Abstract
Background
Atrial fibrillation (AF) is associated with an increased risk of poor cardiovascular outcomes; appropriate rhythm control can reduce the incidence of these adverse events. Therefore, catheter ablation is recommended in symptomatic patients with AF. The aims of this study were to compare AF‐related outcomes according to a baseline symptom scale score and to determine the best treatment strategy for asymptomatic patients with AF.
Methods and Results
This study enrolled all patients who completed a baseline Atrial Fibrillation Effect on Quality‐of‐Life (AFEQT) survey in a prospective observational registry. The patients were divided into 2 groups according to AFEQT score at baseline; scores ≤80 were defined as symptomatic, whereas scores >80 represented asymptomatic patients. The primary outcome was defined as a composite of hospitalization for heart failure, ischemic stroke, or cardiac death. This study included 1515 patients (mean age: 65.7±10.5 years; 998 [65.9%] men). The survival curve showed a poorer outcome in the symptomatic group compared with the asymptomatic group (log‐rank P=0.04). Rhythm control led to a significantly lower risk of a composite outcome in asymptomatic patients (hazard ratio [HR], 0.47 [95% CI, 0.27–0.84], P=0.01). Rhythm control was associated with more favorable composite outcomes in the asymptomatic group with paroxysmal AF, left atrium diameter ≤50 mm, and CHA2DS2‐VASc score ≥3.
Conclusions
Symptomatic patients with AF experienced more adverse outcomes compared with asymptomatic patients. In asymptomatic patients with AF, a strategy of rhythm control improved the outcomes, especially with paroxysmal AF, smaller left atrium size, or higher stroke risk.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02786095.
Keywords: atrial fibrillation, quality of life, treatment outcome
Subject Categories: Atrial Fibrillation, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- AFEQT
Atrial Fibrillation Effect on Quality‐of‐Life
- EAST‐AFNET
Early Treatment of Atrial Fibrillation for Stroke Prevention Trial
Clinical Perspective.
What Is New?
This study reported cardiovascular outcomes according to a baseline symptom scale and found that rhythm control may improve the outcomes in asymptomatic patients with atrial fibrillation.
What Are the Clinical Implications?
A rhythm control strategy might be considered even in asymptomatic patients with atrial fibrillation, especially those with paroxysmal atrial fibrillation, normal left atrium size, and significant risk of stroke.
Atrial fibrillation (AF) is associated with increased risk of heart failure (HF), thromboembolic events, and cardiovascular death. 1 , 2 , 3 Rhythm control (including catheter ablation) for AF is associated with lower rates of both death and HF exacerbation. 4 In addition, rhythm control results in improvements in quality of life (QoL), especially via catheter ablation or electrical cardioversion. 5 , 6 , 7 Therefore, current guidelines recommend that AF‐related symptoms be quantified to aid in rhythm control treatment decisions. 8 The primary indication for rhythm control is need for reduced AF‐related symptoms in symptomatic patients with AF. 8 , 9 However, no trials to date have evaluated whether asymptomatic patients have better outcomes with rhythm control of AF. Previous studies have reported that ≈10% of ischemic strokes are related to unrecognized asymptomatic AF. 10 In addition, progression of asymptomatic subclinical AF has been associated with HF hospitalization. 11
The Atrial Fibrillation Effect on Quality‐of‐Life (AFEQT) survey has been validated to assess the impact of AF on QoL. 12 The objective of this study was to compare AF‐related outcomes according to the baseline symptom scale using AFEQT scores and to determine the best treatment strategy for asymptomatic patients.
METHODS
Data Availability
All supporting data are available within the article and the supplemental file.
Study Population
The CODE‐AF (Comparison Study of Drugs for Symptom Control and Complication Prevention of Atrial Fibrillation) Registry is an ongoing prospective, multicenter, observational registry located at 18 tertiary hospitals in Republic of Korea. The study design and details have been described previously. 13 In brief, this registry contains epidemiological data of patients with AF along with their treatment plans and clinical outcomes. This study was approved by the institutional review board (IRB) of Samsung Medical Center in Republic of Korea (number: 2018–01‐157). All patients provided informed consent and were registered at ClinicalTrials.gov (NCT02786095).
