E/e' ratio was 20.9, and peak tricuspid regurgitation velocity was 286 cm/s, which indicates they had an increased LV filling pressure. The baseline cardiac parameters are listed in Table 3. Overall, the sacubitril/valsartan group had a lower LVEF (31±5% versus 36±4%, P=0.015) and a lower blood pressure, suggesting that nephrologists/cardiologists in our hospitals tend to prescribe sacubitril/valsartan for patients with more severe systolic dysfunction.
Figure 1. Selection of patients with HFrEF and ESKD.
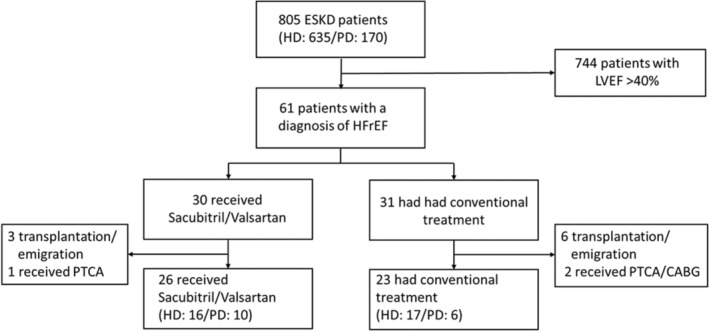
Participants had LVEF <40% and end‐stage kidney disease, and those who were enrolled either had received at least 6 months of hemodialysis or peritoneal dialysis. Patients who had PTCA or CABG in the study period and those without follow‐up echocardiogram were excluded from analysis. CABG indicates coronary artery bypass graft; ESKD, end stage kidney disease; HD, hemodialysis; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; PD, peritoneal dialysis; and PTCA, percutaneous transluminal coronary angioplasty.
Table 1.
Final Dose of Sacubitril/Valsartan in the Study Group
| Final dose | Number of patients (%) |
|---|---|
| 50 mg BID | 7 (26.9%) |
| 100 mg BID | 9 (34.6%) |
| 200 mg BID | 10 (38.5%) |
Table 2.
Demographic Data
| Data categories | Sacubitril/valsartan (n=26) | Conventional (n=23) | P value |
|---|---|---|---|
| Demographics | |||
| Age, y | 60.96±12.09 | 65.17±17.11 | 1.000 |
| Female sex | 7 (26.92%) | 10 (43.48%) | 0.247 |
| Height, cm | 166.30±9.21 | 163.30±8.25 | 1.000 |
| Body weight, kg | 65.23±12.41 | 63.05±19.60 | 1.000 |
| BMI, kg/m2 | 23.51±3.47 | 23.49±6.24 | 1.000 |
| Dialysis vintage, y | 4.92±4.85 | 5.13±3.00 | 1.000 |
| Hemodialysis | 16 (61.54%) | 17 (73.91%) | 0.382 |
| Causes of ESKD | |||
| Hypertension | 3 (11.54%) | 1 (4.35%) | 0.612 |
| Diabetic kidney disease | 13 (50%) | 13 (56.52%) | 0.776 |
| Chronic glomerulonephritis | 6 (23.07%) | 4 (17.39%) | 0.731 |
| SLE | 0 (0) | 2 (8.7%) | 0.215 |
| Polycystic kidney disease | 2 (7.69%) | 1 (4.35%) | 1.000 |
| Others | 2 (7.69%) | 2 (8.7%) | 1.000 |
| Medical history | |||
| Hypertension | 23 (88.46%) | 19 (82.61%) | 0.692 |
| Diabetes | 12 (46.15%) | 13 (56.52%) | 0.571 |
| Coronary artery disease | 13 (50%) | 14 (60.87%) | 0.567 |
| Biochemical data | |||
| Albumin, g/dL | 3.88±0.35 | 3.66±0.51 | 0.432 |
| Hemoglobin, g/dL | 10.29±1.74 | 9.84±2.23 | 1.000 |
| HbA1c, % | 6.08±1.52 | 6.33±2.11 | 1.000 |
| Intact PTH, pg/mL | 319.13±244.55 | 338.82±418.86 | 1.000 |
| Potassium, mmol/L | 4.14±0.65 | 4.59±0.78 | 0.238 |
| Calcium, mg/dL | 9.00±0.79 | 8.77±0.87 | 1.000 |
| Phosphate, mg/dL | 4.94±1.59 | 5.34±1.53 | 1.000 |
| Medication | |||
| ARNI | 26 (100%) | 0 (0%) | 0.000 |
| ACEi or ARB | 0 (0%) | 14 (60.87%) | 0.000 |
| β‐Blocker | 16 (61.54%) | 22 (95.65%) | 0.055 |
| Calcium channel blocker | 5 (19.23%) | 13 (56.52%) | 0.025 |
| MRA | 2 (7.69%) | 4 (17.39%) | 0.400 |
| Ivabradine | 6 (23.08%) | 1 (4.35%) | 0.103 |
| Hydralazine | 1 (3.8%) | 0 (0%) | 1.000 |
| Nitrates | 9 (34.6%) | 5 (21.7%) | 0.36 |
| Digoxin | 4 (15.38%) | 1 (4.35%) | 0.353 |
| Blood pressure | |||
| SBP, mm Hg | 136.04±18.94 | 146.87±13.43 | 0.054 |
| DBP, mm Hg | 72.54±13.80 | 76.17±13.20 | 0.352 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BMI, body mass index; DBP, diastolic blood pressure; ESKD, end‐stage kidney disease; HbA1c, hemoglobin A1c; MRA, mineralocorticoid receptor antagonist; PTH, parathyroid hormone; SBP, systolic blood pressure; and SLE, systemic lupus erythematosus.
