Abstract
Background
To evaluate the prognostic value of plasma YKL‐40 (human cartilage glycoprotein‐39) for acute ischemic stroke.
Methods and Results
We measured plasma YKL‐40 levels in 3377 participants from CATIS (China Antihypertensive Trial in Acute Ischemic Stroke). Study outcome data on death, major disability (modified Rankin Scale score ≥3), and vascular diseases were collected at 3 months after stroke onset. The primary outcome was defined as a combination of death and major disability. During the 3‐month follow‐up, 828 participants (24.5%) experienced major disability or died. After multivariate adjustment, the highest YKL‐40 quartile was associated with an increased risk of the primary outcome (odds ratio, 1.426 [95% CI, 1.105–1.839]; P trend=0.01) compared with the lowest quartile. Each SD increase in log‐transformed YKL‐40 level was associated with a 15.5% (95% CI, 5.6–26.3%) increased risk of the primary outcome. The multivariable‐adjusted spline regression models showed a linear dose–response relationship between YKL‐40 and clinical outcomes. Adding YKL‐40 to a model containing conventional risk factors significantly improved the reclassification power for the primary outcome (net reclassification improvement: 15.61%, P<0.001; integrated discrimination index: 0.37%, P=0.004) and marginally significantly improved the discriminatory power for the primary outcome (area under the receiver operating characteristic curve improved by 0.003, P=0.099).
Conclusions
A higher YKL‐40 level in the acute phase of ischemic stroke was associated with an increased risk of mortality and major disability at 3 months after stroke, indicating that YKL‐40 may play an important role as a prognostic marker of ischemic stroke.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01840072.
Keywords: acute ischemic stroke, prognosis, YKL‐40
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- CATIS
China Antihypertensive Trial in Acute Ischemic Stroke
- mRS
modified Rankin Scale
- YKL‐40
human cartilage glycoprotein‐39
Clinical Perspective.
What Is New?
Elevated plasma YKL‐40 (human cartilage glycoprotein‐39) is associated with major disability and mortality of ischemic stroke.
YKL‐40 has a potential value in predicting the prognosis of patients with ischemic stroke.
What Are the Clinical Implications?
YKL‐40 has potential as a target for ischemic stroke therapy.
Lowering the YKL‐40 levels might improve the clinical outcomes of ischemic stroke.
YKL‐40 (human cartilage glycoprotein‐39), an inflammatory biomarker, has been reported to be involved in the process and progression of atherosclerosis. 1 , 2 It is secreted from macrophages, neutrophils, and vascular smooth muscle cells. 3 , 4 , 5 Previous large‐sample studies have reported that elevated YKL‐40 levels are associated with a higher risk of ischemic stroke. 6 , 7 YKL‐40 was also found to be correlated with infarct volume and admission severity of acute ischemic stroke. 8 However, limited studies with reliable statistical power have investigated the effect of YKL‐40 on the comprehensive prognosis after ischemic stroke including functional outcome, mortality, and vascular events.
Biomarkers that could be used to identify patients at high risk of poor prognosis would increase the efficacy and precision of secondary prevention for ischemic stroke. Well‐designed prospective studies with large sample sizes to investigate the potential relationship between YKL‐40 levels and clinical outcomes of acute ischemic stroke are needed. The objective of this study was to prospectively investigate the association between plasma YKL‐40 levels and prognosis in patients with acute ischemic stroke using data from CATIS (China Antihypertensive Trial in Acute Ischemic Stroke).
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Participants
We conducted this study using CATIS data. CATIS was a multicenter, single‐blind, blinded end point randomized clinical trial conducted in 26 hospitals across China. The design and main results of CATIS have been described in detail previously. 9 Briefly, a total of 4071 patients aged >22 years who had ischemic stroke confirmed by computed tomography or magnetic resonance imaging of the brain within 48 hours of symptom onset and increased systolic blood pressure (BP) between 140 and 220 mm Hg were recruited. The CATIS exclusion criteria were any of the following: (1) systolic BP ≥220 or diastolic BP≥120 mm Hg; (2) severe heart failure, acute myocardial infarction, unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis (≥70%), resistant hypertension, deep coma, or transient ischemic attacks; and (3) treatment with intravenous thrombolytic therapy. For the present study, 623 patients were excluded because they did not offer blood samples or we failed to measure plasma YKL‐40 concentrations. After 3 months, 71 patients were lost to follow‐up, for a follow‐up rate of 97.9%. Finally, a total of 3377 patients with ischemic stroke from CATIS were analyzed in this study. Most baseline characteristics were balanced between the patients who had their plasma YKL‐40 measured and those who did not, indicating that those who were assayed were representative of all CATIS participants (Table S1). The CATIS trial was registered (URL: http://www.clinicaltrials.gov; unique identifier: NCT01840072). The current study was approved by the institutional review boards at Soochow University in China and Tulane University in the United States. Written consent was obtained from all study participants or their immediate family members.