We included all patients who completed a baseline AFEQT survey between June 2016 and January 2021. We excluded any patients who had a short follow‐up period with less than 1 follow‐up visit without available clinical outcome data. The index date was that on which the baseline AFEQT survey was conducted. A follow‐up visit was scheduled every 6 months until the end of the study. Our final analysis included 1515 patients.
AFEQT Survey
The AFEQT 20‐item questionnaire is a validated QoL survey used to assess symptoms related to AF. 12 The questionnaire consists of 4 categories: symptoms, daily activities, treatment concerns, and treatment satisfaction. The overall AFEQT score is calculated using the first 3 domains, which include 18 questions (AFEQT18). Each question is assessed using 7‐point Likert‐type responses, and the raw scores are transformed into an overall score from 0 to 100, with 0 representing the most severe symptoms and 100 representing the best health status with no limitations. The version of the AFEQT used in this study had been linguistically translated into Korean. To calculate the participants' pure AF‐related symptom scores, the first 12 questions in the “symptom” and “daily activities” domains were assessed (AFEQT12). According to a study that validated the European Heart Rhythm Association symptom classification score using AFEQT scores, the average AFEQT score of European Heart Rhythm Association Class 1 classified into the asymptomatic group was 78.4. 14 On this basis, research based on patient QoL questionnaires found that an AFEQT score <80 was defined as symptomatic. 15 Therefore, AFEQT scores <80 were defined as symptomatic. 14 , 15 The AFEQT questionnaire is shown in Data S1.
Data Collection and Study Outcomes
The basic demographic data of each participant were acquired from the database. The patients were divided into 2 groups according to baseline AFEQT score, where a score ≤80 was defined as symptomatic and scores >80 comprised the asymptomatic group. The primary outcome was the composite of the incidence rates of hospitalization for HF, ischemic stroke, and cardiac death. The treatment strategy was also assessed; therapies comprised catheter ablation, electrical cardioversion, antiarrhythmic agents, or rate‐control strategies. We grouped the patients using 2 methods. First, we grouped patients into 2 treatment strategy groups for analysis: the rhythm control group and the rate control group. The rhythm control group was defined as patients treated with antiarrhythmic drugs, electrical cardioversion, and/or catheter ablation. If a patient did not receive any type of rhythm control treatment (including antiarrhythmic agent therapy), the patient was classified into the rate control group. Second, the patients were classified into 2 treatment strategy groups according to catheter ablation: the catheter ablation group and the medical therapy group. If a patient received multiple treatment strategies, including catheter ablation, they were allocated to the catheter ablation group. The medical therapy group was defined as those either treated with antiarrhythmic drugs, electrical cardioversion, and/or with drugs for rate control.
A subgroup analysis was performed based on age, sex, AF type, ejection fraction, left atrial diameter, and CHA2DS2‐VASc score. In each subgroup analysis, the statistical significance (P<0.1) of the interaction between treatment with the specific subgroup was evaluated. Masked adjudication of all relevant events was performed by expert physicians, who reviewed cases in a blinded manner with regard to the study treatment.
Statistical Analysis
The baseline characteristics are presented as the mean±SD for continuous variables and as frequency with percentage for categorical variables. Continuous variables were compared using an unpaired t test, and categorical variables were compared using either the χ2 test or the Fisher's exact test where appropriate. Event rate curves were obtained using a Kaplan–Meier analysis and were compared using the log‐rank test. The risks for the composite outcome were assessed using a Cox proportional hazards model and are presented as the hazard ratio (HR). Proportional hazards assumptions were assessed using Schoenfeld residuals. P values <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences software, version 27.0 (SPSS, Inc., Chicago, IL).
RESULTS
Clinical Characteristics
This study followed 1515 patients (mean age: 65.7±10.5 years; 998 [65.9%] men) with AF. The mean follow‐up duration was 806.9±364.2 days. The average AFEQT18 summary score was 83.4±14.9, and 1020 patients (67.3%) were asymptomatic. The mean AFEQT12 summary score for the symptom and daily activity domains was 83.5±17.0, and 1058 patients (69.8%) were defined as asymptomatic. The baseline characteristics of all participants and the symptom scale results are summarized in Table 1 and Table S1. There were significant differences in age, sex, and CHA2DS2‐VASc score between the 2 groups. The symptomatic group showed an older age and a higher CHA2DS2‐VASc score.