Table 3.
Baseline Cardiac Parameters
| Parameters | Sacubitril/valsartan (n=26) | Conventional (n=23) | P value |
|---|---|---|---|
| Parameters of LV systolic function | |||
| LVEF, % | 31.31±5.53 | 35.73±4.21 | 0.015 |
| LVESV, mL | 95.75±33.30 | 80.11±23.04 | 0.356 |
| LVEDV, mL | 140.00±43.62 | 123.85±36.09 | 0.603 |
| LVIDs, mm | 47.19±6.88 | 44.93±7.24 | 0.603 |
| LVIDd, mm | 57.27±5.97 | 56.40±7.66 | 0.656 |
| Parameters of LV diastolic function | |||
| LAD, mm | 46.85±7.23 | 46.20±6.65 | 1 |
| LAVI, mL/m2 | 51.27±24.59 | 53.06±16.85 | 1 |
| MV E/A | 1.40±0.73 | 1.26±0.53 | 1 |
| E/Lat E' | 16.52±7.62 | 17.14±8.46 | 1 |
| E/Med E' | 26.07±15.59 | 23.55±9.15 | 1 |
| Peak TR Vel, cm/sec | 268.01±80.57 | 308.12±82.97 | 0.702 |
| Parameters of LV hypertrophy | |||
| IVSd, mm | 11.42±2.86 | 11.47±1.75 | 0.951 |
| LVPWd, mm | 10.73±1.97 | 11.52±1.54 | 0.384 |
| LV mass index, g/m2 | 157.28±43.65 | 167.88±48.86 | 0.852 |
| Other parameters | |||
| Aortic root, mm | 33.46±5.52 | 34.03±5.53 | 0.723 |
E/Lat E' indicates Doppler‐derived lateral E/e' ratio; E/Med E', Doppler‐derived medial E/e' ratio; IVSd, interventricular septum thickness in diastole; LAD, left atrial dimension; LAV, left atrial volume; LAVI, left atrial volume index; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular end‐systolic volume; LVESV, left ventricular end‐systolic volume; LVIDd, left ventricular internal diameter at end‐diastole; LVIDs, left ventricular internal diameter at end‐systole; LVPWd, left ventricular posterior wall thickness in diastole; MV E/A, tissue Doppler‐derived E/A ratio across the mitral valve; and Peak TR Vel, peak tricuspid regurgitation velocity.
Systolic Function Changes After 1‐Year Sacubitril/Valsartan Treatment
All patients received regular dialysis treatment and their dry weight remained consistent in the study period. We repeated echocardiographic examinations in all subjects 1 year later. The data showed LVEF was significantly improved (31.3±5.5 to 45.1±11.7%, P<0.0001, Figure 2A) in the sacubitril/valsartan group. LVESV (95.7±33.3 to 70.1±35.7 mL, P=0.006, Figure 2B) and left ventricular internal diameter at end‐systole phase (47.2±6.9 to 40.1±9.8 mm, P=0.005, Figure 2C) also decreased in the sacubitril/valsartan group. These changes were not observed in the conventional treatment group. There were no significant changes in left ventricular internal diameter at end‐diastole phase and LVEDV in both groups (Figure 2D, and 2E). These findings showed that sacubitril/valsartan improves LV systolic function, which appears to be the result of reduced afterload and thus improved stroke volume. Subgroup analysis showed that the LVEF improvement effect of sacubitril/valsartan existed in both hemodialysis and peritoneal dialysis (Figure 2F). In summary, these data suggest sacubitril/valsartan treatment improves LV systolic function in patients with ESKD.
Figure 2. Changes of echocardiographic parameters for systolic function in 2 groups.
