Abstract
Facing cancer diagnosis, patients with cancer are prone to psychological stress and consequent psychological disorders. The association between psychological stress and cancer has long been a subject of high interest. To date, preclinical studies have gradually uncovered the promotive effects of psychological distress on tumor hallmarks. In contrast, eustress may exert suppressive effects on tumorigenesis and beneficial effects on tumor treatment, which brings a practicable means and psychosocial perspective to cancer treatment. However, the underlying mechanisms remain incompletely understood. Here, by focusing on the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, as well as stress-related crucial neurotransmitters and hormones, we highlight the effects of distress and eustress on tumorigenesis, the tumor microenvironment, and tumor treatment. We also discuss the findings of clinical studies on stress management in patients with cancer. Last, we summarize questions that remain to be addressed and provide suggestions for future research directions.
Psychological distress and eustress play opposite roles in tumorigenesis, the tumor microenvironment, and tumor therapy.
INTRODUCTION
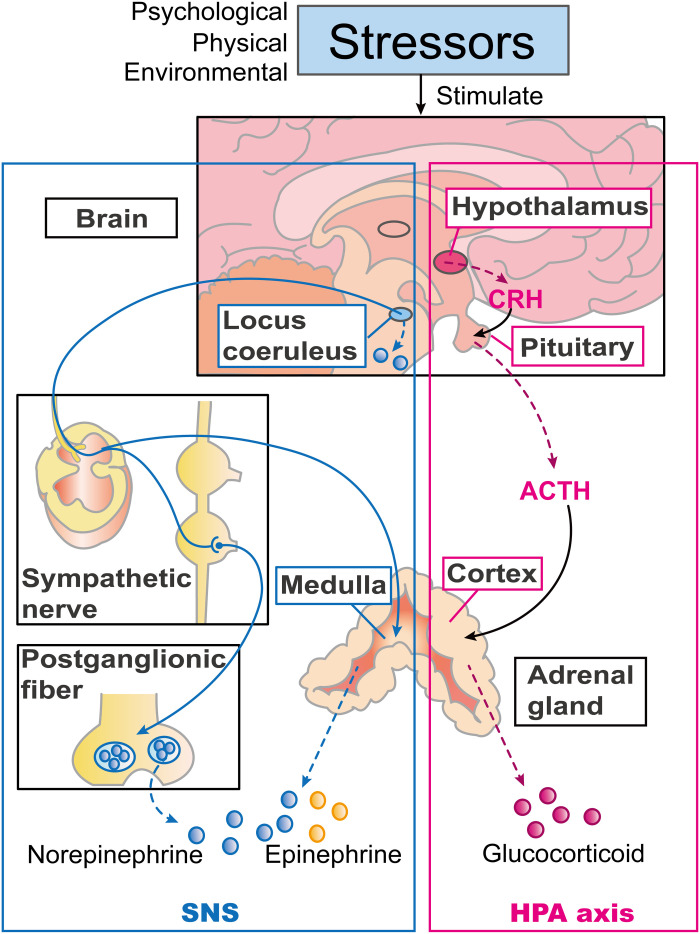
The stress response consists of neuroendocrine cascades mediated by the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis through the release of stress neurotransmitters and hormones, including catecholamines (CAs) and glucocorticoids (GCs) (Fig. 1) (1). The stress response, also known as the “fight-or-flight” response, triggered by psychological, physical, or environmental stressors, can help someone fight against or flee from life-threatening problems (1). In 1974, Selye proposed two forms of stress: distress and eustress (2, 3). When stress is prolonged or exceeds the endurance of organisms, they may experience distress, which may induce a pathological condition. In contrast, moderate stress can help people cope with stressors and adapt to the environment.
Fig. 1. Neuroendocrine mechanisms of the stress response.
Psychological, physical, or environmental stressors can induce the stress response. There are two main neuroendocrine response systems involved, the SNS and HPA axes. The box lined blue shows the components of the SNS. The locus coeruleus can secrete norepinephrine (NE) and activate the SNS in response to stress. Upon activation, the terminals of sympathetic postganglionic nerves secrete vesicles containing NE. Sympathetic nerves also innervate the adrenal medulla, inducing it to synthesize and secrete NE and epinephrine. The box lined pink shows the components of the HPA axis. Corticotropin-releasing hormone (CRH) secreted by the hypothalamus acts on the pituitary gland to stimulate secretion of adrenocorticotropic hormone (ACTH), which promotes the secretion and release of GCs from the adrenal cortex.
On the basis of this theory, more and more researchers are aware of the double-sided effect of stress (4, 5). However, the term stress is still widely used in contexts where it actually refers to distress, i.e., bad stress.
Here, we define the term distress broadly as a negative and unpleasant physical or psychological situation arising when the stress is too overwhelming or persistent. Psychologically, distress can be considered as a negative psychological state under pressure (6). Distress is not the same as mental illness. In the Diagnostic and Statistical Manual of Mental Disorders and the International Classification of Diseases systems, there are no clinical diagnosis criteria for distress, while it mainly serves as an assessment dimension of dysfunction in other psychological disorders (7). In existing research, distress has been assessed by scales such as the profile of mood states short form (8) or general health questionnaire (9). The National Comprehensive Cancer Network considers patients with mental illnesses such as depression at high risk for moderate or severe distress (6). Therefore, distress should include diagnosed mental illnesses, as well as anxiety and depressive symptoms that do not meet the diagnostic criteria.
The term eustress is used less frequently than the term distress and is often misused due to inconsistent definitions in different fields (4). Generally, eustress can be characterized by short duration, optimal amount, and good experience (10). Therefore, we define eustress as the opposite of distress, that is, a positive condition with short-term, moderate, and agreeable stress. Eustress can reduce depression/anxiety-like behavior in stressed mouse models (11, 12), indicating that it may be a protective factor for mental illness.
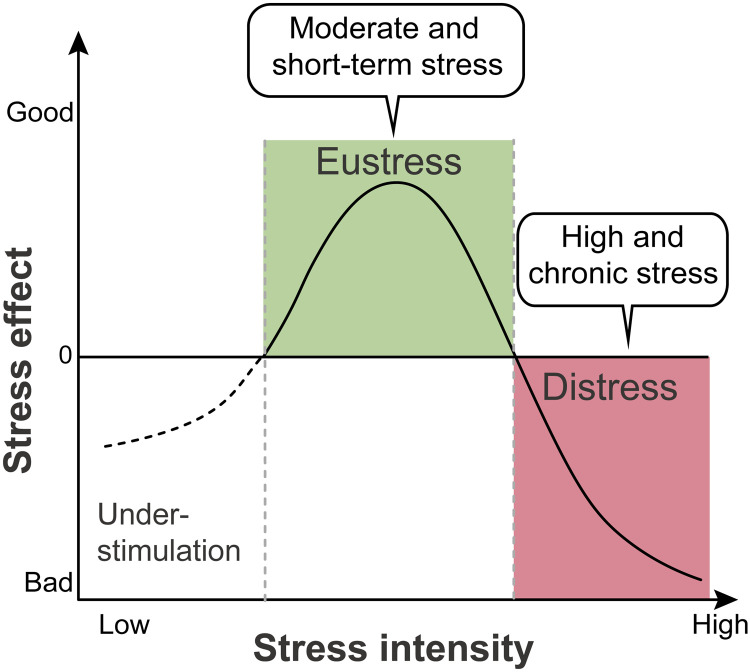
Therefore, the relationship between stress and its effects can be described as an inverted U shape (Fig. 2). Stress below the threshold to trigger a stress response may fail to mobilize the body, while severe or chronic stress may lead to distress and later pathological conditions. Only moderate and short-term stress can serve as eustress and improve adaptability to stressors (2, 3).
Fig. 2. The inverted U–shaped model of stress.
The relationship between stress intensity and effects can be described as an “inverted U” shape. Low-intensity stress does not mobilize the body to cope with stressors. Moderate-intensity and short-term stress can serve as eustress to improve adaptation and show a beneficial effect. On the contrary, chronic exposure to strong distress results in harmful effects.
Cancer diagnosis can become a high and chronic stressor, and thus contribute to persistent psychological distress in patients with cancer (5). On the one hand, cancer patients with psychological distress are more likely to be diagnosed with psychological disorders (13, 14). On the other hand, psychological distress is associated with increased cancer incidence (15, 16) and worse prognosis (9, 17). Preclinical studies have demonstrated that distress can promote tumorigenesis, tumor progression, and metastasis, as well as impair antitumor therapy (5). In contrast, recent animal experiments illustrated that environmental eustress can not only improve chronic stress–induced depression–like behavior (18) but also inhibit tumor growth and attenuate treatment resistance (19, 20).
Here, we summarize the effects and potential mechanisms of both psychological distress and eustress on tumorigenesis, the tumor microenvironment (TME), and tumor therapy. In addition, we review clinical studies of interventions targeting psychological stress in patients with cancer. We also discuss existing limitations and provide suggestions for future research directions.
PSYCHOLOGICAL DISTRESS AND CANCER
Epidemiology
There is evidence that psychological distress may increase the risk of cancer (15, 16). Cancer patients with psychological distress are more prone to psychological disorders (13, 14). Psychological stress may also affect cancer prognosis. Both psychological disorders and distress are related to higher mortality in patients with cancer (9, 17).
Preclinical models
Psychological distress in preclinical models can be induced by physical or social stressors (Table 1). Physical restraint (21–26) is a commonly used physical stressor, and there is also a method based on restraint, exposing mice to predator scent (27, 28). Social stressors include social isolation (21), repeated social defeat (22), and social disruption (29). Witnessing a conspecific mouse receiving an electric shock combines both physical and social stressor to induce stress in mice (30). Moreover, some studies applied unpredictable stressors including physical restraints, light changes, isolation, and crowding randomly and repeatedly to induce distress in mice (31).
Table 1. Psychological distress models in cancer.
| Stress procedure | Details | Duration | Reference | |
| Physical intervention | ||||
| Restraint | Mice were restrained individually in 50-ml ventilated centrifuge tubes. |
Acute stress: 1 hour/day for 3 days |
(21–26) | |
| Chronic stress: 2 hours/day for 21 days | ||||
| Immobilization and exposure to predator scent |
Mice were restrained individually in a ventilated conical vial, which was placed in a box with tissue penetrated by fox urine. | Acute stress: 1 hour | (27, 28) | |
| Chronic stress: 1 hour/day for 7 days | ||||
| Social intervention | ||||
| Social isolation | Mice were housed individually. | During the whole experiment | (21) | |
| Repeated social defeat | A mouse was introduced into a home cage of an aggressive heterospecific mouse (CD-1 mouse) for 10-min physical interaction. Then, a perforated glass divider was placed to physically separate two mice but allowed them sensory interaction for 24 hours (change the aggressor daily to avoid habituation). |
Chronic stress: Exposure/day for 10 consecutive days | (22) | |
| Social disruption | An aggressive mouse was placed periodically into a group of mice that have established social hierarchy (change the aggressor daily to avoid habituation). |
Chronic stress: 2 hours/day for six consecutive days |
(29) | |
| Exposure to conspecific mice being electric shocked |
Mice were placed in two-chambered shuttle boxes with a perforated transparent glass partition to witness a conspecific mouse receiving inescapable foot electric shock. |
Exposure to 26-min foot shock/day on days 1 and 6 |
(30) | |
| Mixed intervention | ||||
| Unpredictable stress | Mice were exposed to different stressors daily, including cage tilt, isolation, crowding, damp bedding, rapid light-dark changes, and overnight illumination. |
Chronic stress: Five consecutive days | (31) | |
Stress does not necessarily translate into psychological distress. Therefore, it is necessary to confirm the distress paradigms by depression/anxiety-like behavior tests (Table 2). In animal models, depression/anxiety-like behavior can be characterized by decreased exploration (22, 30), anhedonia (30, 32), despair (32), and social avoidance (22). The exploratory behavior can be determined by elevated plus mazes (30), open-field test (22, 30), and light-dark box test (22), with the locomotion tracks recorded by the camera for later software evaluation. The sucrose preference test (30, 32) is the most commonly used method to detect anhedonia. Despair behavior can be assessed by forced swimming (32) and tail suspension test (32). Social avoidance (22) is mostly used for evaluating social stressor–induced psychological distress.