Data Collection
Baseline data, including demographic characteristics, clinical characteristics, medical histories, and lifestyle factors, were collected at the time of enrollment. Stroke severity was assessed with the National Institutes of Health Stroke Scale by trained neurologists at admission. 10 According to the symptoms and imaging data of the patients, the ischemic stroke subtype was classified as large artery atherosclerosis, cardiac embolism, or small artery occlusion lacunae. 11 Three BP measurements were also obtained at admission by trained nurses using a standard mercury sphygmomanometer according to the standard procedure recommended by the American Heart Association. In addition, serum lipids, plasma glucose, and other clinical laboratory measurements were obtained at the participating hospitals at admission. Dyslipidemia was defined as total cholesterol ≥6.22 mmol/L or triglycerides ≥2.26 mmol/L or low‐density lipoprotein cholesterol ≥4.14 mmol/L or high‐density lipoprotein cholesterol <1.04 mmol/L or a self‐reported history of physician‐diagnosed dyslipidemia according to Chinese guidelines on the prevention and treatment of dyslipidemia. 12
YKL‐40 Measurements
For patients with ischemic stroke, fasting blood samples were collected within 24 hours of hospital admission. Plasma was separated from the blood sample at the clinical laboratories of the participating hospitals and immediately frozen at −80 °C. We measured the plasma YKL‐40 concentrations of all participants centrally at Soochow University. The plasma YKL‐40 test was performed using a commercial enzyme‐linked immunosorbent assay kit (catalog number DC3L10; R&D Systems, Inc., Minneapolis, MN) according to the manufacturer's instructions. The intra‐ and interassay coefficients of variation were <5% and 7%, respectively. The laboratory technicians were blinded to the clinical characteristics and the clinical outcomes of the patients with ischemic stroke.
Outcome Assessment
Participants were followed up in person at 3 months by trained neurologists who were unaware of the treatment assignment. The primary outcome was a composite outcome of death or major disability (modified Rankin Scale [mRS] score of 3–6) within 3 months. Secondary outcomes were separately those of death and major disability (mRS score of 6 and 3–5, respectively), and vascular diseases (eg, vascular death, nonfatal stroke, nonfatal myocardial infarction, hospitalized and treated angina, hospitalized and treated congestive heart failure, and hospitalized and treated peripheral arterial disease). We further included an ordered 7‐level categorical score of the mRS as an outcome for neurological functional status based on the recommendation for acute stroke trials. 13 Death certificates were obtained for the participants who died, and hospital data were collected for all vascular events. The outcome assessment committee reviewed and adjudicated vascular events based on the criteria established in ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial).
Statistical Analysis
The participants were classified into plasma YKL‐40 level quartiles. Baseline characteristics were compared among quartiles using χ2 tests for categorical variables and analysis of variance (normal distribution) or Wilcoxon signed‐rank test (skewed distribution) for continuous variables, respectively. Multivariable binary logistic regression was used to estimate the risk of clinical outcomes associated with YKL‐40 quartiles. Odds ratios (OR) and 95% CIs were calculated for upper quartiles of YKL‐40 with the lowest quartile as a reference. The covariates included in the multivariable models were age, sex, admission National Institutes of Health Stroke Scale score, antihypertensive treatment, time from onset to hospitalization, current smoking status, alcohol consumption, body mass index, fasting plasma glucose, dyslipidemia, history of hypertension, history of diabetes, family history of stroke, systolic BP at baseline, and ischemic stroke subtype. Tests for linear trends in ORs across YKL‐40 quartiles were conducted with each quartile as the continuous parameter in the model. In addition, we used restricted cubic splines to examine the pattern and magnitude of associations between plasma YKL‐40 and clinical outcomes with 4 knots (at the 5th, 35th, 65th, and 95th percentiles). 14 The effect of the YKL‐40 quartiles on the mRS shift was analyzed using the multivariable ordinal logistic regression model. Furthermore, we calculated the area under the receiver operating characteristic curve, continuous net reclassification index, and integrated discrimination improvement to assess the incremental discriminatory power and reclassification value of plasma YKL‐40 beyond conventional risk factors for prognosis. 15
Because previous studies have reported that YKL‐40 levels are associated with several stroke‐related risk factors (ie, age, sex, hypertension, and diabetes), 16 , 17 , 18 we conducted subgroup analyses to assess the association between YKL‐40 and the primary outcome. Interactions between plasma YKL‐40 and subgroup variables on the primary outcome were tested in the models with interaction terms using the likelihood ratio test, adjusting for the aforementioned covariates unless the variable was used as a subgroup variable. All P values were 2 tailed, and a significance level of 0.05 was used. All statistical analyses were conducted with SAS statistical software (version 9.4, Cary, NC) and R software (version 4.0; The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Among the 3377 patients with ischemic stroke, those with higher plasma YKL‐40 levels tended to be older, female, nonsmokers and nondrinkers; to have higher baseline systolic BP, glucose, National Institutes of Health Stroke Scale score, total cholesterol, and triglycerides; and to have a higher prevalence of a history of diabetes. However, they were also likely to have lower diastolic BP and body mass index, a shorter time from onset to randomization, and a lower prevalence of a history of hypertension (Table 1).