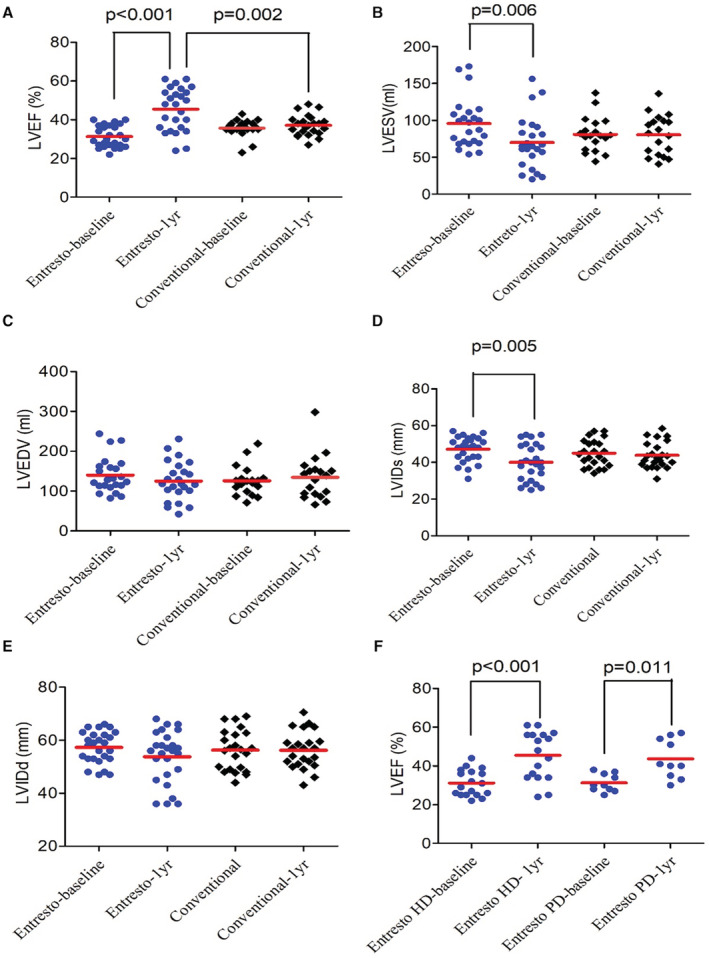
One‐year changes of (A) LVEF, (B) LEVSV, (C) LVEDV, (D) LVIDs, and (E) LVIDd in sacubitril/valsartan (Entresto) and conventional treatment groups. (F) LVEF changes of patients undergoing hemodialysis and peritoneal dialysis in the Entresto group. HD indicates hemodialysis; LVEF, left ventricular end‐systolic volume; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; LVIDs, left ventricular internal diameter at end‐systole; LVIDd, left ventricular internal diameter at end‐diastole; and PD, peritoneal dialysis.
Diastolic Function Changes After 1‐Year Sacubitril/Valsartan Treatment
Unlike LVEF for systolic function quantification, there is no single criterion standard to diagnose diastolic dysfunction by an echocardiogram. In the current study, we used 6 parameters to quantify the LV diastolic function. In the sacubitril/valsartan group, the Doppler‐derived E/A ratio across the mitral valve decreased from 1.3±0.7 to 0.8±0.3 (P=0.009, Figure 3A). The medial E/e' ratio decreased from 25.3±16.2 to 18.8±8.6 (P=0.010, Figure 3B). The tissue Doppler‐derived lateral E/e' ratio decreased from 15.9±7.5 to 12.8±6.4 (P=0.011, Figure 3C). The peak TR velocity decreased from 272.9±78.7 to 234.2±65.4 cm/s (P=0.047, Figure 3D). The left atrial dimension decreased from 46.9±7.2 to 43.7±8.1 mm (P=0.047, Figure 3E) and the left atrial volume index decreased from 53.7±24.1 to 44.4±22.7 mL/m2 (P=0.048, Figure 3F). These changes were not observed in the conventional treatment group. The E/A ratio represents the ratio of peak velocity blood flow from LV relaxation in early diastole (the E wave) to peak velocity flow in late diastole caused by atrial contraction (the A wave). In normal heart, E/A ratio is >1. In the early stage of diastolic dysfunction, the E wave velocity reduces and results in reversed E/A ratio, with progression of LV relaxation impairment and filling pressure elevation, which further results in higher E/A ratio (>1.2–2), again as a pseudo‐normal pattern. In restrictive filling heart with significantly elevated left atrial pressures, the E/A ratio elevates above 2. The reduction of tissue Doppler‐derived lateral E/e' ratio and medial E/e' ratio imply LV filling pressure reduction. The reduction of peak TR velocity suggests improvement in diastolic heart function and secondary pulmonary hypertension. 38 In summary, the data here indicate that the improved afterload by sacubitril/valsartan could also drive the improvement of diastolic function in patients with ESKD.
Figure 3. Changes of echocardiographic parameters for diastolic function in 2 groups.