Table 1.
Baseline Characteristics
| Variables | All patients (N=1515) | AFEQT12 >80 (N=1058) | AFEQT12 ≤80 (N=457) | P value |
|---|---|---|---|---|
| Age, y | 65.7±10.5 | 65.4±10.3 | 66.5±10.9 | 0.044 |
| Sex (male) (n, %) | 998 (65.9%) | 746 (70.5%) | 252 (55.1%) | < 0.001 |
| Hypertension (n, %) | 963 (63.6%) | 674 (63.7%) | 289 (63.2%) | 0.907 |
| Diabetes (n, %) | 343 (22.6%) | 249 (23.5%) | 94 (20.6%) | 0.229 |
| Previous stroke (n, %) | 171 (11.3%) | 109 (10.3%) | 62 (13.6%) | 0.077 |
| Congestive heart failure (n, %) | 121 (8.0%) | 78 (7.4%) | 43 (9.4%) | 0.181 |
| CHA2DS2‐VASc | 2.3±1.6 | 2.2±1.5 | 2.6±1.7 | 0.001 |
| HAS‐BLED | 1.6±1.0 | 1.6±1.0 | 1.6±1.0 | 0.938 |
| Atrial fibrillation type (n, %) | 0.353 | |||
| Paroxysmal | 959 (63.3%) | 678 (64.1%) | 281 (61.5%) | |
| Persistent | 556 (36.7%) | 380 (35.9%) | 176 (38.5%) | |
| Ejection fraction (%) | 61.4±8.9 | 61.6±8.9 | 60.8±8.7 | 0.156 |
| LA diameter, mm | 43.7±15.0 | 44.0±17.1 | 43.1±7.8 | 0.358 |
| LA volume index, mL/m2 | 45.2±19.4 | 44.9±19.0 | 46.0±20.4 | 0.437 |
| Antiarrhythmic drug use only | 562 (37.1%) | 387 (36.6%) | 175 (38.3%) | 0.562 |
| Catheter ablation | 260 (17.2%) | 177 (16.7%) | 83 (18.2%) | 0.505 |
| Rate control only | 584 (38.5%) | 412 (38.9%) | 172 (37.6%) | 0.646 |
AFEQT indicates Atrial Fibrillation Effect on Quality‐of‐Life; and LA, left atrium.
To treat AF, 171 (11.3%) patients underwent electrical cardioversion during follow‐up, 260 (17.2%) received catheter ablation, 781 (51.6%) were treated with an antiarrhythmic agent with or without catheter ablation, and 562 (37.1%) were treated with an antiarrhythmic agent only. In addition, 62 (4.1%) patients in the catheter ablation group received electrical cardioversion compared with 109 (7.2%) in the antiarrhythmic agent use group.
Overall Outcomes According to Symptom Scale
AFEQT12 Scores
A composite outcome of hospitalization for HF, ischemic stroke, and cardiac death events occurred in 48 patients in the asymptomatic group (2.1 per 100 person‐years) and in 31 patients in the symptomatic group (3.3 per 100 person‐years). Symptomatic patients had a significantly higher risk of a composite outcome compared with the asymptomatic group (unadjusted HR, 1.60 [95% CI, 1.02–2.52], P=0.04; adjusted HR, 1.55 [95% CI, 0.99–2.44], P=0.06) (Table 2). The Kaplan–Meier curves for composite hospitalization for HF, ischemic stroke, and cardiac death in the symptomatic group compared with the asymptomatic group are shown in Figure 1A according to the symptom and daily activity scores of the AFEQT12 (log‐rank P=0.04).
Table 2.
Clinical Outcomes and Hazard Ratios for the Composite Outcome According to Symptom Scale
| No. of patients | Ischemic stroke | Hospitalization for heart failure | Cardiac death | Composite outcome* | Unadjusted HR (95% CI) | P value | Adjusted HR† (95% CI) | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events No. | Event rate %/100 pys | Events No. | Event rate %/100 pys | Events No. | Event rate %/100 pys | Events No. | Event rate %/100 pys | ||||||
| Asymptomatic | 1058 | 24 | 2.22 | 7 | 0.49 | 0 | … | 48 | 2.08 | ref | ref | ||
| Symptomatic | 457 | 13 | 4.19 | 36 | 0.93 | 2 | 0.21 | 31 | 3.33 | 1.60 (1.02–2.52) | 0.04 | 1.55 (0.99–2.44) | 0.06 |
HR indicates hazard ratio.
