Abstract
Objective
Previous studies have described several prognostic factors for heart failure (HF); however, these results were derived from registries consisting of conventional age groups, which might not represent the increasingly aging society. The present study explored the prognostic factors for all-cause death in hospitalized patients with HF across different age categories using an acute HF registry that included relatively old patients.
Methods
From a total of 1,971 consecutive patients with HF, 1,136 patients were enrolled. We divided the patients into 4 groups (≤65, 66-75, 76-85, and >85 years old) to evaluate all-cause death and prognostic factors of all-cause death.
Results
During the mean follow-up period of 1,038 days, 445 patients (39.2%) had all-cause death. A Kaplan-Meier analysis demonstrated significantly higher incidence of all-cause death in the elderly groups than in the younger groups (log-rank p<0.001). A Cox proportional-hazard regression analysis revealed that the presence of atrial fibrillation [hazard ratio (HR): 23.3, 95% confidence interval (CI): 2.36-231.1, p=0.007] was a notable predictive factor for all-cause death in the ≤65 years old group, whereas the Clinical Frailty Scale score (HR: 1.33, 95% CI: 1.16-1.52, p<0.001) and hypoalbuminemia (HR: 0.49, 95% CI: 0.31-0.78, p=0.003) were predictors in the >85 years old group.
Conclusions
Atrial fibrillation was a notable predictor of HF in young patients, whereas frailty and low-grade albuminemia were essential predictive factors of HF in elderly patients. With the increasing number of elderly patients with HF, comprehensive multidisciplinary treatment will be necessary.
Keywords: heart failure, mortality, prognostic factor, Japanese registry
Introduction
The number of patients with heart failure (HF) has been increasing rapidly with the aging of the population (1). In Japan, 29% of the population was over 65 years old in 2020. As the incidence of HF increases with age, the HF pandemic is predicted to become evident in Japan by 2035 (2). Although evidence-based medication improved the long-term prognosis of patients with HF until the 2000s (3), the one-year mortality rate remained high and relatively unchanged over the last decade (4). In addition, the complication of a high re-hospitalization rate after discharge (5,6)constitutes a burden on the healthcare system.
Previous studies have shown various prognostic factors for patients with HF including anemia, chronic kidney disease, and hypoalbuminemia (7,8). However, these data are derived from registries that include patients of conventional age groups, which might not represent the increasingly aging society. As the features of HF differ among age groups (9-11), the prognostic factors may differ between younger and older patients. With the gradual aging of the population, it has become important to assess the factors separately by age groups.
Awaji Island is one of the largest isolated islands in Japan; it also has one of the most aged populations in Japan and a low migration rate with a relatively stable population. The total oopulation of Awaji Island is about 130,000, which has decreased slightly over time from 135,147 in 2015 (12) to 130,866 in 2022 (13-15). In 2015, the proportion of the Awaji Island population ≥65 years old was 34.2%, which was higher than the Japanese national average of 26.6% (12). The migration rate in Awaji Island is about 0.3% (13-15), which is markedly lower than the Japanese national average (2.1%) (16).
The present study explored the prognostic factors of all-cause death in patients with HF across age categories using the Kobe University Heart Failure Registry in Awaji Medical Center, the acute heart failure registry; the KUNIUMI Registry acute cohort (2).
Materials and Methods
Study design and population
The KUNIUMI Registry acute cohort is a population-based registry of acute HF on Awaji Island in Japan (2). This registry is a multicenter retrospective study of hospitalized patients with HF in Awaji Island. Six acute-care hospitals (Awaji Medical Center, Seirei Awaji Hospital, Higashiura Heisei Hospital, Junshin Awaji Hospital, Sumoto Itsuki Hospital, and Nakabayashi Hospital) were enrolled in this registry. Since all acute-care hospitals in Awaji Island participated in this registry and no patients with acute HF had been transported off of the Awaji Island to another island, including the main island by ambulance, nearly all patients with acute HF on Awaji Island were considered to have been enrolled.
From this registry, a total of 1,971 consecutive patients with HF who met the Framingham criteria (17) and were hospitalized on Awaji Island between April 2013 and March 2020 were retrospectively enrolled (Fig. 1). After the exclusion of 523 recurrent cases, we assessed the data of the remaining 1,448 patients with de novo acute HF. We also excluded 166 patients with in-hospital death and 146 without sufficient follow-up data; the final enrolment for this study therefore comprised 1,136 patients with de novo acute HF. Patients were divided into 4 groups by age category: 134 patients (11.8%) ≤65 years old, 184 patients (16.2%) 66-75 years old, 400 patients (35.2%) 76-85 years old, and 418 patients (36.8%) >85 years old.
Figure 1.
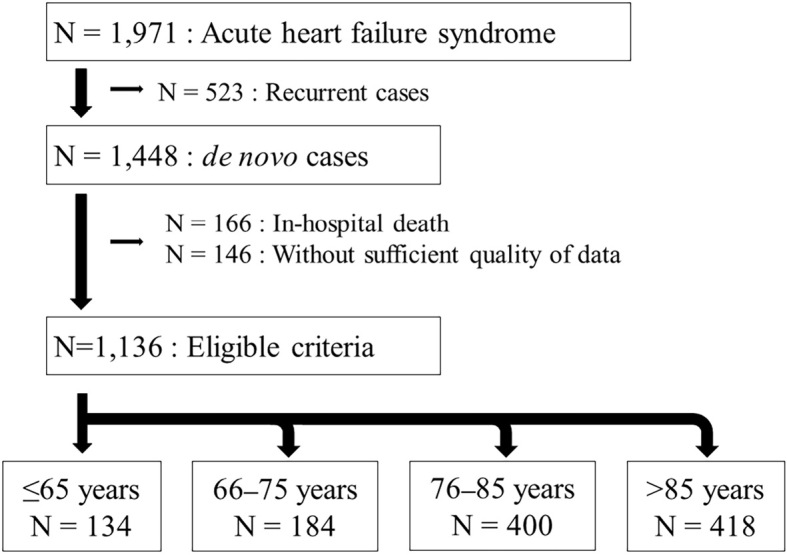
Patient flowchart. Among a total of 1,971 patients with acute heart failure who were hospitalized in Awaji Island, 1,136 patients were finally included and divided into 4 groups.