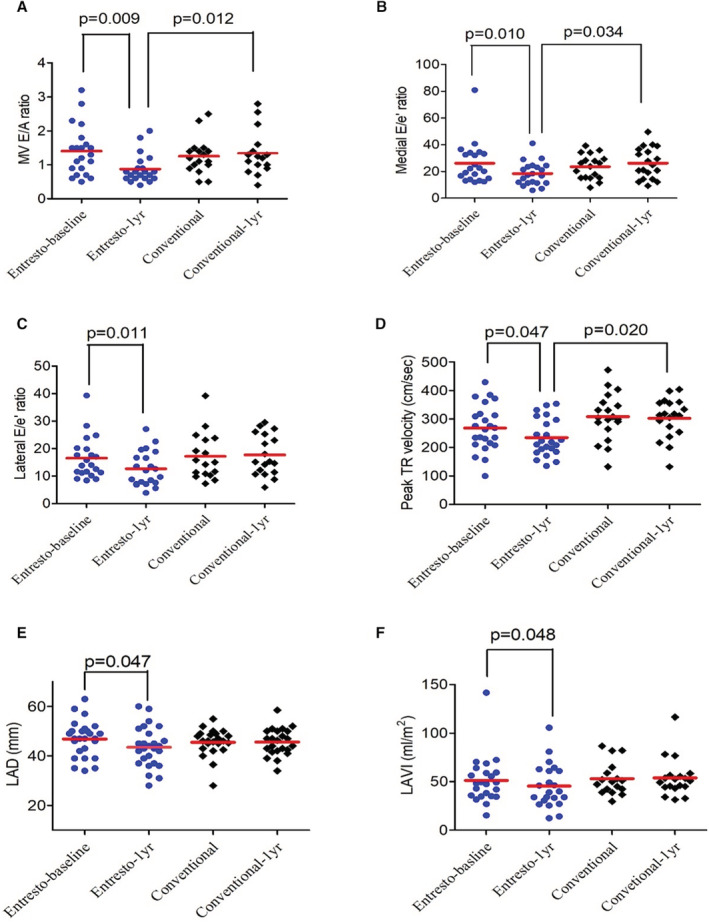
One‐year changes of (A) sacubitril/valsartan mitral valve (MV) E/A ratio, (B) medial E/e' ratio, (C) lateral E/e' ratio, (D) peak TR velocity, (E) LAD, and (F) LAVI in sacubitril/valsartan (Entresto) and conventional treatment groups. E/A ratio indicates the ratio of peak velocity blood flow from left ventricular relaxation in early diastole (the E wave) to peak velocity flow in late diastole caused by atrial contraction (the A wave); E/e' ratio, the ratio between early mitral inflow velocity and mitral annular early diastolic velocity; LAD, left atrial dimension; LAVI, left atrial volume index; and peak TR Vel, peak tricuspid regurgitation velocity.
Cardiac Hypertrophy After 1‐Year Sacubitril/Valsartan Treatment
We used 4 echocardiographic parameters to evaluate the changes of cardiac hypertrophy. In the sacubitril/valsartan group, there was no significant interval change in LV mass index (157.28±43.65 to 144.41±46.86 mg/m2, P=0.531, Figure 4A), interventricular septum thickness in diastole phase (11.5±2.9 to 11.4±2.6 mm, P=0.898, Figure 4B), and left ventricular posterior wall thickness in diastole phase (10.9±2.0 to 11.2±2.7 mm, P=0.898, Figure 4C). These parameters did not change significantly in the conventional group either.
Figure 4. Changes of echocardiographic parameters for cardiac hypertrophy in 2 groups.
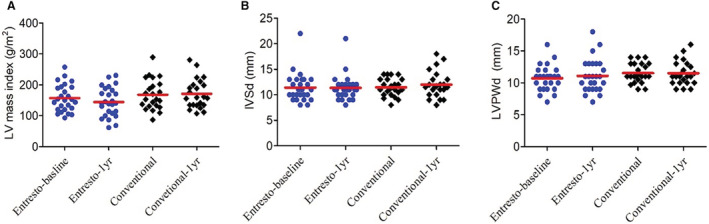
One‐year changes of (A) LV mass, (B) IVSd, and (C) LVPWd in sacubitril/valsartan (Entresto) and conventional treatment groups. IVSd indicates interventricular septum thickness in diastole; LV, left ventricular; and LVPWd, left ventricular posterior wall thickness in diastole.
Safety of Sacubitril/Valsartan
The adverse events of both groups are summarized in Table 4. There was no difference between the sacubitril/valsartan group and the conventional group in new‐onset intradialytic hypotension, hyperkalemia, abnormal liver biochemistry, and hospitalization rate of cardiovascular diseases or other causes. No patient in the sacubitril/valsartan group reported angioedema or dry cough. Sacubitril/valsartan did not change systolic (136.0±19.0 to 132.0±20.9 mm Hg, P=0.337) or diastolic blood pressure (72.5±13.8 to 70.8±14.4 mm Hg, P=0.337). The predialysis serum potassium level did not increase after sacubitril/valsartan treatment (4.6±0.8 to 4.5±0.7 mEq/dL, P=0.765).
Table 4.
