Abstract
The catalytic asymmetric geminal bis-nucleophilic addition to nonreactive functional groups is a type of highly desirable yet challenging transformation in organic chemistry. Here, we report the first catalytic asymmetric reductive/deoxygenative alkynylation of secondary amides. The method is based on a multicatalysis strategy that merges iridium/copper relay catalysis with organocatalysis. A further combination with the palladium-catalyzed alkyne hydrogenation allows the one-pot enantioselective reductive alkylation of secondary amides. This versatile protocol allows the efficient synthesis of four types of α-branched chiral amines, which are prevalent structural motifs of active pharmaceutical ingredients. The protocol also features excellent enantioselectivity, chemoselectivity, and functional group tolerance to be compatible with more reactive functional groups such as ketone and aldehyde. The synthetic utility of the method was further demonstrated by the late-stage functionalization of two drug derivatives and the concise, first catalytic asymmetric approach to the κ-opioid antagonist aticaprant.
The efficient methodology for the highly enantioselective synthesis of four types of α-branched chiral amines is described.
INTRODUCTION
Despite the substantial advance made over the past decades on the catalytic asymmetric reactions, the catalytic enantioselective reductive functionalization of nonreactive functional groups at a high oxidation state such as amides and nitriles remains highly challenging (Fig. 1A). The challenge originates not only from the low reactivity of those functional groups but also from the need to undertake a geminal bis-addition of two different nucleophiles onto the same carbon in a highly enantioselective manner [>90% enantiomeric excess (ee)] and in one pot. For the functionalization of nitriles, Hoveyda and colleagues reported in 2019 an elegant catalytic enantioselective reductive allylation methodology to yield chiral α,β-disubstituted homoallylic primary amines (cf. Fig. 1A) (1).
Fig. 1. The challenge and significance of the topic.
(A) The challenge. (B) State of the art. (C) This work: versatile catalytic enantioselective transformations of secondary amides. (D) Significance of structurally diverse α-branched amines for medicinal chemistry/pharmaceutical industry. GARPHOS, ligand containing the skeleton of (4,4’,6,6’-tetramethoxy-[1,1’-biphenyl]-2,2’-diyl)bis(diphenylphosphane).
The catalytic, asymmetric reductive functionalization of highly stable amides represents an unsolved challenge. Because of the delocalization of the nitrogen lone pair into the carbonyl group, the carboxamide group is known as one of the least electrophilic carboxylic acid derivatives. Hence, the amide group is prevalent in proteins, peptides, medicinal agents, and materials (2). Amides are readily available from natural sources and through a variety of established methods from carboxylic acids and amines, as well as recently developed strategies such as amide-directed C─H bond activation and asymmetric coupling (3–5). These salient features of amides make them highly attractive starting materials for organic synthesis (6–12). Nevertheless, the inertness of amides renders the direct and chemoselective transformation of amides (9, 13, 14) a long-standing challenge in organic synthesis and multistep protocols have to be used (6, 7). This is the case of the reductive alkylation of amides, an indispensable transformation for the synthesis of many biologically active alkaloids (6–12). During the past decade, considerable advancements on the direct transformations of amides have been achieved (14–18) either via the electrophilic activation of amides (19–31) or via catalytic partial reduction of amides (32–40). However, very few examples of the asymmetric transformations of amides have been reported (41–44), and those involving the catalytic asymmetric reductive transformation of the amide group itself is even more scarce (42, 43). Recently, we disclosed the catalytic asymmetric reductive cyanation and phosphonylation of secondary amides using iridium/chiral thiourea relay catalysis (42). More recently, a highly enantioselective, catalytic reductive alkynylation of tertiary benzamides has been achieved by the collaborative research of Wang’s and Huang’s groups (43). The method consists of Vaska’s complex [IrCl(CO)(PPh3)2]-catalyzed hydrosilylation of amides with tetramethyldisiloxane, followed by CuI/chiral diphosphine ligand-catalyzed asymmetric alkynylation of the iminium ion intermediates (i) to give tertiary propargylamines in up to 98% ee (Fig. 1B). Despite these notable progresses, several limitations exist, including the failure on secondary amides, incompatibility with the synthesis of α-branched chiral primary, secondary, and cyclic tertiary amines, and moderate functional group tolerance (43). These limitations originate from the fact that tertiary, secondary, and primary amides behave differently in many reactions. Many transformations developed for tertiary amides are not applicable to secondary amides (3, 19, 23, 25, 26) and vice versa (4, 5, 21, 22, 24).
Thus, the catalytic, asymmetric reductive alkynylation and reductive alkylation of secondary amides remain unconquered. Nevertheless, such transformations are in high demand, given the easy availability (4, 5, 45, 46) and widespread use of secondary amides in organic synthesis (7), and the presence of chiral α-branched secondary propargylamines (47–51) and chiral α-branched secondary amine motifs (52) in many medicinal agents (Fig. 1D) (53), bioactive alkaloids (54), and chiral catalyst (55). In the list of the top 200 brand-name drugs by total U.S. prescriptions in 2021, 26 contain a chiral secondary amine motif (53). Moreover, the chiral α-branched secondary propargylamines could be converted in one pot into chiral α-branched primary amines or cyclic tertiary amines (Fig. 1C), namely, nitrogen heterocycles, which are also active pharmaceutical ingredients (APIs) (Fig. 1D). The importance of the latter is highlighted by the fact that in U.S. Food and Drug Administration–approved drugs, 59% of unique small-molecule drugs contain a nitrogen heterocycle (56).
On the basis of abovementioned considerations, we decided to explore a catalytic, enantioselective reductive alkynylation of secondary amides. However, such transformations present several challenges: (i) After the planned Ir-catalyzed hydrosilation of a secondary amide, the presumed imine intermediate is much less reactive than the iminium ion (i in Fig. 1B) generated from a tertiary amide; (ii) the compatibility of all the reagents used in a one-pot environment to realize the fully catalytic and highly chemo- and enantioselective reaction; and (iii) high versatility and functional group tolerance are needed to cover both aromatic (benzamide-type) and aliphatic amides and diverse functionalized terminal alkynes.