Composite outcome includes ischemic stroke, heart failure hospitalization, and cardiac death.
Adjusted for age, sex, and underlying disease (diabetes, hypertension).
Figure 1. Kaplan–Meier curves of the primary outcomes according to AFEQT score.
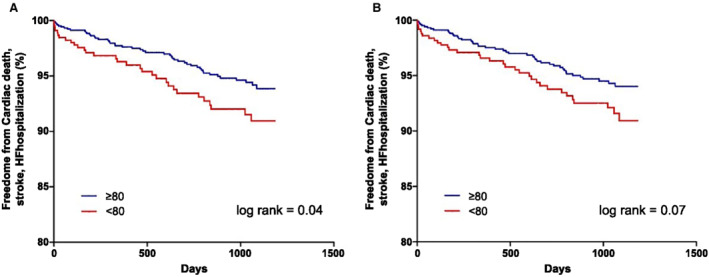
A, The AFEQT12 scores, which were related to symptoms and daily activities. B, The overall AFEQT18 scores. AFEQT indicates Atrial Fibrillation Effect on Quality‐of‐Life; and HF, heart failure.
AFEQT18 Scores
A composite outcome event occurred in 46 patients in the asymptomatic group (2.1 per 100 person‐years) and in 33 patients in the symptomatic group (3.2 per 100 person‐years). Patients who were symptomatic demonstrated a trend for a higher risk of a composite outcome compared with the asymptomatic group (unadjusted HR, 1.52 [95% CI, 0.97–2.37], P=0.07; adjusted HR, 1.49 [95% CI, 0.95–2.33], P=0.08). The Kaplan–Meier curves are shown in Figure 1B (log‐rank P=0.07).
AFEQT Scores by Treatment Strategy Group
An outcome analysis was performed according to treatment strategy in asymptomatic patients (those with AFEQT12 score >80). The rhythm control group showed a significantly lower risk of a composite outcome compared with the rate control group (unadjusted HR, 0.47 [95% CI, 0.27–0.84], P=0.01; adjusted HR, 0.52 [95% CI, 0.29–0.93], P=0.03). The survival curve demonstrated a significant reduction in composite outcome in the rhythm control group compared with the rate control group (log‐rank P=0.01, Figure 2). The survival curves showed a similar reduction in outcome for the catheter ablation group compared with the participants who underwent any other medical treatment type in asymptomatic patients (log‐rank P=0.04, Figure 2). The Kaplan–Meier curves (Figure S1) demonstrated a significant reduction in the primary outcome in the rhythm control group compared with the rate control group in symptomatic patients (AFEQT ≤80). The composite outcomes according to symptom scale in patients who received catheter ablation and medical therapy were analyzed in all patients (Figure S2). The survival curve showed no significant differences based on symptom scale in the catheter ablation group and the medical therapy group.
Figure 2. Kaplan–Meier curves of the primary outcomes in the asymptomatic group according to treatment strategy.