Table 1.
Characteristics of Participants According to Quartiles of Plasma YKL‐40
| Characteristics | YKL‐40, ng/mL | |||||
|---|---|---|---|---|---|---|
| Total | <37.4751 | 37.4751–64.37 | 64.37–133.4822 | ≥133.4822 | P trend | |
| No. of participants | 3377 | 846 | 846 | 845 | 840 | |
| Age, y | 61.6±10.8 | 56.8±10.0 | 59.5±9.9 | 62.7±10.0 | 67.2±10.4 | <0.001 |
| Male sex, n (%) | 2171 (64.3) | 597 (70.6) | 587 (69.4) | 498 (58.9) | 489 (58.2) | <0.001 |
| Time from onset to randomization, hour | 10.3 (5–24) | 12 (5–24) | 12 (5–24) | 11 (5–24) | 8.3 (4–24) | <0.001 |
| Admission systolic BP, mm Hg | 166.1±16.8 | 164.1±16.5 | 166.4±17.1 | 166.5±16.6 | 167.3±16.7 | <0.001 |
| Admission diastolic BP, mm Hg | 96.6±11.0 | 98±11.2 | 97.6±11.2 | 96.3±10.4 | 94.6±11 | <0.001 |
| Admission National Institute of Health Stroke Scale score | 4 (2–7) | 4 (2–7) | 4 (2–7) | 4 (3–7) | 5 (3–9) | <0.001 |
| Body mass index, kg/m2 | 25.0±3.1 | 25.1±3.1 | 25.1±2.9 | 24.9±3.1 | 24.7±3.4 | 0.02 |
| Total cholesterol, mmol/L | 5.1±1.2 | 4.9±1.1 | 5.1±1.1 | 5.1±1.2 | 5.1±1.3 | 0.001 |
| Triglycerides, mmol/L | 1.9±2.7 | 1.7±1.1 | 1.8±1.3 | 1.9±1.4 | 2.1±5.1 | 0.004 |
| Low‐density lipoprotein cholesterol, mmol/L | 2.9±0.9 | 2.9±0.9 | 2.9±1.0 | 3.0±1.0 | 2.9±0.9 | 0.63 |
| High‐density lipoprotein cholesterol, mmol/L | 1.3±0.5 | 1.3±0.5 | 1.3±0.5 | 1.3±0.4 | 1.3±0.4 | 0.66 |
| Glucose, mmol/L | 6.7±2.8 | 6.4±2.4 | 6.7±2.8 | 6.7±3.0 | 6.9±2.8 | 0.002 |
| Current cigarette smoking, n (%) | 1248 (37.0) | 351 (41.5) | 349 (41.3) | 257 (30.4) | 291 (34.6) | <0.001 |
| Current alcohol drinking, n (%) | 1068 (31.6) | 309 (36.5) | 296 (35.0) | 241 (28.5) | 222 (26.4) | <0.001 |
| Dyslipidemia, n (%) | 1799 (53.3) | 431 (51.0) | 454 (53.7) | 463 (54.8) | 451 (53.7) | 0.22 |
| History of hypertension, n (%) | 2652 (78.5) | 676 (79.9) | 678 (80.1) | 669 (79.2) | 629 (74.9) | 0.01 |
| History of diabetes, n (%) | 596 (17.7) | 141 (16.7) | 138 (16.3) | 147 (17.4) | 170 (20.2) | 0.04 |
| Family history of stroke, n (%) | 634 (18.8) | 169 (20.0) | 153 (18.1) | 162 (19.2) | 150 (17.9) | 0.38 |
| Antihypertensive medications, n (%) | 1687 (50.0) | 417 (49.3) | 413 (48.8) | 426 (50.4) | 431 (51.3) | 0.32 |
| Ischemic stroke subtype, n (%) | ||||||
| Large artery atherosclerosis | 2612 (77.4) | 673 (79.6) | 652 (77.1) | 655 (77.5) | 632 (75.2) | 0.05 |
| Cardiac embolism | 150 (4.4) | 40 (4.7) | 27 (3.2) | 35 (4.1) | 48 (5.7) | 0.22 |
| Small artery occlusion lacunae | 615 (18.2) | 133 (15.7) | 167 (19.7) | 155 (18.3) | 160 (19.1) | 0.15 |
Continuous variables are expressed as mean±SD or median (interquartile range). Categorical variables are expressed as number (percentage). BP indicates blood pressure; and YKL‐40, human cartilage glycoprotein‐39.
