Abstract
Background
The association between chronic obstructive pulmonary disease exacerbations and increased cardiovascular event risk has not been adequately studied in a heterogenous population with both low and high cardiovascular risk.
Methods and Results
This post hoc analysis of the IMPACT (Informing the Pathway of COPD Treatment) trial (N=10 355 symptomatic patients with chronic obstructive pulmonary disease at risk of exacerbations) evaluated time‐dependent risk of cardiovascular adverse events of special interest (CVAESI) following exacerbations and impact of exacerbation history, cardiovascular risk factors, and study treatment on this association. Risk (time‐to‐first) of CVAESI or CVAESI resulting in hospitalization or death was assessed during and 1 to 30, 31 to 90, and 91 to 365 days after resolution of moderate or severe exacerbations. CVAESI risk was compared between the period before and during/after exacerbation. CVAESI risk increased significantly during a moderate (hazard ratio [HR], 2.63 [95% CI, 2.08–3.32]) or severe (HR, 21.84 [95% CI, 17.71–26.93]) exacerbation and remained elevated for 30 days following an exacerbation (moderate: HR, 1.63 [95% CI, 1.28–2.08]; severe: HR, 1.75 [95% CI, 0.99–3.11; nonsignificant]) and decreased over time, returning to baseline by 90 days. Risk of CVAESI resulting in hospitalization or death also increased during an exacerbation (moderate: HR, 2.46 [95% CI, 1.53–3.97]; severe: HR, 41.29 [95% CI, 30.43–56.03]) and decreased in a similar time‐dependent pattern. Results were consistent regardless of exacerbation history, cardiovascular risk at screening, or study treatment.
Conclusions
Overall risk of cardiovascular events was higher during and in the 30 days following chronic obstructive pulmonary disease exacerbations, even among those with low cardiovascular risk, highlighting the need for exacerbation prevention and vigilance for cardiovascular events following exacerbations.
Registration
URL: https://clinicaltrials.gov/ct2/show/NCT02164513; Unique identifier: NCT02164513
Keywords: cardiovascular disease, chronic obstructive pulmonary disease, exacerbations, LAMA/LABA, triple therapy
Subject Categories: Clinical Studies, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- CVAESI
cardiovascular adverse event of special interest
- FF
fluticasone furoate
- FEV1
forced expiratory volume in one second
- UMEC
umeclidinium
- VI
vilanterol
Clinical Perspective.
What Is New?
The association between chronic obstructive pulmonary disease (COPD) exacerbations and increased cardiovascular event risk has not been fully elucidated.
This post hoc analysis of IMPACT (Informing the Pathway of COPD Treatment; n=10 355 patients with COPD at risk for COPD exacerbations) showed that cardiovascular event risk is higher during and in the 30 days following COPD exacerbations, returning to baseline by 90 days.
This risk was especially strong during hospitalized exacerbations (hazard ratio, 21.8 [95% CI, 17.7– 26.9]).
The association between COPD exacerbations and cardiovascular event risk did not differ by exacerbation history, cardiovascular risk at screening, or study treatment.
What Are the Clinical Implications?
Providers and patients with COPD should be vigilant for cardiovascular events during and following COPD exacerbations, particularly in exacerbations requiring hospitalization.
The natural history of chronic obstructive pulmonary disease (COPD) is characterized by acute exacerbations that, over time, contribute to poorer health outcomes, including reduced lung function 1 , 2 and increased mortality. 3 , 4 Cardiovascular disease (CVD) is a common comorbidity in patients with COPD, affecting between 28% and 70% of patients, 5 and COPD has also been shown to increase the risk of CVD. 5 , 6 Tobacco smoke exposure is a common risk factor for both COPD and CVD (including coronary heart disease, stroke, and vascular disease), 7 and atherosclerosis is exaggerated in smokers with COPD compared with smokers without COPD and nonsmokers with COPD. 8 In addition, several common cardiovascular comorbidities including heart failure, hypertension, and peripheral vascular disease are associated with increased COPD exacerbation risk. 9 , 10 Consequently, COPD with comorbid CVD is associated with worse outcomes than with COPD or CVD alone. 11 , 12 CVD is a frequent cause of mortality among patients with COPD, 5 , 11 and patients with COPD who experience cardiovascular events, specifically myocardial infarction (MI), are at greater risk of death and hospital readmissions for recurrent MI than patients without COPD. 12
Mechanisms behind the increased CVD risk in COPD remain to be fully elucidated, but heightened systemic inflammation may be one pathway, given that it is common in COPD and is linked to higher CVD risk, 13 , 14 , 15 , 16 , 17 and reducing levels of inflammatory mediators has been shown to improve cardiovascular outcomes. 18 The SUMMIT (Study to Understand Mortality and Morbidity in COPD) trial 19 was a prospective, double‐blind, event‐driven study that assessed the effects of treatment with fluticasone furoate/vilanterol (FF/VI) compared with placebo on survival in patients with moderate COPD who had a history of, or were at increased risk of, CVD. 19 Previous studies, including a post hoc analysis of the SUMMIT trial, suggest a link between COPD exacerbations and an increased risk of subsequent cardiovascular events, with a significantly elevated risk of serious cardiovascular events. 20 , 21 , 22 , 23 , 24 , 25 However, the generalizability and/or validity of many of these studies were limited by use of only administrative data and lack of prospective, standardized cardiovascular event ascertainment procedures or inclusion of COPD populations only at particularly high risk of cardiovascular events. 20 , 21 , 23 , 24 , 25
The IMPACT (Informing the Pathway of COPD Treatment) trial evaluated the effects of single‐inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC) triple therapy versus FF/VI and UMEC/VI dual therapy on exacerbations in a large population of patients with symptomatic COPD at risk of exacerbations. Results showed a significant reduction in the rate and risk of moderate/severe exacerbations with triple versus both dual therapies. 26 The IMPACT trial was designed to reflect real‐world clinical practice more closely, allowing enrollment of patients with or without significant CVD. 26 The objective of this post hoc analysis of the IMPACT trial was to quantify the risk of cardiovascular events during and over various time periods up to a year following an exacerbation. It also evaluated the risk based on preexisting cardiovascular risk factors, exacerbation history, and study treatment.