This study was approved by the Ethics Committee of Hyogo prefectural Awaji Medical Center (No. 20-11) and was conducted in accordance with the principles of the Declaration of Helsinki. The study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000038637).
Study outcomes
The study endpoints were all-cause death, cardiovascular (CV) death, non-CV death, and HF re-hospitalization. CV death was defined as death from HF, myocardial infarction, or stroke; cardiac death; or any documented sudden death without an apparent non-CV cause (18). All patients were followed up by telephone and medical record reviews. We defined frailty using the Clinical Frailty Scale (CFS), which is a semiquantitative tool that provides a generally accepted clinical definition of frailty, ranging from 1 (very fit) to 9 (terminally ill) at discharge (19).
Statistical analyses
Continuous variables are expressed as means±standard deviations or medians (25-75th percentile) and compared using a one-way analysis of variance followed by a Scheffe post-hoc analysis for normally distributed data and the Kruskal-Wallis test followed by a Conover post-hoc analysis for non-normally distributed data. Categorical variables are presented as numbers or frequencies (%) and compared using the χ2 test or Fisher's exact test. The cumulative incidence of the endpoint was calculated using the Kaplan-Meier method with the log-rank test. Baseline characteristics, medication, laboratory data at discharge, echocardiographic data, CFS at discharge, all-cause deaths, CV deaths, non-CV deaths, and HF re-hospitalizations were compared among the four groups.
To examine the predictive factors for all-cause death in each group, we used the Cox proportional-hazards model to estimate the hazard ratio (HR) for endpoints and their 95% confidence interval (CI) using univariate and multivariate models. First, a univariate analysis was performed with certain clinical factors, and then the interaction between age and these factors was investigated as a whole; if the interaction was significant, the clinical factors were investigated separately for the age group. All of the variables were included in the multiple regression analysis using forced entry method. All analyses were performed using the MedCalc software program version 19.8 (MedCalc Software, Ostend, Belgium). A two-sided p value of <0.05 was considered statistically significant.
Results
Patient characteristics
In the overall population, the mean age was 79.8±11.8 years old, and the percentage of men was 54.3%. Table 1 summarizes the baseline characteristics, medication, laboratory data at discharge, and echocardiographic data. The proportion of women increased with increasing age, and the body mass index (BMI) was lower in the >85 years group than other younger groups. The prevalence of comorbidities, such as diabetes mellitus, atrial fibrillation (AF), hemodialysis, ischemic disease, and cognitive disorder, differed significantly among groups. The usage of β-blockers and angiotensin-converting enzyme inhibitors or angiotensin-II receptor blockers was higher in the younger groups, whereas the usage of diuretics including loop diuretic and tolvaptan was higher in the older groups. Older patients showed higher levels of blood urea nitrogen (BUN) and lower levels of serum hemoglobin and albumin than younger patients. The left ventricular end-diastolic and end-systolic diameters were significantly larger and the left ventricular ejection fraction significantly lower in younger patients than in older patients. Elderly patients showed higher E/e′ values than younger patients. The CFS score at discharge was significantly higher in the older patients than in the younger patients. More than 80% of the patients >65 years old were undergoing rehabilitation during hospitalization.
Table 1.
A Comparison of the Patients’ Baseline Characteristics, Medication, Laboratory Data at Discharge, and Echocardiographic Data.
| ≤65 years N=134 | 66-75 years N=184 | 76-85 years N=400 | >85 years N=418 | p value | Post hoc analysis* | |
|---|---|---|---|---|---|---|
| Age, years | 55.7±9.2 | 70.8±3.0 | 81.1±2.8 | 90.1±3.3 | <0.001 | a,b,c,d,e,f |