Adverse Events of the 2 Groups
| Adverse events | Sacubitril/valsartan (n=26) | Conventional (n=23) | P value |
|---|---|---|---|
| New‐onset intradialytic hypotension* | 2/26 (7.7%) | 1/23 (4.3%) | 1.000 |
| Hyperkalemia (>5.5 mEq/L) | 3/26 (11.5%) | 4/23 (17.4%) | 0.692 |
| Cough | 0/26 (0%) | 0/23 (0%) | 1.000 |
| Angio‐edema | 0/26 (0%) | 0/23 (0%) | 1.000 |
| Abnormal AST/ALT (>40 U/L) | 1/26 (3.8%) | 0/23 (0%) | 1.000 |
| Hospitalization of all causes | 13/26 (50%) | 13/23 (56.5%) | 0.776 |
| Hospitalization because of cardiovascular diseases | 6/26 (23.1%) | 7/23 (30.4%) | 0.747 |
ALT indicates alanine aminotransferase; and AST, aspartate aminotransferase.
Intradialytic hypotension defined as decrease in systolic blood pressure ≥20 mm Hg or mean blood pressure ≥10 mm Hg during dialysis treatment with associated symptoms (cramping, headache, lightheadedness, vomiting, or chest pain) or need for intervention (reduction in ultrafiltration, administration of fluids or blood pump flow reduction).
DISCUSSION
Sacubitril/valsartan is the first agent to be approved in the angiotensin receptor neprilysin inhibitor (ARNI) class. The medication is US Food and Drug Administration–approved to treat patients with chronic heart failure with HFrEF because it reduces the mortality and heart failure hospitalization rate. Nevertheless, patients with advanced kidney disease were excluded from these trials and its efficacy in patients with advanced kidney disease is therefore uncertain. The current study is the first case–control study that showed sacubitril/valsartan could effectively improve LV systolic and diastolic function in patients with HFrEF and ESKD. Our data also suggest that the concerns about hyperkalemia and hypotension should not be a barrier to patients with CKD to receive ARNI treatment.
Over the past 10 years, many new treatment strategies against heart failure have been developing. PARADIGM‐HF (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) 18 and PIONEER‐HF (Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT‐proBNP in Patients Stabilized From an Acute Heart Failure Episode) 39 are landmark trials illustrating that sacubitril/valsartan can reduce mortality and hospitalization rates in patients with HFrEF. The VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial shows vericiguat, an oral guanylate cyclase stimulator, can reduce by 10% the cardiovascular mortality rate in patients with HFrEF. 39 The EMPA‐REG (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients), 40 CANVAS (CANagliflozin cardioVascular Assessment Study), 41 and DECLARE‐TIMI (Dapagliflozin on the Incidence of Cardiovascular Events) 58 42 trials point out the beneficial effect of sodium‐glucose cotransporter 2 inhibitors on cardiovascular mortality and heart failure hospitalization rate. However, because of pharmacokinetic concern and possible side effects, patients with advanced kidney disease (estimated glomerular filtration rate <30 mL/min) were all excluded from these trials. The treatment strategies of HFrEF in patients with advanced kidney disease are based on subgroup analysis or observation studies. 31
Echocardiography has an essential role in investigation of LV structure and function. Hinderliter et al studied 211 patients with HFrEF and found LVEF and left atrial volume index are strong predictors of all‐cause mortality, even after adjusting for NT‐pro‐BNP (N‐terminal proB‐type natriuretic peptide) level and other clinical variables. 43 The diagnostic accuracy of an echocardiogram to detect heart failure is believed to be higher than NT‐pro‐BNP. 44 Kidney Disease Outcomes Quality Initiative guidelines recommend echocardiograms to be performed in all patients at the initiation of dialysis and at 3‐yearly intervals thereafter, 45 and ≈87% of patients with ESKD have major abnormalities on echocardiography. 46 Compared with sodium‐glucose cotransporter 2 inhibitors, sacubitril/valsartan has shown not only clinical benefit but also evidence of reverse cardiac remodeling in HFrEF. In patients with preserved kidney function, sacubitril/valsartan treatment improves LVEF, and decreases LVEDV, LVESV, left atrial volume index, E/e' and E/A ratio 47 , 48 In our study, parameters of both systolic and diastolic function improved after sacubitril/valsartan treatment; nevertheless, parameters of internal diameter/volume at end‐diastole phase (left ventricular internal diameter at end‐diastole phase/LVEDV) and cardiac hypertrophy were not reduced. NT‐pro‐BNP is another useful biomarker of HF in the general population. The PARAMOUNT (Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion) phase II trial used NT‐pro‐BNP as the primary outcome and showed that a 12‐week sacubitril/valsartan treatment reduced NT‐proBNP by 23%. 49 Phase III of the PARAMOUNT trial illustrated that this reduction of NT‐pro‐BNP can be translated to clinical benefit. 19 Although NT‐pro‐BNP is still used as a surrogate marker of HF in early‐stage kidney disease, 50 , 51 patients with advanced kidney disease usually have a very high NT‐pro‐BNP level, and there is therefore no evidence of NT‐pro‐BNP in the diagnosis of HF, either to rule it in or rule it out. In patients with mild‐to‐moderate CKD (eGFR 30 to 60 mL/min), subgroup analysis of PARADIGM‐HF showed that sacubitril/valsartan reduced cardiovascular death and HF hospitalization rate by 21%, 52 which is a noninferior absolute risk reduction compared with those without CKD. 18 , 52 Our data further extend the treatment to patients with ESKD. The fact that 1‐year treatment with sacubitril/valsartan significantly improved LV systolic (LVEF 31% to 45%) and diastolic function suggests that sacubitril/valsartan is a powerful treatment against HFrEF regardless of kidney function.