METHODS
Data Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Study Design
The IMPACT trial (study CTT116855, NCT02164513) was a Phase III, multicenter, randomized, double‐blind, parallel‐group study conducted in 37 countries between June 2014 and July 2017. 26 Following a 2‐week run‐in period, during which patients continued their existing COPD medications, patients were randomized (2:2:1) to receive triple therapy with FF/UMEC/VI 100/62.5/25 μg or dual therapy with FF/VI 100/25 μg or VI 62.5/25 μg for 52 weeks, all administered once daily via the Ellipta dry powder inhaler. 26 , 27 Rescue medication (salbutamol) was available on an as‐needed basis.
Study Population
Full eligibility criteria for the IMPACT trial have been reported previously. 26 , 27 Patients were ≥40 years of age with symptomatic COPD (COPD Assessment Test score ≥10) and either had a postbronchodilator forced expiratory volume in 1 second (FEV1) <50% predicted and a history of ≥1 moderate/severe exacerbation, or a postbronchodilator FEV1 50% to <80% predicted and ≥2 moderate or ≥1 severe exacerbation in the previous 12 months. Exclusion criteria included a concomitant diagnosis of asthma or other respiratory disorders and pneumonia or other respiratory tract infections not resolved ≤14 days or ≤7 days before screening, respectively. Patients who had unstable or life‐threatening cardiovascular disease were excluded, although participants could enroll if previous MI was >6 months before screening, heart failure was New York Heart Association Class 1 to 3, or previous unstable/life‐threatening arrhythmia requiring intervention was >3 months before screening. Patients using ≤3 L/min of supplemental oxygen at rest at screening were eligible to participate.
All patients provided written informed consent. The study was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki and received approval by local ethics review boards of the participating sites.
Outcomes
Outcomes assessed in this analysis were risk (time‐to‐first) of cardiovascular adverse events of special interest (CVAESI) or CVAESI resulting in or prolonging hospitalization or resulting in death during and 1 to 30, 31 to 90, and 91 to 365 days after resolution of a moderate or severe exacerbation. CVAESI resulting in or prolonging hospitalization or resulting in death are referred to as serious CVAESI hereafter.
Investigators or site staff were responsible for documenting or reporting these cardiovascular events, and serious CVAESIs were adjudicated by an independent clinical end point committee. CVAESIs included in this analysis were defined by Standardized Medical Dictionary for Regulatory Activities Queries, 28 which are groups of validated, predetermined sets of Preferred Terms aiming to capture all plausible events linked to a disease process. 29 CVAESIs included new or worsened cardiac arrhythmia, cardiac failure, ischemic heart disease, hypertension, and central nervous system hemorrhages and cerebrovascular conditions. CVAESIs were determined from investigator‐assessed adverse events or serious adverse events reported during IMPACT, with serious adverse events and deaths independently adjudicated. A sensitivity analysis of time‐to‐first CVAESI and time‐to‐first serious CVAESI, excluding hypertension events during and after moderate and severe exacerbation was also conducted.
Moderate exacerbations were defined as exacerbations requiring treatment with oral/systemic corticosteroids and/or antibiotics (not involving hospitalization or resulting in death). Severe exacerbations were defined as exacerbations requiring hospitalization or resulting in death. Exacerbation duration was determined by investigators' assessment based on guidance in the trial protocol and recorded in electronic clinical records. The date of onset was the first of at least 2 consecutive days of worsening COPD symptoms, confirmed by the investigator. The date of resolution was based on when the investigator and/or patient determined that the COPD symptoms had returned to preexacerbation levels or to a new exacerbation‐free period. Cardiovascular events occurring between the start and resolution of the exacerbation were considered as occurring during the exacerbation event.
Time‐to‐first CVAESI during and following moderate and severe exacerbations was also assessed in subgroups of patients derived from the intention‐to‐treat (ITT) population based on cardiovascular risk factors at screening (no risk factors; 1 risk factor, or ≥2 risk factors), exacerbation history (<2 moderate exacerbations in year prior; or ≥2 moderate or ≥1 severe), and treatment group (FF/UMEC/VI, FF/VI, or UMEC/VI). The following past or current medical conditions were classed as cardiovascular risk factors: angina pectoris, coronary artery disease, MI, arrhythmia, congestive heart failure, hypertension, cerebrovascular accident, carotid or aortofemoral vascular disease, diabetes, and hypercholesterolemia.
Statistical Analysis
The ITT population was defined as all patients randomized to treatment, except those randomized in error. 27 Time‐to‐first on‐treatment CVAESI and time‐to‐first on‐treatment serious CVAESI following an exacerbation were analyzed using a time‐dependent‐covariate Cox model with covariates for time period following exacerbation, study treatment, age, sex, body mass index, race, ethnicity, region, cardiovascular risk factors, smoking status, prior exacerbation history, and percentage of predicted postbronchodilator FEV1. For this post hoc analysis, the hazard for a CVAESI was compared between the period between randomization and the first exacerbation (termed the “exacerbation‐free on study treatment” period—referred to as “exacerbation‐free period” throughout) and the period during or after an exacerbation over the trial duration (Figure 1); these analyses were repeated for each subgroup. Subgroup analyses by IMPACT study cardiovascular risk factor at screening and exacerbation history were performed using the same model and covariates, with the exclusion of the covariates of cardiovascular risk factors and exacerbation history, respectively. The hazard was compared using 2 different methods of analysis: the primary analysis method assumed that for exacerbations and CVAESIs that occurred on the same day, the exacerbation occurred first. The secondary method assumed that the CVAESI occurred before the exacerbation.
Figure 1. Graphic representation of analysis of order of exacerbation and CVAESIs.