| Male, n (%) | 102 (76.1) | 123 (66.8) | 226 (56.5) | 166 (39.7) | <0.001 | b,c,d,e,f |
| BMI, km/m2 | 24.8±6.6 | 21.7±4.4 | 21.1±3.7 | 19.8±3.7 | <0.001 | a,b,c,e,f |
| Comorbidities | ||||||
| Hypertension, n (%) | 82 (61.2) | 119 (64.7) | 246 (61.5) | 283 (67.7) | 0.26 | |
| Diabetes mellitus, n (%) | 59 (44.0) | 78 (42.4) | 117 (29.3) | 71 (17.0) | <0.001 | b,c,d,e,f |
| Dyslipidemia, n (%) | 38 (28.4) | 49 (26.6) | 88 (22.0) | 79 (18.9) | 0.059 | |
| Atrial fibrillation, n (%) | 43 (32.1) | 102 (55.4) | 215 (53.8) | 230 (55.0) | <0.001 | a,b,c |
| Lung disease, n (%) | 17 (12.7) | 23 (12.5) | 78 (19.5) | 66 (15.8) | 0.096 | |
| Hemodialysis, n (%) | 15 (11.2) | 19 (10.3) | 22 (5.5) | 9 (2.2) | <0.001 | b,c,d,e,f |
| Ischemic heart disease, n (%) | 31 (23.1) | 70 (38.0) | 126 (31.5) | 85 (20.3) | <0.001 | a,e,f |
| Cognitive disorder, n (%) | 3 (2.2) | 8 (4.3) | 51 (12.8) | 106 (25.4) | <0.001 | b,c,d,e,f |
| Clinical presentation | ||||||
| Systolic blood pressure, mmHg | 151.1±42.0 | 145.4±34.6 | 142.0±30.8 | 145.0±32.9 | 0.089 | |
| Diastolic blood pressure, mmHg | 95.3±26.2 | 88.8±21.4 | 79.7±20.5 | 81.1±64.6 | 0.005 | b,c |
| Heart rate, bpm | 108.9±30.4 | 100.7±29.1 | 89.3±26.7 | 90.6±27.2 | <0.001 | b,c,d,e |
| NYHA | 0.60 | |||||
| II, n (%) | 7 (5.2) | 11 (6.0) | 20 (5.0) | 12 (2.9) | ||
| III, n (%) | 26 (19.4) | 40 (21.7) | 88 (22.0) | 88 (21.1) | ||
| IV, n (%) | 101 (75.4) | 133 (72.3) | 292 (73.0) | 318 (76.1) | ||
| Etiology | ||||||
| Ischemic cardiomyopathy, n (%) | 31 (23.1) | 70 (38.0) | 126 (31.5) | 85 (20.3) | <0.001 | a,e,f |
| Hospitalization length, days | 22.3±19.4 | 26.3±20.6 | 24.4±18.2 | 26.2±18.9 | 0.14 | |
| Rehabilitation during hospitalization, n (%) | 91 (67.9) | 148 (80.4) | 332 (83.0) | 360 (86.1) | <0.001 | a,b,c |
| Treatment | ||||||
| Intubation, n (%) | 22 (16.4) | 26 (14.1) | 34 (8.5) | 23 (5.5) | <0.001 | b,c,d,e |
| NIPPV, n (%) | 30 (22.4) | 39 (21.2) | 83 (20.8) | 89 (21.3) | 0.98 | |
| IABP, n (%) | 15 (11.2) | 11 (6.0) | 20 (5.0) | 8 (1.9) | <0.001 | b,c,e,f |
| Dobutamine, n (%) | 28 (20.9) | 34 (18.5) | 58 (14.5) | 66 (15.8) | 0.29 | |
| Nitroglycerine, n (%) | 43 (32.1) | 46 (25.0) | 90 (22.5) | 105 (25.1) | 0.18 | |
| hANP, n (%) | 15 (11.2) | 13 (7.1) | 40 (10.0) | 22 (5.3) | 0.036 | c,f |
| β-blocker, n (%) | 108 (80.6) | 160 (87.0) | 304 (76.0) | 296 (70.8) | <0.001 | c,d,e |
| ACEi/ARB, n (%) | 105 (78.3) | 135 (73.4) | 287 (71.8) | 277 (66.3) | 0.035 | c |
| MRA, n (%) | 61 (45.5) | 95 (51.6) | 166 (41.5) | 179 (42.8) | 0.13 | |
| Loop diuretic, n (%) | 86 (64.2) | 121 (65.8) | 297 (74.3) | 344 (82.3) | <0.001 | b,c,d,e,f |
| Tolvaptan, n (%) | 20 (14.9) | 34 (18.5) | 79 (19.8) | 114 (27.3) | 0.0041 | c,e,f |
| Pimobendan, n (%) | 0 (0.0) | 0 (0.0) | 8 (2.0) | 10 (2.4) | 0.062 | |
| Digitalis, n (%) | 1 (0.7) | 2 (1.1) | 9 (2.3) | 8 (1.9) | 0.60 | |
| Laboratory data | ||||||
| Hb, g/dL | 12.8±2.6 | 12.2±2.3 | 11.4±2.0 | 11.0±1.8 | <0.001 | b,c,d,e |
| Alb, g/dL | 3.3±0.5 | 3.3±0.5 | 3.1±0.6 | 3.0±0.5 | <0.001 | b,c,d,e |
| T-bil, mg/dL | 0.71±0.49 | 0.80±0.89 | 0.67±0.41 | 0.61±0.40 | 0.003 | e |
| BUN, mg/dL | 22.5±12.7 | 23.1±12.5 | 27.6±18.6 | 29.0±18.3 | <0.001 | b,c,d,e |
| Cre, mg/dL | 1.4±1.5 | 1.3±1.1 | 1.3±1.0 | 1.6±8.0 | 0.83 | |
| BNP, pg/mL (median, IQR) | 197.8 (79.4- 534.4) |
267.1 (152.2- 609.7) |
285.5 (152.7- 587.0) |
307.2 (172.1- 525.1) |
0.22 | |
| Echocardiographic data | ||||||
| LVDd, mm | 56.0±9.1 | 52.7±9.1 | 49.9±8.8 | 46.1±8.0 | <0.001 | a,b,c,d,e,f |
| LVDs, mm | 44.6±11.5 | 40.8±11.2 | 36.8±10.2 | 33.0±9.0 | <0.001 | a,b,c,d,e,f |
| LVEF, % | 41.1±13.3 | 43.4±13.8 | 48.0±13.4 | 49.7±12.9 | <0.001 | b,c,d,e |
| LAD, mm | 43.8±7.7 | 44.2±8.8 | 44.9±20.0 | 42.7±7.5 | 0.17 | |
| E/e’ | 15.3±6.9 | 17.5±9.2 | 17.0±8.0 | 17.7±7.6 | 0.025 | c |
| Clinical Frailty Scale at discharge | <0.001 | b,c,d,e,f | ||||
| 1-3, n (%) | 108 (80.6) | 141 (76.6) | 196 (49.0) | 100 (23.9) | ||
| 4, n (%) | 8 (6.0) | 17 (9.2) | 77 (19.3) | 79 (18.9) | ||
| 5, n (%) | 9 (6.7) | 13 (7.1) | 52 (13.0) | 101 (24.2) | ||
| 6, n (%) | 2 (1.5) | 2 (1.1) | 23 (5.8) | 45 (10.8) | ||
| 7-9, n (%) | 7 (5.2) | 11 (6.0) | 52 (13.0) | 93 (22.2) |
Mean±standard deviation for continuous variables; frequency count n (%) for categorical variables.