RESULTS
Development of an enantioselective reductive alkynylation of secondary amides
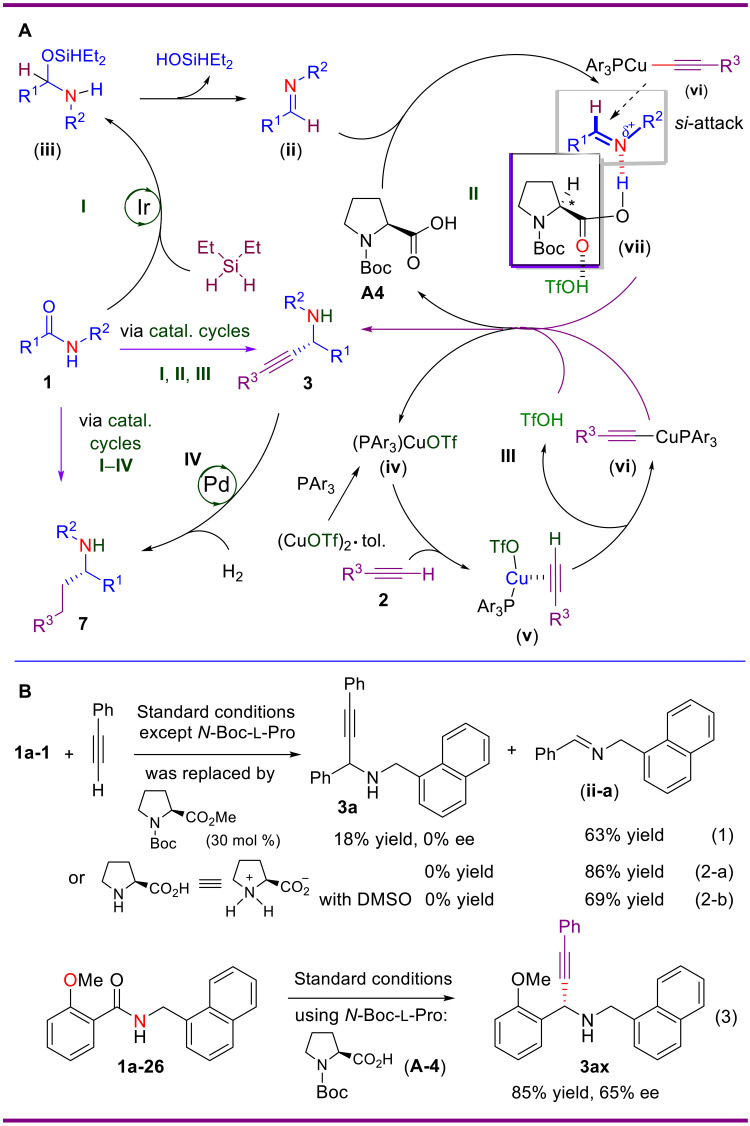
To tackle the abovementioned multiple challenges, a multicatalysis (57–60) approach was envisioned. The multicatalysis is an emerging strategy for developing unprecedented and challenging transformations that could not be achieved by a single catalytic system. Here, we disclose the results of this investigation, which include the first direct asymmetric transformation of secondary amides into α-chiral secondary propargylamines or chiral α-branched secondary amines. The methodology relies on the catalysis in trio or in quartet, namely, by merging Ir/Cu relay catalysis with Cu/N-protected l-proline cooperative catalysis, or further with sequential Pd-catalyzed hydrogenation of the chiral propargylamines (Fig. 1C).
In view of the frequent occurrence of N-naphthylmethyl and N-benzyl secondary amine motifs in medicinal agents (cf. Fig. 1D), we opted for the reductive alkynylation of N-(naphthalen-1-ylmethyl)benzamide (1a) with phenylacetylene (2a) as the model reaction. Jacobsen’s chiral thiourea C1 and chiral phosphoric acid C2 were first evaluated as organocatalysts (42, 57) in our multicatalysis strategy (Table 1). Disappointedly, the desired propargylamine 3a was not observed, only the imine intermediate was obtained in 84 and 76% yield, respectively (Table 1, entries 1 and 2). Encouragingly, when N-Ac-l-proline (A1) was used both as a chiral catalyst and as an imine activator, the desired deoxygenative alkynylation reaction occurred to give the α-chiral propargylamine 3a in 47% yield with 63% ee (Table 1, entry 3).
Table 1. Optimization of reaction conditions.
| Entry | Cu catal. | Organocatal. | Additive | Yield (%)* | ee %† |
| 1 | CuOTf | C1 | – | (84)‡ | – |
| 2 | CuOTf | C2 | – | (76)§ | – |
| 3 | CuOTf | A1 | – | 47 | 63 |
| 4 | CuOTf | A1 | P1 | 52 | 91|| |
| 5 | CuOTf | A2 | P1 | 52 | 76|| |
| 6 | CuOTf | A3 | P1 | 74 | 84|| |
| 7 | CuOTf | A4 | P1 | 65 | 93|| |
| 8 | CuOTf | A5 | P1 | 73 | 89|| |
| 9 | CuOTf | A6 | P1 | 53 | 87|| |
| 10 | CuOTf | A4 | P2 | 31 | 73|| |
| 11 | CuOTf | A4 | P3 | 81 | 73|| |
| 12 | CuOTf | A4 | P4 | 67 | 63|| |
| 13 | CuOTf | A4 | P1 | 76 | 93 |
| 14 | (CuOTf)2•toluene | A4 | P1 | 78 | 93 |
| 15 | (CuOTf)2 | A4 | P1 | 70 | 92 |
| 16 | (CuOTf)4PF6 | A4 | P1 | 77 | 93 |
| 17 | CUBr | A4 | P1 | (87)¶ | – |
*Determined by GC using n-dodecane as an internal standard.
†Determined by chiral high-performance liquid chromatography.
‡The imine intermediate obtained in 84% yield.
§The imine intermediate obtained in 76% yield.
||Twenty mole percent of additive was used.
¶The imine intermediate obtained in 87% yield.