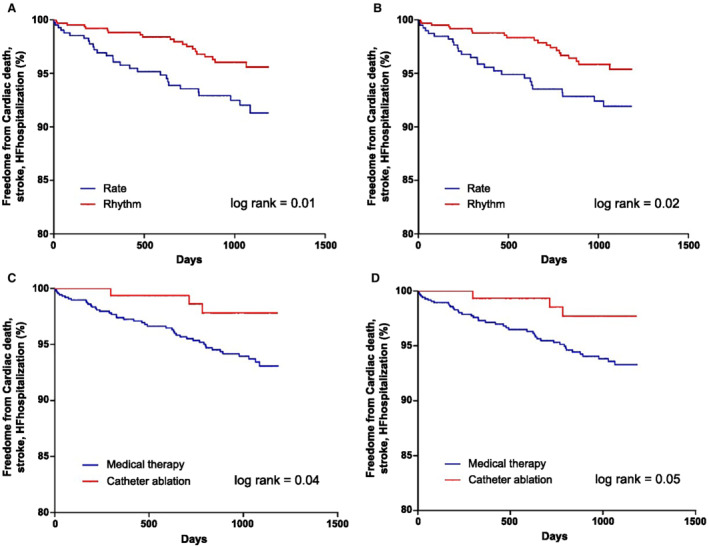
A, Rhythm control vs rate control therapy in patients with AFEQT12 score >80. B, Rhythm control vs rate control in patients with AFEQT18 score >80. C, Catheter ablation vs medical therapy in patients with AFEQT12 score >80, which was related with symptoms and daily activities. D, Catheter ablation vs medical therapy in patients with AFEQT18 score >80. AF indicates atrial fibrillation; AFEQT, Atrial Fibrillation Effect on Quality‐of‐Life; HF, heart failure; and HR, hazard ratio.
A subgroup analysis in the asymptomatic group was carried out according to treatment strategy. For the composite outcomes, the benefit of rhythm control compared with rate control showed a significant interaction in patients with left atrium (LA) diameter >50 compared with ≤50 and also for those with CHA2DS2‐VASc ≥3 versus <3 subgroups. Rhythm control had a more favorable composite outcome in paroxysmal AF (HR, 0.31 [95% CI, 0.14–0.69]), LA diameter ≤50 (HR, 0.36 [95% CI, 0.17–0.77]), and CHA2DS2‐VASc score ≥3 (HR, 0.49 [95% CI, 0.24–0.99]) subgroups (Figure 3).
Figure 3. The hazard ratios of composite outcomes of ischemic stroke, HF hospitalization, and cardiac death by subgroup compared with rhythm and rate control in asymptomatic patients.
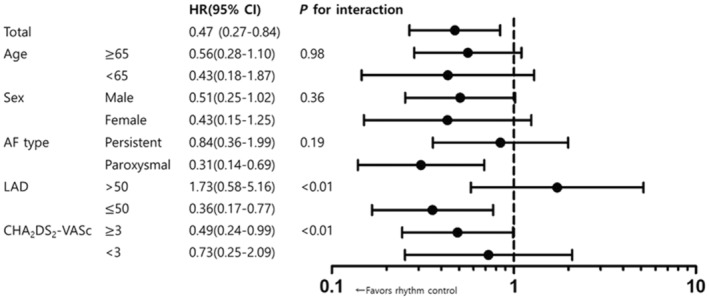
AF indicates atrial fibrillation; HF, heart failure; and LAD, left atrial diameter.
DISCUSSION
This is the first investigation of a patient outcome analysis according to a baseline AF symptom scale. The main findings were as follows. First, symptomatic patients with AF showed more adverse outcomes than asymptomatic patients regardless of treatment strategy. Second, rhythm control demonstrated a significant reduction in adverse cardiovascular events in the asymptomatic group. Third, rhythm control produced a favorable outcome in asymptomatic patients with paroxysmal AF, LA size ≤50 mm, and CHA2DS2‐VASc score ≥3.
Symptoms and Adverse Cardiovascular Events
AF symptoms range from none to disabling and can be nonspecific. Several scales are currently used to characterize symptom severity, such as the European Heart Rhythm Association symptom scale, the AFEQT score, and the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. 12 , 14 , 16 This scale is a simple semiquantitative validated instrument that is practical for clinical use. 17 In our study, we used the AFEQT scores to distinguish symptoms in detail through quantitative and detailed questions. Symptom improvement has been demonstrated with rhythm control treatment (including cardioversion or catheter ablation), so symptom status affects treatment strategy. 5 , 18 Recent reports suggest that an initial treatment strategy using cryoballoon catheter ablation significantly improved AF‐related symptoms and QoL, with a mean 8.32‐point difference in AFEQT scores. 19 However, little is known about adverse cardiovascular outcomes in asymptomatic patients with AF. In aspects of ventricular premature depolarization, the incidence of left ventricular dysfunction associated with ventricular premature depolarization was significantly higher in the asymptomatic group. 20 There is some evidence that the increased subclinical burden in patients with AF is associated with hospitalization for HF. 11 In our study, the symptomatic group demonstrated worse outcomes based on rates of hospitalization for HF, ischemic stroke, or cardiac death. In addition, rhythm control produced a more favorable outcome than rate control in symptomatic patients. These results were consistent in the asymptomatic group. We also found that rhythm control reduced the rate of adverse outcomes in patients with paroxysmal AF, smaller LA size, and higher stroke risk. Previous studies have suggested that subclinical asymptomatic AF is associated with increased risk of ischemic stroke or systemic embolism, and early screening of AF is important in patients with higher stroke risk. 21 , 22 Our study results support early screening of AF and rhythm control in such patients, especially those with higher CHA2DS2‐VASc score to reduce adverse cardiovascular outcomes.