Table 2. Depression/anxiety-like behavior tests in mouse models.
| Behavior tests | Details |
Parameter and its correlation with anxiety/ depression |
Reference | |
| Exploratory behavior | ||||
| Elevated plus-maze test | Mice were placed in an elevated plus-maze, which is a cross-shaped apparatus elevated above the floor, consisting of two open arms, two closed arms and a central square area. Mice were allowed to explore freely in the maze for 5 min. The time each mouse spent in the open arms was recorded. |
The time mice spent in the open arms: Negative correlation |
(30) | |
| Open-field test | Mice were placed in the corner of open boxes individually and allowed to explore freely for 0.5 or 1 hour under dim light conditions (5 or 10 lux). The locomotion of tracks of each mouse was recorded by camera and evaluated by software. |
Total locomotion (length of the track): Negative correlation |
(22, 30) | |
| Light-dark box test | Mice were placed in the box with a dark chamber and a light chamber (~200 lux). Two compartments were connected with a door. The locomotion tracks of each mouse in the box and the time they stayed in the light chamber were recorded by a camera and evaluated by software. |
Light chamber locomotion (length of the track): Negative correlation |
(22) | |
| Anhedonia | ||||
| Sucrose preference test | Mice were housed individually in cages and supplied with equal- volume pure water and 1% sucrose solution for 24 hours (with food) or 3 hours (without food). The consumption of both liquids was recorded. |
Sucrose preference [sucrose consumption/(sucrose + water consumption) × 100%]: Negative correlation |
(30, 32) | |
| Despair behavior | ||||
| Forced swimming test | Mice were individually placed in a transparent vertical cylinder with water (about 25°C) for 6 min. The duration of immobility of each mouse after 1-min habituation was recorded. |
Immobility duration: Positive correlation |
(32) | |
| Tail suspension test | Mice were hung upside down by their tails that were fixed at a certain height. The duration of immobility of each mouse after 1-min habituation was recorded. |
Immobility duration: Positive correlation |
(32) | |
| Social behavior | ||||
| Social avoidance tests | Mice were placed in open-field arenas individually with an empty wire cage under dim light condition (5 lux) for 150 s. Later, an aggressive CD-1 mouse was placed in the wire cage. Their interaction was evaluated by the duration the mice spent in the area projecting 8 cm around the wire cage with a CD-1 mouse. |
Interaction ratio [(time spent in the area around the cage with CD-1 mouse/time spent in the area around the empty cage) × 100%]: Negative correlation |
(22) | |
Model animals are subjected to stressors of different duration and frequencies to mimic acute or chronic stress. There are no specific definitions and time criteria for acute and chronic stress (5). In preclinical studies, duration or frequencies of stimulation for chronic or acute stress paradigms vary by the type of stressor. For example, 2-hour daily restraint for 21 days (23) or unpredictable stressors for 6 days (31) can be used for inducing chronic stress in animal models. Previous studies illustrated that both acute and chronic stress could induce depression/anxiety-like behavior and promote tumor growth in mice (21, 22, 27). However, the difference in biological effects on tumors caused by acute and chronic stress is unclear. Apart from duration, the sequence of establishing distress models and transplanting tumor cells should be taken into consideration. In preclinical studies, mice have been exposed to stress before tumor inoculation (29, 33), after tumor formation (21, 23), or both (24, 25). Spontaneous tumor mice such as Hi-Myc mice (27) and LSL-Kras+/G12D;LSL-Trp53+/R172H;Pdx1-Cre (KPC) mice (28) were also used in studies to observe the effects of distress. These models can be used for exploring the impact of distress at different stages of tumorigenesis and disease progression.
In distress-tumor models, distress can be biologically defined as an excessive stress response that is strong enough to induce depression/anxiety-like behaviors and a series of biological processes in vivo such as DNA damage, angiogenesis, and immune suppression (5). The details will be described in the following sections.
Effects on tumorigenesis, tumor progression, and metastasis
Tumorigenesis
Although the clinical evidence of the association between psychological distress and tumorigenesis is still controversial (34), some preclinical studies have suggested the possibility of distress involved in tumorigenesis. DNA damage can cause somatic mutations and genomic instability, which may promote tumorigenesis (35). A potential mechanism by which psychological distress promotes the initiation of cancer is that distress may induce DNA damage through β-adrenergic receptor (β-AR) signaling. Stress-related norepinephrine (NE) can induce DNA damage in the presence or absence of carcinogens (36, 37) and prevent the repair of damaged DNA (38). β2-AR–mediated attenuation of p53 levels can increase the accumulation of DNA damage in response to chronic stress (39). Elevated GC levels can also suppress p53 function, which may induce chronic stress–induced tumorigenesis (40). In addition to inducing DNA damage, chronic stress facilitated lung tumorigenesis by enhanced exocytosis of insulin-like growth factor 2 in lung epithelial cells through phosphorylation of L-type voltage-dependent calcium channels induced by β-AR signaling (41).
Tumor progression
During tumorigenesis, tumor had acquired various characteristics and capabilities. Preclinical studies have indicated that distress may promote tumor progression by enhancing hallmarks of cancer, including inhibiting apoptosis, promoting angiogenesis, and regulating energy utilization (35).
Distress may help tumor cells evade apoptosis. Through β-AR signaling, distress up-regulates the expression of antiapoptotic myeloid cell leukemia 1, B cell lymphoma-2 (BCL-2), BCL-XL, and BCL-XL/BCL-2–associated death promoter (42), thus reducing tumor cell apoptosis. Moreover, chronic stress can enhance stem cell properties of breast cancer cells with up-regulated expression of self-renewal–related genes to promote tumor growth (24).
Animal studies showed that chronic stress promoted tumor angiogenesis in mice with ovarian carcinoma by up-regulating vascular endothelial growth factor (VEGF) expression in tumor tissue through β2-AR–activated cyclic adenosine 3′,5′-monophosphate (cAMP)–protein kinase A (PKA) signaling (43). This result was consistent with the conclusion from an in vitro study that treatment with NE, a main player involved in the stress response, can promote the expression and secretion of angiogenesis-related cytokines, such as VEGF, interleukin-8 (IL-8), and IL-6 by melanoma cells through the pathway mentioned (43, 44).
According to the Warburg effect, tumor cells rely primarily on anaerobic glycolysis for energy supply (35). Distress may promote tumor energy utilization by elevating the level of lactate dehydrogenase A, which executes the final step of the Warburg effect (24).
As mentioned above, β2-AR signaling has been shown to promote tumor progression in multiple ways. The feed-forward loops between tumor and nerve can enhance this effect. In a mouse model of typically highly innervated pancreatic ductal adenocarcinoma, stress activated the β2-AR/PKA pathway and elevated the secretion of nerve growth factor and brain-derived neurotrophic factor (BDNF), which induced axonogenesis with subsequent increased NE accumulation in the TME, which promoted tumor growth (28).
Tumor metastasis
Psychological distress may regulate the TME to promote tumor invasion and metastasis. Matrix metalloproteinases (MMPs) are related to extracellular matrix degradation and tumor cell migration (35). Administration of CAs can promote MMP-2 and MMP-9 secretion in various tumor cell lines (45, 46). Increased MMP activity may be related to β-AR–induced signal transducers and activators of transcription 3 (STAT3), an important convergence point for signaling pathways in tumors (46, 47). Moreover, activated β2-AR signaling can lead to epithelial mesenchymal transition (EMT) promoting metastasis of tongue squamous cell carcinoma through the β2-AR/IL-6/STAT3 pathway (48).
Furthermore, distress can promote tumor metastasis by establishing a premetastatic niche. Chronic stress increased lung colonization and metastasis in a breast cancer model by increasing monocyte output in the premetastatic phase and macrophage infiltration in the premetastatic lung (31). Stress can increase M2 macrophage infiltration and the expression of macrophage-derived prometastatic molecules such as prostaglandin-endoperoxide synthase 2 (PTGS2), MMP-9, and VEGF to mediate stress-enhanced metastasis (49). Moreover, stress increased myeloid-derived suppressor cell (MDSC) infiltration in tumor and lung metastases, which up-regulated transforming growth factor–β, VEGF, and IL-10 to promote EMT and tumor metastasis (50).
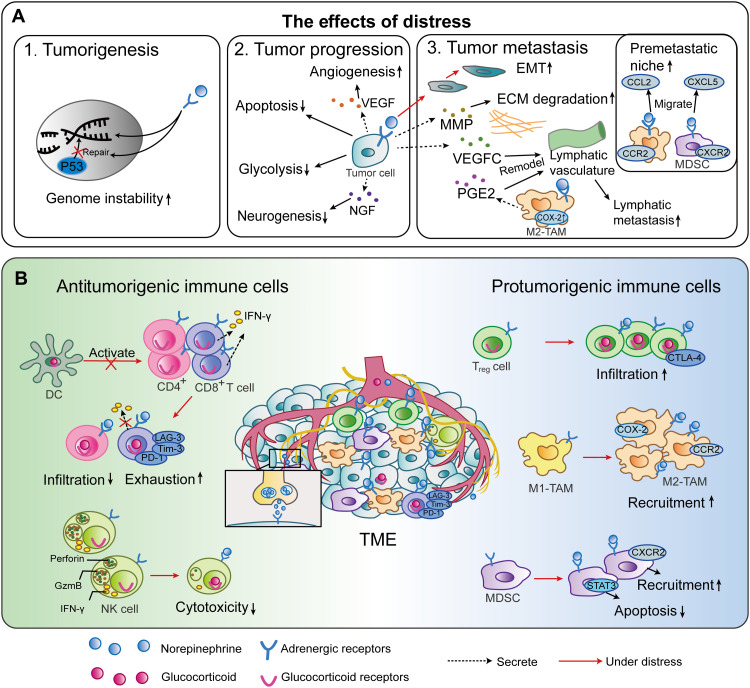
Tumor cells can migrate through lymphatic vessels, and the remodeling of lymphatics may be an important step in the lymphatic metastatic process (35). The synergy of cyclooxygenase 2 (COX-2) expressed by macrophages and AR-activated VEGFC-VEGFR3 signaling may be involved in stress-induced lymph vasculature remodeling in mice, which can promote tumor cell dissemination (51). In summary, distress promotes tumor genesis, progression, and metastasis (Fig. 3A).
Effects influencing the function and infiltration of immune cells
T cells
Distress can affect T cell numbers in secondary lymphoid organs (52) and the TME (21, 26). Social isolation stress shortened survival in a breast cancer mouse model, which was associated with a reduction in activated T cells and splenic CD8+ cells (21). Moreover, stress accelerated pancreatic cancer growth in young mice by down-regulating tumor-infiltrating CD4+ T cells (52). The decrease in T cell numbers may be due to distress suppressing the migration of T cells from lymph nodes to tumors and impairing T cell proliferation. β2-AR agonists reduced the motility of T cells in lymph nodes (53). This was due to local vasoconstriction induced by β2-AR signaling causing hypoxia in lymph nodes, triggering rapid calcium signaling in leukocytes and inhibiting cell motility. β-AR signaling can impair the proliferative capacity of antigen-specific T cells in mice with lymphoma (54). This is consistent with the reduced proliferation capacity of T cells from lymph nodes of stressed mice (55).
Distress can also promote T cell exhaustion, characterized by reduced cytokine secretion, decreased effector function, and elevated inhibitory receptor expression (56). β-AR signaling impairs the cytotoxic effects of T cells (53). In contrast, blocking β-AR signaling in CD8+ tumor-infiltrating lymphocytes (TILs) isolated from stressed mice increases the secretion of interferon-γ (IFN-γ), granzyme B, and IL-12a (56). Glucocorticoid receptor (GR) activation can also notably suppress T cells. Elevated GCs can inhibit T cell responses through GR-induced transcription of immunosuppressive genes (57). By up-regulating immunosuppressive Tsc22d3 expression in dendritic cells (DCs), social defeat stress–induced GCs suppressed IFN-γ–positive T cell activation and inhibited type I IFN responses, which are necessary for antitumor immune surveillance (22). Distress can increase the expression of inhibitory receptors on T cells. In restraint-stressed mice, programmed cell death protein 1 (PD-1) and LAG-3 were up-regulated on intratumoral CD8+ TILs (26). Blocking β-AR signaling in stressed mice decreased PD-1, LAG-3, and Tim-3 expressed on CD8+ TILs (56).