Notably, when P(1-naphthyl)3 was introduced as an additive, an excellent ee of 91% and a good yield of 52% were obtained (Table 1, entry 4). A survey of the effect of other N-protected l-proline derivatives A2 to A6 showed that all were effective affording 3a in good to excellent ee’s (76 to 93%). However, eight other P additives (cf. P2 to P4 and table S3 in the Supplementary Materials) turned out to be less effective for the reaction. Further examining the effect of copper catalyst showed that many copper salts such as CuOTf, (CuOTf)2·toluene, Cu(OTf)2, and Cu(MeCN)4PF6 were equally effective for the reaction, affording propargylamine 3a in excellent ee’s (92 to 93%) and good yields (70 to 78%). Unexpectedly, CuBr, which is the catalyst of choice for the deoxygenative alkynylation of tert amides (38), was totally ineffective for the current reaction. The striking difference between CuBr and CuOTf in the asymmetric induction may be explained by the acidity of corresponding acid (HBr versus TfOH) generated from an alkyne and CuBr/CuOTf. TfOH (pKa = −15) is a much stronger acid than HBr (pKa = −9) and forms a strong H-bond with the carbonyl of N-Boc-l-proline (A4). This hydrogen bonding enhances the imine N···H hydrogen bonding in the intermediate vii, thus enhancing the asymmetric induction (Fig. 8A). Assessment of other reaction parameters including the amounts of reagents and additive, catalyst loadings as well as reaction temperature and time allowed defining the optimal reaction conditions (Table 1, entry 14). Several N-protected proline derivatives (entries 4 and 7 to 9) and copper salts (entries 13 to 16) are promising and efficient in catalyzing the enantioselective reaction. These results showed that the reaction is robust. Because (CuOTf)2·toluene is cheaper than the other copper salts and a stable solid, it was used for the subsequent investigations.
Fig. 8. Plausible reaction mechanisms.
(A) Plausible mechanisms for the multicatalytic, one-pot asymmetric reductive alkynylation/alkylation of secondary amides. (B) Control experiments to probe the dual roles of N-Boc-l-proline (A4) in the catalytic asymmetric reductive alkynylation of secondary amides. DMSO, dimethyl sulfoxide.
Scope of the reaction and chemoselectivity
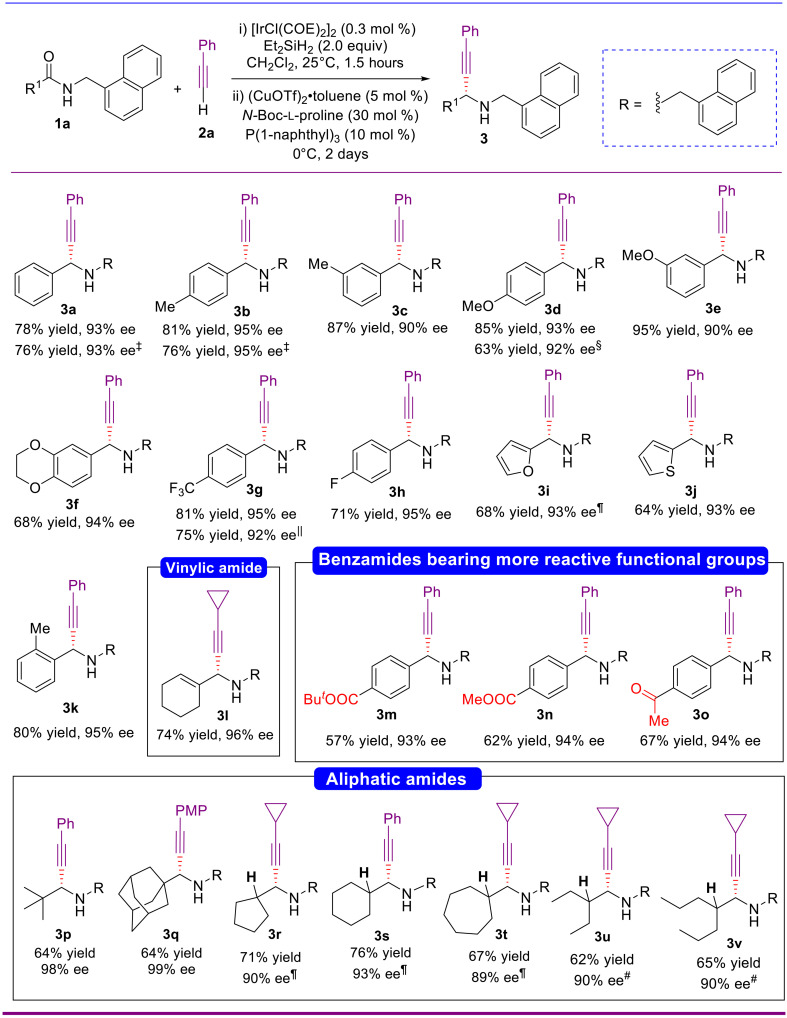
With the optimal reaction conditions in hands, we turned our attention to examine the reaction scope of this catalytic enantioselective reductive alkynylation reaction. The scope with respect to the amide was first examined. As shown in Fig. 2, the reaction proceeded with simple aryl amides such as p-tolyl (1a-2) and m-tolyl (1a-3) and was compatible with benzamides bearing either strong electron-donating (1a-4 to 1a-6) (the structures of all amides used are listed in table S1) or electron-withdrawing groups (1a-7 and 1a-8) at the para- or meta-position of the phenyl ring providing the corresponding propargylamines 3b to 3h in good to excellent yields (68 to 95%) and with high enantioselectivities (90 to 95% ee). Heteroaromatic amides were also viable substrates providing the corresponding α-chiral propargylamines (3i and 3j) in 68 and 64% yields and with high enantioselectivity (93% ee). The reaction of 2-methylbenzamide also proceeded smoothly to give the desired propargylamine (3k) in excellent enantioselectivity (80% yield, 95% ee). In view of the importance of trifluoromethyl, fluoro, and heteroaromatic groups in biological and medicinal chemistry as well as in the agrochemical field, the smooth enantioselective synthesis of amines 3g to 3j is of value. The enantioselective reductive alkynylation reaction showed remarkable chemoselectivity and functional group tolerance. For example, the amide substrates containing an ester moiety (1a-13 and 1a-14) or a keto group (1a-15) took place chemoselectively at the less electrophilic amide group, leaving the more reactive ester and ketone groups intact. The CuOTf, Cu(OTf)2, and Cu(MeCN)4PF6 catalyzed syntheses of 3a, 3b, 3d, and 3g showed that they are also effective catalysts for the alkynylation.
