Abstract
Bone marrow necrosis (BMN) has various underlying diseases. In hematological malignancies, both lymphoid and myeloid neoplasms have been shown to cause BMN. Charcot-Leyden crystals (CLCs) are bipyramidal crystals that have been found in patients with immune system diseases, tumors, skin diseases, asthma, infections, and intestinal diseases. Because the combination of CLCs and acute myeloid leukemia (AML) is rare, the relationship between BMN, CLCs, and AML remains largely unexplored. We herein report a suspected case of AML that was difficult to diagnose morphologically because of complete BMN with CLCs but achieved complete hematologic remission with treatment similar to that for AML.
Keywords: acute myeloid leukemia, bone marrow necrosis, Charcot-Leyden crystals
Introduction
Bone marrow necrosis (BMN) has various underlying diseases. In hematological malignancies, both lymphoid and myeloid neoplasms have been shown to cause BMN (1). Charcot-Leyden crystals (CLCs) are bipyramidal crystals that have been found in patients with immune system diseases, tumors, skin diseases, asthma, infections, and intestinal diseases (2). Khrizman et al. reported CLCs in BMN in patients with acute myeloid leukemia (AML) without evidence of eosinophilia (3). Because this combination is rare, the relationship between BMN, CLCs, and AML remains largely unexplored.
We herein report a suspected case of AML that was difficult to diagnose morphologically because of complete BMN with CLCs. This study was approved by the Institutional Review Board of Kurashiki Central Hospital and was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from the patient for the publication of this case report and accompanying images.
Case Report
A 46-year-old man presented to a local hospital with severe chest pain. Laboratory data showed coagulopathy (fibrin/fibrinogen degradation products, 15.1 μg/mL; D-dimer, 9.8 μg/mL). An electrocardiogram, transthoracic echocardiogram, cardiac computed tomography, and contrast-enhanced computed tomography revealed neither thrombosis nor cardiovascular disease. However, he became unable to walk because of pain in the left buttock and both lower limbs. Because magnetic resonance imaging showed diffuse signal alterations in the bone marrow, he was referred and admitted to our hospital for specialist treatment.
Table shows the laboratory data on admission. Some of the coagulation markers were consistent with the hematopoietic disorder type of disseminated intravascular coagulation according to the Japanese Society on Thrombosis and Hemostasis criteria (4). 18F-fluorodeoxyglucose positron emission tomography/computed tomography showed the following three major findings: a diffuse 18F-fluorodeoxyglucose uptake in the bone marrow, hepatosplenomegaly, and an increased 18F-fluorodeoxyglucose uptake in the spleen, both kidneys, and several muscles (Fig. 1).
Table.
Laboratory Data on Admission.
| Blood count | Biochemistry | Coagulation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 2,600 | /μL | TP | 7 | g/dL | PT-INR | 1.07 | ||||||
| Neut | 58 | % | Alb | 3.5 | g/dL | APTT | 46 | s | |||||
| Eos | 0 | % | T-Bil | 0.9 | mg/dL | Fbg | 525 | mg/dL | |||||
| Lymph | 28 | % | AST | 22 | U/L | FDP | 114.2 | μg/mL | |||||
| Mono | 10 | % | ALT | 18 | U/L | D-dimer | 60.7 | μg/mL | |||||
| Myelo | 3 | % | LDH | 1,240 | U/L | ||||||||
| Blast | 1 | % | Ferritin | 6,530 | ng/mL | PLG | 107 | % | |||||
| Hb | 13.6 | g/dL | ALP | 1,094 | U/L | α2-PI | 113 | % | |||||
| Plt | 72,000 | /μL | γ-GTP | 36 | U/L | PIC | 2.7 | μg/mL | |||||
| BUN | 21 | mg/dL | TAT | 24.9 | ng/mL | ||||||||
| UA | 5.8 | mg/dL | AT-3 | 107 | % | ||||||||
| Cre | 0.64 | mg/dL | |||||||||||
| Na | 135 | mmol/L | WT-1 mRNA | 3,100 | copies/μgRNA | ||||||||
| K | 3.8 | mmol/L | JAK2 V617F mutation | Negative | |||||||||
| Cl | 93 | mmol/L | |||||||||||
| CRP | 24.75 | mg/dL | |||||||||||
WBC: white blood cell, Neut: neutrophils, Eos: eosinophils, Mono: monocytes, Myelo: myelocytes, Hb: hemoglobin, Plt: platelet, TP: total protein, Alb: albumin, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, UA: uric acid, Cre: creatinine, Na: natrium, K: potassium, Cl: chlorine, CRP: C-reactive protein, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time, Fbg: fibrinogen, FDP: fibrin/fibrinogen degradation products, PLG: plasminogen, α2-PI: alpha-2 plasmin inhibitor, PIC: plasmin-α2 plasmin inhibitor complex, TAT: thrombin・antithrombin III complex, AT-3: antithrombin III, WT-1 mRNA: Wilm’s tumor 1 mRNA, JAK2 V617F mutation: Janus kinase 2 V617F mutation
Figure 1.