The mechanisms underlying the promotion of the T cell–exhausted phenotype are unclear, but suppressed T cell metabolic reprogramming (56) and activated kisspeptin/Gpr54 signaling (26) may be involved. Activated T cells require large amounts of energy supplied by glycolysis and oxidative phosphorylation, and the process increasing cellular metabolism is called metabolic reprogramming (58). In vitro experiments revealed that treatment with adrenergic agonists inhibited the metabolism in CD8+ T cells (58). Restraint-induced psychological distress also impaired glycolysis and oxidative phosphorylation in naive CD4+ T cells isolated from mouse spleens (59). Blocking β-AR signaling with propranolol in stressed mice promoted glycolysis and mitochondrial oxidative phosphorylation in CD8+ TILs and reduced the proportion of exhausted cells expressing inhibitory receptors (56). Stress-induced purine metabolism disorder in peripheral CD4+ T lymphocytes may be responsible for stress-derived depression–like behavior (59). In addition to regulating metabolism, distress may also promote T cell exhaustion through other pathways. Restraint stress increased not only the level of plasma kisspeptin, a neuropeptide that could affect T cell function, but also the expression of its receptor Gpr54 on T cells in tumor, spleen, and hypothalamus (26). The knockdown of Gpr54 inhibited lung cancer growth by suppressing T cell dysfunction and exhaustion.
NK cells
Distress was found to be associated with lower cytotoxicity of natural killer (NK) cells in patients with ovarian cancer (8). CAs can reduce NK cytotoxicity through β2-AR signaling (60). However, in social disruption models, NK cells in the spleen or lung have been activated through β2-AR receptor signaling (61).
Besides CAs, GCs can suppress the cytotoxicity of NK cells and down-regulate the expression of perforin, granzyme, and IFN-γ by GR-altered gene transcription or epigenetic modifications (62). However, the inhibition of NK cells by cortisol relied on the mediation of CAs and/or prostaglandins, as the effect could be reversed by blocking NE or prostaglandins rather than GR (63).
Regulatory T cells
Regulatory T (Treg) cells are a subset of CD4+ T cells that suppress immune responses, thereby maintaining homeostasis and self-tolerance (64). Activation of β2-AR signaling enhanced the immunosuppressive effect of Treg cells by promoting Treg cell–mediated conversion of CD4+ Foxp3− T cells to Foxp3+-induced Treg cells and up-regulating the expression of cytotoxic T lymphocyte (CTL)–associated protein 4, an immune checkpoint, on Treg cells through the β2-AR/cAMP/PKA pathway (65). In a mouse model of squamous cell carcinoma, chronic stress–induced high corticosterone levels increased Treg cell infiltration in tumors through up-regulating C-C motif chemokine ligand 22 (CCL22), while they decreased the numbers of CTLs and helper T cells in tumors through down-regulating cutaneous T-cell-attracting chemokine (CTACK)/CCL27 (66).
Myeloid-derived suppressor cells
Chronic stress–induced β2-AR activation has been found to lead to an increase in MDSCs (67) and their accumulation in the spleen and tumor, promoting tumor growth, metastasis, and vascularization (68). Stress-induced β2-AR signaling inhibited MDSC apoptosis and promoted MDSC survival through regulating STAT3 and the Fas-FasL interaction, respectively. In addition, β-AR activation up-regulated immunosuppressive arginase-1 and programmed death ligand 1 (PD-L1) expression in MDSCs, thereby altering their ability to inhibit T cell proliferation (68).
Chronic stress can also promote the mobilization of MDSCs. Through activating β2-AR signaling, chronic restraint stress up-regulated the expression of C-X-C motif chemokine receptor 2 (CXCR2) and phosphorylation of extracellular-regulated kinase in MDSCs in the bone marrow and chemokine C-X-C motif ligand 5 (CXCL5) in tumors (25). Through the β2-AR/CXCL5-CXCR2/Erk pathway, chronic stress mobilized MDSCs from bone marrow to spleen and tumor and promoted hepatocellular carcinoma growth in mice.
Tumor-associated macrophages
Tumor escape is associated with the switch of macrophages from the proinflammatory M1 type toward the anti-inflammatory M2 type (69). M2 polarization can be induced by GCs (69). In vitro, isoprenaline can promote precursor cell M2-like polarization in the presence of the M2 polarization stimulator IL-4, which can be inhibited by β2-AR blockade (70). Stress increased M2–tumor-associated macrophage (TAM) polarization through β2-AR signaling and promoted breast cancer growth and metastasis (49). In summary, distress disturbs antitumor immunity (Fig. 3B) by inducing hyperactivated SNS and HPA axes.
Fig. 3. Effects of psychological distress on the TME and underlying mechanisms.
(A) Schematic diagram of the effects of distress on biological behaviors of tumor. GCs and CAs produced by the hyperactivated neuroendocrine system are involved in tumor regulation in the following aspects by binding to their receptors. 1. Distress may promote tumorigenesis through DNA damage. 2. Distress can facilitate tumor progression through reducing tumor cell apoptosis and promoting angiogenesis, glycolysis, and neurogenesis. 3. Distress can promote tumor metastasis through establishing a prometastatic microenvironment and premetastatic niches and remodeling the lymphatic vasculature. (B) Schematic diagram of the effects of distress on tumor immune microenvironment. Distress can induce a suppressive TME through reducing infiltration and function of effector immune cells, such as T cells, DCs, and NK cells, and promoting infiltration and function of suppressive cells, including Treg cells, M2-TAMs, and MDSCs. NGF, nerve growth factor; ECM, extracellular matrix; PGE2, prostaglandin E2; CCR2, C-C motif chemokine receptor; GzmB, granzyme B; PD-L1, programmed death-ligand 1.
Effects on cancer treatment
Psychological distress can also impair the efficacy of various types of cancer treatments.
Surgery
Surgery is a radical cancer treatment, but it is also a strong stressor. Surgical stress has been associated with tumor progression and metastasis in both animals (71) and human patients (72). Psychological distress during surgery may affect the prognosis of patients after surgery. An 11-year follow-up study showed that greater postsurgical depressive symptoms in patients were associated with shorter survival (73). Operation, anesthetics, analgesics, and psychological factors can induce dysregulation of the neuroendocrine-immune system, which affects the prognosis of tumor patients (74). Therefore, the perioperative period is a critical window for physiological and psychological intervention to improve the prognosis of patients with tumor.
Chemotherapy
Distress has been shown to impair the efficacy of cytotoxic agents by inhibiting chemotherapy-induced tumor cell apoptosis. β2-AR signaling impaired paclitaxel-induced apoptosis in ovarian cancer cells by up-regulating dual-specificity protein phosphatase 1 expression to inhibit c-Jun N-terminal kinase–mediated c-Jun phosphorylation (75). Stress-activated β2-AR signaling regulates the levels of Bcl-2 family proapoptotic molecules, which contribute to the resistance of apoptotic effects to chemotherapy (27, 42).
Distress can also weaken chemotherapy effects by inducing DNA damage, thus perhaps modulating the chemotherapy-induced DNA damage response. Cell line experiments have shown that stress-induced DNA double-strand breaks reduce DNA damage caused by cisplatin and diminish the therapeutic effect of cisplatin (37). Stress hormone–induced DNA damage and phosphorylation of ataxia-telangiectasia-mutated-and-Rad3-related kinase (ATR) and its major downstream effector checkpoint kinase 1 (CHK1) further up-regulated the G1 cell kinase inhibitor p21 to halt breast tumor cells in the G0-G1 phase. Stress thus impaired the effect of paclitaxel, which targets cells in the S phase of the cell cycle (76). Furthermore, animal studies showed that distress can impair the antitumor efficacy of immunogenic cell death inducers, such as oxaliplatin and mitoxantrone, through inducing intratumoral and systemic immunosuppressive effects (22).
Immunotherapy
As previously mentioned, distress can modulate tumor infiltration and function of various immune cells. Therefore, it is not unexpected that distress can affect the efficacy of immunotherapy. Preclinical studies have found that distress impairs the effects of immune checkpoint inhibitors, tumor vaccines, and immune-stimulating agents.
Distress may influence the efficacy of immune checkpoint inhibitors via AR- or GR-regulated T cell function or the expression of immune checkpoints and their ligands. Activated β2-AR signaling reduced the response to anti–PD-1 and anti–4-1BB monoclonal antibodies (mAbs) in mice with lymphoma by suppressing proliferation and function of CD8+ T cells (54). In a solid tumor model of stress-induced resistance to anti–PD-1 mAbs, β2-AR blockade up-regulated the ratio of effector CD8+ T cells to CD4+ Treg cells, decreased CD8+ TILs expressing PD-1, and thus reduced treatment resistance in the stressed mice (77). GR signaling has been shown to cause increased PD-L1 and decreased major histocompatibility complex I expression in pancreatic ductal adenocarcinoma models, thus promoting tumor immune escape and impairing the effects of anti–PD-1 treatment (78). GR blockade can induce an immunologically active TME to reverse the resistance to anti–PD-1 mAbs in mice caused by GC administration or social distress (22, 78). Distress attenuated the antitumor effects of CpG-C, a novel Toll-like receptor-9 immunostimulatory agent, in metastatic tumor models by impairing CpG-C–induced NK-cell activity, which could be reversed by simultaneous inhibition of COX-2, as well as GR and β-AR signaling (33).
Psychological distress can also affect the efficacy of tumor vaccines. The potential mechanism is inhibiting effector T cell function directly and/or indirectly preventing T cell activation. By preventing DCs from migrating into lymph nodes and activating CD8+ T cells, distress reduced IFN-γ–producing CD8+ T cells and CTL-mediated killing, which may account for the resistance to poly(d, l-lactide-co-glycolide) microsphere–based cancer vaccines in mice (29). Repeated social defeat stress negatively affected the response to prophylactic tumor cell vaccination by up-regulating the expression of GC-inducible factor Tsc22d3 in DCs, which can inhibit DC function and IFN-γ+ T cell activation, and such resistance can be reversed when a GR antagonist is present (22).
Radiotherapy
Distress may induce resistance to radiotherapy by suppressing radiation-induced antitumor immunity. Cool housing temperature stress impaired the response to irradiation in mouse models with a decrease in the percentage of CD4+ and CD8+ T cells expressing IFN-γ and granzyme B in tumors (79). Moreover, such stress can inhibit tumor responses outside the irradiated field, namely, the radiation-induced abscopal effect (80). Through β2-AR signaling, stress down-regulates T cell effector function and migration-related gene expression, hence decreasing IFN-γ, tumor necrosis factor–α (TNF-α), and granzyme B secretion and inhibiting CXCR3/CXCL9 signaling, while β2-AR signaling blockade can enhance T cell–mediated antitumor immune responses in both irradiated and distant unirradiated tumors (80).
EMT is associated with increased tumor invasion and contributes to tumor metastasis (35). Distress may also affect the efficacy of radiotherapy by regulating EMT-related pathways. Psychological stress–induced tumor progression and radiation resistance in mice with lung cancer may be the result of adrenergic-activated Wnt/β-catenin signaling with up-regulated expression of Wnt1, drosha, and vimentin and down-regulated E-cadherin in tumors (30).
Targeted therapy
Distress can affect the effects of antiangiogenic drugs and targeted inhibition of the epidermal growth factor receptor (EGFR) in mouse tumor models. For example, sunitinib exerts antitumor effects by inhibiting tumor angiogenesis. NE can attenuate the efficacy of sunitinib by up-regulating proangiogenic VEGF, IL-8, and IL-6 (81). Restraint stress impaired sunitinib antitumor effects through the same mechanisms in mouse colorectal tumor models, which can be reversed by propranolol (23).