Fig. 2. Scope of secondary amides.
The structures of all amides and alkynes used are listed in tables S1 and S2. Reaction conditions: amide (0.25 mmol), [Ir(COE)2Cl]2 (0.3 mol %), Et2SiH2 (0.5 mmol), CH2Cl2 (1.25 ml), 25°C, 1.5 hours, then (CuOTf)2•toluene (5 mol %), N-Boc-l-proline (30 mol %), P(1-naphthyl)3 (10 mol %), alkyne (1.0 mmol), 0°C, 2 days. ‡CuOTf instead of (CuOTf)2•toluene. §Cu(OTf)2 instead of (CuOTf)2•toluene. ||Cu(MeCN)4PF6 instead of (CuOTf)2•toluene. ¶Time for the Ir-catalyzed reduction: 0.5 hour. #0.5 mol % of [Ir(COE)2Cl]2 used. PMP, p-methoxyphenyl.
The catalytic reductive alkynylation of aliphatic amides in high enantioselectivity presents a formidable challenge because of the absence of a rigid aromatic ring that appears to be a valuable control element for the enantioselective addition. Moreover, for α-hydrogen–containing amides, the situation may be further complicated by the possible isomerization of the imine intermediates to unreactive enamines. The reported enantioselective reductive alkynylation of tertiary amides is only compatible with benzamide derivatives (43).
To our delight, the current catalytic enantioselective reaction tolerated both α,β-unsaturated amide 1a-12 and aliphatic amides. For 1a-12, alkenyl propargylamine 3l was obtained in 74% yield with 96% ee (Fig. 2). For aliphatic amides, not only hindered pivalamide 1a-16 and adamantane-1-carboxamide 1a-17 but also less hindered α-hydrogen–containing secondary amides 1a-18 to 1a-22 reacted to afford the desired secondary propargylamines 3p to 3v in good yields (62 to 76%) and excellent enantioselectivities (89 to 99% ee) (Fig. 2). To the best of our knowledge, the synthesis of 3r to 3v represents the first examples of catalytic asymmetric synthesis of α-hydrogen–containing secondary propargylamines in high enantioselectivity. Note that for the syntheses of 3i and 3r to 3t, the reaction time of the Ir-catalyzed hydrosilylation was shortened to 0.5 hours to avoid over reduction.
Scope of alkynes and functional group tolerance of the reaction
The alkyne coupling partner also tolerated variation. The reactions were compatible with phenylacetylene derivatives bearing substituents at the para- (2b, 2e, and 2i), meta- (2c, 2f), and ortho-position (2d and 2g) of the benzene ring and 2-ethynylthiophene (2h) (Fig. 3) (the structures of all alkynes used are listed in table S2). Notably, phenylacetylene derivatives that contain on the benzene ring a chloro (2j), trifluoromethyl (2k), ester (2l), or even an aldehyde (2m) group all reacted successfully to give the desired products 3ae to 3ah in good yields and excellent enantioselectivities. Last, alkylacetylenes (2n and 2o) were proven to be viable substrates.
Fig. 3. Scope of alkynes.
For reaction conditions, see footnote in Fig. 2.
Extension to N-benzylamides
Besides N-(naphthalen-1-ylmethyl)amides, N-benzylamides were also suitable substrates for the catalytic enantioselective reductive alkynylation reaction to afford the corresponding chiral propargylamines (3ak to 3as) in 63 to 88% yields and 90 to 92% ee (Fig. 4). The absolute configuration of the propargylamines was determined as R by comparing the specific optical rotation data of amine 3aq with that reported in (51).
Fig. 4. The catalytic asymmetric reductive alkynylation of N-benzyl amides.
For reaction conditions, see footnote in Fig. 2.
Synthetic applications of the reaction
The practical utility of our method was demonstrated by the preparation of propargylamine 3a in multigram scale. The 10-mmol scale reaction afforded 2.359 g of 3a (68% yield) without losing enantioselectivity (93% ee) (Fig. 5A). The absolute configuration of propargylamine 3a, a representative of the series of N-(naphthalen-1-ylmethyl)-propargylamines, was determined as R by comparing the specific optical rotation data of its hydrogenation product (S)-4 (Fig. 5B) with that reported for its enantiomer (see the Supplementary Materials). To showcase the versatility of propargylamines in organic synthesis, derivatization of 3a was performed. Thus, 3a was partially hydrogenated with Lindlar’s catalyst to allylic amine (S)-5 in 84% yield and 93% ee (Fig. 5C). By using functionalized alkyne 2t, the resulting propargylamine 3av was synthesized in excellent ee (96%) (Fig. 5D), which was further converted, in one pot, into 2-substituted piperidine (S)-6 in 94% ee, demonstrating the value of current method for the asymmetric synthesis of chiral, cyclic tertiary amines in high enantiomeric excess. Note that motifs such as α-substituted tertiary amine (S)-6 are inaccessible by the method previously developed for tertiary benzamides (43), and the present synthesis is among the most efficient methods for its asymmetric synthesis in high enantiopurity (52).
Fig. 5. Synthetic applications of the reaction.
(A) Multigram-scale synthesis. (B) Determination of absolute configuration of propargylamine 3a. (C) Selective synthesis of allylic amine (S)-5. (D) Two-step synthesis of 2-substituted piperidine (S)-6. wt %, weight %. DMF, Dimethylformamide; RT, room temperature; EDA, ethylenediamine.
To further demonstrate the synthetic utility of the method, the late-stage deoxygenative alkynylations of amide derivatives (1a-23 and 1a-24) of two drugs SR11237 and adapalene were undertaken. Under the standard conditions, the catalytic asymmetric alkynylation reactions proceeded without incident to yield the desired propargylamines 3at and 3au in 78 and 73% yield and in 90 and 94% ee, respectively (Fig. 6). Next, we addressed the catalytic asymmetric synthesis of aticaprant (S)-8 (formerly known as LY-2456302 and CERC-501), a high-affinity and selective κ-opioid receptor antagonist (61, 62). Under standard conditions, the catalytic asymmetric reductive alkynylation of functionalized amide 1a-25 with 3,3-diethoxyprop-1-yne (2u) produced the desired propargylamine 3aw in 63% yield and in 88% ee. Pd/C-catalyzed catalytic hydrogenation, hydrogenolysis, and reductive alkylation under acidic conditions proceeded in tandem to afford 2-arylpyrrolidine (S)-7 in 84% yield. Because racemic 7 has previously been converted into aticaprant (S)-8 by a two-step protocol followed by resolution (61, 62), this work constitutes the first formal catalytic asymmetric total synthesis of aticaprant (S)-8.