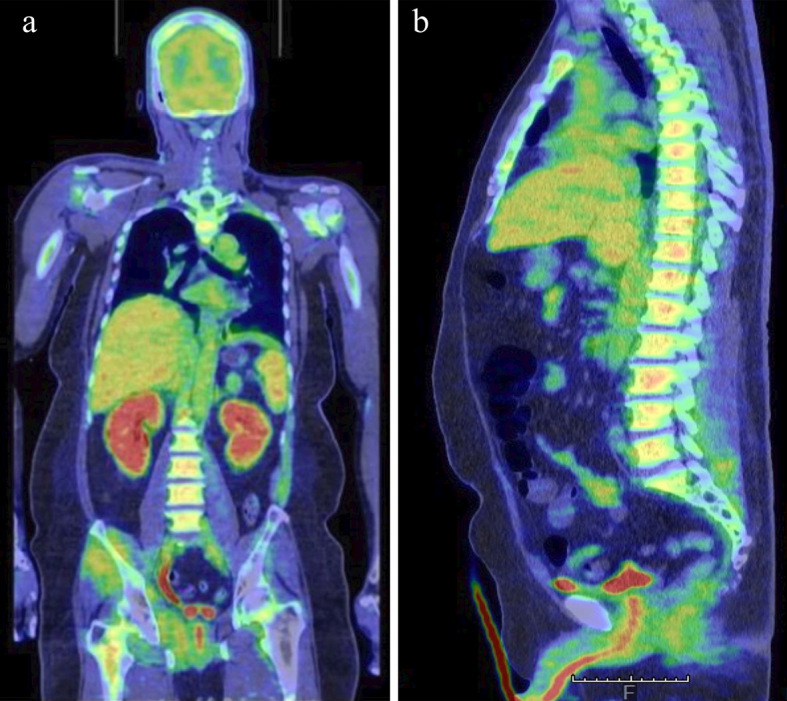
Coronal (a) and sagittal (b) 18F-fluorodeoxyglucose positron emission tomography/computed tomography images showing a diffuse 18F-fluorodeoxyglucose uptake in the bone marrow, hepatosplenomegaly, and an increased 18F-fluorodeoxyglucose uptake in the spleen, both kidneys, and several muscles.
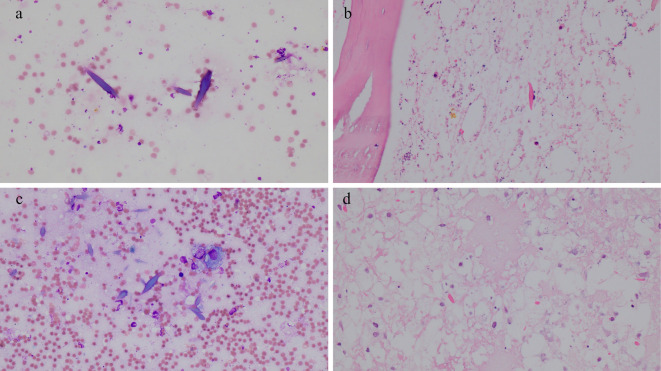
Bone marrow aspiration from the bilateral posterior superior iliac spines and a bone marrow biopsy from the right posterior superior iliac spine showed complete BMN with CLCs (Fig. 2a, b). A sternal biopsy resulted in a dry tap. A biopsy of the right gluteus maximus muscle, one of the muscles showing an increased 18F-fluorodeoxyglucose uptake, revealed no malignant cells.
Figure 2.
Pathological findings. Bone marrow aspiration performed before induction therapy showing CLCs (May-Giemsa staining, ×40 magnification) (a); a bone marrow biopsy performed before induction therapy showing CLCs [Hematoxylin and Eosin (H&E) staining, ×40 magnification] (b); bone marrow aspiration performed after induction therapy showing CLCs and macrophages (May-Giemsa staining, ×40 magnification) (c); and a bone marrow biopsy performed after induction therapy showing CLCs in necrotic tissues (H&E staining, ×40 magnification) (d). CLCs: Charcot-Leyden crystals
A cytogenetic analysis of the peripheral blood showed a normal karyotype (46, XY). However, the white blood cell count gradually increased. On hospital day 12, the count was 27,000 /μL (neutrophils, 22%; lymphocytes, 7%; eosinophils, 0.0%; basophils, 0.0%; monocytes, 21%; metamyelocytes, 1%; myelocytes, 41%; and blast cells, 8%), and flow cytometry data on peripheral blood were as follows: CD13, 80%; CD33, 86%; and CD34, 1%.