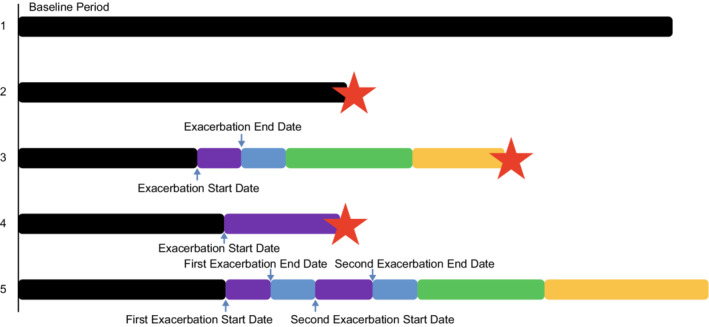
Black bars start at study randomization for all patients and indicate exacerbation‐free on study treatment periods. Blue arrows indicate exacerbation start and end dates. Purple bars indicate exacerbation duration. Blue, green, and yellow bars indicate fixed‐length postexacerbation risk periods of 1 to 30 days, 31 to 90 days, and 91 days to 1 year after exacerbation, respectively. Red stars indicate cardiovascular events, no further risk periods are applicable after a cardiovascular event. The representations depict scenarios of event patterns in this analysis. (1) No exacerbation and no cardiovascular event; (2) no exacerbation, but cardiovascular event in exacerbation‐free period; (3) exacerbation (start and end date indicated with blue arrows) with cardiovascular event in the fixed‐length postexacerbation risk period; (4) exacerbation with cardiovascular event during exacerbation period; and (5) multiple exacerbations with no cardiovascular event – the fixed‐length postexacerbation events are reset following each exacerbation. CVAESI indicates cardiovascular adverse events of special interest.
RESULTS
Study Population
The ITT population included 10 355 patients. Baseline demographics and clinical characteristics are reported in Table 1. Overall, 1.9% of patients experienced a CVAESI during a moderate exacerbation, and 11.3% of patients experienced a CVAESI during a severe exacerbation (Table 2).
Table 1.
Patients Demographics and Baseline Characteristics
| Demographic/characteristic |
FF/UMEC/VI N=4151 |
FF/VI N=4134 |
UMEC/VI N=2070 |
Overall N=10 355 |
|---|---|---|---|---|
| Age, y, mean (SD) | 65.3 (8.2) | 65.3 (8.3) | 65.2 (8.3) | 65.3 (8.3) |
| Sex, male, n (%) | 2766 (67) | 2748 (66) | 1356 (66) | 6870 (66) |
| Body mass index, mean (SD), kg/m2 | 26.6 (6.2) | 26.7 (6.1) | 26.6 (5.9) | 26.6 (6.1) |
| Race, n (%) | ||||
| American Indian or Alaskan Native | 87 (2) | 86 (2) | 45 (2) | 218 (2) |
| Asian | 668 (16) | 676 (16) | 335 (16) | 1679 (16) |
| Black | 122 (3) | 99 (2) | 43 (2) | 264 (3) |
| Native Hawaiian or other Pacific Islander | 2 (<1) | 3 (<1) | 2 (<1) | 7 (<1) |
| White | 3231 (78) | 3224 (78) | 1628 (79) | 8083 (78) |
| Multiple | 41 (<1) | 45 (1) | 17 (<1) | 103 (<1) |
| Smoking status, n (%) | ||||
| Current smoker | 1436 (35) | 1423 (34) | 728 (35) | 3587 (35) |
| Former smoker | 2715 (65) | 2711 (66) | 1342 (65) | 6768 (65) |
| Chronic obstructive pulmonary disease exacerbations in previous year, n (%) | ||||
| <2 moderate and no severe | 1198 (29) | 1242 (30) | 616 (30) | 3056 (30) |
| ≥2 moderate or ≥1 severe | 2953 (71) | 2892 (70) | 1454 (70) | 7299 (70) |
| Postbronchodilator forced expiratory volume in 1 second % predicted, mean (SD) | 45.7 (15.0) | 45.5 (14.8) | 45.4 (14.7) | 45.5 (14.8) |
| Cardiovascular risk factors at screening*, n (%) | ||||
| 0 | 1365 (33) | 1322 (32) | 656 (32) | 3343 (32) |
| 1 | 1147 (28) | 1158 (28) | 580 (28) | 2885 (28) |
| ≥2 | 1639 (39) | 1654 (40) | 834 (40) | 4127 (40) |
FF indicates fluticasone furoate; UMEC, umeclidinium; and VI, vilanterol.
As captured in the electronic case report form.
Table 2.
Number of Patients Experiencing Cardiovascular Events During and Following Exacerbation Events
| First CVAESI | First CVAESI resulting in hospitalization or death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n | Patients experiencing cardiovascular event, n (%) | Total time at risk (patient‐years) | Event rate per 100 patient‐years | Primary analysis method* HR (95% CI) (time point vs baseline) | Secondary analysis method† HR (95% CI) (time point vs baseline) | Total n | Patients experiencing cardiovascular event, n (%) | Total time at‐risk (patient‐years) | Event rate per 100 patient‐years | Primary analysis method* HR (95% CI) (time point vs baseline) | Secondary analysis method† HR (95% CI) (time point vs baseline) | |
| Exacerbation‐free “baseline” “on study treatment” period | 10 340 | 681 (6.6) | 5916 | 11.5 | … | … | 10 340 | 174 (1.7) | 6139 | 2.8 | … | … |
| Moderate exacerbations | ||||||||||||
| Exacerbation event | 4207 | 80 (1.9) | 267 | 29.9 | 2.63 (2.08–3.32) | 2.01 (1.55–2.60) | 4361 | 19 (0.4) | 283 | 6.7 | 2.46 (1.53–3.97) | 1.55 (0.90–2.67) |
| 1–30 d post event | 3874 | 74 (1.9) | 466 | 15.9 | 1.63 (1.28–2.08) | 1.66 (1.32–2.10) | 4050 | 26 (0.6) | 495 | 5.3 | 2.00 (1.32–3.05) | 1.97 (1.33–2.92) |
| 31–90 d post event | 3410 | 49 (1.4) | 614 | 8.0 | 0.90 (0.67–1.21) | 0.91 (0.69–1.22) | 3599 | 22 (0.6) | 655 | 3.4 | 1.36 (0.86–2.13) | 1.22 (0.79–1.89) |
| 91–365 d post event | 2548 | 61 (2.4) | 785 | 7.8 | 0.95 (0.72–1.26) | 0.98 (0.75–1.28) | 2710 | 18 (0.7) | 836 | 2.2 | 0.90 (0.55–1.50) | 0.92 (0.57–1.47) |
| Severe exacerbations | ||||||||||||
| Exacerbation event | 1099 | 124 (11.3) | 52 | 238.9 | 21.84 (17.71–26.93) | 11.75 (9.11–15.16) | 1160 | 64 (5.5) | 58 | 111.1 | 41.29 (30.43–56.03) | 15.12 (10.11–22.63) |
| 1–30 d post event | 790 | 12 (1.5) | 68 | 17.8 | 1.75 (0.99–3.11) | 1.77 (1.02–3.07) | 873 | 9 (1.0) | 75 | 12.0 | 4.39 (2.24–8.60) | 4.09 (2.17–7.71) |
| 31–90 d post event | 600 | 13 (2.2) | 86 | 15.1 | 1.61 (0.93–2.81) | 1.95 (1.20–3.18) | 667 | 2 (0.3) | 97 | 2.1 | 0.81 (0.20–3.26) | 2.01 (0.89–4.56) |
| 91–365 d post event | 386 | 10 (2.6) | 106 | 9.5 | 1.12 (0.60–2.10) | 1.15 (0.63–2.11) | 435 | 6 (1.4) | 119 | 5.0 | 2.06 (0.90–4.73) | 1.74 (0.76–3.99) |
CVAESI indicates cardiovascular adverse events of special interest; and HR, hazard ratio.