During the 3 months of follow‐up, 736 patients (21.8%) experienced major disability and 92 patients (2.7%) died. As shown in Table 2, the multivariable‐adjusted ORs (95% CI) associated with the highest vs. lowest quartile of YKL‐40 were 1.426 (1.105–1.839), 1.329 (1.018–1.735), and 2.15 (1.135–4.075) for the primary outcome (P for trend=0.01), major disability (P for trend=0.05), and death (P for trend=0.003), respectively. Each 1 SD higher log‐transformed YKL‐40 level was significantly associated with a 15.5%, 11.3%, and 56.4% increased risk for the primary outcome, major disability, and death, respectively. Multivariable‐adjusted spline regression models showed a linear association between the YKL‐40 level and the primary outcome (P for linearity<0.001), major disability (P for linearity=0.03), and death (P for linearity<0.001) (Figure 1). No significant association between plasma YKL‐40 and vascular events was observed. Multivariable ordinal logistic regression analysis showed a significantly worse shift in the distribution of mRS scores with higher YKL‐40 quartiles in patients with ischemic stroke (OR, 1.08 [95% CI, 1.02–1.15]; P=0.01; Figure 2). In the subgroup analyses, the 3377 participants were stratified by age, sex, current smoking status, alcohol consumption, history of hypertension, history of diabetes, and receiving immediate BP reduction. A significant association between YKL‐40 and the risk of the primary outcome was observed in most of the subgroups (ORs [95% CIs] for quartiles 2 and 3 were not shown). These variables did not substantially modify the effect of YKL‐40 level on the risk of the primary outcome (Figure 3).
Table 2.
ORs (95% CIs) of Clinical Outcomes According to Quartiles of Plasma YKL‐40 in the Acute Phase of Ischemic Stroke
| Outcome | YKL‐40, ng/mL | |||||
|---|---|---|---|---|---|---|
| <37.4751 | 37.4751–64.37 | 64.37–133.4822 | ≥133.4822 | P trend | Each 1‐SD (0.852 ng/mL) increase in logYKL‐40 | |
| Primary outcome: death or major disability (mRS 3–6) | ||||||
| Case, n (%) | 161 (19.03) | 188 (22.22) | 208 (24.62) | 271 (32.26) | <0.001 | 828 (24.52) |
| Unadjusted | 1.00 | 1.215 (0.96–1.539) | 1.389 (1.101–1.752) | 2.026 (1.619–2.536) | <0.001 | 1.321 (1.22–1.431) |
| Multiple‐adjusted | 1.00 | 1.178 (0.914–1.517) | 1.19 (0.925–1.532) | 1.426 (1.105–1.839) | 0.01 | 1.155 (1.056–1.263) |
| Secondary outcomes | ||||||
| Major disability (mRS 3–5) | ||||||
| Case, n (%) | 146 (17.57) | 176 (21.1) | 189 (22.88) | 225 (28.34) | <0.001 | 736 (22.40) |
| Unadjusted | 1.00 | 1.255 (0.983–1.602) | 1.392 (1.093–1.772) | 1.855 (1.465–2.349) | <0.001 | 1.265 (1.163–1.375) |
| Multiple‐adjusted | 1.00 | 1.215 (0.936–1.578) | 1.197 (0.921–1.555) | 1.329 (1.018–1.735) | 0.05 | 1.113 (1.014–1.223) |
| Death | ||||||
| Case, n (%) | 15 (1.77) | 12 (1.42) | 19 (2.25) | 46 (5.48) | <0.001 | 92 (2.72) |
| Unadjusted | 1.00 | 0.797 (0.371–1.713) | 1.274 (0.643–2.525) | 3.21 (1.778–5.795) | <0.001 | 1.831 (1.475–2.273) |
| Multiple‐adjusted | 1.00 | 0.731 (0.336–1.59) | 1.044 (0.516–2.11) | 2.15 (1.135–4.075) | 0.003 | 1.564 (1.237–1.976) |
| Vascular events | ||||||
| Case, n (%) | 16 (1.89) | 22 (2.6) | 25 (2.96) | 27 (3.21) | 0.08 | 90 (2.67) |
| Unadjusted | 1.00 | 1.385 (0.722–2.656) | 1.582 (0.838–2.984) | 1.723 (0.921–3.221) | 0.08 | 1.21 (0.982–1.492) |
| Multiple‐adjusted | 1.00 | 1.212 (0.626–2.348) | 1.232 (0.64–2.373) | 1.172 (0.6–2.289) | 0.69 | 1.052 (0.838–1.321) |
Multiple‐adjusted covariates included age, sex, admission National Institutes of Health Stroke Scale score, antihypertensive treatment, time from onset to hospitalization, current smoking, alcohol consumption, body mass index, fasting plasma glucose, dyslipidemia, history of hypertension, history of diabetes, family history of stroke, systolic blood pressure at baseline, and ischemic stroke subtypes. mRS indicates modified Rankin scale; and YKL‐40, human cartilage glycoprotein‐39.