ACEi: angiotensin-converting enzyme inhibitor, Alb: albumin, ARB: angiotensin II receptor blocker, BMI: body mass index, BNP: brain natriuretic peptide, BUN: blood urea nitrogen, Cre: ceatinine, hANP: human atrial natriuretic peptide, Hb: hemoglobin, IABP: intra-aortic balloon pumping, LAD: left atrial dimension, LVDd: left ventricular end-diastolic diameter, LVDs: left ventricular end-systolic diameter, LVEF: left ventricular ejection fraction, MRA: mineralocorticoid receptor antagonist, NIPPV: non-invasive positive pressure ventilation, NYHA: New York Heart Association, T-bil: total bilirubin
* Significant difference (p<0.05) between a: ≤65 and 66-75 years, b: ≤65 and 76-85 years, c: ≤65 and >85 years, d: 66-75 and 76-85 years, e: 66-75 and >85 years, f: 76-85 and >85 years
The percentage of patients prescribed anticoagulants for AF is shown in Supplementary material 1, which also shows the rate of patients underdosed with direct oral anticoagulant (DOAC).
Clinical outcomes
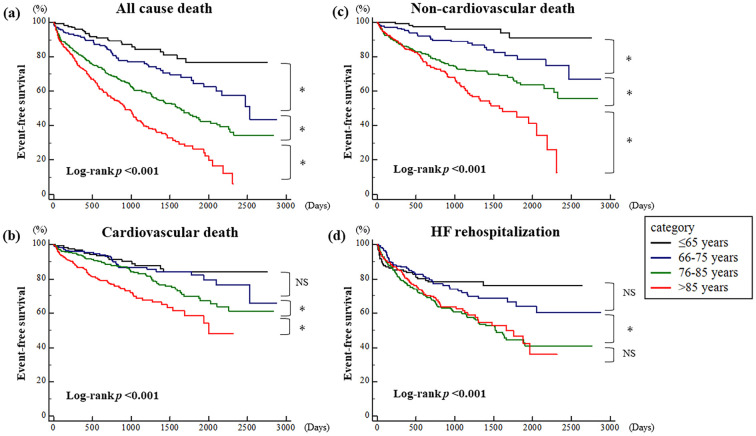
During the mean follow-up period of 1,038±716 days after hospital discharge, 445 patients (39.2%) had all-cause death, including 199 (17.5%) who suffered CV deaths. The number of all-cause deaths among patients by age category was 21 (≤65 years old), 51 (66-75 years old), 170 (76-85 years old), and 203 (>85 years old). A total of 322 patients (28.3%) were re-hospitalized for HF. The 1- and 3-year mortality rates were 18.4% and 39.4%, respectively. A Kaplan-Meier analysis showed a significantly higher incidence of all-cause death, CV death, and non-CV death in the older groups than in the younger groups (log-rank p<0.001) (Fig. 2a-c). Regarding HF re-hospitalization, there was no significant difference in the incidence between the ≤65 and 66-75 years old groups (HR: 1.26, 95% CI: 0.87-1.83) and between the 76-85 years and >85 years old groups (HR: 1.09, 95% CI: 0.83-1.43); however, the incidence was significantly higher in the 76-85 years old group than in the 66-75 years old group (HR: 1.70, 95% CI: 1.25-2.32) (Fig. 2d).
Figure 2.
Results of a Kaplan-Meier analysis for (a) all-cause death, (b) cardiovascular death, (c) non-cardiovascular death, and (d) heart failure re-hospitalization among patients aged ≤65 years old, 66-75 years old, 76-85 years old, and >85 years. *p<0.05. HF: heart failure, NS: not significant
Cause of death
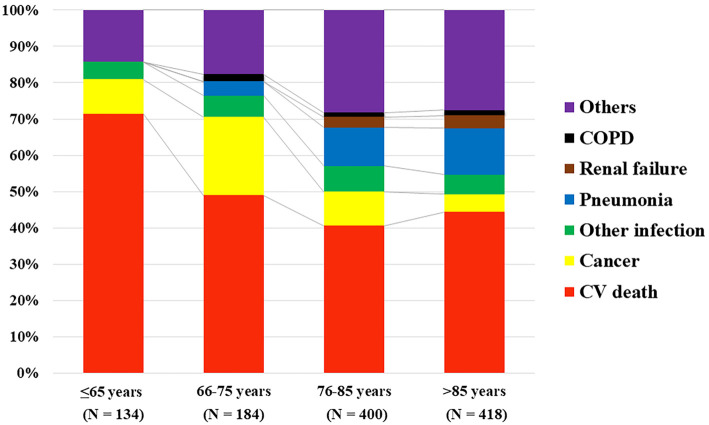
The causes of death are compared by age category in Fig. 3. In all age categories, CV death was the main cause of death in patients with HF; however, the proportion of CV death decreased with age (71.4%, 49.0%, 40.6%, and 44.3% in ≤65 years old, 66-75 years old, 76-85 years old, and >85 years old groups, respectively, p=0.054). In contrast, the older groups had a higher rate of pneumonia and other infections than the younger groups. Cancer deaths were frequent in the 66-75 years old group (21.6%), although the proportion tended to decrease in the 76-85 (9.4%) and >85 (4.9%) years old groups.
Figure 3.