The primary method of analysis assumed that if a cardiovascular event occurred on the same day as an exacerbation, the exacerbation occurred first.
The secondary method of analysis assumed that if a cardiovascular event occurred on the same day as an exacerbation, the cardiovascular event occurred first.
Risk of CVAESI in ITT Population
CVAESI risk increased significantly from an exacerbation‐free period level during a moderate exacerbation (hazard ratio [HR], 2.63 [95% CI, 2.08–3.32]), and decreased over time from 1.6‐fold increased risk 1 to 30 days after a moderate exacerbation to exacerbation‐free period risk levels 91 to 365 days after exacerbation. Risk of serious CVAESI also increased significantly during a moderate exacerbation (HR, 2.46 [95% CI, 1.53–3.97]) and decreased over time from 2‐fold increased risk 1 to 30 days after a moderate exacerbation to exacerbation‐free period risk levels 91 to 365 days after exacerbation (Figure 2; Table 2).
Figure 2. Risk of a first cardiovascular event during and following a moderate exacerbation (primary analysis method) ***P<0.001; **P<0.01 versus exacerbation‐free period.
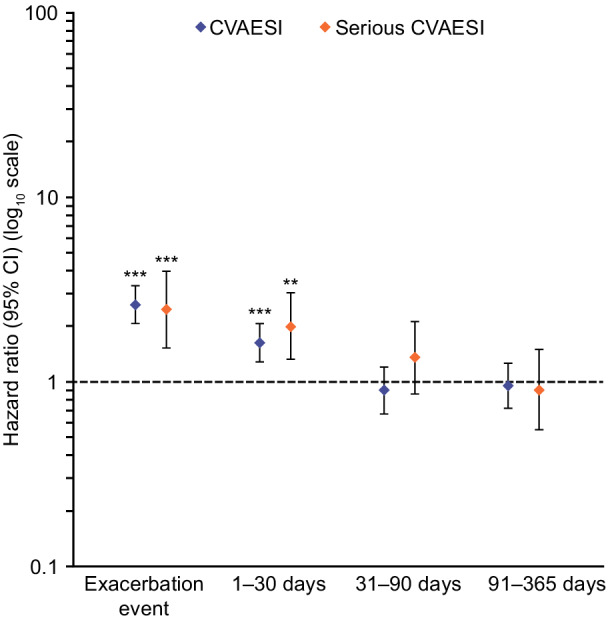
The hazard for a CVAESI was compared assuming that for exacerbations and CVAESIs that occurred on the same day, the exacerbation occurred first. Serious CVAESI are CVAESI resulting in or prolonging hospitalization or resulting in death. CVAESI indicates cardiovascular adverse events of special interest.
CVAESI risk was markedly increased during a severe exacerbation (HR, 21.84 [95% CI, 17.71–26.93]) and followed a similar temporal pattern, decreasing to ≈1.7‐fold 1 to 30 days after an exacerbation, returning to exacerbation‐free period levels after 30 days. The risk of serious CVAESI increased over 40‐fold during a severe exacerbation (HR, 41.29 [95% CI, 30.43–56.03]) declining to 4.4‐fold 1 to 30 days following the exacerbation and returning to exacerbation‐free period levels after 30 days (Figure 3; Table 2).
Figure 3. Risk of a first cardiovascular event during and following a severe exacerbation (primary analysis method) ***P<0.001 versus exacerbation‐free period.
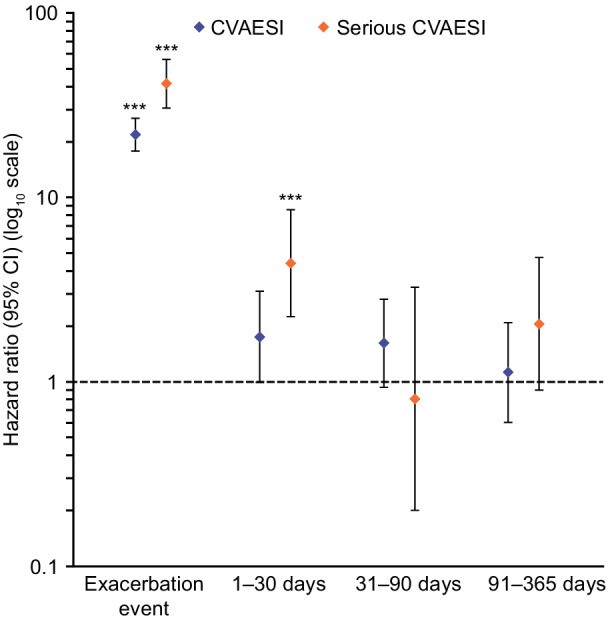
The hazard for a CVAESI was compared assuming that for exacerbations and CVAESIs that occurred on the same day, the exacerbation occurred first. Serious CVAESI are CVAESI resulting in or prolonging hospitalization or resulting in death. CVAESI indicates cardiovascular adverse events of special interest.
The type of CVAESIs experienced during and after exacerbations are shown in Table S1. The breakdown of events was similar regardless of exacerbation severity, and cardiac arrythmia, cardiac failure, and hypertension comprised most events. There were 26 patients experiencing cardiac arrythmia events, 26 experiencing cardiac failure events, and 14 experiencing hypertension events during moderate exacerbations (Table S1). There were 44 patients experiencing cardiac arrythmia events, 45 experiencing cardiac failure events, and 25 experiencing hypertension events during severe exacerbations (Table S1). These numbers decreased sharply post event and remained low in all postexacerbation periods.