Fig. 6. Synthetic applications of the reaction to drug molecules.
(A) Late-stage functionalization of drug derivatives and (B) catalytic asymmetric formal synthesis of the key intermediate of κ-opioid antagonist aticaprant. RXR, retinoid X receptor.
Catalytic enantioselective reductive alkylation of secondary amides
Considering that the catalytic asymmetric alkylation of amides remains unknown, we investigated the catalytic alkynylation, followed by complete reduction in one pot. Thus, after the catalytic alkynylation under standard conditions, the resulting reaction mixture was subjected to Pd/C-catalyzed hydrogenation under H2 (1 atm). In this manner, α-substituted chiral amines 9a to 9f were obtained in good yields and excellent enantioselectivity (Fig. 7). Notably, more reactive functional groups such as ester, ketone, and aldehyde on the amide or alkyne coupling partner were tolerated.
Fig. 7. One-pot, chemoselective, catalytic enantioselective reductive alkylation of secondary amides.
Reaction conditions: amide (0.5 mmol), [Ir(COE)2Cl]2 (0.3 mol %), Et2SiH2 (1.0 mmol), CH2Cl2 (2.5 ml), 25°C, 1.5 hours, then (CuOTf)2•toluene (5 mol %), N-Boc-l-proline (30 mol %), P(1-naphthyl)3 (10 mol %), alkyne (2.0 mmol), 0°C, 2 days, and then 10% Pd/C, H2, MeOH.
Plausible mechanism of the reaction
Plausible mechanisms for the multicatalysis-based asymmetric reductive alkynylation/alkylation of secondary amides are outlined in Fig. 8A. For the asymmetric reductive alkynylation of secondary amides, it involves three catalytic cycles: (i) Ir-catalyzed hydrosilylation of a secondary amide with diethylsilane to give O-silyl hemiaminal intermediate iii, which eliminates diethylsilanol to generate imine intermediate ii; (ii) copper-catalyzed in situ generation of nucleophilic Cu-alkynylide species vi; and (iii) N-Boc l-proline–catalyzed asymmetric alkynylation of imine ii in which N-Boc-l-proline (A4) serves as a Brønsted acid to activate the nonreactive imine intermediate ii via hydrogen bonding (see vii) and as an asymmetric inducer to block re-face of vii. The addition of Cu-alkynylide vi to reactive intermediate vii then occurs preferentially from si-face to afford (R)-propargylamine 3. Further merging of this tris-catalysis with Pd-catalyzed hydrogenation of the alkynyl group affords α-alkylated amine 7 in one pot.
To confirm the dual roles of N-Boc-l-proline (A4), we carried out the reductive alkynylation reactions of secondary amide 1a-1 with N-Boc-l-proline methyl ester or l-proline instead of N-Boc-l-proline (A4) (Fig. 8B-1). In the first case, the expected propargylamine 3a was obtained in only 18% yield in racemic form along with imine ii-a in 63% yield (Fig. 8B-1). In the second case, only imine ii-a was obtained in 86% yield (Fig. 8B-2a). To increase the solubility of proline, in the alkynation step, a 20% (v/v) of dimethyl sulfoxide was added as a cosolvent. However, only imine was formed in 69% yield (Fig. 8B-2b). These control experiments showed that neither Boc-l-proline methyl ester nor l-proline (being a zwitterion in the reaction medium) can promote the alkynylation or provide any asymmetric induction even when the alkynylation occurs as a side reaction, likely because none of them can activate imine ii through H-bonding. An additional evidence for the H-bond asymmetric catalysis was provided by the catalytic reductive alkynylation of o-methoxybenzamide 1a-26, a substrate bearing an additional H-bond acceptor (OMe) at the ortho-position of the phenyl group (Fig. 8B-3). This amide reacted with moderate stereoselectivity (65% ee) reflecting the competing H-bonding effect of the appropriately positioned OMe (Fig. 8B-3).
DISCUSSION
By designing a multicatalysis system, we have achieved the direct asymmetric reductive alkynylation and reductive alkylation of secondary amides to yield chiral α-branched secondary propargylamines and α-branched secondary amines, respectively. The method is characterized by mild reaction conditions, wide scope for both amides and alkynes, good yields, and high enantioselectivities. Another notable feature of our method is the exceptional chemoselectivity and functional group tolerance to allow the reactions to take place preferentially at the less reactive amide group over the more reactive ester, ketone, and aldehyde moieties. The observed unusual chemoselectivity can be understood from two aspects. First, the multicatalysis protocol avoids the direct nucleophilic addition to amides, instead, it involves an Ir-catalyzed O-silylation of the amide carbonyl (partial reduction) as the first step of the reaction sequence. Second, due to the delocalization of the nitrogen lone pair of an amide, the oxygen of the amide C═O is more electron rich as compared with those of aldehyde, ketone, and ester and thus is more reactive vis-à-vis electrophilic silyl species (34). Consequently, the amide carbonyl can be chemoselectively hydrosilylated, leading to the chemoselective asymmetric alkynylation reaction. The products can be elaborated in one step into two other types of α-branched amines: chiral primary amines and chiral tertiary aza-heterocycles. This method is expected to find applications in the total synthesis of alkaloids and N-containing medicinal agents, and the multicatalysis strategy will be useful for the catalytic asymmetric transformations of other carboxylic acid derivatives.