A higher AFEQT score indicates fewer limitations in daily activities and fewer symptoms. Therefore, this group included healthier patients with fewer comorbidities. In other words, these patients may be in a relatively early stage of AF disease progression or have a smaller AF burden. The most recent AF innovation study, the EAST‐AFNET (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial), demonstrated that early rhythm control therapy was associated with a lower risk of adverse cardiovascular outcomes than usual care. 23 In this regard, our study supports a similar hypothesis that rhythm control can improve cardiovascular outcomes even in patients with fewer symptoms and limitations. However, we acknowledge that not all asymptomatic patients can be assumed to be in the early stage of AF.
The average AFEQT score was relatively higher in our cohort. Most other studies have enrolled patients who were scheduled to begin rhythm control treatment, which suggests that their participants likely included more symptomatic patients. In contrast, our cohort included all patients diagnosed with AF and was designed to assess clinical epidemiology, therapeutic processes, and clinical outcomes. Therefore, our study was more likely to include asymptomatic patients. In addition, one registry that was designed to collect clinical variables and outcomes from patients with AF showed that 54.6% of patients were asymptomatic based on AFEQT score >80. 14 Therefore, our registry likely better reflects real‐world conditions instead of the characteristics of patients who plan to begin an intervention for AF symptoms.
Limitations
This study had some limitations. First, as this was an observational study design, there was potential for selection bias and residual confounding factors owing to incomplete adjustment. However, our study included a relatively larger scale of patients and a long‐term follow‐up duration that was comparable with other randomized controlled studies. Second, we could not find any verified follow‐up symptom scale that was organized according to treatment strategy. Furthermore, the presence of missing follow‐up data reduced statistical power in outcome analysis. However, because this registry is an ongoing prospective observational registry, there is a lower chance for event loss. The symptomatic group showed older age and higher CHA2DS2‐VASc score, which might have produced selection bias and influenced the outcome analysis. However, it would have not affected the analysis of the asymptomatic group. In contrast, these differences in baseline characteristics suggest that the asymptomatic group was likely in the early stage of AF. Finally, we were unable to analyze the outcomes according to rhythm status. Our study results indicate that a rhythm control strategy can improve outcome even in asymptomatic patients with AF regardless of the maintenance of a normal sinus rhythm and suggest the principle of treatment to be applied in this population. Despite these limitations, this study presents real‐world clinical cardiovascular outcomes according to a baseline symptom scale and suggests some recommendations for treatment guidelines in asymptomatic patients.
CONCLUSIONS
This study revealed worthwhile observational data on cardiovascular outcomes according to a baseline symptom scale. Our observation suggests that rhythm control may improve outcomes even in asymptomatic patients. Further, a strategy of rhythm control might be considered in asymptomatic patients with paroxysmal AF, smaller LA size, or higher stroke risk. Based on this consideration, a randomized clinical trial will be necessary to confirm our observations.
Sources of Funding
This research was supported by a grant of the Patient‐Centered Clinical Research Coordinating Center funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HC19C013).
Disclosures
None.
Supporting information
Data S1
Table S1
Figures S1–S2
J. Y. Kim and H. Park contributed equally.
For Sources of Funding and Disclosures, see page 7.
Contributor Information
Boyoung Joung, Email: cby6908@yuhs.ac.
Kyoung‐Min Park, Email: kyoungmin.park@samsung.com.