A comparison of cause of death among the age categories. COPD: chronic obstructive pulmonary disease, CV: cardiovascular
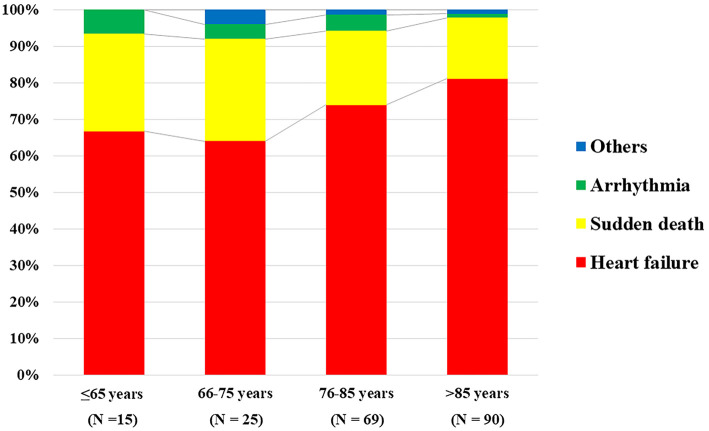
Fig. 4 shows the comparison of the cause of CV deaths in each age category. HF was the main cause of CV deaths in all groups (66.7%, 64.0%, 73.9%, and 81.1%, in the ≤65, 66-75, 76-85, and >85 years old groups, respectively, p=0.26), and sudden death was the second-most common cause of CV deaths (26.7%, 28.0%, 20.3%, and 16.7%, in the ≤65, 66-75, 76-85, and >85 years old groups, respectively, p=0.56).
Figure 4.
A comparison of cause of CV death among the age categories. CV: cardiovascular
Predictive factors for all-cause death
A Cox proportional-hazard regression analysis identified the predictors of all-cause death in each age category (Table 2). Since an interaction between age and several factors was found in all patients, we investigated these factors separately for each age group.
Table 2.
Predictive Factors of the All-cause Death in Each Age Category.
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR [95% CI] | p value | HR [95% CI] | p value | ||||
| ≥65 years | |||||||
| Male | 0.84 [0.31-2.30] | 0.73 | 0.73 [0.22-8.92] | 0.73 | |||
| BMI | 0.91 [0.83-1.01] | 0.08 | 1.00 [0.84-1.18] | 0.97 | |||
| HT | 0.63 [0.27-1.49] | 0.29 | 0.28 [0.03-2.37] | 0.24 | |||
| DM | 1.20 [0.51-2.82] | 0.68 | 1.06 [0.18-6.19] | 0.95 | |||
| AF | 4.78 [1.92-11.9] | <0.001 | 23.3 [2.36-231.1] | 0.007 | |||
| ACEi/ARB | 0.54 [0.22-1.31] | 0.17 | 0.99 [0.17-5.84] | 0.99 | |||
| β-blocker | 0.61 [0.22-1.66] | 0.33 | 0.07 [0.01-0.82] | 0.034 | |||
| Diuretic | 1.84 [0.67-5.03] | 0.23 | 8.03 [0.35-184.0] | 0.19 | |||
| Hb | 0.91 [0.77-1.08] | 0.28 | 0.59 [0.35-1.01] | 0.056 | |||
| Alb | 0.86 [0.34-2.16] | 0.74 | 2.26 [0.35-14.6] | 0.39 | |||
| T-bil | 1.08 [0.43-2.70] | 0.87 | 3.21 [0.40-25.5] | 0.27 | |||
| Cre | 1.09 [0.98-1.22] | 0.10 | 1.57 [1.10-2.25] | 0.013 | |||
| EF | 1.01 [0.98-1.05] | 0.41 | 0.94 [0.86-1.02] | 0.12 | |||
| ICM | 1.28 [0.50-3.30] | 0.61 | 3.93 [0.59-26.2] | 0.16 | |||
| CFS | 1.30 [0.96-1.76] | 0.11 | 0.81 [0.40-1.62] | 0.55 | |||
| 66-75 years | |||||||
| Male | 1.24 [0.69-2.23] | 0.47 | 2.34 [0.76-7.20] | 0.14 | |||
| BMI | 0.90 [0.83-0.97] | 0.006 | 0.86 [0.76-0.98] | 0.025 | |||
| HT | 0.98 [0.55-1.73] | 0.94 | 0.52 [0.21-1.27] | 0.15 | |||
| DM | 1.37 [0.79-2.39] | 0.26 | 1.22 [0.48-3.12] | 0.68 | |||
| AF | 0.99 [0.57-1.72] | 0.96 | 1.25 [0.54-2.91] | 0.61 | |||
| ACEi/ARB | 1.21 [0.62-2.36] | 0.58 | 1.44 [0.49-4.24] | 0.50 | |||
| β-blocker | 0.53 [0.26-1.09] | 0.083 | 0.40 [0.11-1.51] | 0.18 | |||
| Diuretic | 1.02 [0.58-1.80] | 0.95 | 1.12 [0.45-2.79] | 0.80 | |||
| Hb | 0.82 [0.72-0.93] | 0.002 | 0.94 [0.81-1.09] | 0.39 | |||
| Alb | 0.90 [0.49-1.64] | 0.73 | 1.03 [0.78-1.36] | 0.85 | |||
| T-bil | 0.96 [0.73-1.40] | 0.96 | 1.69 [0.61-4.70] | 0.32 | |||
| Cre | 1.15 [1.04-1.28] | 0.008 | 1.22 [1.01-1.47] | 0.044 | |||
| EF | 1.01 [0.99-1.03] | 0.55 | 0.99 [0.96-1.02] | 0.59 | |||
| ICM | 1.22 [0.70-2.11] | 0.49 | 1.30 [0.54-3.16] | 0.56 | |||
| CFS | 1.36 [1.32-1.64] | 0.001 | 1.72 [1.24-2.40] | 0.001 | |||
| 76-85 years | |||||||
| Male | 1.38 [1.01-1.88] | 0.044 | 2.15 [1.35-3.44] | 0.001 | |||
| BMI | 0.95 [0.91-1.00] | 0.039 | 0.95 [0.89-1.01] | 0.10 | |||
| HT | 1.02 [0.75-1.40] | 0.88 | 0.96 [0.61-1.51] | 0.86 | |||