MATERIALS AND METHODS
General procedure A (catalytic asymmetric reductive alkynylation of secondary amides)
In a glove box, to a stirring solution of a secondary amide 1 (0.25 mmol, 1.0 equiv) and [IrCl(COE)2]2 [0.75 μmol, 0.3 mole percent (mol %)] (0.5 mol % for amides 1a-21 and 1a-22) in anhydrous CH2Cl2 (1.25 ml, 0.25 M) was added Et2SiH2 (0.5 mmol, 2.0 equiv, 65 μl) at room temperature under an Ar atmosphere. After being stirred for 1.5 hours (30 min for amides 1a-9, 1a-18, 1a-19, and 1a-20), the resulting mixture was transferred out of the glove box and added to a mixture of N-Boc-l-proline (0.075 mmol, 30 mol %), (CuOTf)2·toluene (0.0125 mmol, 5 mol %), and P(1-naphthyl)3 (0.025 mmol, 10 mol %) and stirred for 30 min at 0 °C. To the resultant mixture, an alkyne (1.0 mmol, 4 equiv) was added, and the reaction mixture was stirred at 0 °C for 2 days. The solvent was evaporated under vacuum, and the residue was purified by flash column chromatography on silica gel to give the desired enantioenriched α-chiral propargylamine 3.
General procedure B (catalytic asymmetric reductive alkylation of secondary amides)
In a glove box, to a stirring solution of a secondary amide (0.25 mmol, 1.0 equiv) and [IrCl(COE)2]2 (0.75 μmol, 0.3 mol %) in anhydrous CH2Cl2 (1.25 ml, 0.25 M) was added EtSiH2 (0.5 mmol, 2.0 equiv, 65 μl) at room temperature under an Ar atmosphere. After being stirred for 1.5 hours, the resulting mixture was transferred out of the glove box and added to a mixture of N-Boc-l-proline (0.075 mmol, 30 mol %), (CuOTf)2·toluene (0.0125 mmol, 5 mol %), and P(1-naphthyl)3 (0.025 mmol, 10 mol %) and stirred for 30 min at 0 °C. To the resultant mixture, an alkyne (1.0 mmol, 4 equiv) was added, and the reaction mixture was stirred at 0 °C for 2 days. Then, CH2Cl2 was removed, and Pd/C (10 weight %) and MeOH (2 ml) were added. The mixture was stirred under a H2 atmosphere at room temperature until the propargylamine intermediate was completely consumed. The solvent was removed under vacuum, and the residue was purified by flash column chromatography on silica gel to give the desired enantioenriched chiral α-branched amine 7.
Acknowledgments
We thank H. -C. Xu and H.-H. Huo for discussion.
Funding: Financial support from the National Natural Science Foundation of China (no. 21931010) and the National Key R&D Program of China (2017YFA0207302) is acknowledged.
Author contributions: P.-Q.H. conceived and directed the project and wrote the paper with assistance from H.C. and Z.-Z.W. H.C. contributed to the reaction design. H.C. and Z.-Z.W. performed the experiments and analyzed the data. D.-Y.S. participated in part in the preparation of racemic compounds. All authors discussed the results and commented on the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Sections S1 to S8
Tables S1 to S4
Figs. S1 to S141
References
REFERENCES AND NOTES
- 1.Zhang S., del Pozo J., Romiti F., Mu Y., Torker S., Hoveyda A., Delayed catalyst function enables direct enantioselective conversion of nitriles to NH2-amines. Science 364, 45–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A. Greenberg, C. M. Breneman, J. F. Liebman, Eds., The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science (Wiley, 2003). [Google Scholar]
- 3.Huo H.-H., Gorsline B. J., Fu G. C., Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles. Science 367, 559–564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu R.-Y., Farmer M. E., Chen Y.-Q., Yu J.-Q., A simple and versatile amide directing group for C-H functionalizations. Angew. Chem. Int. Ed. 55, 10578–10599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X.-C., Gong W., Fang L.-Z., Zhu R.-Y., Li S.-H., Engle K. M., Yu J.-Q., Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heathcock C. H., The enchanting alkaloids of Yuzuriha. Angew. Chem. Int. Ed. 31, 665–681 (1992). [Google Scholar]
- 7.Lee A. S., Liau B. B., Shair M. D., A unified strategy for the synthesis of 7-membered-ring-containing Lycopodium alkaloids. J. Am. Chem. Soc. 136, 13442–13452 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Piemontesi C., Wang Q., Zhu J., Enantioselective total synthesis of (−)-terengganensine A. Angew. Chem. Int. Ed. 55, 6556–6560 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Yoritate M., Takahashi Y., Tajima H., Ogihara C., Yokoyama T., Soda Y., Oishi T., Sato T., Chida N., Unified total synthesis of stemoamide-type alkaloids by chemoselective assembly of five-membered building blocks. J. Am. Chem. Soc. 139, 18386–18391 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Guo L.-D., Hou J.-P., Tu W.-T., Zhang Y., Zhang Y., Chen L.-X., Xu J., Total synthesis of dapholdhamine B and dapholdhamine B lactone. J. Am. Chem. Soc. 141, 11713–11720 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Huang X.-Z., Gao L.-H., Huang P.-Q., Enantioselective total syntheses of (+)-stemofoline and three congeners based on a biogenetic hypothesis. Nat. Commun. 11, 5314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabriel P., Almehmadi Y. A., Wong Z. R., Dixon D. J., A general Iridium-catalyzed reductive dienamine synthesis allows a five-step synthesis of catharanthine via the elusive dehydrosecodine. J. Am. Chem. Soc. 143, 10828–10835 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peruzzi M. T., Mei Q., Leeb S. J., Gagné M. R., Chemoselective amide reductions by heteroleptic fluoroaryl boron Lewis acids. Chem. Commun. 54, 5855–5858 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Pace V., Holzer W., Chemoselective activation strategies of amidic carbonyls towards nucleophilic reagents. Aust. J. Chem. 66, 507–510 (2013). [Google Scholar]
- 15.Pace V., Holzer W., Olofsson B., Increasing the reactivity of amides towards organometallic reagents: An overview. Adv. Synth. Catal. 356, 3697–3736 (2014). [Google Scholar]
- 16.Sato T., Yoritate M., Tajima H., Chida N., Total synthesis of complex alkaloids by nucleophilic addition to amides. Org. Biomol. Chem. 16, 3864–3875 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kaiser D., Bauer A., Lemmerera M., Maulide N., Amide activation: An emerging tool for chemoselective synthesis. Chem. Soc. Rev. 47, 7899–7925 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Czerwiński P. J., Furman B., Reductive functionalization of amides in synthesis and for modification of bioactive compounds. Front. Chem. 9, 655849 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falmagne J.-B., Escudero J., Taleb-Sahraoui S., Ghosez L., Cyclobutanone and cyclobutenone derivatives by reaction of tertiary amides with alkenes or alkynes. Angew. Chem. Int. Ed. 20, 879–880 (1981). [Google Scholar]