EGFR–tyrosine kinase inhibitors (EGFR-TKIs) can suppress tumor cell proliferation through inhibiting EGFR autophosphorylation and blocking signal conduction. IL-6 serves as a main mediator in T790M-independent EGFR-TKI resistance (82). Stress hormones can promote IL-6–mediated EGFR-TKI resistance in both lung cancer cell lines and mouse models by activating the β2-AR/protein kinase C/liver kinase B1/cAMP response element-binding protein axis, which can be reversed by β-AR inhibitors or IL-6–neutralizing antibodies (83). In summary, distress can impair antitumor efficacy of different therapies (Table 3).
Table 3. Effects and potential underlying mechanisms of distress on cancer treatment in preclinical studies.
↑, increase; ↓, decrease; →, causal; E, epinephrine; MCL-1, myeloid cell leukemia 1; BAD, Bcl-xL/Bcl-2–associated death promoter; ISO, isoprenaline; PLC, phospholipase C; PKC, protein kinase C; CREB, cAMP response element-binding protein; DUSP1, dual-specificity protein phosphatase-1; JNK, c-Jun N-terminal kinase; Chk1, checkpoint kinase 1; NSCLC, non–small cell lung cancer; APC, antigen-presenting cells; GzmB, granzyme B; LKB1, liver kinase B1.
| Treatments | Role of stress | Cancer type | Model | Stress | Specific effects or pathway | Reference |
| Chemotherapy | ||||||
| Cisplatin | Antiapoptosis | Pancreatic cancer | Mice | Cold stress (22°C versus 30°C) |
β2-AR → ↑anti-apoptotic molecules (MCL-1, BCL-2, and BCL-XL) |
(42) |
| PI3K inhibitor, bicalutamide | Antiapoptosis | Prostate cancer | Mice | Immobilization stress; stress hormone (E) |
β2-AR/PKA/BAD antiapoptotic signaling pathway |
(27) |
| Paclitaxel, cisplatin, or docetaxel | Antiapoptosis | Ovarian cancer | Cell lines; mice | Stress hormones (NE); β-AR agonist (ISO); restraint stress |
β2-AR/cAMP/PLC/PKC/CREB→ ↑DUSP1 → ↓JNK-mediated phosphorylation of c-Jun |
(75) |
| Cisplatin | DNA damage | Epithelial ovarian cancer | Cell lines | Stress hormone (NE) | β2-AR → ↑DNA double strand breaks | (37) |
| Paclitaxel | DNA damage | Triple-negative breast cancer | Cell lines; mice | Stress hormones (cortisol, NE);restraint stress |
Stress hormone → DNA damage → ATR/Chk1/p21 → tumor cell cycle halt in the G1 phase. (Paclitaxel targets cells in S phase) |
(76) |
| Immunotherapy | ||||||
| Immunogenic cell death inducer; tumor vaccination, anti–PD-1 mAb |
Immunosuppression | NSCLC; fibrosarcomas; colorectal cancer | Mice | Repeated social defeat stress; acute restraint stress |
GC → ↑Tsc22d3 → ↓type I IFN responses in DC, activation of IFN-γ+ T cell |
(22) |
| Tumor vaccination | Immunosuppression | Melanoma | Mice | Social disruption stress | Stress→ ↓DC function and migration to draining lymph nodes, APC priming→↓IFN-γ + CD8+ T cell, CTL-mediated target cell killing |
(29) |
| CpG-C | Immunosuppression | Mammary carcinoma; colon tumor with liver metastasis; melanoma |
Rats and mice | Wet cage stress | Stress → β2-AR, GR, COX-2 → ↓ cytotoxicity of NK cells by CpG-C |
(33) |
| Anti–PD-1 mAb, anti–4-1BB mAb | Immunosuppression | B cell lymphoma | Mice | β-AR agonist (ISO) | β2-AR → ↓proliferation, IFN-γ production, and cytolytic killing capacity of antigen-specific CD8+ T cells. | (54) |
| Anti–PD-1 mAb | Immunosuppression | Mammary carcinoma; melanoma | Mice | Cold stress (22°C versus 30°C) |
β-AR → ↓the ratio of effector CD8+ T cell and CD4+ regulatory T cell ratio (IFN+ CD8+ T cell: Treg); ↑PD-1 expression in effector CD8+ TILs |
(77) |
| Radiotherapy | ||||||
| Local irradiation | Immunosuppression | Colon tumors; melanoma; mammary carcinoma |
Mice | Cold stress (22°C versus 30°C) |
β2-AR → ↓CD8+ T cell migration (CXCR3/CXCL9) and function (T-bet, IFN-γ, TNF-α, and GzmB) |
(80) |
| Irradiation | EMT | Lung cancer | Mice | Exposure to a conspecific mouse receiving inescapable foot shocks |
β2-AR → ↑Biomarker of EMT expressed in tumor: ↑Wnt1, Drosha, and vimentin, ↓E-cadherin |
(30) |
| Irradiation | Immunosuppression | Colon adenocarcinoma | Mice | Cold stress (22°C versus 30°C) |
β2-AR → ↓the percentage of IFN-γ+
GzmB+ CD4+ and CD8+ in tumor |
(79) |
| Targeted therapy | ||||||
| Sunitinib | Angiogenesis | Colorectal cancer; colon carcinoma |
Mice | Chronic restraint stress; stress hormone (NE) |
β-AR/cAMP/PKA → ↑VEGF, IL-8 | (23) |
| EGFR-TKIs | NSCLC | Cell lines; mice | Stress hormone (NE) | β2-AR/PKC/LKB1/CREB → IL-6– mediated EGFR TKI resistance |
(83) | |
PSYCHOLOGICAL EUSTRESS AND CANCER
Epidemiology
The concept of eustress is rarely mentioned in epidemiological studies and is mainly investigated in animal experiments. However, some positive lifestyles can be considered as eustress and may influence cancer incidence and mortality. For example, leisure-time exercise has been associated with a lower risk for 13 cancers (84). Social support may be related to a lower risk of breast and ovarian cancer and better prognosis of patients with cancer (11, 12).
Preclinical models
Most current studies use an enriched environment (EE) to model eustress, which is a well-studied modeling approach in psychiatry studies and has gradually been applied in tumor studies (19, 85, 86). EE consists of a variety of toys, such as climbing frames, wheels or shelters, and sufficient social communication (87).
EE can regulate the expression of receptors such as the β-AR (19) and the GR (18, 88) and of neurotransmitters such as serotonin and dopamine (89, 90). Living in EE can reduce stress responses and depression in mice (91, 92).
Effects on tumorigenesis and tumor progression
Tumorigenesis
Preclinical studies have found that environmental eustress can inhibit tumorigenesis after tumor cell inoculation or carcinogen induction. EE delayed tumorigenesis after subcutaneous injection of B16 melanoma or MC38 colon cancer cells and even completely abrogated tumor growth in some mice, the potential mechanism of which was EE decreasing a mitogenic factor, leptin (85). Compared to the standard environment, EE decreased the genesis of hepatocellular carcinoma induced by carcinogens, which may be associated with up-regulated antitumor immunity (19).
However, the underlying mechanisms are not yet fully understood. The protumorigenic effect of chronic distress may be related to the accumulation of DNA damage (5). In contrast to distress, eustress exhibited a protective effect against DNA damage. For example, EE improved the response to DNA damage and the rate of DNA repair after radiation exposure (93).
Tumor progression
Preclinical models of tumor-bearing mice housed in EE illustrated that eustress can suppress tumor progression by regulating the secretome of adipocytes and oxidative metabolism in tumor cells. Some preclinical studies found that EE eustress activated the hypothalamic-sympathoneural-adipocyte axis and thus decreased leptin secreted by white adipocytes (85), whose role in promoting tumor development and metastasis had been demonstrated (94). In mice inoculated subcutaneously with melanoma or colon cancer cells, EE up-regulated hypothalamic BDNF expression, which reduced the expression and production of leptin in white adipocytes (85). β-AR signaling served as a peripheral pathway synergistically involved in these antitumor effects of EE by reducing leptin (85). In addition, by up-regulating brain BDNF, EE also reduced microglia/macrophage activation in an intracranial glioma model (86).
In addition to decreasing leptin secretion, eustress can also inhibit tumor growth through inducing interorgan signaling cross-talk and adipokine/cytokine secretion (95). Spontaneous physical activity of obese mice housed in EE limited mammary tumor growth. Multiple factorial analysis showed cross-talk of signaling pathways and of adipokine/cytokine secretion of tumor, adipose tissue, and muscles, decreasing the antioxidative response and inflammation in tumor tissue. Similarly, another study of mouse mammary tumor models showed that EE-suppressed tumor growth was associated with increased adiponectin/leptin ratio in blood plasma and decreased COX-2, an inflammatory factor and a crucial enzyme in the metabolic pathway leading to prostaglandin formation in tumors (96).
EE may also regulate intracellular oxidative metabolism in tumor tissues. Being housed in EE significantly reduced subcutaneous and orthotopic pancreatic tumor growth in mice. Integrative transcriptomic and proteomic analysis of dissected tumor tissue revealed that EE mainly down-regulated genes localized to mitochondria and related to oxidative phosphorylation and the citric acid cycle, which is a key metabolic pathway linking carbohydrate, adipose tissue, and protein metabolism (97). In addition to the above mechanisms, eustress can also inhibit tumor growth by promoting antitumor immunity, which will be described below.
Effects influencing the function and infiltration of immune cells
T cells
In contrast to distress, eustress can promote antitumor immunity through activating the SNS and HPA axes. This is consistent with the view that the moderate stress response in eustress can have protective effects.
In a melanoma model, CD8+ T cells were required to mediate the anticancer effects of an EE. EE increased the proportion of CD8+ CTL in secondary lymphoid tissue with no significant alteration in CD8+ T cells in the TME (98). The modulation of T cell immunity by EE was reversed by BDNF knockdown, β-AR, or GR blockade, indicating the involvement of the SNS and HPA axis.
In a hepatocellular carcinoma model, EE eustress also induced CD8+ T cell–dependent tumor suppression. Through the β-AR/CCL2 axis, EE increased CD8+ T cell infiltration and decreased M-MDSCs, G-MDSCs, and M2 tumor–associated macrophages in the TME (19).
NK cells
In general, eustress seems to promote cytotoxicity and infiltration of NK cells in tumor models. EE promoted maturation and proliferation of NK cells in blood, bone marrow, and spleen in a pancreatic cancer mouse model (99). A potential mechanism is that EE can up-regulate receptors or cytokines related to NK cell activation and proliferation.
NKG2D, an activating receptor expressed on NK cells and some T cell subsets, plays an important role in tumor immunosurveillance (100). EE-housed mice showed enhanced antitumor effects and tumor infiltration of NK cells with up-regulated expression of NKG2D and C-C chemokine receptor 5 on NK cells, which could be reversed by blocking β-AR signaling or chemical sympathectomy (20).
IL-15 can induce differentiation and proliferation of NK cells (100). By up-regulating brain IL-15, EE enhanced antitumor activity and levels of NK cells both in TME and peripheral blood of mice with intracranial glioma (86).
Effects on cancer treatment
Although there are few studies in this field, the existing studies show that eustress can significantly promote the efficacy of antitumor treatments. EE eustress can synergize with chemotherapy and immunotherapy. EE enhanced the response to 5-fluorouracil or gemcitabine in pancreatic tumor models (101). Microarray analysis showed that EE down-regulated expression of the tumoral adenosine triphosphate–binding cassette transporter A8b gene. Living in EE promoted CD8+ T cell–mediated antitumor immunity through β-AR/CCL2 axis and enhanced the response to PD-1 mAb in a PD-1–insensitive hepatocellular carcinoma model (19).
Exercise can be a factor of eustress and can improve the efficacy of chemo- and radiotherapy. Physical exercise can enhance tumor blood flow and reduce tumor hypoxia, which may decrease tumor aggressiveness and facilitate antitumor drug delivery (102). Exercise promoted chemotherapy efficacy and suppressed tumor growth in mice, which was associated with improved tumor perfusion (102). Moreover, physical exercise may up-regulate NK cell infiltration to enhance the antitumor efficacy of radiotherapy (103). Overall, studies showed that eustress may inhibit tumorigenesis and tumor progression and enhance antitumor treatments (Table 4).
Table 4. Effects and potential mechanisms of eustress on cancer treatment in EE models.