| DM | 0.93 [0.68-1.26] | 0.63 | 0.85 [0.52-1.38] | 0.51 | |||
| AF | 0.99 [0.73-1.33] | 0.93 | 1.03 [0.65-1.63] | 0.91 | |||
| ACEi/ARB | 0.75 [0.54-1.05] | 0.096 | 0.77 [0.48-1.22] | 0.26 | |||
| β-blocker | 1.01 [0.71-1.44] | 0.94 | 1.00 [0.62-1.61] | 0.99 | |||
| Diuretic | 0.89 [0.63-1.24] | 0.49 | 0.86 [0.55-1.42] | 0.61 | |||
| Hb | 0.78 [0.72-0.85] | <0.001 | 0.77 [0.67-0.88] | <0.001 | |||
| Alb | 0.48 [0.36-0.66] | <0.001 | 0.90 [0.60-1.37] | 0.90 | |||
| T-bil | 0.87 [0.55-1.38] | 0.56 | 1.54 [0.97-2.44] | 0.069 | |||
| Cre | 1.20 [1.12-1.28] | <0.001 | 1.17 [1.06-1.29] | 0.002 | |||
| EF | 1.00 [0.99-1.02] | 0.46 | 1.00 [0.98-1.01] | 0.72 | |||
| ICM | 0.96 [0.69-1.33] | 0.81 | 0.84 [0.50-1.42] | 0.52 | |||
| CFS | 1.31 [1.19-1.44] | <0.001 | 1.30 [1.12-1.51] | <0.001 | |||
| >85 years | |||||||
| Male | 1.49 [1.13-1.96] | 0.005 | 1.61 [1.10-2.35] | 0.014 | |||
| BMI | 0.93 [0.89-0.97] | 0.001 | 0.94 [0.88-0.99] | 0.026 | |||
| HT | 0.93 [0.69-1.25] | 0.63 | 1.23 [0.82-1.84] | 0.32 | |||
| DM | 0.79 [0.53-1.17] | 0.23 | 1.00 [0.58-1.71] | 0.99 | |||
| AF | 1.04 [0.79-1.38] | 0.77 | 0.87 [0.58-1.29] | 0.48 | |||
| ACEi/ARB | 0.72 [0.54-0.96] | 0.024 | 0.54 [0.37-0.80] | 0.002 | |||
| β-blocker | 1.07 [0.79-1.46] | 0.65 | 1.13 [0.73-1.75] | 0.57 | |||
| Diuretic | 1.19 [0.83-1.72] | 0.34 | 1.39 [0.84-2.30] | 0.19 | |||
| Hb | 0.89 [0.82-0.96] | 0.003 | 0.94 [0.84-1.05] | 0.29 | |||
| Alb | 0.39 [0.28-0.54] | <0.001 | 0.49 [0.31-0.78] | 0.003 | |||
| T-bil | 0.97 [0.60-1.55] | 0.89 | 1.12 [0.70-1.81] | 0.63 | |||
| Cre | 1.01 [1.00-1.02] | 0.019 | 1.01 [1.00-1.02] | 0.13 | |||
| EF | 1.00 [0.98-1.01] | 0.37 | 1.01 [0.99-1.02] | 0.39 | |||
| ICM | 1.08 [0.78-1.50] | 0.64 | 1.15 [0.73-1.83] | 0.54 | |||
| CFS | 1.35 [1.23-1.48] | <0.001 | 1.33 [1.16-1.52] | <0.001 | |||
Data given as mean for continuous variables, or %.
ACEi/ARB: angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, AF: atrial fibrillation, Alb: albumin, β-blocker: beta-blocker, BMI: body mass index, CI: confidence interval, CFS: Clinical Frailty Scale, Cre: creatinine, DM: diabetes mellitus, EF: ejection fraction, Hb: hemoglobin, HR: hazard’s ratio, HT: hypertension, ICM: ischemic cardiomyopathy, T-bil: total bilirubin
In the ≤65 years old group, the presence of AF was a notable predictor among patients with HF (HR: 23.3, 95% CI: 2.36-231.1, p=0.007). Creatinine was an independent predictor in the 3 youngest groups: ≤65 years old (HR: 1.57, 95% CI: 1.10-2.25, p=0.013), 66-75 years old (HR: 1.22, 95% CI: 1.01-1.47, p=0.044), and 76-85 years old (HR: 1.17, 95% CI: 1.06-1.29, p=0.002). CFS was an independent predictive factor in the three oldest groups: 66-75 years old (HR: 1.72, 95% CI: 1.24-2.40, p=0.001), 76-85 years old (HR: 1.30, 95% CI: 1.12-1.51, p<0.001), and >85 years old (HR: 1.33, 95% CI: 1.16-1.52, p<0.001). In the >85 years old group, low-grade albuminemia was also an independent predictor of all-cause death (HR: 0.49, 95% CI: 0.31-0.78, p=0.003). Men were associated with all-cause death in the 76-85 (HR: 2.15, 95% CI: 1.35-3.44, p=0.001) and >85 (HR: 1.61, 95% CI: 1.10-2.35, p=0.014) years old groups. The BMI was an independent predictive factor in the 66-75 (HR: 0.86, 95% CI: 0.76-0.98, p=0.025) and >85 (HR: 0.94, 95% CI: 0.88-0.99, p=0.026) years old age groups. No significant difference was found in the univariate analysis in the etiology (ischemic cardiomyopathy or not).