Risk of Nonhypertension CVAESIs in ITT Population: Sensitivity Analysis
Results from the sensitivity analysis excluding hypertension events, conducted using the primary analysis method, were consistent with the primary analysis that included all CVAESI. Nonhypertension CVAESI risk increased significantly during a moderate exacerbation (HR, 2.77 [95% CI, 2.14–3.58]) and decreased over time from ≈1.7‐fold increased risk 1 to 30 days after a moderate exacerbation to exacerbation‐free period risk levels 91 to 365 days after exacerbation. Serious nonhypertension CVAESI risk was also significantly increased during a moderate exacerbation (HR, 2.40 [95% CI, 1.47–3.92]) and decreased over time from 2.1‐fold 1 to 30 days after a moderate exacerbation to exacerbation‐free period risk levels 91 to 365 days after exacerbation (Figure S1).
In this sensitivity analysis, nonhypertension CVAESI risk was also markedly increased during a severe exacerbation (HR, 23.47 [95% CI, 18.73–29.40]), decreasing to 2.2‐fold 1 to 30 days after exacerbation, 1.9‐fold 31 to 90 days after exacerbation, and returning to exacerbation‐free period levels after 90 days. The risk of serious nonhypertension CVAESI increased 40‐fold during a severe exacerbation (HR, 40.40 [95% CI, 29.66–55.02]) declining to 4.5‐fold 1 to 30 days following the exacerbation and returning to exacerbation‐free period levels after 30 days (Figure S2).
Risk of CVAESIs in ITT Population: Secondary Analyses
The secondary analysis method, which assumed that the CVAESI occurred before the exacerbation for events occurring on the same day, produced results generally consistent with the primary method. CVAESI risk during a moderate exacerbation increased significantly (HR, 2.01 [95% CI, 1.55–2.60]) and decreased over time, returning to exacerbation‐free period levels after 30 days. Risk of serious CVAESI was not significantly increased during a moderate exacerbation (HR, 1.55 [95% CI, 0.90–2.67]) but increased 2‐fold 1 to 30 days after exacerbation, returning to exacerbation‐free period levels after 30 days (Table 2 and Figure S3).
During a severe exacerbation, CVAESI risk increased 12‐fold (HR, 11.75 [95% CI, 9.11–15.16]), and decreased over time returning to exacerbation‐free period levels after 90 days. Risk of serious CVAESI increased 15‐fold during a severe exacerbation event (HR, 15.12 [95% CI, 10.11–22.63]), declining to a 4‐fold risk 1 to 30 days after exacerbation and returning to exacerbation‐free period levels after 30 days (Table 2 and Figure S4).
Subgroup Analyses
Subgroup analyses suggested a trend consistent with the overall ITT population across all subgroups; CVAESI risk was highest during the exacerbation and declined over time. Increases in risk during a severe exacerbation were numerically greater compared with those during a moderate exacerbation (Figure S5 through S7). The risk of CVAESI was similar across all study treatment groups, during or following moderate or severe exacerbations (Figure S5). CVAESI risk during or following a moderate or severe exacerbation was also similar regardless of patients' exacerbation history in the previous year (Figure S6), or cardiovascular risk factors at screening (Figure S7), and 95% CIs overlapped at all time points.
DISCUSSION
This analysis of cardiovascular risk during and following COPD exacerbations was conducted in a population of patients with symptomatic COPD at risk of exacerbation. Results demonstrated that on average, the risk of patients experiencing a CVAESI or a serious CVAESI during and after an exacerbation event was significantly higher and decreased over time. Importantly, this analysis shows that these findings remained consistent irrespective of cardiovascular risk factors, exacerbation history, and randomized treatment. Although patients on FF/UMEC/VI in IMPACT experienced fewer exacerbations than those on FF/VI or UMEC/VI, 26 once a patient had experienced an exacerbation, the risk of experiencing a CVAESI was similar.
This higher risk of CVAESI observed across the IMPACT patient population during and in the first 30 days following an exacerbation highlights a need for exacerbation prevention and for physicians and patients to be vigilant for cardiovascular events following exacerbations, particularly severe exacerbations. Indeed, building on findings from previous studies, 20 , 21 , 22 this post hoc analysis showed that severe exacerbations confer a higher risk than moderate exacerbations. The increase in risk of CVAESIs during severe exacerbations was 21.8‐fold versus 2.6‐fold during moderate exacerbations, and the increase in the risk of serious CVAESI was 41.3‐fold during severe exacerbations versus 2.5‐fold during moderate exacerbations. It is possible that severe exacerbations improve detection of CVAESIs because of closer observation of hospitalized patients versus detection when the patient is at home; for example, in a hospital setting it is possible to detect elevated troponins indicating myocardial injury 30 or atrial fibrillation on a routine electrocardiogram during admission. This suggests a possible methodological contribution to the observed elevated risk following a severe versus moderate exacerbation.