↑, increase; ↓, decrease; →, causal; NR, not report; ATP, adenosine triphosphate; CCR5, C-C chemokine receptor 5; M2-TAM, M2 tumor–associated macrophages.
| Cancer type | Effects on tumor | Effects on TME | Mechanism | Reference |
| Melanoma, colon cancer | ↓ Tumorigenesis, growth | ↓VEGF ↑NK cell, CD8+ function |
↑Hypothalamic-derived BDFN → ↑β-AR → ↓leptin ↑lipocalin production in adipose tissue |
(85) |
| Intracranial glioma | ↓Tumor growth | ↑IL-15, BDFN ↓NK cell, microglia/macrophage |
↑Brain IL-15 → ↑NK cell ↑brain BDFN → ↓microglia/macrophage infiltration and activation |
(86) |
| Mammary cancer | ↓Tumor growth | NR | Cross-talk between changed signaling pathways and adipokine/cytokine secretions in muscle, adipose tissue, and tumor |
(95) |
| Mammary cancer | ↓Tumor growth | COX-2 expression | ↓Intratumoral COX-2 → inflammatory state ↓plasma ratio of adiponectin and leptin |
(96) |
| Pancreatic cancer | ↓Tumor growth | NR | ↓Mitochondria-related genes (encoding key enzymes of the citrate cycle and pyruvate decarboxylation) in cancer cells |
(97) |
| Pancreatic cancer, lung cancer | ↓Tumor growth | ↑ NK cell | β-AR↑ → ↑expression of CCR5 and NKG2D on NK cell → NK cell function |
(20) |
| Hepatocellular carcinoma | ↓ Tumorigenesis, growth ↑response to anti–PD-1 mAb |
↓Immune suppression (↑CD8+ T; ↓MDSC, M2-TAM) |
SNS → β-AR → ↓CCL2/CCR2 → ↓chemotaxis of MDSC and M2-TAM |
(19) |
| Pancreatic cancer | ↑Response to chemotherapy | NR | ↓Tumoral ATP-binding cassette transporter A8b |
(101) |
INTERVENTIONS TARGETING PSYCHOLOGICAL DISTRESS IN PATIENTS WITH CANCER
Psychological intervention
Because of the prevalence of mental illness among patients with cancer and the potential promotion of cancer progression by distress, distress management is necessary and important. Psychological management significantly mitigates psychological distress and improves quality of life (QoL) of patients with cancer (Table 5) (104–110). However, the effects on long-term survival are still controversial. Psychological intervention can improve survival and reduce mortality and recurrence in patients with breast cancer (111, 112). However, in other studies, psychological interventions only reduced psychological distress but did not significantly improve survival of patients with cancer (113–118).
Table 5. Randomized controlled trials on psychological distress management in patients with cancer.
NS, not significant; HR, hazard rate; MBSR, mindfulness-based stress reduction; MBCR, mindfulness-based cancer recovery; SET, supportive expressive group therapy; MCGP, meaning-centered group psychotherapy; CALM, cancer and living meaningfully; SGP, supportive group psychotherapy; PCS, physical component scale.
| Study | Patients (type; feature) | Intervention(n); time | Laboratory examinations | Psychological outcome | Survival effect | Other benefits | Reference |
| Psychotherapy | |||||||
| Kissane et al.
(2004) |
Breast cancer; early stage |
Cognitive-existential group therapy (n = 154); 20 weeks control (n = 149); 20 weeks |
NR | ↓Anxiety | NS (HR for death, 1.35, 95% CI, 0.76–2.39; P = 0.31) |
↑Family functioning |
(117) |
| Andersen et al.
(2008) |
Breast cancer; postsurgery |
Psychological intervention (n = 114); 1 year control (n = 113); 1 year |
NR | NR | ↓Risk of breast cancer recurrence (HR, 0.55; P = 0.034); risk of death from breast cancer (HR, 0.44; P = 0.016) |
NR | (111) |
| Hoffman et al.
(2012) |
Breast cancer; postsurgery |
MBSR (n = 114); 8 weeks control group (n = 115); 8 weeks |
NR | ↓: Total mood disturbance, anxiety |
NR | ↑: Breast cancer-related QoL and endocrine symptoms, well-being* |
(104) |
| Carlson et al.
(2013) |
Breast cancer; with clinically meaningful distress |
MBCR (n = 113); 8 weeks |
MBCR, SET: Steep diurnal cortisol slope |
MBCR: ↓Stress level |
NR | MBCR: ↑QoL, social support |
(108) |
| SET (n = 104); 12 weeks | |||||||
| Control (stress management seminar) (n = 54); 1 day | |||||||
| Witek Janusek et al.
(2019) |
Breast cancer; newly diagnosed |
MBSR (n = 84); 8 weeks | ↑TNF-α, IL-6; ↓IFN-γ |
↓Perceived stress, depressive symptoms |
NR | ↓Fatigue, sleep disturbance |
(122) |
| Control (n = 80); 8 weeks | |||||||
| Breitbart et al.
(2015) |
Various cancers; advanced |
MCGP (n = 132); 8 weeks |
NR | ↓Depression, hopelessness, desire for hastened death |
NR | ↓Physical symptom distress |
(105) |
| SGP (n = 121); 8 weeks | NS: Anxiety | ||||||
| Rodin et al. (2018) | Various cancers; advanced |
CALM (n = 151); 3 to 6 months usual care (n = 154); 3 to 6 months |
NR | ↓Depressive symptoms |
NR | NR | (109) |
| Psychosocial support | |||||||
| Spiegel et al. (1989) | Breast cancer; metastatic |
SGP (n = 50); 1 year | NR | NR | ↑Mean survival: 36.6 months versus 18.8 months (P < 0.00001, Cox; P < 0. 005, log-rank) |
NR | (112) |
| Control (n = 36); 1 year | |||||||
| Goodwin et al.
(2001) |
Breast cancer; metastatic |
SET (n = 158); ≥1 year |
NR | ↓Psychological symptoms |
NS (univariate analysis: HR, 1.06; 95% CI, 0.78–1.45; P = 0.72; multivariate analysis: HR, 1.23; 95% CI 0.88–1.72; P = 0.22) |
↓Pain | (113) |
| Control (n = 77); ≥1 year | |||||||
| Wenzel et al.
(2015) |
Cervical cancer; ≥ 9 and <30 months from diagnosis |
Psychosocial telephone counseling (n = 115); five weekly sessions and a 1-month booster |
NS: IL-4, IL-5, IL-13, IL-10 |
↓Depression, gynecologic and cancer-specific concerns |
NR | NS: QoL | (110) |
| Control (n = 89); 5 weeks and 1 month | NS: Anxiety | ||||||
| Physical relaxation | |||||||
| Kiecolt-Glaser et al. (2014) |
Breast cancer; survivor |
Yoga (n = 100); 3 months |
↓IL-6, TNF-α, IL-1β |
NS | NR | ↑Vitality | (123) |
| Control (n = 100); 3 months |
NS: Fatigue | ||||||
| Chandwani et al.
(2014) |
Breast cancer; undergoing radiotherapy |
Yoga (n = 53); 6 weeks | Yoga: steep diurnal cortisol slope |
NS | NR | Yoga: ↑PCS, physical functioning |
(121) |
| Stretch (n = 56); 6 weeks |
Yoga and stretch: ↓Fatigue |
||||||
| Waitlist (n = 54); 6 weeks | |||||||
| Other | |||||||
| Sharpe et al.
(2014) |
Good prognosis cancers; with major depression |
Depression care for people with cancer (n = 253); 4 months Usual care (n = 247); 4 months |
NR | ↑Responded to anti-depression treatment; ↓Depression, anxiety |
NS (HR, 1.02; 95% CI, 0.72–1·42; P = 0·93) |
↑QoL; ↓Pain, fatigue |
(114, 118) |
| Mulick, et al.
(2018) | |||||||
| Walker et al.
(2014) |
Lung cancer; with major depression |
Depression care for people with lung cancer (n = 68); 4 months |
NR | ↓Average depression severity |
NS (HR, 0.82; 95% CI, 0.56–1.18; P = 0·28) |
↑QoL | (107, 118) |
| Mulick et al. (2018) | Usual care (n = 74); 4 months |
NS: Pain, fatigue, physical; functioning, social functioning |
|||||
*Evaluated by the World Health Organization–Five Well-Being Index (WHO-5).
A flattened diurnal curve of cortisol rhythm is associated with psychological distress and even poor prognosis in patients with cancer (119, 120). Psychological intervention can maintain the diurnal cortisol profile with a steep slope (108, 121). Psychological intervention can also down-regulate the levels of stress-related inflammatory cytokines, such as IL-6 and TNF-α, and up-regulate antitumor immunomodulatory factors, such as IFN-γ (122, 123).
Pharmacological blockade of the stress response
On the basis of the stress response theory, targeting stress response mediators may prevent cascades induced by distress and improve antitumor effects in patients with cancer. There are not many studies in this field, and most of them have explored the role of β-AR blockade. β-AR blockers reduced expression of inflammatory genes induced by acute social psychological stress in healthy volunteers (124). A phase 1 clinical trial showed the safety, tolerability, and promising activity of the combination of propranolol and pembrolizumab in patients with melanoma (125). In some stress-prone phases, blocking the β-AR seems to be particularly useful. The peritransplant period is a stress-prone phase in patients undergoing hematopoietic cell transplantation. Blocking the β-AR in this context is safe and feasible and can reduce stress-induced risk markers (126, 127).
In summary, most clinical studies on psychological stress management in patients with cancer have applied short-term interventions, with changes in psychological distress, physical discomfort, and QoL as the primary and secondary outcomes. By contrast, blocking stress-related signaling, especially in stress-prone phases, can improve the effects of antitumor therapies.
SUMMARY AND FUTURE CONSIDERATIONS
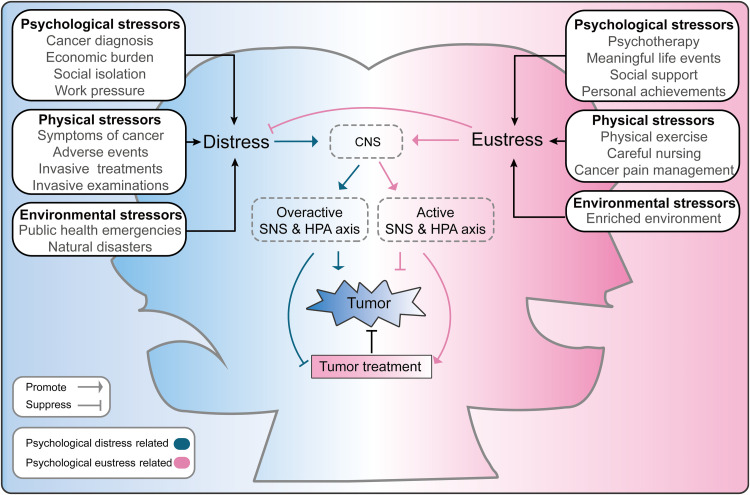
Clinical and preclinical findings suggest virtually opposite effects of psychological distress and eustress on malignant tumors (Fig. 4). These effects are largely mediated by changes in the neuroendocrine-immune system, with the SNS and HPA axes appearing to be the most important mediators. However, there are still limitations in current studies on the effects of psychological distress and eustress on malignant tumors.
Fig. 4. Schematic diagram of the effects of psychological distress and eustress on tumors.
The boxes on either side of the image show examples of psychological, physical, and environmental stressors that can induce psychological distress or eustress. Distress can overactivate the central nervous system (CNS) and thus promote release of large amounts of stress-related neurotransmitters or hormones through the activated SNS and HPA axes. In these ways, psychological distress not only promotes tumorigenesis, tumor growth, and metastasis but also suppresses the efficacy of tumor treatments. Positive stressors can induce eustress and may reduce distress. In addition, positive stressors can activate the neuroendocrine system at a moderate range to suppress tumor progression and enhance the effects of tumor treatments.
In preclinical studies of distress, there is still a lack of models that can mimic the distress experienced by patients, which is complex and unpredictable. Moreover, the timing of stimulation is seldom taken into consideration. For eustress, EE is a widely accepted modeling method. However, specific settings of EE vary in different studies. One question, therefore, is whether a standard and simplified EE model with comparable effects can be developed to improve the reproducibility.