We performed similar analyses among 3 groups (≤65 years old, 66-85 years old, and >85 years) for sensitivity; the independent predictive factors were not markedly different from those identified in the four main groups. In addition, the results of the univariate and multivariate analyses were similar even when 80% of the patients were randomly selected. Therefore, the robustness of our data has been proven.
Discussion
We presented the age distribution of hospitalized patients with HF, their prognosis, and the difference in predictors of all-cause death according to the age category using the KUNIUMI Registry acute cohort. The main findings were as follows: 1) the incidence of all-cause death increased with advancing age, and the proportion of CV death decreased, whereas the proportion of pneumonia and other infections increased with increasing age; 2) in addition to well-known predictors, such as chronic kidney disease and anemia, in the ≤65 years old group, the presence of AF was a notable predictive factor of all-cause death, whereas CFS and hypoalbuminemia were stronger predictors of all-cause death in the aging population.
In Japan, some acute HF cohorts have revealed that in-hospital mortality decreased over time, whereas the 1-year mortality after re-hospitalization remained unchanged, accounting for approximately 20% of deaths (4). Our registry revealed similar mortality rates to a previous HF registry. However, as we excluded in-hospital deaths (166 patients), the 1-year mortality rate of our registry may have been higher than that of previous registries (4). In addition, as previously reported, older patients showed worse mortality rates than younger patients hospitalized for acute HF (20) in our registry. This trend was observed for CV deaths, non-CV deaths, and all-cause deaths, possibly indicating that the 1-year mortality will increase in the future as the population ages. Therefore, identifying prognostic factors and the cause of death is important, especially in elderly patients with HF compared with younger patients with HF.
AF is the most common arrhythmia and often coexists with HF. Previous studies have revealed that AF is associated with a poor prognosis in high-risk patients with HF, such as elderly patients and those with a reduced estimated glomerular filtration rate, increased B-type natriuretic peptide levels, and low left ventricular ejection fraction (21). The incidence of AF in our study was higher than that in previous studies, occurring in more than 50% of patients >65 years old. Remarkably, only in young patients (≤65 years) was AF an independent predictive factor. The incidence of AF is strongly influenced by aging; however, it can be both a mediator and a surrogate of HF severity, especially in younger patients. Recently, the CASTLE-AF trial revealed that catheter ablation for AF in patients with HF was associated with a significantly lower rate of composite endpoint of all-cause death and worsening HF than medical therapy (22). A subgroup analysis showed that the benefit of catheter ablation was greater in the younger group (≤65 years old) than in the older group (>65 years old). Given the present findings, a positive consideration of catheter ablation may improve the prognosis of younger patients with HF and AF.
The incidence of diabetes mellitus in patients ≤65 years old was higher in our registry than in that of the United States (44.0% vs. 38.1%, respectively) (23). This is probably because East Asian patients, even non-obese and younger patients, are generally prone to developing type 2 diabetes mellitus due to β cell dysfunction (24). Since diabetes mellitus is a well-established risk factor of stroke, especially ischemic stroke (25), catheter ablation in younger patients should be positively considered, and compliance with anticoagulant agents should be improved. The percentage of anticoagulant prescriptions in our registry was lower than that in a previous report (26), and the percentage of DOAC among anticoagulants was also lower. Since the bleeding risk was greater than the embolization risk due to the older age of the patients or their extremely poor general condition, anticoagulants prescriptions were avoided. Furthermore, in our study, 14.0% of the patients who were prescribed DOACs were given less than the recommended dosage. A similar tendency was noted in a previous study (26). We should be careful not to underdose DOACs in patients, as an adequate dose can contribute to a reduction in embolic events and mortality.
Frailty is a state of increased vulnerability to genetic, biological, physical, psychological, social, and environmental stressors due to age-related impairments (27,28). Previous studies have demonstrated that frailty is common in patients with HF and is associated with mortality (29,30). The CFS is a semi-quantitative tool for evaluating patients' frailty and has been widely used because of its simplicity and usefulness (31). Our study demonstrated that the CFS is an independent prognostic factor for HF in elderly patients. There are complex mechanisms underlying the association between HF and frailty; for example, a low cardiac output leads to tissue hypoxia and cell apoptosis, which activate inflammatory cytokines, increase oxidative stress, and are related to mitochondrial dysfunction (32). Neurohumoral factors, such as the renin-angiotensin-aldosterone system, are also believed to activate the inflammatory state. These phenomena lead to increased muscle catabolism, which results in sarcopenia or physical frailty. Physically frail patients with HF had significantly lower cardiac outputs and higher heart rates than non-frail patients, and their HF was often difficult to control (33). Thus, physical frailty and HF complexly interact in what is referred to as the “frailty cycle” (34). Indeed, our study showed a higher BUN/Cr ratio in elderly patients than in younger patients, which may reflect hyper-catabolism in elderly patients. Undoubtedly, the management of frailty is essential for elderly patients with HF. As recent research has shown that appropriate rehabilitation implementation can prevent a decline in activities of daily living in elderly patients with HF (35), early and active rehabilitation may help prevent physical frailty, which is a risk factor for poor outcomes in elderly patients with HF.
Malnutrition as well as a low BMI are associated with an increased risk of mortality in patients with HF (36). As BMI values are much lower in the general Japanese population than in the Western population (37), nutritional management is also important in elderly patients with HF, especially in Japan. Indeed, our study revealed that low-grade albuminemia was a predictive factor for a poor prognosis in elderly patients with HF. In the absence of renal dysfunction (chronic kidney disease grade ≥3b), protein-focused nutritional management is necessary to compensate for the increased protein metabolism (38,39). A recent report revealed that the assessment of dietary intake tailored to the patient's nutritional status improves the mortality risk in elderly patients with HF (40); therefore, careful management of the dietary intake is necessary for elderly patients with HF in daily practice.