In the current study, the fact that sacubitril/valsartan decreased LVESV but had no effect on LVEDV suggests that the main contributor of LV function improvement in the ESKD population is afterload reduction; nevertheless, we did not observe more intradialytic hypotension events in the sacubitril/valsartan group. Although blood pressure is usually used as a surrogate of afterload, it is not synonymous with afterload. Natriuretic peptides can regulate LV pre‐ and afterload via not only acting on body fluid homeostasis but also vascular tone modulation, thereby resulting in decreased systemic vascular resistance favoring cardiac reverse remodeling. 53 Sacubitril/valsartan not only inhibits angiotensin II but also simultaneously augments the natriuretic peptide system, which may favor afterload reduction resulting in attenuated cardiac remodeling as observed in our present study. In fact, a recent study that used strain echocardiography demonstrated that 6‐month sacubitril/valsartan treatment can improve LV global longitudinal strain, twist, and apical and basal rotations; in other words, it can relieve myocardial wall tension. 54
Hyperkalemia is always a concern regarding renin‐angiotensin‐aldosterone system inhibition, especially in patients with kidney disease. However, in patients already on renin‐angiotensin‐aldosterone system inhibitor, add‐on neprilysin seems not to increase hyperkalemia risk. A meta‐analysis in patients with HFrEF showed that the hyperkalemia rate was lower in patients receiving ARNI treatment relative to those in patients taking ACEi's. 55 A randomized clinical trial of sacubitril/valsartan, including patients with eGFR as low as 20 mL/min, demonstrated that its safety and efficacy are similar to that of irbesartan. 56 In the current study, the hyperkalemia rate was not increased after initiating sacubitril/valsartan treatment. Based on this evidence, we believe sacubitril/valsartan is well tolerated in patients with CKD.
There are several limitations in our study. First, it is not a randomized controlled trial. The sacubitril/valsartan group in our study had worse heart function at baseline; nevertheless, we compared 15 echocardiographic parameters before and after sacubitril/valsartan treatment, and our data showed that sacubitril/valsartan improves systolic and diastolic function in patients with HFrEF and ESKD. Second, the sample size is small. We only followed up 49 patients with HFrEF‐ESKD and analyzed their longitudinal heart function changes. Although the beneficial effect of sacubitril/valsartan is highly statistically significant in our cohort, trials with larger sample size are warranted. Third, the ACEi/ARB prescription rate in the conventional treatment group is only 61%. Nevertheless, although ACEi/ARB are standard treatment for patients with HF with normal kidney function, large trials showed that ACEi/ARB failed to reduce cardiovascular events in patients undergoing dialysis, 57 , 58 which reflects the fact that high‐quality data are lacking for ACEi/ARB in patients with ESKD. 59 Finally, the patients were followed up for only 1 year. Therefore, we do not have long‐term clinical outcome data. However, despite these limitations, our study shows that sacubitril/valsartan can effectively improve heart function in patients with HFrEF and ESKD. Randomized control trials with larger sample size are required to validate their clinical effect.
CONCLUSIONS
The current case–control study shows the effectiveness and safety of sacubitril/valsartan in patients with ESKD. Sacubitril/valsartan could improve systolic and diastolic function in patients with HFrEF and ESKD. Larger‐scale prospective studies are warranted to survey whether this cardiac function improvement translates to clinical outcomes.
Sources of Funding
This work was supported by the Ministry of Science and Technology R.O.C MOST 110‐2314‐B‐075‐028‐MY3.
Disclosures
None.
Acknowledgments
We sincerely thank all the HD and PD nurses in the Taipei Veterans General Hospital for their hard work over the past 20 years and their contributions to this project.