REFERENCES
- 1. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013;128:2192–2201. doi: 10.1161/circulationaha.112.000491 [DOI] [PubMed] [Google Scholar]
- 2. Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6 [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.Cir.0000072767.89944.6e [DOI] [PubMed] [Google Scholar]
- 4. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 5. Sandhu RK, Smigorowsky M, Lockwood E, Savu A, Kaul P, McAlister FA. Impact of electrical cardioversion on quality of life for the treatment of atrial fibrillation. Can J Cardiol. 2017;33:450–455. doi: 10.1016/j.cjca.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 6. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. doi: 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blomström‐Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, Rubulis A, Malmborg H, Raatikainen P, Lönnerholm S, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019;321:1059–1068. doi: 10.1001/jama.2019.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 9. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/cir.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 10. Wallenhorst C, Martinez C, Freedman B. Risk of ischemic stroke in asymptomatic atrial fibrillation incidentally detected in primary care compared with other clinical presentations. Thromb Haemost. 2021;122:277–285. doi: 10.1055/a-1541-3885 [DOI] [PubMed] [Google Scholar]
- 11. Wong JA, Conen D, Van Gelder IC, McIntyre WF, Crijns HJ, Wang J, Gold MR, Hohnloser SH, Lau CP, Capucci A, et al. Progression of device‐detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. 2018;71:2603–2611. doi: 10.1016/j.jacc.2018.03.519 [DOI] [PubMed] [Google Scholar]
- 12. Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, Lakkireddy DR, Wimmer AP, Bhandari A, Burk C. Development and validation of the Atrial Fibrillation Effect on QualiTy‐of‐Life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15–25. doi: 10.1161/circep.110.958033 [DOI] [PubMed] [Google Scholar]
- 13. Kim H, Kim TH, Cha MJ, Lee JM, Park J, Park JK, Kang KW, Shim J, Uhm JS, Kim J, et al. A Prospective survey of atrial fibrillation management for real‐world guideline adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE‐AF) Registry. Korean Circ J. 2017;47:877–887. doi: 10.4070/kcj.2017.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof P, Gupta D. The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace. 2014;16:965–972. doi: 10.1093/europace/eut395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katsumata Y, Kimura T, Kohsaka S, Ikemura N, Ueda I, Fujisawa T, Nakajima K, Nishiyama T, Aizawa Y, Oki T, et al. Discrepancy in recognition of symptom burden among patients with atrial fibrillation. Am Heart J. 2020;226:240–249. doi: 10.1016/j.ahj.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 16. Dorian P, Cvitkovic SS, Kerr CR, Crystal E, Gillis AM, Guerra PG, Mitchell LB, Roy D, Skanes AC, Wyse DG. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: the CCS‐SAF scale. Can J Cardiol. 2006;22:383–386. doi: 10.1016/s0828-282x(06)70922-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorian P, Guerra PG, Kerr CR, O'Donnell SS, Crystal E, Gillis AM, Mitchell LB, Roy D, Skanes AC, Rose MS, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol. 2009;2:218–224. doi: 10.1161/CIRCEP.108.812347 [DOI] [PubMed] [Google Scholar]
- 18. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- 19. Andrade JG, Wazni OM, Kuniss M, Hawkins NM, Deyell MW, Chierchia G‐B, Nissen S, Verma A, Wells GA, Turgeon RD. Cryoballoon ablation as initial treatment for atrial fibrillation. J Am Coll Cardiol. 2021;78:914–930. doi: 10.1016/j.jacc.2021.06.038 [DOI] [PubMed] [Google Scholar]
- 20. Park KM, Im SI, Chun KJ, Hwang JK, Park SJ, Kim JS, On YK. Asymptomatic ventricular premature depolarizations are not necessarily benign. Europace. 2016;18:881–887. doi: 10.1093/europace/euv112 [DOI] [PubMed] [Google Scholar]
- 21. Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, et al. Duration of device‐detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. doi: 10.1093/eurheartj/ehx042 [DOI] [PubMed] [Google Scholar]
- 22. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE‐AF study. Circulation. 2017;136:1784–1794. doi: 10.1161/circulationaha.117.030583 [DOI] [PubMed] [Google Scholar]
- 23. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figures S1–S2
Data Availability Statement
All supporting data are available within the article and the supplemental file.