Because further examinations were difficult to perform due to the patient's worsening condition, we decided to give him treatment similar to that for AML. He was started on induction therapy with daunorubicin hydrochloride (50 mg/m2 for 5 days) and cytosine arabinoside (100 mg/m2/day for 7 days) at a full dose because his organ function had been well preserved. Targeted sequencing of the peripheral blood showed a frameshift insertion of NPM1 (p.L287fs) and two different nonsynonymous single nucleotide variants of TET2 (p.H1382Y, p.C1378Y). The FLT3 mutation was negative.
Disseminated intravascular coagulation gradually improved after induction therapy. His bone pain improved temporarily but worsened again as his white blood cell count gradually returned to normal. Bone marrow aspiration and a biopsy after induction therapy showed complete BMN with CLCs again (Fig. 2c, d). Because of the necrotic features of the bone marrow specimen, we were unable to determine whether or not he was in remission. Based on the normalization of his complete white blood cell count and decrease in WT-1 mRNA (200 copies/μg RNA) and lactate dehydrogenase (LDH) (113 U/L), complete hematologic remission was suspected.
Because his activities of daily living had deteriorated, allogeneic stem cell transplantation was not indicated for him, so we decided to treat him with chemotherapy alone. The first cycle of consolidation therapy (mitoxantrone dihydrochloride and cytosine arabinoside) was initiated. Although his bone pain remained, it was well controlled with regular acetaminophen. Bone marrow aspiration after the first consolidation therapy session also resulted in complete necrosis. After the second cycle of consolidation therapy (daunorubicin hydrochloride and cytosine arabinoside), bone marrow aspiration showed the disappearance of necrosis and CLCs for the first time, and hematologic complete remission was confirmed with 3.5% myeloblasts. He remained in remission after the final consolidation therapy session (aclarubicin and cytosine arabinoside). Targeted sequencing of peripheral blood 10 months after the completion of treatment showed no mutations of NPM1 or TET2. Bone marrow aspiration performed 29 months after the completion of treatment also showed hematologic complete remission with 0.2% myeloblasts, and necrosis and CLCs were no longer observed.
Discussion
BMN is a rare phenomenon that accounts for between 0.3% and 2% of total antemortem bone marrow biopsy samples, depending on the patient population (1,5,6). It makes the diagnosis of hematologic malignancies difficult by reducing the visibility of the cell morphology. The main clinical features of BMN are as follows: bone pain, a fever, cytopenia, elevated LDH, alkaline phosphatase and ferritin, and leukoerythroblastosis (1,7). Although BMN in hematologic malignancies seems to be associated with microvascular failure due to hypercellular bone marrow and the extrinsic pathway of apoptosis, its pathophysiology has not been fully elucidated (1,8). In patients with hematologic malignancies with BMN, lymphoid malignancy is dominant over myeloid malignancy. This is possibly due to the highly proliferative characteristic of lymphoid cells. Most AML cases with BMN have monocytic features (4).
CLCs are overexpressed in eosinophils and also identified in basophils and macrophages (2). Lao et al. identified CLCs in tissue macrophages and the extracellular matrix of an eosinophil-rich cutaneous lesion of mastocytoma using electron microscopy (9). This suggests that there is a pathophysiological relationship between mast cells, eosinophils, and macrophages (9). According to the World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, CLCs may be present in any tissue of myeloid/lymphoid neoplasms with eosinophilia (10). By contrast, in the setting of AML, CLCs are rare but often found in BMN, irrespective of the presence or absence of eosinophilia (3). The role of CLCs in BMN in hematologic malignancies remains poorly understood. Rodríguez-Alcázar et al. report that CLCs induce the release of the highly proinflammatory cytokine interluekin-1β (11). They produced CLCs from whole-cell lysates of AML14.3D10 cells, a subclone of the AML14 cell line that was established from a 68-year-old man with French-American-British M2 AML (12). Their discovery suggests that CLC-induced interluekin-1β release contributes to BMN.