Figure 1. Association between plasma YKL‐40 levels and adverse clinical outcomes after ischemic stroke.
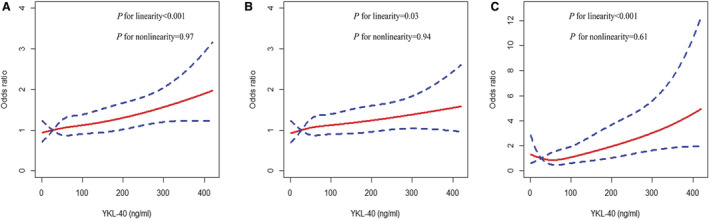
A, Primary outcome. B, Major disability. C, Death. Odds ratios (red solid line) and 95% CIs (blue dotted line) derived from restricted cubic spline regression, with knots placed at the 5th, 35th, 65th, and 95th percentiles of the distribution of plasma YKL‐40. ORs were adjusted for age, sex, admission NIHSS score, antihypertensive treatment, time from onset to hospitalization, current smoking status, alcohol consumption, body mass index, fasting plasma glucose, dyslipidemia, history of hypertension, history of diabetes, family history of stroke, systolic blood pressure at baseline, and ischemic stroke subtype. NIHSS indicates National Institutes of Health Stroke Scale; OR, odds ratio; and YKL‐40, human cartilage glycoprotein‐39.
Figure 2. Distribution of modified Rankin Scale (mRS) score at 3 months according to plasma YKL‐40 quartiles in patients with ischemic stroke.

YKL‐40 indicates human cartilage glycoprotein‐39.
Figure 3. Subgroup analyses of the association between plasma YKL‐40 and primary outcome (quartile 4 vs quartile 1, data for quartiles 2 and 3 not shown).

The odds ratios and 95% CIs were calculated after adjusting for the same confounding factors in Table 2 unless the variable was used as a subgroup variable. BP indicates blood pressure; OR, odds ratio; and YKL‐40, human cartilage glycoprotein‐39.
Among all patients with ischemic stroke, adding plasma YKL‐40 to a model containing conventional risk factors significantly improved the risk reclassification for the clinical outcomes (primary outcome: net reclassification index =15.61%, P<0.001, integrated discrimination improvement =0.37%, P=0.004; major disability: net reclassification index =11.35%, P=0.005, integrated discrimination improvement =0.17%, P=0.06; death: net reclassification index =42.75%, P<0.001, integrated discrimination improvement =1.37%, P=0.001). It also improved the discriminatory power for the prognosis to some degree (primary outcome: the area under the receiver operating characteristic curve improved by 0.003, P=0.099; major disability: the area under the receiver operating characteristic curve improved by 0.002, P=0.158; death: the area under the receiver operating characteristic curve improved by 0.026, P=0.049) (Table 3).
Table 3.