Clinical findings on the effects of psychological stress on tumor control are still controversial. There are reciprocal, interactive, and bidirectional effects between psychological factors and tumors in patients with cancer. Therefore, the establishment of long-term and prospective clinical cohorts is important to uncover the influence of psychological distress and eustress on the risk and prognosis of cancer. Moreover, since the psychological stress faced by patients with tumor is complex, real-world studies are recommended to explore the association between psychological stress and patients’ outcomes.
To elucidate the mechanisms underlying the effects of psychological stress on malignant disease, multiomics studies, such as cytomics, genomics, metabolomics, proteomics, and bioinformatics analytical approaches, can be applied to explore the key mediators of psychological distress and eustress in malignant diseases and to develop relevant targeted therapies or to find prognostic biomarkers. In addition to the SNS and HPA axes, other molecules associated with stress coping may also have effects on malignant tumors, including dopamine, serotonin, and oxytocin (89, 90, 128). And the impact of these mediators on malignancies could be of interest in future studies.
Recently, polymorphic microbiomes have been added to the “hallmarks of cancer,” emphasizing the potential to regulate the antitumor immune response and other hallmarks of cancer (129). The microbiome of the host, e.g., the gut microbiome, can interact with the nervous system through the microbiota-gut-brain axis, which can be influenced by psychological factors (130). Apart from the gut, microbes also reside in other organs, such as the lung. A recently published study illustrated that lung microbiomes can affect brain immunity by regulating microglia, pointing toward a role of the lung-brain axis (131). Therefore, the interaction between microbiomes and the stress response may be one of the mechanisms by which psychological factors modulate tumorigenesis and antitumor immunity.
Acknowledgments
We would like to thank the Thoracic Oncology Ward, Cancer Center and State Key Laboratory of Biotherapy of West China Hospital for providing assistance during writing.
Funding: This work was supported by National Natural Science Foundation of China (no. 81872478).
Author contributions: J.X. conceived the study. Y.W., X.Z., and L.Z. collected the literature. Y.W., X.Z., and L.Z. co-wrote the manuscript. Y.W., X.Z., and X.Y. prepared the figures and tables. G.N. assisted with the language editing and reviewing. J.X. supervised the project. All authors discussed and confirmed the final version of the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Chrousos G. P., Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Selye H., Stress without distress. Brux. Med. 56, 205–210 (1976). [PubMed] [Google Scholar]
- 3.Selye H., Confusion and controversy in the stress field. J. Human Stress 1, 37–44 (1975). [DOI] [PubMed] [Google Scholar]
- 4.Bienertova-Vasku J., Lenart P., Scheringer M., Eustress and distress: Neither good nor bad, but rather the same? Bioessays 42, 1900238 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Eckerling A., Ricon-Becker I., Sorski L., Sandbank E., Ben-Eliyahu S., Stress and cancer: Mechanisms, significance and future directions. Nat. Rev. Cancer 21, 767–785 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Riba M. B., Donovan K. A., Andersen B., Braun I. I., Breitbart W. S., Brewer B. W., Buchmann L. O., Clark M. M., Collins M., Corbett C., Fleishman S., Garcia S., Greenberg D. B., Handzo R. G. F., Hoofring L., Huang C.-H., Lally R., Martin S., Guffey L. M., Mitchell W., Morrison L. J., Pailler M., Palesh O., Parnes F., Pazar J. P., Ralston L., Salman J., Shannon-Dudley M. M., Valentine A. D., Millian N. R. M., Darlow S. D., Distress management, version 3.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 17, 1229–1249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips M. R., Is distress a symptom of mental disorders, a marker of impairment, both or neither? World Psychiatry 8, 91–92 (2009). [PMC free article] [PubMed] [Google Scholar]
- 8.Lutgendorf S. K., Sood A. K., Anderson B., McGinn S., Maiseri H., Dao M., Sorosky J. I., de Geest K., Ritchie J., Lubaroff D. M., Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 23, 7105–7113 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Batty G. D., Russ T. C., Stamatakis E., Kivimäki M., Psychological distress in relation to site specific cancer mortality: Pooling of unpublished data from 16 prospective cohort studies. BMJ 356, j108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D. L. Nelson, B. L. Simmons, Eustress: An elusive construct, an engaging pursuit. Research in Occupational Stress and Well-being 3, 265–322 (2003).
- 11.Lutgendorf S. K., de Geest K., Bender D., Ahmed A., Goodheart M. J., Dahmoush L., Zimmerman M. B., Penedo F. J., Lucci J. A. III, Ganjei-Azar P., Thaker P. H., Mendez L., Lubaroff D. M., Slavich G. M., Cole S. W., Sood A. K., Social influences on clinical outcomes of patients with ovarian cancer. J. Clin. Oncol. 30, 2885–2890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coughlin S. S., Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res. Treat. 177, 537–548 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Mitchell A. J., Chan M., Bhatti H., Halton M., Grassi L., Johansen C., Meader N., Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 12, 160–174 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Lu D., Andersson T. M. L., Fall K., Hultman C. M., Czene K., Valdimarsdóttir U., Fang F., Clinical diagnosis of mental disorders immediately before and after cancer diagnosis. JAMA Oncol. 2, 1188–1196 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Yang T., Qiao Y., Xiang S., Li W., Gan Y., Chen Y., Work stress and the risk of cancer: A meta-analysis of observational studies. Int. J. Cancer 144, 2390–2400 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Kruk J., Aboul-Enein H. Y., Psychological stress and the risk of breast cancer: A case–control study. Cancer Detect. Prev. 28, 399–408 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Wang Y.-H., Li J.-Q., Shi J.-F., Que J.-Y., Liu J.-J., Lappin J. M., Leung J., Ravindran A. V., Chen W.-Q., Qiao Y.-L., Shi J., Lu L., Bao Y.-P., Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 25, 1487–1499 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Shilpa B. M., Bhagya V., Harish G., Srinivas Bharath M. M., Shankaranarayana Rao B. S., Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog. Neuropsychopharmacol. Biol Psychiatry 76, 88–100 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Liu C., Yang Y., Chen C., Li L., Li J., Wang X., Chu Q., Qiu L., Ba Q., Li X., Wang H., Environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat. Commun. 12, 5725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y., Gan Y., Wang Q., Meng Z., Li G., Shen Y., Wu Y., Li P., Yao M., Gu J., Tu H., Enriching the housing environment for mice enhances their NK Cell antitumor immunity via sympathetic nerve–dependent regulation of NKG2D and CCR5. Cancer Res. 77, 1611–1622 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Budiu R. A., Vlad A. M., Nazario L., Bathula C., Cooper K. L., Edmed J., Thaker P. H., Urban J., Kalinski P., Lee A. V., Elishaev E. L., Conrads T. P., Flint M. S., Restraint and social isolation stressors differentially regulate adaptive immunity and tumor angiogenesis in a breast cancer mouse model. Cancer Clin. Oncol. 6, 12–24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H., Xia L., Chen J., Zhang S., Martin V., Li Q., Lin S., Chen J., Calmette J., Lu M., Fu L., Yang J., Pan Z., Yu K., He J., Morand E., Schlecht-Louf G., Krzysiek R., Zitvogel L., Kang B., Zhang Z., Leader A., Zhou P., Lanfumey L., Shi M., Kroemer G., Ma Y., Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 25, 1428–1441 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Deng G.-H., Zhang J., Wang Y., Xia X.-Y., Luo X.-M., Deng Y.-T., He S.-S., Mao Y.-Y., Peng X.-C., Wei Y.-Q., Jiang Y., The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 52, 130–142 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Cui B., Luo Y., Tian P., Peng F., Lu J., Yang Y., Su Q., Liu B., Yu J., Luo X., Yin L., Cheng W., An F., He B., Liang D., Wu S., Chu P., Song L., Liu X., Luo H., Xu J., Pan Y., Wang Y., Li D., Huang P., Yang Q., Zhang L., Zhou B. P., Liu S., Xu G., Lam E. W.-F., Kelley K. W., Liu Q., Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Invest. 129, 1030–1046 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao M., Huang W., Chen Y., Li G., Liu N., Wu Y., Wang G., Li Q., Kong D., Xue T., Yang N., Liu Y., Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through β-adrenergic-activated CXCL5-CXCR2-Erk signaling cascades. Int. J. Cancer 149, 460–472 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Zhang S., Yu F., Che A., Tan B., Huang C., Chen Y., Liu X., Huang Q., Zhang W., Ma C., Qian M., Liu M., Qin J., du B., Neuroendocrine regulation of stress-induced T cell dysfunction during lung cancer immunosurveillance via the kisspeptin/GPR54 signaling pathway. Adv. Sci. 9, 2104132 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan S., Karpova Y., Baiz D., Yancey D., Pullikuth A., Flores A., Register T., Cline J. M., D’Agostino R Jr, Danial N., Datta S. R., Kulik G., Behavioral stress accelerates prostate cancer development in mice. J. Clin. Invest. 123, 874–886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renz B. W., Takahashi R., Tanaka T., Macchini M., Hayakawa Y., Dantes Z., Maurer H. C., Chen X., Jiang Z., Westphalen C. B., Ilmer M., Valenti G., Mohanta S. K., Habenicht A. J. R., Middelhoff M., Chu T., Nagar K., Tailor Y., Casadei R., Marco M. D., Kleespies A., Friedman R. A., Remotti H., Reichert M., Worthley D. L., Neumann J., Werner J., Iuga A. C., Olive K. P., Wang T. C., β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell 33, 75–90.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sommershof A., Scheuermann L., Koerner J., Groettrup M., Chronic stress suppresses anti-tumor TCD8+ responses and tumor regression following cancer immunotherapy in a mouse model of melanoma. Brain Behav. Immun. 65, 140–149 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Zanos P., Jackson I. L., Zhang X., Zhu X., Gould T., Vujaskovic Z., Psychological stress enhances tumor growth and diminishes radiation response in preclinical model of lung cancer. Radiother. Oncol. 146, 126–135 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Chen H., Liu D., Guo L., Cheng X., Guo N., Shi M., Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J. Pathol. 244, 49–60 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Pan C., Wu J., Zheng S., Sun H., Fang Y., Huang Z., Shi M., Liang L., Bin J., Liao Y., Chen J., Liao W., Depression accelerates gastric cancer invasion and metastasis by inducing a neuroendocrine phenotype via the catecholamine/β2-AR/MACC1 axis. Cancer Commun. (Lond) 41, 1049–1070 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levi B., Matzner P., Goldfarb Y., Sorski L., Shaashua L., Melamed R., Rosenne E., Page G. G., Ben-Eliyahu S., Stress impairs the efficacy of immune stimulation by CpG-C: Potential neuroendocrine mediating mechanisms and significance to tumor metastasis and the perioperative period. Brain Behav. Immun. 56, 209–220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falcinelli M., Thaker P. H., Lutgendorf S. K., Conzen S. D., Flaherty R. L., Flint M. S., The role of psychologic stress in cancer initiation: Clinical relevance and potential molecular mechanisms. Cancer Res. 81, 5131–5140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Valente V. B., de Melo Cardoso D., Kayahara G. M., Nunes G. B., Tjioe K. C., Biasoli É. R., Miyahara G. I., Oliveira S. H. P., Mingoti G. Z., Bernabé D. G., Stress hormones promote DNA damage in human oral keratinocytes. Sci. Rep. 11, 19701 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamboy-Caraballo R., Ortiz-Sanchez C., Acevedo-Santiago A., Matta J., N. A. Monteiro A., N. Armaiz-Pena G., Norepinephrine-induced DNA damage in ovarian cancer cells. Int. J. Mol. Sci. 21, 2250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flint M. S., Baum A., Chambers W. H., Jenkins F. J., Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 32, 470–479 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Hara M. R., Kovacs J. J., Whalen E. J., Rajagopal S., Strachan R. T., Grant W., Towers A. J., Williams B., Lam C. M., Xiao K., Shenoy S. K., Gregory S. G., Ahn S., Duckett D. R., Lefkowitz R. J., A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 477, 349–353 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Z., Liu L., Zhang C., Zheng T., Wang J., Lin M., Zhao Y., Wang X., Levine A. J., Hu W., Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 109, 7013–7018 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang H. J., Boo H. J., Lee H. J., Min H. Y., Lee H. Y., Chronic stress facilitates lung tumorigenesis by promoting exocytosis of IGF2 in lung epithelial cells. Cancer Res. 76, 6607–6619 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Eng J. W.