- 20.Charette A. B., Grenon M., Spectroscopic studies of the electrophilic activation of amides with triflic anhydride and pyridine. Can. J. Chem. 79, 1694–1703 (2001). [Google Scholar]
- 21.Movassaghi M., Hill M. D., Synthesis of substituted pyridine derivatives via the ruthenium-catalyzed cycloisomerization of 3-azadienynes. J. Am. Chem. Soc. 128, 4592–4593 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Movassaghi M., Hill M. D., Ahmad O. K., Direct synthesis of pyridine derivatives. J. Am. Chem. Soc. 129, 10096–10097 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Barbe G., Charette A. B., Highly chemoselective metal-free reduction of tertiary amides. J. Am. Chem. Soc. 130, 18–19 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Pelletier G., Bechara W. S., Charette A. B., Controlled and chemoselective reduction of secondary amides. J. Am. Chem. Soc. 132, 12817–12819 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Madelaine C., Valerio V., Maulide N., Unexpected electrophilic rearrangements of amides: A stereoselective entry to challenging substituted lactones. Angew. Chem. Int. Ed. 49, 1583–1586 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Xiao K.-J., Luo J.-M., Ye K.-Y., Wang Y., Huang P.-Q., Direct, one-pot sequential reductive alkylation of lactams/amides with Grignard and organolithium reagents through lactam/amide activation. Angew. Chem. Int. Ed. 49, 3037–3040 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Shirokane K., Kurosaki Y., Sato T., Chida N., A direct entry to substituted N-methoxyamines from N-methoxyamides via N-oxyiminium Ions. Angew. Chem. Int. Ed. 49, 6369–6372 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Bechara W. S., Pelletier G., Charette A. B., Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nat. Chem. 4, 228–234 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Mewald M., Medley J. W., Movassaghi M., Concise and enantioselective total synthesis of (−)-mehranine, (−)-methylenebismehranine, and related Aspidosperma alkaloids. Angew. Chem. Int. Ed. 53, 11634–11639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heindl S., Riomet M., Matyasovsky J., Lemmerer M., Malzer N., Maulide N., Chemoselective γ-oxidation of β,γ-unsaturated amides with TEMPO. Angew. Chem. Int. Ed. 60, 19123–19127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao J., Wang X.-M., Merging electron transfer with 1,2-metalate rearrangement: Deoxygenative arylation of aromatic amides with arylboronic esters. Angew. Chem. Int. Ed. 60, 17088–17093 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Donnelly L. J., Berthet J.-C., Cantat T., Selective reduction of secondary amides to imines catalysed by Schwartz’s reagent. Angew. Chem. Int. Ed. 61, e202206170 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Matheau-Raven D., Gabriel P., Leitch J. A., Almehmadi Y. A., Yamazaki K., Dixon D. J., Catalytic reductive functionalization of tertiary amides using Vaska’s complex: Synthesis of complex tertiary amine building blocks and natural products. ACS Catal. 10, 8880–8897 (2020). [Google Scholar]
- 34.Cheng C., Brookhart M., Iridium-catalyzed reduction of secondary amides to secondary amines and imines by diethylsilane. J. Am. Chem. Soc. 134, 11304–11307 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Gregory A. W., Chambers A., Hawkins A., Jakubec P., Dixon D., Iridium-catalyzed reductive nitro-Mannich cyclization. Chem. Eur. J. 21, 111–114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima M., Sato T., Chida N., Iridium-catalyzed chemoselective reductive nucleophilic addition to N-methoxyamides. Org. Lett. 17, 1696–1699 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Katahara S., Kobayashi S., Fujita K., Matsumoto T., Sato T., Chida N., An iridium-catalyzed reductive approach to nitrones from N-hydroxyamides. J. Am. Chem. Soc. 138, 5246–5249 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Huang P.-Q., Ou W., Han F., Chemoselective reductive alkynylation of tertiary amides by Ir and Cu(I) bis-metal sequential catalysis. Chem. Commun. 52, 11967–11970 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Fuentes de Arriba Á. L., Lenci E., Sonawane M., Formery O., Dixon D., Iridium-catalyzed reductive Strecker reaction for late-stage amide and lactam cyanation. Angew. Chem. Int. Ed. 56, 3655–3659 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Xie L.-G., Dixon D. J., Iridium-catalyzed reductive Ugi-type reactions of tertiary amides. Nat. Commun. 9, 2841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Berger M., Zawodny W., Simaan M., Maulide N., A chemoselective α-oxytriflation enables the direct asymmetric arylation of amides. Chem 5, 1883–1891 (2019). [Google Scholar]
- 42.Chen D. H., Sun W. T., Zhu C. J., Lu G. S., Wu D. P., Wang A. E., Huang P.-Q., Enantioselective reductive cyanation and phosphonylation of secondary amides by iridium and chiral thiourea sequential catalysis. Angew. Chem. Int. Ed. 60, 8827–8831 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Li Z., Zhao F., Ou W., Huang P.-Q., Wang X. M., Asymmetric deoxygenative alkynylation of tertiary amides enabled by iridium/copper bimetallic relay catalysis. Angew. Chem. Int. Ed. 60, 26604–26609 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Feng M., Mosiagin I., Kaiser D., Maryasin B., Maulide N., Deployment of sulfinimines in charge-accelerated sulfonium rearrangement enables a surrogate asymmetric Mannich reaction. J. Am. Chem. Soc. 144, 13044–13049 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Do H.-Q., Bachman S., Bissember A. C., Peters J. C., Fu G. C., Photoinduced, copper-catalyzed alkylation of amides with unactivated secondary alkyl halides at room temperature. J. Am. Chem. Soc. 136, 2162–2167 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Lundberg H., Tinnis F., Selander N., Adolfsson H., Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 43, 2714–2742 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Peshkov V. A., Pereshivko O. P., Nechaev A. A., Peshkovc A. A., Eycken E. V. V., Reactions of secondary propargylamines with heteroallenes for the synthesis of diverse heterocycles. Chem. Soc. Rev. 47, 3861–3898 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Lauder K., Toscani A., Scalacci N., Castagnolo D., Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 117, 14091–14200 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Yoo W.-J., Zhao L., Li C.-J., The A3-coupling (aldehyde–alkyne–amine) reaction: A versatile method for the preparation of propargylamines. Aldrichimica Acta 44, 43–51 (2011). [Google Scholar]