Discrimination and Reclassification Statistics for 3‐Month Clinical Outcomes by Plasma YKL‐40 Among Patients With Acute Ischemic Stroke
| AUC | NRI | IDI | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI), % | P value | Estimate (95% CI), % | P value | Estimate (95% CI), % | P value | |
| Death or major disability (mRS score 3–6) | ||||||
| Conventional model | 0.747 (0.732–0.761) | Reference | Reference | |||
| Conventional model+YKL‐40 | 0.75 (0.735–0.764) | 0.099 | 15.61 (8.02–23.21) | <0.001 | 0.37 (0.12–0.62) | 0.004 |
| Major disability (mRS score 3–5) | ||||||
| Conventional model | 0.745 (0.73–0.76) | Reference | Reference | |||
| Conventional model+YKL‐40 | 0.747 (0.732–0.762) | 0.158 | 11.35 (3.44–19.26) | 0.005 | 0.17 (−0.01–0.34) | 0.06 |
| Death | ||||||
| Conventional model | 0.732 (0.717–0.747) | Reference | Reference | |||
| Conventional model+YKL‐40 | 0.758 (0.743–0.772) | 0.049 | 42.75 (22.09–63.42) | <0.001 | 1.37 (0.54–2.2) | 0.001 |
Conventional model included age, sex, admission National Institutes of Health Stroke Scale score, antihypertensive treatment, time from onset to hospitalization, current smoking, alcohol consumption, body mass index, dyslipidemia, fasting plasma glucose, history of hypertension, history of diabetes, family history of stroke, systolic blood pressure at baseline, and ischemic stroke subtypes. AUC indicates area under the receiver operating characteristic curve; IDI, integrated discrimination index; mRS, modified Rankin Scale; NRI, net reclassification improvement; and YKL‐40, human cartilage glycoprotein‐39.
DISCUSSION
In this large prospective multicenter CATIS study, we observed dose–response associations between higher YKL‐40 levels in ischemic stroke and an increased risk of major disability and death after adjusting for other established risk factors. The addition of YKL‐40 level to a model containing conventional risk factors substantially improved the prediction power for ischemic stroke prognosis. Our findings suggest that plasma YK‐40 is an effective prognostic marker for ischemic stroke.
There are limited data on the effect of YKL‐40 levels on poor prognosis in patients with ischemic stroke. Chen et al. reported that YKL‐40 levels were higher in patients with ischemic stroke than those in controls. It was also reported that YKL‐40 level was associated with the 3‐month functional outcome (OR, 6.47 [95% CI, 1.36–30.76]) and improved the prognostic accuracy of the conventional factors in predicting functional outcome among 141 patients with large artery atherosclerotic stroke. 19 In another study based on 105 patients with ischemic stroke, the YKL‐40 level was able to better discriminate acute ischemic stroke from controls compared with C‐reactive protein. YKL‐40 on the second day after admission was positively correlated with the National Institutes of Health Stroke Scale score, infarct volume, and 3‐month mRS, and it was also associated with poor functional outcome (OR, 5.73, P=0.03) after adjusting for age, sex, hypertension, diabetes, hypercholesterolemia, smoking, previous stroke, and C‐reactive protein. 8 However, these studies had small sample sizes with limited statistical power. Our study included 3377 patients with ischemic stroke from the multicenter CATIS study; this large‐sample size resulted in sufficient statistical power. Additionally, standardized protocols and rigid quality control procedures were adopted in the data collection and outcome assessment. Furthermore, comprehensive information about potential confounders was adjusted in the multivariate models. Thus, the study findings provide a valid appraisal of the relationship between YKL‐40 levels and the clinical outcomes of ischemic stroke. This study has clinical implications for better understanding the progression of ischemic stroke. A previous study found that CHI3L1 (chitinase‐3‐like protein 1) deletion decreased the amyloid plaque burden and increased the periplaque expression of the microglial lysosomal marker CD68 in a mouse APP/PS1 model of Alzheimer's disease. 20 Therefore, YKL‐40/CHI3L1 is expected to be used as a target for the treatment of neurological diseases. Lowering the YKL‐40 levels might improve the clinical outcomes of patients with ischemic stroke.