These results validate and build upon the current understanding of cardiovascular risk associated with COPD exacerbations. Previous studies have shown an elevated risk of major adverse cardiovascular events following an exacerbation. 20 , 21 , 22 , 23 For example, a study of patients with COPD from a database in Taiwan, investigating the association between acute exacerbations in the prior year and mortality of cardiovascular events, found that hospitalizations for acute exacerbations within the previous year were associated with higher risk of 90‐day and overall mortality of cardiovascular events. 25
In the SUMMIT study, the patient population had a mean age of 65 years, a mean body mass index of 28 kg/m2, and 47% were current smokers. 20 Results from this study showed a 3.8‐fold higher risk of experiencing a cardiovascular event in the 30 days following the start of an exacerbation, and 9.9‐fold higher risk in the 30 days following the start of an exacerbation requiring hospitalization, with both returning to exacerbation‐free period levels by 1 year after exacerbation. 20 The current analysis supports this time‐dependent risk of cardiovascular events following COPD exacerbations, although the cardiovascular risk returned to exacerbation‐free period levels by day 90 after the investigator‐determined end of the exacerbation event. It should be noted that the risk periods for cardiovascular events were not defined using the end dates of an exacerbation event for the analysis in SUMMIT, limiting the ability to estimate the risk during versus after resolution of the exacerbation, as has been done uniquely in the current study. 20 , 26 It is also worth noting that this analysis captured a broader range of CVAESIs than SUMMIT, which assessed only the risk of major adverse cardiovascular events. 20 Mean age, body mass index, and smoking status were similar in the IMPACT and SUMMIT patient populations. However, exacerbation history differed, with 54% and 15% of patients, experiencing ≥2 moderate or severe exacerbations respectively, in the previous year. 20 , 26 Furthermore, SUMMIT was enriched for patients with heightened cardiovascular risk, as this was an inclusion criterion, with all patients having CVD or increased CVD risk. 20 IMPACT also included patients with a heightened cardiovascular risk but had broader cardiovascular inclusion criteria than SUMMIT meaning that patients with low cardiovascular risk were also included; 32% of patients in IMPACT had no cardiovascular risk factors at screening. 26
Whereas previous studies have examined the risk association between COPD exacerbations and cardiovascular events, these analyses of IMPACT are the first to show that the increase in risk is not significantly affected by preexisting cardiovascular risk factors or exacerbation history. This may indicate that a patient does not require a history of frequent or severe exacerbation events or a high cardiovascular risk to have an elevated cardiovascular risk after an exacerbation and further reinforces the need to prevent exacerbations as stated in the Global Initiative for Chronic Obstructive Lung Disease report 31 and the importance of physicians and patients to be vigilant for cardiovascular events following an exacerbation. Studies are required to determine optimal methods to monitor for cardiovascular events, in addition to identification of interventions that can reduce the risk of these events. Results from the analysis presented here contrast with a previous analysis of the UPLIFT (Understanding Potential Long‐Term Impacts on Function With Tiotropium) trial, in which patients with a baseline cardiac disorder were at a greater increased risk of cardiovascular event following exacerbation than those without. 23 This could potentially be attributed to the differences between the studies in both the type of cardiac disease in the trial population at baseline, the types of cardiac adverse event being recorded following exacerbations, or the criteria used to define cardiac adverse events. In this study, the distribution of cardiovascular event type observed during and after exacerbations was similar to that observed in the baseline population, regardless of exacerbation severity. Cardiac arrhythmia, cardiac failure, and hypertension were the most frequently reported events and occurred in similar proportions, suggesting that no one type of event was disproportionately driving the higher risk of a CVAESI following exacerbation. Furthermore, the sensitivity analysis excluding hypertension events, which were potentially less clinically meaningful events, confirmed the findings of the overall analysis.
Although this study did not investigate mechanisms driving the greater risk of cardiovascular events during or following severe compared with moderate exacerbations, several hypotheses may explain these findings. Acute inflammation is a highly prothrombotic event, 13 and the severity of inflammation may account for the difference in risk seen between severe and moderate exacerbations in this analysis. 32 Accordingly, increased concentrations of procalcitonin, an inflammatory biomarker, following exacerbations have shown to correlate with severity of the preceding exacerbation. 33 Furthermore, COPD exacerbations are also often associated with hypoxemia, with cardiac output increasing as a compensatory mechanism, and a hypoxic state is associated with an elevated inflammatory response. 32 COPD treatment could also influence the risk of cardiovascular events following an exacerbation. Besides the causal explanation of systemic inflammation and prothrombotic risk, beta‐agonists may contribute to cardiovascular risk. 34 Furthermore, short‐acting beta2‐agonists are used for short‐term symptomatic relief in exacerbating patients, and it is possible that their elevated use in patients around the time of exacerbation could contribute to the risk of cardiac events associated with exacerbations. 35 , 36 , 37 Corticosteroid use has also been linked to an increased risk of MI in patients with COPD, 38 although it should be noted that patients with COPD who receive inhaled corticosteroids generally have more severe COPD, 31 which itself is associated with increased cardiovascular risk. 39
This analysis had several strengths including the fact that it considered a large population of patients with symptomatic COPD at risk for exacerbation from multiple international sites, with diverse clinical history and baseline characteristics, including a range of cardiovascular risk factors. 20 , 21 , 22 Furthermore, the IMPACT trial used broad inclusion criteria and allowed enrollment of patients with significant concurrent CVD. Additionally, the current analysis examines a broader range of CVAESIs not limited to major adverse cardiovascular events. 20 , 21 , 22 Nevertheless, some limitations should also be considered. Although the analyses are based on controlled clinical trial data, only serious adverse events and deaths were independently adjudicated in the IMPACT trial. Furthermore, although the trial was randomized by treatment, the analyses presented here are between nonrandomized groups of patients (those who exacerbate and those who do not) making it difficult to establish causality between exacerbations and cardiovascular events. However, the modeling analysis was adjusted for cardiovascular and exacerbation risk factors, and the reduction in the estimated cardiovascular risk back to exacerbation‐free period levels for patients who experienced an exacerbation provides confidence that the differences between patients who exacerbate, and those who do not, have largely been adjusted out. Additionally, overall cardiovascular event rates were low and this can lead to imprecision in the risk estimated; although it is important to recognize that exacerbations are associated with a significantly increased risk of subsequent cardiovascular events so extra vigilance over these patients should be exercised, this should be considered in the context that, in terms of absolute numbers, most patients did not experience cardiovascular events following an exacerbation. Characterizing the critical risk period for these clinically significant cardiovascular events following an exacerbation can inform clinical practice, in terms of physician and patient vigilance and treatment, and could help refine clinical care for patients. Future studies could investigate postexacerbation interventions that act to decrease or prevent this risk elevation. Finally, this analysis relied on investigator‐reported events; measurements for specific cardiac biomarkers during and after the index exacerbation would facilitate an increased understanding of drivers behind cardiovascular events.
Interpretation
In this post hoc analysis of the IMPACT trial, the risk of experiencing a CVAESI or a serious CVAESI was on average significantly higher during a moderate or severe exacerbation and remained elevated in the 30 days following an exacerbation. This was observed regardless of cardiovascular risk at screening or exacerbation history. These results extend our understanding of the increased risk of cardiovascular events during and in the period following an exacerbation seen in other studies, 20 , 21 , 22 , 23 highlighting a need for exacerbation prevention and physicians and patients to be vigilant for adverse cardiovascular events following exacerbation events.