- 50.Wei C., Li C.-J., Enantioselective direct-addition of terminal alkynes to imines catalyzed by Copper(I)pybox complex in water and in toluene. J. Am. Chem. Soc. 124, 5638–5639 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Lu Y.-D., Johnstone T. C., Arndtsen B. A., Hydrogen-bonding asymmetric metal catalysis with α-amino acids: A simple and tunable approach to high enantioinduction. J. Am. Chem. Soc. 131, 11284–11285 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Cabré A., Verdaguer X., Riera A., Recent advances in the enantioselective synthesis of chiral amines via transition metal-catalyzed asymmetric hydrogenation. Chem. Rev. 122, 269–339 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGrath N. A., Brichacek M., Njardarson J. T., A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 87, 1348–1349 (2010). [Google Scholar]
- 54.Daly J. W., Spande T. F., Garraffo H. M., Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 68, 1556–1575 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Banik S. M., Levina A., Hyde A. M., Jacobsen E. N., Lewis acid enhancement by hydrogen-bond donors for asymmetric catalysis. Science 358, 761–764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vitaku E., Smith D. T., Njardarson J. T., Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA–approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Martínez S., Veth L., Lainer B., Dydio P., Challenges and opportunities in multicatalysis. ACS Catal. 11, 3891–3915 (2021). [Google Scholar]
- 58.Allen A. E., MacMillan D. W. C., Synergistic catalysis: A powerful synthetic strategy for new reaction development. Chem. Sci. 3, 633–658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuang Y., Wang K., Shi X., Huang X., Meggers E., Wu J., Asymmetric synthesis of 1,4-dicarbonyl compounds from aldehydes by hydrogen atom transfer photocatalysis and chiral Lewis acid catalysis. Angew. Chem. Int. Ed. 58, 16859–16863 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Zhou L., Wu X., Yang X., Mou C., Song R., Yu S., Chai H., Pan L., Jin Z., Chi Y. R., Gold and carbene relay catalytic enantioselective cycloisomerization/cyclization reactions of ynamides and enals. Angew. Chem. Int. Ed. 59, 1557–1561 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Wang J., Song Q., Xu A., Bao Y., Xu Y., Zhu Q., Design, synthesis and biological evaluation of aminobenzyloxyarylamide derivatives as selective κ opioid receptor antagonists. Eur. J. Med. Chem. 130, 15–25 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Mitch C. H., Quimby S. J., Diaz N., Pedregal C., de la Torre M. G., Jimenez A., Shi Q., Canada E. J., Kahl S. D., Statnick M. A., McKinzie D. L., Benesh D. R., Rash K. S., Barth V. N., Discovery of aminobenzyloxyarylamides as κ-opioid receptor selective antagonists: Application to preclinical development of a κ opioid receptor antagonist receptor occupancy tracer. J. Med. Chem. 54, 8000–8012 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Das J., Banerjee D., Nickel-catalyzed phosphine free direct N-alkylation of amides with alcohols. J. Org. Chem. 83, 3378–3384 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Xu P., Han F.-S., Wang Y.-H., Copper(I)-catalyzed reductive cross-coupling of N-tosylhydrazones with amides: A straightforward method for the construction of C(sp3)-N amide bonds from aldehydes. Adv. Synth. Catal. 357, 3441–3446 (2015). [Google Scholar]
- 65.Cellitti J., Zhang Z.-M., Wang S., Wu B.-N., Yuan H.-B., Hasegawa P., Guiney D. G., Pellecchia M., Small molecule dnak modulators targeting the β domain. Chem. Biol. Drug Des. 74, 349–357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welker A., Kersten C., Müller C., Madhugiri R., Zimmer C., Müller P., Zimmermann R., Hammerschmidt S., Maus H., Ziebuhr J., Sotriffer C., Schirmeister T., Structure-activity relationships of benzamides and isoindolines designed as SARS-CoV protease inhibitors effective against SARS-CoV-2. ChemMedChem 16, 340–354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molander G. A., Beaumard F., Cross-coupling of mesylated phenol derivatives with potassium ammonio- and amidomethyltrifluoroborates. Org. Lett. 13, 1242–1245 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corre Y., Trivelli X., Capet F., Djukic J.-P., Agbossou-Niedercorn F., Michon C., Efficient and selective hydrosilylation of secondary and tertiary amides catalyzed by an iridium(III) metallacycle: Development and mechanistic investigation. ChemCatChem 9, 2009–2017 (2017). [Google Scholar]
- 69.Klapars A., Antilla J. C., Huang X.-H., Buchwald S. L., A general and efficient copper catalyst for the amidation of aryl halides and the N-arylation of nitrogen heterocycles. J. Am. Chem. Soc. 123, 7727–7729 (2001). [DOI] [PubMed] [Google Scholar]
- 70.Lavrov M. I., Lapteva V. L., Grigor’ev V. V., Palyulin V. A., Bachurin S. O., Zefirov N. S., Synthesis and AMPA-receptor modulating activity of benzodioxanecarboxylic and piperonylic acid derivatives. Pharm. Chem. J. 46, 92–95 (2012). [Google Scholar]
- 71.Ye P.-Q., Shao Y.-L., Ye X.-Z., Zhang F.-J., Li R.-H., Sun J.-N., Xu B.-H., Chen J.-X., Homoleptic bis(trimethylsilyl)amides of Yttrium complexes catalyzed hydroboration reduction of amides to amines. Org. Lett. 22, 1306–1310 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Blay G., Cardona L., Climent E., Pedro J. R., Highly enantioselective zinc/binol-catalyzed alkynylation of N-sulfonyl aldimines. Angew. Chem. Int. Ed. 47, 5593–5596 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sections S1 to S8
Tables S1 to S4
Figs. S1 to S141
References