The exact mechanisms underlying the observed association between higher plasma YKL‐40 and an increased risk of poor prognosis for ischemic stroke are unclear. YKL‐40 is a glycoprotein produced by neutrophils and macrophages in response to inflammation. 21 The inflammatory process is involved in the pathological development of ischemic stroke, from prestroke arteriosclerosis to poststroke brain damage. 22 , 23 , 24 Inflammation is an important risk factor for death and major disability among patients with various diseases (eg, cardiovascular diseases, cancers, and multiple sclerosis), 25 , 26 which is consistent with our findings of the association between the YKL‐40 and death and major disability among patients with ischemic stroke. YKL‐40 plays a role in endothelial dysfunction in relation to cell migration, reorganization, and tissue remodeling during atherogenesis. 27 An elevation in the YKL‐40 level activates endothelial cells to express VCAM‐1 (vascular adhesion molecule‐1) and ICAM‐1 (intercellular adhesion molecule‐1), further damaging vascular endothelial cells and accelerating the progression of atherosclerosis. 28
Our study has several limitations that should be mentioned. First, this study was a post hoc analysis of the data from CATIS trial and was not a real‐world study. Some patients with extreme hypertension (BP ≥220/120 mm Hg) or those treated with intravenous thrombolytic therapy at admission were excluded, which might have induced a selection bias. However, the baseline characteristics of the participants in this study were similar to those from the China National Stroke Registry. 29 Most of the baseline characteristics did not significantly differ between the included and excluded patients. Thus, the selection bias was small. The BP reduction intervention in CATIS may have influenced our results but we controlled for the covariate of antihypertensive treatment in addition to other important factors in the analyses. Therefore, the influence is also likely slight. Second, plasma YKL‐40 concentrations were assayed only once at baseline. A previous study suggested that YKL‐40 levels in patients with acute ischemic stroke fluctuated after admission, 8 but we did not assess the YKL‐40 levels over time during hospitalization or during follow‐up. Therefore, we had no data to examine the association between YKL‐40 changes and clinical outcomes of patients with acute ischemic stroke. Third, ischemic stroke patients with cardiac embolism had higher levels of YKL‐40 than those with large artery atherosclerosis or small artery occlusion lacunae in our study. However, most stroke patients were caused due to large artery atherosclerosis and there were very few patients in this study who had stroke from cardioembolism or caused by small vessel occlusion (lacunar subtype). It was difficult for us to evaluate the prognostic value of YKL‐40 for the different subtypes of ischemic stroke. Future cohort studies focusing on specific ischemic stroke subtype would be helpful to elucidate any association. Finally, because all of the patients were from China, the external validity of these findings is limited. Further prospective studies conducted among different populations are needed to validate our findings.
CONCLUSIONS
In conclusion, elevated plasma YKL‐40 was associated with poor prognosis in patients with acute ischemic stroke. The plasma YKL‐40 level may have potential value in predicting the prognosis of patients with ischemic stroke.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (Grants: 82073627), the China Postdoctoral Science Foundation funded project (Grants: 2020M671564), the Postdoctoral Science Foundation of Jiangsu Province funded project (Grants: 2020Z039), and the Open Project of Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases (Grants: KJS1971).
Disclosures
None.
Supporting information
Table S1.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.026263
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Rathcke CN, Vestergaard H. YKL‐40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. 2006;55:221–227. doi: 10.1007/s00011-006-0076-y [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Chasman DI, Rose L, Loscalzo J, Elias JA. Plasma levels of the proinflammatory chitin‐binding glycoprotein YKL‐40, variation in the chitinase 3‐like 1 gene (chi3l1), and incident cardiovascular events. J Am Heart Assoc. 2014;3:e000897. doi: 10.1161/JAHA.114.000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang H, Fang Z, Sun Y, Li B, Shi Z, Chen J, Zhang T, Xiu Q. YKL‐40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin e. Eur Respir J. 2010;35:757–760. doi: 10.1183/09031936.00034409 [DOI] [PubMed] [Google Scholar]
- 4. Bakirci EM, Degirmenci H, Hamur H, Gunay M, Gulhan B, Aydin M, Kucuksu Z, Ceyhun G, Topal E. New inflammatory markers for prediction of non‐dipper blood pressure pattern in patients with essential hypertension: serum YKL‐40/chitinase 3‐like protein 1 levels and echocardiographic epicardial adipose tissue thickness. Clin Exp Hypertens. 2015;37:505–510. doi: 10.3109/10641963.2015.1013122 [DOI] [PubMed] [Google Scholar]