-L., Reed C. B., Kokolus K. M., Pitoniak R., Utley A., Bucsek M. J., Ma W. W., Repasky E. A., Hylander B. L., Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat. Commun. 6, 6426 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thaker P. H., Han L. Y., Kamat A. A., Arevalo J. M., Takahashi R., Lu C., Jennings N. B., Armaiz-Pena G., Bankson J. A., Ravoori M., Merritt W. M., Lin Y. G., Mangala L. S., Kim T. J., Coleman R. L., Landen C. N., Li Y., Felix E., Sanguino A. M., Newman R. A., Lloyd M., Gershenson D. M., Kundra V., Lopez-Berestein G., Lutgendorf S. K., Cole S. W., Sood A. K., Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 12, 939–944 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Yang E. V., Kim S.-J., Donovan E. L., Chen M., Gross A. C., Webster Marketon J. I., Barsky S. H., Glaser R., Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: Implications for stress-related enhancement of tumor progression. Brain Behav. Immun. 23, 267–275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang E. V., Sood A. K., Chen M., Li Y., Eubank T. D., Marsh C. B., Jewell S., Flavahan N. A., Morrison C., Yeh P.-E., Lemeshow S., Glaser R., Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 66, 10357–10364 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Landen Jr. C. N., Lin Y. G., Pena G. N. A., Das P. D., Arevalo J. M., Kamat A. A., Han L. Y., Jennings N. B., Spannuth W. A., Thaker P. H., Lutgendorf S. K., Savary C. A., Sanguino A. M., Lopez-Berestein G., Cole S. W., Sood A. K., Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 67, 10389–10396 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Shi M., Liu D., Duan H., Han C., Wei B., Qian L., Chen C., Guo L., Hu M., Yu M., Song L., Shen B., Guo N., Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol. Cancer 9, 269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H., Wang C., Xie N., Zhuang Z., Liu X., Hou J., Huang H., Activation of adrenergic receptor β2 promotes tumor progression and epithelial mesenchymal transition in tongue squamous cell carcinoma. Int. J. Mol. Med. 41, 147–154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sloan E. K., Priceman S. J., Cox B. F., Yu S., Pimentel M. A., Tangkanangnukul V., Arevalo J. M. G., Morizono K., Karanikolas B. D. W., Wu L., Sood A. K., Cole S. W., The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma X., Wang M., Yin T., Zhao Y., Wei X., Myeloid-derived suppressor cells promote metastasis in breast cancer after the stress of operative removal of the primary cancer. Front. Oncol. 9, 855 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le C. P., Nowell C. J., Kim-Fuchs C., Botteri E., Hiller J. G., Ismail H., Pimentel M. A., Chai M. G., Karnezis T., Rotmensz N., Renne G., Gandini S., Pouton C. W., Ferrari D., Möller A., Stacker S. A., Sloan E. K., Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 7, 10634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellinger D. L., Dulcich M. S., Molinaro C., Gifford P., Lorton D., Gridley D. S., Hartman R. E., Psychosocial stress and age influence depression and anxiety-related behavior, drive tumor inflammatory cytokines and accelerate prostate cancer growth in mice. Front Oncol. 11, 703848 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devi S., Alexandre Y. O., Loi J. K., Gillis R., Ghazanfari N., Creed S. J., Holz L. E., Shackleford D., Mackay L. K., Heath W. R., Sloan E. K., Mueller S. N., Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230.e7 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Nissen M. D., Sloan E. K., Mattarollo S. R., β-adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol. Res. 6, 98–109 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Frick L. R., Barreiro Arcos M. L., Rapanelli M., Zappia M. P., Brocco M., Mongini C., Genaro A. M., Cremaschi G. A., Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress 12, 134–143 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Qiao G., Chen M., Mohammadpour H., MacDonald C. R., Bucsek M. J., Hylander B. L., Barbi J. J., Repasky E. A., Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol. Res. 9, 651–664 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taves M. D., Ashwell J. D., Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 21, 233–243 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Qiao G., Bucsek M. J., Winder N. M., Chen M., Giridharan T., Olejniczak S. H., Hylander B. L., Repasky E. A., β-adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: A mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. 68, 11–22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan K.-Q., Li Y.-Y., Wang H.-L., Mao X.-T., Guo J.-X., Wang F., Huang L.-J., Li Y.-N., Ma X.-Y., Gao Z.-J., Chen W., Qian D.-D., Xue W.-J., Cao Q., Zhang L., Shen L., Zhang L., Tong C., Jin J., Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179, 864–879.e19 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Sun Z., Hou D., Liu S., Fu W., Wang J., Liang Z., Norepinephrine inhibits the cytotoxicity of NK92-MI cells via the β2-adrenoceptor/cAMP/PKA/p-CREB signaling pathway. Mol. Med. Rep. 17, 8530–8535 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Tarr A. J., Powell N. D., Reader B. F., Bhave N. S., Roloson A. L., Carson W. E. III, Sheridan J. F., β-Adrenergic receptor mediated increases in activation and function of natural killer cells following repeated social disruption. Brain Behav. Immun. 26, 1226–1238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krukowski K., Eddy J., Kosik K. L., Konley T., Janusek L. W., Mathews H. L., Glucocorticoid dysregulation of natural killer cell function through epigenetic modification. Brain Behav. Immun. 25, 239–249 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenne E., Sorski L., Shaashua L., Neeman E., Matzner P., Levi B., Ben-Eliyahu S., In vivo suppression of NK cell cytotoxicity by stress and surgery: Glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav. Immun. 37, 207–219 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Togashi Y., Shitara K., Nishikawa H., Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Guereschi M. G., Araujo L. P., Maricato J. T., Takenaka M. C., Nascimento V. M., Vivanco B. C., Reis V. O., Keller A. C., Brum P. C., Basso A. S., Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur. J. Immunol. 43, 1001–1012 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Dhabhar F. S., Saul A. N., Holmes T. H., Daugherty C., Neri E., Tillie J. M., Kusewitt D., Oberyszyn T. M., High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLOS ONE 7, e33069 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin J., Wang X., Wang Q., Guo X., Cao J., Zhang X., Zhu T., Zhang D., Wang W., Wang J., Shen B., Gao X., Shi Y., Zhang J., Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLOS ONE 8, e74497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohammadpour H., MacDonald C. R., Qiao G., Chen M., Dong B., Hylander B. L., McCarthy P. L., Abrams S. I., Repasky E. A., β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 129, 5537–5552 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez F. O., Sica A., Mantovani A., Locati M., Macrophage activation and polarization. Front. Biosci. 13, 453–461 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Fjæstad K. Y., Rømer A. M. A., Goitea V., Johansen A. Z., Thorseth M. L., Carretta M., Engelholm L. H., Grøntved L., Junker N., Madsen D. H., Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 41, 1364–1375 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J.-W., Shahzad M. M. K., Lin Y. G., Armaiz-Pena G., Mangala L. S., Han H.-D., Kim H.-S., Nam E. J., Jennings N. B., Halder J., Nick A. M., Stone R. L., Lu C., Lutgendorf S. K., Cole S. W., Lokshin A. E., Sood A. K., Surgical stress promotes tumor growth in ovarian carcinoma. Clin. Cancer Res. 15, 2695–2702 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peeters C. F., de Waal R. M., Wobbes T., Westphal J. R., Ruers T. J. M., Outgrowth of human liver metastases after resection of the primary colorectal tumor: A shift in the balance between apoptosis and proliferation. Int. J. Cancer 119, 1249–1253 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Antoni M. H., Jacobs J. M., Bouchard L. C., Lechner S. C., Jutagir D. R., Gudenkauf L. M., Blomberg B. B., Glück S., Carver C. S., Post-surgical depressive symptoms and long-term survival in non-metastatic breast cancer patients at 11-year follow-up. Gen. Hosp. Psychiatry 44, 16–21 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matzner P., Sandbank E., Neeman E., Zmora O., Gottumukkala V., Ben-Eliyahu S., Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat. Rev. Clin. Oncol. 17, 313–326 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Kang Y., Nagaraja A. S., Armaiz-Pena G. N., Dorniak P. L., Hu W., Rupaimoole R., Liu T., Gharpure K. M., Previs R. A., Hansen J. M., Rodriguez-Aguayo C., Ivan C., Ram P., Sehgal V., Lopez-Berestein G., Lutgendorf S. K., Cole S. W., Sood A. K., Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin. Cancer Res. 22, 1713–1724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reeder A., Attar M., Nazario L., Bathula C., Zhang A., Hochbaum D., Roy E., Cooper K. L., Oesterreich S., Davidson N. E., Neumann C. A., Flint M. S., Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br. J. Cancer 112, 1461–1470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bucsek M. J., Qiao G., Donald C. R. M., Giridharan T., Evans L., Niedzwecki B., Liu H., Kokolus K. M., Eng J. W.-L., Messmer M. N., Attwood K., Abrams S. I., Hylander B. L., Repasky E. A., β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 77, 5639–5651 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deng Y., Xia X., Zhao Y., Zhao Z., Martinez C., Yin W., Yao J., Hang Q., Wu W., Zhang J., Yu Y., Xia W., Yao F., Zhao D., Sun Y., Ying H., Hung M.-C., Ma L., Glucocorticoid receptor regulates PD-L1 and MHC-I in pancreatic cancer cells to promote immune evasion and immunotherapy resistance. Nat. Commun. 12, 7041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacDonald C. R., Bucsek M. J., Qiao G., Chen M., Evans L., Greenberg D. J., Uccello T. P., Battaglia N. G., Hylander B. L., Singh A. K., Lord E. M., Gerberc S. A., Repasky E. A., Adrenergic receptor signaling regulates the response of tumors to ionizing radiation. Radiat. Res. 191, 585–589 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen M., Qiao G., Hylander B. L., Mohammadpour H., Wang X. Y., Subjeck J. R., Singh A. K., Repasky E. A., Adrenergic stress constrains the development of anti-tumor immunity and abscopal responses following local radiation. Nat. Commun. 11, 1821 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng G. H., Liu J., Zhang J., Wang Y., Peng X. C., Wei Y. Q., Jiang Y., Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J. Exp. Clin. Cancer Res. 33, 21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao Z., Fenoglio S., Gao D. C., Camiolo M., Stiles B., Lindsted T., Schlederer M., Johns C., Altorki N., Mittal V., Kenner L., Sordella R., TGF-β IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc. Natl. Acad. Sci. U.S.A. 107, 15535–15540 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nilsson M. B., Sun H., Diao L., Tong P., Liu D., Li L., Fan Y., Poteete A., Lim S.-O., Howells K., Haddad V., Gomez D., Tran H., Pena G. A., Sequist L. V., Yang J. C., Wang J., Kim E. S., Herbst R. S., Lee J. J., Hong W. K., Wistuba I., Hung M.-C., Sood A. K., Heymach J. V., Stress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with β-blockers. Sci. Transl. Med. 9, eaao4307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moore S. C., Lee I.-M., Weiderpass E., Campbell P. T., Sampson J. N., Kitahara C. M., Keadle S. K., Arem H., de Gonzalez A. B., Hartge P., Adami H.-O., Blair C. K., Borch K. B., Boyd E., Check D. P., Fournier A., Freedman N. D., Gunter M., Johannson M., Khaw K.-T., Linet M. S., Orsini N., Park Y., Riboli E., Robien K., Schairer C., Sesso H., Spriggs M., Van Dusen R., Wolk A., Matthews C. E., Patel A. V., Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 176, 816–825 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao L., Liu X., Lin E.-J. D., Wang C., Choi E. Y., Riban V., Lin B., During M. J., Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142, 52–64 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garofalo S., D’Alessandro G., Chece G., Brau F., Maggi L., Rosa A., Porzia A., Mainiero F., Esposito V., Lauro C., Benigni G., Bernardini G., Santoni A., Limatola C., Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat. Commun. 