C.H.H. and S.Z.L. designed the study and applied for the initial IRB approval. S.F.Y., S.M.O., and C.C.L. participated in patient enrollment and data interpretation. C.H.W., P.H.H., and C.L.H. analyzed echocardiographic data. C.H.H. designed the figures and wrote the initial draft of the manuscript. C.L.H. and S.Y. L. reviewed and edited the manuscript. All authors read and approved the final manuscript.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–1995. doi: 10.1016/S0140-6736(17)31071-1 [DOI] [PubMed] [Google Scholar]
- 3. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324:488–504. doi: 10.1001/jama.2020.10262 [DOI] [PubMed] [Google Scholar]
- 4. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, Mebazaa A. Acute heart failure. Nat Rev Dis Primers. 2020;6:16. doi: 10.1038/s41572-020-0151-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 8. National Clinical Guideline C. National institute for health and clinical excellence: guidance . Chronic heart failure: National clinical guideline for diagnosis and management in primary and secondary care: partial update. London: Royal College of Physicians (UK)Copyright © 2010, National Clinical Guideline Centre; 2010. [Google Scholar]
- 9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 10. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, et al. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC heart failure long‐term registry. Eur J Heart Fail. 2017;19:1574–1585. doi: 10.1002/ejhf.813 [DOI] [PubMed] [Google Scholar]
- 11. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in olmsted county, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, Kadam UT, Kwok CS, Clark AB, Murchie P, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care‐based cohort study with 10‐year follow‐up in Scotland. Eur J Heart Fail. 2017;19:1095–1104. doi: 10.1002/ejhf.822 [DOI] [PubMed] [Google Scholar]
- 14. Askoxylakis V, Thieke C, Pleger ST, Most P, Tanner J, Lindel K, Katus HA, Debus J, Bischof M. Long‐term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer. 2010;10:105. doi: 10.1186/1471-2407-10-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farmakis D, Stafylas P, Giamouzis G, Maniadakis N, Parissis J. The medical and socioeconomic burden of heart failure: a comparative delineation with cancer. Int J Cardiol. 2016;203:279–281. doi: 10.1016/j.ijcard.2015.10.172 [DOI] [PubMed] [Google Scholar]
- 16. Haselhuhn LR, Brotman DJ, Wittstein IS. Heart failure guidelines: what you need to know about the 2017 focused update. Cleve Clin J Med. 2019;86:123–139. doi: 10.3949/ccjm.86a.18022 [DOI] [PubMed] [Google Scholar]
- 17. van der Meer P, Gaggin HK, Dec GW. ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J Am Coll Cardiol. 2019;73:2756–2768. doi: 10.1016/j.jacc.2019.03.478 [DOI] [PubMed] [Google Scholar]
- 18. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 19. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 20. Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA‐REG OUTCOME trial. Circulation. 2019;139:1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santos‐Gallego CG, Vargas‐Delgado AP, Requena‐Ibanez JA, Garcia‐Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel‐Perez F, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255. doi: 10.1016/j.jacc.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 22. Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016;102:1342–1347. doi: 10.1136/heartjnl-2014-306775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sible AM, Nawarskas JJ, Alajajian D, Anderson JR. Sacubitril/valsartan: a novel cardiovascular combination agent. Cardiol Rev. 2016;24:41–47. doi: 10.1097/CRD.0000000000000093 [DOI] [PubMed] [Google Scholar]
- 24. Sauer AJ, Cole R, Jensen BC, Pal J, Sharma N, Yehya A, Vader J. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev. 2019;24:167–176. doi: 10.1007/s10741-018-9757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haynes R, Zhu D, Judge PK, Herrington WG, Kalra PA, Baigent C. Chronic kidney disease, heart failure and neprilysin inhibition. Nephrol Dial Transplant. 2020;35:558–564. doi: 10.1093/ndt/gfz058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV. Sacubitril/valsartan: neprilysin inhibition 5 years after paradigm‐hf. JACC Heart Fail. 2020;8:800–810. doi: 10.1016/j.jchf.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA. The role of the kidney in heart failure. Eur Heart J. 2012;33:2135–2142. doi: 10.1093/eurheartj/ehs205 [DOI] [PubMed] [Google Scholar]
- 28. Lofman I, Szummer K, Hagerman I, Dahlstrom U, Lund LH, Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016;3:e000324. doi: 10.1136/openhrt-2015-000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884–1891. doi: 10.1001/archinte.166.17.1884 [DOI] [PubMed] [Google Scholar]
- 30. He J, Shlipak M, Anderson A, Roy JA, Feldman HI, Kallem RR, Kanthety R, Kusek JW, Ojo A, Rahman M, et al. Risk factors for heart failure in patients with chronic kidney disease: the CRIC (chronic renal insufficiency cohort) study. J Am Heart Assoc. 2017;6. doi: 10.1161/JAHA.116.005336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, Kasiske BL, Deswal A, deFilippi CR, Cleland JGF, et al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:1304–1317. doi: 10.1016/j.kint.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 32. Buglioni A, Burnett JC Jr. Pathophysiology and the cardiorenal connection in heart failure. Circulating hormones: biomarkers or mediators. Clin Chim Acta. 2015;443:3–8. doi: 10.1016/j.cca.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nasrallah R, Hassouneh R, Hébert RL. PGE2, kidney disease, and cardiovascular risk: beyond hypertension and diabetes. J Am Soc Nephrol. 2016;27:666–676. doi: 10.1681/ASN.2015050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tan K, Sethi SK. Biomarkers in cardiorenal syndromes. Transl Res. 2014;164:122–134. doi: 10.1016/j.trsl.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 35. Agharazii M, St‐Louis R, Gautier‐Bastien A, Ung RV, Mokas S, Larivière R, Richard DE. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease‐related vascular calcification. Am J Hypertens. 2015;28:746–755. doi: 10.1093/ajh/hpu225 [DOI] [PubMed] [Google Scholar]