Prognostic factors for BMN largely depend on the patient's age and the nature of the underlying disease. Estimating the prognosis of patients with BMN is often difficult when BMN is extensive, as shown in our case. Chen et al. retrospectively analyzed the outcomes of 23 patients with BMN and reported that the 2-week and 2-year cumulative survivals were 56.5% and 47.4%, respectively (13). This indicates that patients with BMN are at the highest risk of death within two weeks. In their study, all four patients with unknown etiology died within two months. Diagnosing the underlying disease is therefore of great importance.
BMN with CLCs in patients with NPM1-positive AML has been described in two case reports thus far, with patients achieving complete remission through conventional chemotherapy (14,15). Because of the rarity of the phenomenon, the relationship between BMN, CLCs, and NPM1 is still unknown. NPM1-positive AML is a provisional entity according to the 2016 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (16). In this category, a favorable prognosis is expected, especially in the absence of an FLT3 mutation. Of the two patients described in the literature above, one with mutated NPM1 remained in morphological and molecular remission six months after the completion of chemotherapy (14), and the other who was positive for NPM1 and negative for FLT3 remained in complete remission five months after presentation (15). In our case, targeted sequencing of the peripheral blood showed mutations, including NPM1, in the absence of FLT3 at the time of the diagnosis, and no mutations, including NPM1, at 10 months after the completion of treatment.
Twenty-nine months have passed since the completion of treatment, and the patient remains in remission. Although the utility of targeted sequencing in the diagnosis and prognostication of AML with BMN has yet to be established, it allowed us to predict a favorable prognosis in the present case, as mutations, including NPM1, were seen in the absence of FLT3. The further accumulation of sequencing data will aid in making a diagnosis of AML with BMN in cases that are difficult to diagnose morphologically.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Deucher A, Wool GD. How I investigate bone marrow necrosis. Int J Lab Hematol 41: 585-592, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Su J. A brief history of Charcot-Leyden crystal protein/galectin-10 research. Molecules 23: 2931, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khrizman P, Altman JK, Mohtashamian A, Peterson L, Chen YH, Tallman MS. Charcot-Leyden crystals associated with acute myeloid leukemia: case report and literature review. Leuk Res 34: e336-e338, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Wool GD, Deucher A. Bone marrow necrosis: ten-year retrospective review of bone marrow biopsy specimens. Am J Clin Pathol 143: 201-213, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Asakura H, Takahashi H, Uchiyama T, et al. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J 14: 42, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paydas S, Ergin M, Baslamisli F, et al. Bone marrow necrosis: clinicopathologic analysis of 20 cases and review of the literature. Am J Hematol 70: 300-305, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Janssens AM, Offner FC, Van Hove WZ. Bone marrow necrosis. Cancer 88: 1769-1780, 2000. [PubMed] [Google Scholar]
- 8.Moritake H, Obara M, Sameshima N, et al. Analysis of the molecular mechanism underlying bone marrow necrosis with acute lymphoblastic leukemia. Int J Hematol 102: 349-356, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Lao LM, Kumakiri M, Nakagawa K, et al. The ultrastructural findings of Charcot-Leyden crystals in stroma of mastocytoma. J Dermatol Sci 17: 198-204, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Bain BJ, Horny HP, Arber DA, et al. Myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB or FGR1, or with PCM1-JAK2. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. International Agency for Research on Cancer, Lyon, 2017: 72-79. [Google Scholar]
- 11.Rodríguez-Alcázar JF, Ataide MA, Engels G, et al. Charcot-Leyden crystals activate the NLRP3 inflammasome and cause IL-1β inflammation in human macrophages. J Immunol 202: 550-558, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Baumann MA, Paul CC. The AML14 and AML14.3D10 cell lines: a long-overdue model for the study of eosinophils and more. Stem Cells 16: 16-24, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Wu J, Yang J, et al. Analysis and clinical characteristics of 23 cases of bone marrow necrosis. Clin Lymphoma Myeloma Leuk 21: e356-e364, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Taylor G, Ivey A, Milner B, Grimwade D, Culligan D. Acute myeloid leukaemia with mutated NPM1 presenting with extensive bone marrow necrosis and Charcot-Leyden crystals. Int J Hematol 98: 267-268, 2013. [DOI] [PubMed] [Google Scholar]
- 15.van de Kerkhof D, Scharnhorst V, Huysentruyt CJ, Brands-Nijenhuis AV, Ermens AA. Charcot-Leyden crystals in acute myeloid leukemia. Int J Lab Hematol 37: e100-e102, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Arber DA, Brunning RD, Le Beau MM, et al. Acute myeloid leukaemia and related precursor neoplasms. In: WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Swerdlow SH, Campo E, Harris NLet al. , Eds. IARC Press, Lyon, 2017: 130-171. [Google Scholar]