6, 6623 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slater A. M., Cao L., A protocol for housing mice in an enriched environment. J. Vis. Exp. 52874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sampedro-Piquero P., Begega A., Arias J. L., Increase of glucocorticoid receptor expression after environmental enrichment: Relations to spatial memory, exploration and anxiety-related behaviors. Physiol. Behav. 129, 118–129 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Darna M., Beckmann J. S., Gipson C. D., Bardo M. T., Dwoskin L. P., Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res. 1599, 115–125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Del Arco A., Segovia G., Garrido P., de Blas M., Mora F., Stress, prefrontal cortex and environmental enrichment: Studies on dopamine and acetylcholine release and working memory performance in rats. Behav. Brain Res. 176, 267–273 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Ashokan A., Hegde A., Balasingham A., Mitra R., Housing environment influences stress-related hippocampal substrates and depression-like behavior. Brain Res. 1683, 78–85 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Novaes L. S., Dos Santos N. B., Batalhote R. F. P., Malta M. B., Camarini R., Scavone C., Munhoz C. D., Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology 113, 457–466 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Sakama S., Kurusu K., Morita M., Oizumi T., Masugata S., Oka S., Yokomizo S., Nishimura M., Morioka T., Kakinuma S., Shimada Y., Nakamura A. J., An enriched environment alters DNA repair and inflammatory responses after radiation exposure. Front. Immunol. 12, 760322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andò S., Catalano S., The multifactorial role of leptin in driving the breast cancer microenvironment. Nat. Rev. Endocrinol. 8, 263–275 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Le Guennec D., Hatte V., Farges M.-C., Rougé S., Goepp M., Caldefie-Chezet F., Vasson M.-P., Rossary A., Modulation of inter-organ signalling in obese mice by spontaneous physical activity during mammary cancer development. Sci. Rep. 10, 8794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nachat-Kappes R., Pinel A., Combe K., Lamas B., Farges M.-C., Rossary A., Goncalves-Mendes N., Caldefie-Chezet F., Vasson M.-P., Basu S., Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLOS ONE 7, e51525 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li G., Gan Y., Fan Y., Wu Y., Lin H., Song Y., Cai X., Yu X., Pan W., Yao M., Gu J., Tu H., Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci. Rep. 5, 7856 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao R., Bergin S. M., Huang W., Slater A. M., Liu X., Judd R. T., Lin E.-J. D., Widstrom K. J., Scoville S. D., Yu J., Caligiuri M. A., Cao L., Environmental and genetic activation of hypothalamic BDNF modulates T-cell immunity to exert an anticancer phenotype. Cancer Immunol. Res. 4, 488–497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meng Z., Liu T., Song Y., Wang Q., Xu D., Jiang J., Li M., Qiao J., Luo X., Gu J., Tu H., Gan Y., Exposure to an enriched environment promotes the terminal maturation and proliferation of natural killer cells in mice. Brain Behav. Immun. 77, 150–160 (2019). [DOI] [PubMed] [Google Scholar]
- 100.Duan S., Guo W., Xu Z., He Y., Liang C., Mo Y., Wang Y., Xiong F., Guo C., Li Y., Li X., Li G., Zeng Z., Xiong W., Wang F., Natural killer group 2D receptor and its ligands in cancer immune escape. Mol. Cancer 18, 29 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Y., Gan Y., Yuan H., Wang Q., Fan Y., Li G., Zhang J., Yao M., Gu J., Tu H., Enriched environment housing enhances the sensitivity of mouse pancreatic cancer to chemotherapeutic agents. Biochem. Biophys. Res. Commun. 473, 593–599 (2016). [DOI] [PubMed] [Google Scholar]
- 102.Betof A. S., Lascola C. D., Weitzel D., Landon C., Scarbrough P. M., Devi G. R., Palmer G., Jones L. W., Dewhirst M. W., Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J. Natl. Cancer Inst. 107, djv040 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dufresne S., Guéritat J., Chiavassa S., Noblet C., Assi M., Rioux-Leclercq N., Rannou-Bekono F., Lefeuvre-Orfila L., Paris F., Rébillard A., Exercise training improves radiotherapy efficiency in a murine model of prostate cancer. FASEB J. 34, 4984–4996 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Hoffman C. J., Ersser S. J., Hopkinson J. B., Nicholls P. G., Harrington J. E., Thomas P. W., Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: A randomized, controlled trial. J. Clin. Oncol. 30, 1335–1342 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Breitbart W., Rosenfeld B., Pessin H., Applebaum A., Kulikowski J., Lichtenthal W. G., Meaning-centered group psychotherapy: An effective intervention for improving psychological well-being in patients with advanced cancer. J. Clin. Oncol. 33, 749–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Faller H., Schuler M., Richard M., Heckl U., Weis J., Küffner R., Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta-analysis. J. Clin. Oncol. 31, 782–793 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Walker J., Hansen C. H., Martin P., Symeonides S., Gourley C., Wall L., Weller D., Murray G., Sharpe M.; SMaRT (Symptom Management Research Trials) Oncology-3 Team , Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): A multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 15, 1168–1176 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Carlson L. E., Doll R., Stephen J., Faris P., Tamagawa R., Drysdale E., Speca M., Randomized controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer (MINDSET). J. Clin. Oncol. 31, 3119–3126 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Rodin G., Lo C., Rydall A., Shnall J., Malfitano C., Chiu A., Panday T., Watt S., An E., Nissim R., Li M., Zimmermann C., Hales S., Managing cancer and living meaningfully (CALM): A randomized controlled trial of a psychological intervention for patients with advanced cancer. J. Clin. Oncol. 36, 2422–2432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wenzel L., Osann K., Hsieh S., Tucker J. A., Monk B. J., Nelson E. L., Psychosocial telephone counseling for survivors of cervical cancer: Results of a randomized biobehavioral trial. J. Clin. Oncol. 33, 1171–1179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andersen B. L., Yang H.-C., Farrar W. B., Golden-Kreutz D. M., Emery C. F., Thornton L. M., Young D. C., Carson W. E. III, Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer 113, 3450–3458 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spiegel D., Bloom J. R., Kraemer H. C., Gottheil E., Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 2, 888–891 (1989). [DOI] [PubMed] [Google Scholar]
- 113.Goodwin P. J., Leszcz M., Ennis M., Koopmans J., Vincent L., Guther H., Drysdale E., Hundleby M., Chochinov H. M., Navarro M., Speca M., Masterson J., Dohan L., Sela R., Warren B., Paterson A., Pritchard K. I., Arnold A., Doll R., O’Reilly S. E., Quirt G., Hood N., Hunter J., The effect of group psychosocial support on survival in metastatic breast cancer. N. Engl. J. Med. 345, 1719–1726 (2001). [DOI] [PubMed] [Google Scholar]
- 114.Sharpe M., Walker J., Holm Hansen C., Martin P., Symeonides S., Gourley C., Wall L., Weller D., Murray G.; SMaRT (Symptom Management Research Trials) Oncology-2 Team , Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): A multicentre randomised controlled effectiveness trial. Lancet 384, 1099–1108 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Jassim G. A., Whitford D. L., Hickey A., Carter B., Psychological interventions for women with non-metastatic breast cancer. Cochrane Database Syst. Rev. CD008729 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Mustafa M., Carson-Stevens A., Gillespie D., Edwards A. G., Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst. Rev. Cd004253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kissane D. W., Love A., Hatton A., Bloch S., Smith G., Clarke D. M., Miach P., Ikin J., Ranieri N., Snyder R. D., Effect of cognitive-existential group therapy on survival in early-stage breast cancer. J. Clin. Oncol. 22, 4255–4260 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Mulick A., Walker J., Puntis S., Burke K., Symeonides S., Gourley C., Wanat M., Frost C., Sharpe M., Does depression treatment improve the survival of depressed patients with cancer? A long-term follow-up of participants in the SMaRT Oncology-2 and 3 trials. Lancet Psychiatry 5, 321–326 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Sephton S. E., Lush E., Dedert E. A., Floyd A. R., Rebholz W. N., Dhabhar F. S., Spiegel D., Salmon P., Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun. 30, S163–S170 (2013). [DOI] [PubMed] [Google Scholar]
- 120.Adam E. K., Quinn M. E., Tavernier R., McQuillan M. T., Dahlke K. A., Gilbert K. E., Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 83, 25–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chandwani K. D., Perkins G., Nagendra H. R., Raghuram N. V., Spelman A., Nagarathna R., Johnson K., Fortier A., Arun B., Wei Q., Kirschbaum C., Haddad R., Morris G. S., Scheetz J., Chaoul A., Cohen L., Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J. Clin. Oncol. 32, 1058–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Witek Janusek L., Tell D., Mathews H. L., Mindfulness based stress reduction provides psychological benefit and restores immune function of women newly diagnosed with breast cancer: A randomized trial with active control. Brain Behav. Immun. 80, 358–373 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Kiecolt-Glaser J. K., Bennett J. M., Andridge R., Peng J., Shapiro C. L., Malarkey W. B., Emery C. F., Layman R., Mrozek E. E., Glaser R., Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. J. Clin. Oncol. 32, 1040–1049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacCormack J. K., Gaudier-Diaz M. M., Armstrong-Carter E. L., Arevalo J. M. G., Meltzer-Brody S., Sloan E. K., Cole S. W., Muscatell K. A., Beta-adrenergic blockade blunts inflammatory and antiviral/antibody gene expression responses to acute psychosocial stress. Neuropsychopharmacology 46, 756–762 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gandhi S., Pandey M. R., Attwood K., Ji W., Witkiewicz A. K., Knudsen E. S., Allen C., Tario J. D., Wallace P. K., Cedeno C. D., Levis M., Stack S., Funchain P., Drabick J. J., Bucsek M. J., Puzanov I., Mohammadpour H., Repasky E. A., Ernstoff M. S., Phase i clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: Safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 27, 87–95 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knight J. M., Rizzo J. D., Hari P., Pasquini M. C., Giles K. E., D’Souza A., Logan B. R., Hamadani M., Chhabra S., Dhakal B., Shah N., Sriram D., Horowitz M. M., Cole S. W., Propranolol inhibits molecular risk markers in HCT recipients: A phase 2 randomized controlled biomarker trial. Blood Adv. 4, 467–476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Knight J. M., Kerswill S. A., Hari P., Cole S. W., Logan B. R., D’Souza A., Shah N. N., Horowitz M. M., Stolley M. R., Sloan E. K., Giles K. E., Costanzo E. S., Hamadani M., Chhabra S., Dhakal B., Rizzo J. D., Repurposing existing medications as cancer therapy: Design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer 18, 593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pan S., Yin K., Tang Z., Wang S., Chen Z., Wang Y., Zhu H., Han Y., Liu M., Jiang M., Xu N., Zhang G., Stimulation of hypothalamic oxytocin neurons suppresses colorectal cancer progression in mice. eLife 10, e67535 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hanahan D., Hallmarks of cancer: New dimensions. Cancer Discov. 12, 31–46 (2022). [DOI] [PubMed] [Google Scholar]
- 130.Cryan J. F., O’Riordan K. J., Cowan C. S. M., Sandhu K. V., Bastiaanssen T. F. S., Boehme M., Codagnone M. G., Cussotto S., Fulling C., Golubeva A. V., Guzzetta K. E., Jaggar M., Long-Smith C. M., Lyte J. M., Martin J. A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O’Connor R., Cruz-Pereira J. S., Peterson V. L., Rea K., Ritz N. L., Sherwin E., Spichak S., Teichman E. M., van de Wouw M., Ventura-Silva A. P., Wallace-Fitzsimons S. E., Hyland N., Clarke G., Dinan T. G., The microbiota-gut-brain axis. Physiol Rev. 99, 1877–2013 (2019). [DOI] [PubMed] [Google Scholar]
- 131.Hosang L., Canals R. C., van der Flier F. J., Hollensteiner J., Daniel R., Flügel A., Odoardi F., The lung microbiome regulates brain autoimmunity. Nature 603, 138–144 (2022). [DOI] [PubMed] [Google Scholar]