Sources of Funding
This study was funded by GSK (study number CTT116855; NCT02164513). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit for publication. Ellipta is owned by or licensed to the GSK group of companies. Dave Singh is supported by the National Institute for Health Research Manchester Biomedical Research Centre.
Disclosures
All authors report other and nonfinancial support from GSK (funding the study and funding medical writing support by Ellen Barker, PhD, and Alexandra Berry, PhD at Fishawack Indicia Ltd, UK). Mark T Dransfield received personal fees from AstraZeneca, Boehringer Ingelheim, PneumRx/BTG, Quark Pharmaceuticals, Teva, and GSK; grant support from the American Lung Association, Department of Defense, Department of Veterans Affairs, and the National Institutes of Health; and contracted clinical trial support from Boehringer Ingelheim, Novartis, AstraZeneca, Yungjin, PneumRx/BTG, Pulmonx, Boston Scientific, Gala, Nuvaira, and GSK. Gerard J Criner received personal fees from Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Broncus Medical, Chiesi, CSA Medical, Eolo, Gala Therapeutics, GSK, Helios Medical, Medtronic, Merck, Mereo BioPharma, NGM Pharmaceuticals, Novartis, Nuvaira, Olympus, Philips Respironics, Pulmonx, Respivant Sciences, The Implementation Group, and Verona and has ownership interest in HGE Technologies. David MG Halpin received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Pfizer and Sanofi, and nonfinancial support from Boehringer Ingelheim and Novartis. MeiLan K Han has received personal fees from GSK, AstraZeneca, Boehringer Inhgelheim, Novartis, Pulmonx, Teva, Verona, Merck, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, Cipla, and Chiesi. She has received stock options from Meissa Vaccines and Altessa Biopharma. She has received either in kind research support or funds paid to the institution from the National Institutes of Health, Novartis, Sunovion, Nuvaira, Sanofi, Astrazeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. She has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. Ravi Kalhan reports grants from the National Heart, Lung, and Blood Institute during the conduct of the study, grants and personal fees from Boehringer Ingelheim, grants from PneumRx (BTG), grants from Spiration, grants and personal fees from AstraZeneca, personal fees from CVS Caremark, personal fees from Aptus Health, grants and personal fees from GlaxoSmithKline, personal fees from Boston Scientific, and personal fees from Boston Consulting Group. David A Lipson and Dawn Midwinter are employees of GSK and hold stocks/shares in GSK. Peter Lange has received personal fees from GSK, AstraZeneca, and Boehringer Ingelheim and grant support from Boehringer Ingelheim and GlaxoSmithKline. Fernando J Martinez has received personal fees and nonfinancial support from the American College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, Continuing Education, ConCert, Genentech, GSK, Inova Fairfax Health System, Miller Communications, National Society for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, Puerto Rico Respiratory Society, Chiesi, Roche, Sunovion, Theravance, Potomac, University of Alabama Birmingham, Physicians Education Resource, Canadian Respiratory Network, and Teva; nonfinancial support from ProterrixBio, Gilead, Nitto, and Zambon; and personal fees from Columbia University, Integritas, MD Magazine, Methodist Hospital Brooklyn, New York University, Unity, UpToDate, WedMD/MedScape, Western Connecticut Health Network, Academic CME, Patara, PlatformIQ, American Thoracic Society, Rockpointe, and France Foundation; grant support from National Institutes of Health, Rare Disease Health Communications, and ProMedior; and is a member of steering committees for Afferent/Merck, Biogen, Veracyte, Prometic, Bayer, and Bridge Biotherapeutics. Dave Singh has received personal fees from GSK, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Theravance, and Verona and grant support from AstraZeneca, Boehringer Ingelheim, Chiesi, Glenmark, Menarini, Mundipharma, Novartis, Pfizer, Pulmatrix, Theravance, and Verona. Robert Wise reports consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers, Chemerx, Chiesi, Clinipace, Contrafect, FSD, Galderma, Pulmonx, Puretech, Merck, GSK, Savara, Vaxart, Verona, and AbbVie and grant support from AstraZeneca, Boehringer Ingelheim, Sanofi‐Aventis and Verona. Ken M Kunisaki reports personal fees from Nuvaira and Allergan and grants from National Institutes of Health, Department of Veterans Affairs, and Department of Defense. Ben Hartley is a contingent worker at GSK.
Supporting information
Table S1
Figures S1–S7
Acknowledgments
Guarantor: Mark T Dransfield agrees to take responsibility for the final content of the article, including the data and analysis.
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the article, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Mark T Dransfield, David MG Halpin, and Gerard J Criner contributed to the acquisition of data; Benjamin Hartley, Mark T Dransfield, David MG Halpin, MeiLan K Han, David A Lipson, Peter Lange, Fernando J Martinez, Dawn Midwinter, Dave Singh, Robert Wise, Ravi Kalhan, Ken M Kunisaki, and Gerard J Criner contributed to the data analysis and interpretation; David A Lipson contributed to the conception and design of the study. All authors were involved in the development of the article and provided approval of the final draft to be published.
Other Contributions: Editorial support (in the form of writing assistance, including preparation of the initial draft under the direction and guidance of the authors, collating and incorporating authors' comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Ellen Barker, PhD, and Alexandra Berry, PhD, at Fishawack Indicia Ltd., UK, part of Fishawack Health, and was funded by GlaxoSmithKline.
See Editorial by Sethi et al.
Presented in part at CHEST Annual Meeting 2020 held virtually from October 18‐21, 2020 and published in abstract form [CHEST. 158;A1722‐A1726 or https://doi.org/10.1016/j.chest.2020.08.1526].