- 5. Ma WH, Wang XL, Du YM, Wang YB, Zhang Y, Wei DE, Guo LL, Bu PL. Association between human cartilage glycoprotein 39 (YKL‐40) and arterial stiffness in essential hypertension. BMC Cardiovasc Disord. 2012;12:35. doi: 10.1186/1471-2261-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Elevated plasma YKL‐40, lipids and lipoproteins, and ischemic vascular disease in the general population. Stroke. 2015;46:329–335. doi: 10.1161/STROKEAHA.114.007657 [DOI] [PubMed] [Google Scholar]
- 7. Kjaergaard AD, Bojesen SE, Johansen JS, Nordestgaard BG. Elevated plasma YKL‐40 levels and ischemic stroke in the general population. Ann Neurol. 2010;68:672–680. doi: 10.1002/ana.22220 [DOI] [PubMed] [Google Scholar]
- 8. Park HY, Jun CD, Jeon SJ, Choi SS, Kim HR, Choi DB, Kwak S, Lee HS, Cheong JS, So HS, et al. Serum YKL‐40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS One. 2012;7:e51722. doi: 10.1371/journal.pone.0051722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, Tong W, Liu C, Xu T, Ju Z, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the catis randomized clinical trial. JAMA. 2014;311:479–489. doi: 10.1001/jama.2013.282543 [DOI] [PubMed] [Google Scholar]
- 10. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 11. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 12. Joint Committee for Developing Chinese guidelines on P, Treatment of Dyslipidemia in A. [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua xin xue guan bing za zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 13. Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, Howard G, Saver JL, European Stroke Organisation Outcomes Working Group . Statistical analysis of the primary outcome in acute stroke trials. Stroke. 2012;43:1171–1178. doi: 10.1161/STROKEAHA.111.641456 [DOI] [PubMed] [Google Scholar]
- 14. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 15. Pencina MJ, D'Agostino RB, Sr. , D'Agostino RB, Jr. , Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 16. Xu T, Zhong C, Wang A, Guo Z, Bu X, Zhou Y, Tian Y, HuangFu X, Zhu Z, Zhang Y. YKL‐40 level and hypertension incidence: a population‐based nested case‐control study in China. J Am Heart Assoc. 2016;5:e004534. doi: 10.1161/JAHA.116.004534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo W, Zhang L, Sheng L, Zhang Z, Yang Z. Increased levels of YKL‐40 in patients with diabetes mellitus: a systematic review and meta‐analysis. Diabetol Metab Syndr. 2021;13:6. doi: 10.1186/s13098-021-00624-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laucyte‐Cibulskiene A, Ward LJ, Ebert T, Tosti G, Tucci C, Hernandez L, Kautzky‐Willer A, Herrero MT, Norris CM, Pilote L, et al. Role of GDF‐15, YKL‐40 and MMP 9 in patients with end‐stage kidney disease: focus on sex‐specific associations with vascular outcomes and all‐cause mortality. Biol Sex Differ. 2021;12:50. doi: 10.1186/s13293-021-00393-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen XL, Li Q, Huang WS, Lin YS, Xue J, Wang B, Jin KL, Shao B. Serum YKL‐40, a prognostic marker in patients with large‐artery atherosclerotic stroke. Acta Neurol Scand. 2017;136:97–102. doi: 10.1111/ane.12688 [DOI] [PubMed] [Google Scholar]
- 20. Lananna BV, McKee CA, King MW, Del‐Aguila JL, Dimitry JM, Farias FHG, Nadarajah CJ, Xiong DD, Guo C, Cammack AJ, et al. Chi3l1/YKL‐40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer's disease pathogenesis. Sci Transl Med. 2020;12:eaax3519. doi: 10.1126/scitranslmed.aax3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeo IJ, Lee CK, Han SB, Yun J, Hong JT. Roles of chitinase 3‐like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther. 2019;203:107394. doi: 10.1016/j.pharmthera.2019.107394 [DOI] [PubMed] [Google Scholar]
- 22. Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, Zhou F, Duan R, Chen W, Huang T, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post‐thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18:51. doi: 10.1186/s12974-021-02090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lambertsen KL, Finsen B, Clausen BH. Post‐stroke inflammation‐target or tool for therapy? Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. doi: 10.1016/S1474-4422(19)30078-X [DOI] [PubMed] [Google Scholar]
- 25. Johansen JS, Bojesen SE, Tybjaerg‐Hansen A, Mylin AK, Price PA, Nordestgaard BG. Plasma ykl‐40 and total and disease‐specific mortality in the general population. Clin Chem. 2010;56:1580–1591. doi: 10.1373/clinchem.2010.146530 [DOI] [PubMed] [Google Scholar]
- 26. Hughes J, Jokubaitis V, Lugaresi A, Hupperts R, Izquierdo G, Prat A, Girard M, Duquette P, Grand'Maison F, Grammond P, et al. Association of inflammation and disability accrual in patients with progressive‐onset multiple sclerosis. JAMA Neurol. 2018;75:1407–1415. doi: 10.1001/jamaneurol.2018.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511 [DOI] [PubMed] [Google Scholar]
- 28. Yasuda T, Kaneto H, Katakami N, Kuroda A, Matsuoka TA, Yamasaki Y, Matsuhisa M, Shimomura I. Ykl‐40, a new biomarker of endothelial dysfunction, is independently associated with albuminuria in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91:e50–e52. doi: 10.1016/j.diabres.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 29. Luo Y, Wang X, Matsushita K, Wang C, Zhao X, Hu B, Liu L, Li H, Liu G, Jia Q, et al. Associations between estimated glomerular filtration rate and stroke outcomes in diabetic versus nondiabetic patients. Stroke. 2014;45:2887–2893. doi: 10.1161/STROKEAHA.114.005380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