- 36. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–944. doi: 10.1161/CIRCULATIONAHA.117.028814 [DOI] [PubMed] [Google Scholar]
- 37. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 38. Augustine DX, Coates‐Bradshaw LD, Willis J, Harkness A, Ring L, Grapsa J, Coghlan G, Kaye N, Oxborough D, Robinson S, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the british society of echocardiography. Echo Res Pract. 2018;5:G11–g24. doi: 10.1530/ERP-17-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 40. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 41. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 42. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 43. Hinderliter AL, Blumenthal JA, O'Conner C, Adams KF, Dupree CS, Waugh RA, Bensimhon D, Christenson RH, Sherwood A. Independent prognostic value of echocardiography and n‐terminal pro‐b‐type natriuretic peptide in patients with heart failure. Am Heart J. 2008;156:1191–1195. doi: 10.1016/j.ahj.2008.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knebel F, Eddicks S, Schimke I, Bierbaum M, Schattke S, Beling M, Raab V, Baumann G, Borges AC. Myocardial tissue doppler echocardiography and N‐terminal B‐type natriuretic peptide (NT‐proBNP) in diastolic and systolic heart failure. Cardiovasc Ultrasound. 2008;6:45. doi: 10.1186/1476-7120-6-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Workgroup KD. K/doqi clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–S153. [PubMed] [Google Scholar]
- 46. McCullough PA, Roberts WC. Influence of chronic renal failure on cardiac structure. J Am Coll Cardiol. 2016;67:1183–1185. doi: 10.1016/j.jacc.2015.11.065 [DOI] [PubMed] [Google Scholar]
- 47. Januzzi JL, Jr., Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, et al. Association of change in n‐terminal pro‐b‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–1095. doi: 10.1001/jama.2019.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF, et al. Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1077–1084. doi: 10.1001/jama.2019.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher‐Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, et al. The angiotensin receptor neprilysin inhibitor lcz696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6 [DOI] [PubMed] [Google Scholar]
- 50. Wang AY. Clinical utility of natriuretic peptides in dialysis patients. Semin Dial. 2012;25:326–333. doi: 10.1111/j.1525-139X.2012.01079.x [DOI] [PubMed] [Google Scholar]
- 51. Group UH‐IC . Randomized multicentre pilot study of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease: United Kingdom heart and renal protection (HARP)‐ III‐rationale, trial design and baseline data. Nephrol Dial Transplant. 2017;32:2043–2051. doi: 10.1093/ndt/gfw321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, et al. Renal effects and associated outcomes during angiotensin‐neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 53. Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008;268:59–93. doi: 10.1016/S1937-6448(08)00803-4 [DOI] [PubMed] [Google Scholar]
- 54. Elshafey WEH, Al Khoufi EA, Elmelegy EK. Effects of sacubitril/valsartan treatment on left ventricular myocardial torsion mechanics in patients with heart failure reduced ejection fraction 2D speckle tracking echocardiography. J Cardiovasc Echogr. 2021;31:59–67. doi: 10.4103/jcecho.jcecho_118_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Solomon SD, Claggett B, McMurray JJ, Hernandez AF, Fonarow GC. Combined neprilysin and renin‐angiotensin system inhibition in heart failure with reduced ejection fraction: a meta‐analysis. Eur J Heart Fail. 2016;18:1238–1243. doi: 10.1002/ejhf.603 [DOI] [PubMed] [Google Scholar]
- 56. Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138:1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818 [DOI] [PubMed] [Google Scholar]
- 57. Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, Lechat P. Prevention of cardiovascular events in end‐stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. doi: 10.1038/sj.ki.5001657 [DOI] [PubMed] [Google Scholar]
- 58. Iseki K, Arima H, Kohagura K, Komiya I, Ueda S, Tokuyama K, Shiohira Y, Uehara H, Toma S. Olmesartan Clinical Trial in Okinawan Patients Under OG. Effects of angiotensin receptor blockade (arb) on mortality and cardiovascular outcomes in patients with long‐term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28:1579–1589. doi: 10.1093/ndt/gfs590 [DOI] [PubMed] [Google Scholar]
- 59. Liu Y, Ma X, Zheng J, Jia J, Yan T. Effects of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on cardiovascular events and residual renal function in dialysis patients: a meta‐analysis of randomised controlled trials. BMC Nephrol. 2017;18:206. doi: 10.1186/s12882-017-0605-7 [DOI] [PMC free article] [PubMed] [Google Scholar]