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024350
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, Criner GJ, Curtis JL, Hanania NA, Nath H, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19:113–118. doi: 10.1183/09059180.00002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blasi F, Cesana G, Conti S, Chiodini V, Aliberti S, Fornari C, Mantovani LG. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLOS One. 2014;9:e101228. doi: 10.1371/journal.pone.0101228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soler‐Cataluna JJ, Martinez‐Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178. doi: 10.1378/chest.12-2847 [DOI] [PubMed] [Google Scholar]
- 6. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 7. Kc R, Shukla SD, Gautam SS, Hansbro PM, O'Toole RF. The role of environmental exposure to non‐cigarette smoke in lung disease. Clin Transl Med. 2018;7:39. doi: 10.1186/s40169-018-0217-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iwamoto H, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, Hattori N, Hara H, Kohno N. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med. 2009;179:35–40. doi: 10.1164/rccm.200804-560OC [DOI] [PubMed] [Google Scholar]
- 9. Westerik JA, Metting EI, van Boven JF, Tiersma W, Kocks JW, Schermer TR. Associations between chronic comorbidity and exacerbation risk in primary care patients with COPD. Respir Res. 2017;18:31. doi: 10.1186/s12931-017-0512-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246–1258. doi: 10.4239/wjd.v6.i13.1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shibata Y, Inoue S, Igarashi A, Yamauchi K, Abe S, Aida Y, Nunomiya K, Sato M, Nakano H, Sato K, et al. A lower level of forced expiratory volume in 1 second is a risk factor for all‐cause and cardiovascular mortality in a Japanese population: the Takahata study. PLOS One. 2013;8:e83725. doi: 10.1371/journal.pone.0083725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campo G, Guastaroba P, Marzocchi A, Santarelli A, Varani E, Vignali L, Sangiorgio P, Tondi S, Serenelli C, De Palma R, et al. Impact of COPD on long‐term outcome after ST‐segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest. 2013;144:750–757. doi: 10.1378/chest.12-2313 [DOI] [PubMed] [Google Scholar]
- 13. Polosa R, Malerba M, Cacciola RR, Morjaria JB, Maugeri C, Prosperini G, Gullo R, Spicuzza L, Radaeli A, Di Maria GU. Effect of acute exacerbations on circulating endothelial, clotting and fibrinolytic markers in COPD patients. Inter Emerg Med. 2013;8:567–574. doi: 10.1007/s11739-011-0636-1 [DOI] [PubMed] [Google Scholar]
- 14. Agusti A, Edwards LD, Rennard SI, MacNee W, Tal‐Singer R, Miller BE, Vestbo J, Lomas DA, Calverley PM, Wouters E, et al. Evaluation of CLtIPSEI. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLOS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967 [DOI] [PubMed] [Google Scholar]
- 16. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of c‐reactive protein and low‐density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993 [DOI] [PubMed] [Google Scholar]
- 17. Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:982–988. doi: 10.1164/rccm.201206-1113OC [DOI] [PubMed] [Google Scholar]
- 18. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 19. Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, Martinez F, Yates J, Newby DE. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double‐blind randomised controlled trial. Lancet. 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1 [DOI] [PubMed] [Google Scholar]
- 20. Kunisaki KM, Dransfield MT, Anderson JA, Brook RD, Calverley PMA, Celli BR, Crim C, Hartley BF, Martinez FJ, Newby DE, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198:51–57. doi: 10.1164/rccm.201711-2239OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reilev M, Pottegard A, Lykkegaard J, Sondergaard J, Ingebrigtsen TS, Hallas J. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology. 2019;24:1183–1190. doi: 10.1111/resp.13620 [DOI] [PubMed] [Google Scholar]
- 22. Rothnie KJ, Connell O, Mullerova H, Smeeth L, Pearce N, Douglas I, Quint JK. Myocardial infarction and ischemic stroke after exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15:935–946. doi: 10.1513/AnnalsATS.201710-815OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Halpin DM, Decramer M, Celli B, Kesten S, Leimer I, Tashkin DP. Risk of nonlower respiratory serious adverse events following COPD exacerbations in the 4‐year UPLIFT(r) trial. Lung. 2011;189:261–268. doi: 10.1007/s00408-011-9301-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137:1091–1097. doi: 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 25. Wang M, Lin EP, Huang LC, Li CY, Shyr Y, Lai CH. Mortality of cardiovascular events in patients with COPD and preceding hospitalization for acute exacerbation. Chest. 2020;158:973–985. doi: 10.1016/j.chest.2020.02.046 [DOI] [PubMed] [Google Scholar]
- 26. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, et al. Once‐daily single‐inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 27. Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, Dransfield MT, Pavord I, Barnes N. A phase III randomised controlled trial of single‐dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48:320–330. doi: 10.1183/13993003.02165-2015 [DOI] [PubMed] [Google Scholar]
- 28. Day NC, Kumar S, Criner G, Dransfield M, Halpin DMG, Han MK, Jones CE, Kaisermann MC, Kilbride S, Lange P, et al. Single‐inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol and umeclidinium/vilanterol in patients with COPD: results on cardiovascular safety from the IMPACT trial. Respir Res. 2020;21:139. doi: 10.1186/s12931-020-01398-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medical Dictionary for Regulatory Activities . Patient‐friendly term list. Available at: https://www.meddra.org/patient‐friendly‐term‐list Accessed 4th March 2022
- 30. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task force for the Universal Definition of Myocardial I . Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 31. Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2022. Available at: https://goldcopd.org/2022‐gold‐reports‐2/ Accessed 4th March 2022
- 32. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pazarli AC, Koseoglu HI, Doruk S, Sahin S, Etikan I, Celikel S, Berktas B. Procalcitonin: is it a predictor of noninvasive positive pressure ventilation necessity in acute chronic obstructive pulmonary disease exacerbation? J Res Med Sci. 2012;17:1047–1051. [PMC free article] [PubMed] [Google Scholar]
- 34. Cazzola M, Matera MG, Donner CF. Inhaled beta2‐adrenoceptor agonists: cardiovascular safety in patients with obstructive lung disease. Drugs. 2005;65:1595–1610. doi: 10.2165/00003495-200565120-00001 [DOI] [PubMed] [Google Scholar]
- 35. European Medicines Agency (EMA) . Blenrep ‐ summary of product characterstics (SMPC). 2020. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/blenrep Accessed on 4th March 2022
- 36. Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta‐agonists in patients with asthma and COPD: a meta‐analysis. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309 [DOI] [PubMed] [Google Scholar]
- 37. Sestini P, Renzoni E, Robinson S, Poole P, Ram FS. Short‐acting beta 2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;2010:CD001495. doi: 10.1002/14651858.CD001495 [DOI] [PubMed] [Google Scholar]
- 38. Huiart L, Ernst P, Ranouil X, Suissa S. Oral corticosteroid use and the risk of acute myocardial infarction in chronic obstructive pulmonary disease. Can Respir J. 2006;13:134–138. doi: 10.1155/2006/935718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27:180057. doi: 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S7
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.