Akita et al. reported that an underdose of guideline-based medication therapy, including renin-angiotensin system inhibitors and β-blockers, was seen in elderly patients with acute HF in their West Tokyo Heart Failure cohort. However, although guideline-based medication therapy at discharge was associated with a reduction in HF readmission or in the composite endpoint of cardiac death and HF readmission in the younger group, this association was not observed in the elderly group (41). This is because the prognosis of elderly patients with HF is associated with not only conventional medical therapy but also multifaceted factors, such as frailty and malnutrition. In fact, Rich et al. reported that a multidisciplinary intervention, such as comprehensive education of the patient and family, the assessment of energy intake, social-service consultation, and intensive follow-up, can reduce the risk of readmission among elderly patients with congestive HF (42). Our study revealed that a high CFS score and low-grade albuminemia were predictive factors for a poor prognosis in elderly patients with HF (>85 years old group). Furthermore, our registry indicated that the rate of non-CV deaths was likely to increase as the population ages. Regarding non-CV deaths, deaths from infections, such as pneumonia, are particularly increasing. Therefore, elderly patients with HF may require comprehensive multidisciplinary interventions, including vaccination against infection (43,44) and prevention of frailty and malnutrition, using social and environmental approaches, such as the use of nursing-care services or family support.
Several limitations associated with the present study warrant mention. First, since this is a retrospective observational registry, unmeasured or unknown factors may have influenced the outcomes. Second, the number of deaths differed among the age groups, with the number in the ≤65 years old group being particularly small. Therefore, the width of the 95% CI in the multivariate analysis (Table 2) was extremely large in the ≤65 years old group. This is a statistical limitation; however, we would still like to present the result as a reference value. Third, although the CFS is a relatively objective assessment tool for frailty, some bias might have occurred, as scoring was performed using a retrospective medical record review. Fourth, as shown above, we only evaluated physical frailty. Mental and social frailty are essential for determining the risk and prognosis of patients with HF. Further considerations, such as of housing conditions, are needed. Furthermore, because of the observational design of this study, we were unable to prove a cause-and-effect relationship. Therefore, whether or not comprehensive multidisciplinary interventions, including prevention of frailty and malnutrition, can improve the prognosis of elderly patients with HF remains unclear. Further research is warranted to establish evidence supporting comprehensive multidisciplinary interventions for elderly patients with HF in Japan.
Conclusion
In addition to well-known risk factors, including chronic kidney disease and anemia, AF was a notable predictor of HF in young patients, and frailty and low-grade albuminemia were essential predictive factors of HF in elderly patients. As the number of elderly patients with HF increases in the future, comprehensive multidisciplinary treatment will be necessary.
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
A comparison of prescribed anticoagulants among patients with AF
References
- 1.Konishi M, Ishida J, Springer J, et al. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Heart Fail 3: 145-151, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto W, Toh R, Takegami M, et al. Estimating incidence of acute heart failure syndromes in Japan - an analysis from the KUNIUMI Registry. Circ J 85: 1860-1868, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Ushigome R, Sakata Y, Nochioka K, et al. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan -- report from the CHART Studies. Circ J 79: 2396-2407, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Shiraishi Y, Kohsaka S, Sato N, et al. 9-year trend in the management of acute heart failure in Japan: a report from the National Consortium of Acute Heart Failure Registries. J Am Heart Assoc 7: e008687, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su K, Kato T, Toyofuku M, et al. Association of previous hospitalization for heart failure with increased mortality in patients hospitalized for acute decompensated heart failure. Circ Rep 1: 517-524, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto W, Konishi A, Iwasaki M, et al. Precipitating factors and clinical impact of early rehospitalization for heart failure in patients with heart failure in Awaji Island, Japan. J Cardiol 77: 645-651, 2021. [DOI] [PubMed] [Google Scholar]
- 7.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, et al. Anemia is an independent predictor of long-term adverse outcomes in patients hospitalized with heart failure in Japan. A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 73: 1901-1908, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi S, Kinugawa S, Goto D, et al. Predictors of long-term adverse outcomes in elderly patients over 80 years hospitalized with heart failure - a report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 75: 2403-2410, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko H, Itoh H, Yotsumoto H, et al. Characteristics and outcomes of super-elderly patients (aged ≥90 years) hospitalized for heart failure - analysis of a nationwide inpatient database. Circ Rep 2: 393-399, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Savarese G, Dahlström U, Lund LH, Fu M. Age-dependent differences in clinical phenotype and prognosis in heart failure with mid-range ejection compared with heart failure with reduced or preserved ejection fraction. Clin Res Cardiol 108: 1394-1405, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno M, Kajimoto K, Sato N, et al. Clinical profile, management, and mortality in very-elderly patients hospitalized with acute decompensated heart failure: an analysis from the ATTEND registry. Eur J Intern Med 27: 80-85, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Statistics Bureau, Ministry of Internal Affairs and Communications. Japanese national census in 2015 [Internet]. [cited 2022 Feb 11]. Available from: https://www.stat.go.jp/data/kokusei/2015/kekka.html (in Japanese)
- 13.Sumoto City web site [Internet]. [cited 2022 Feb 11]. Available from: https://www.city.sumoto.lg.jp (in Japanese)
- 14.Awaji City web site [Internet]. [cited 2022 Feb 11]. Available from: https://www.city.awaji.lg.jp (in Japanese)
- 15.Minami Awaji City web site [Internet]. [cited 2022 Feb 11]. Available from: https://www.city.minamiawaji.hyogo.jp (in Japanese)
- 16. Ministry of Internal Affairs and Communications. Basic resident register [Internet]. [cited 2022 Feb 11]. Available from: http://www.stat.go.jp/data/idou/2019np/shousai/pdf/youyaku.pdf (in Japanese)
- 17.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 285: 1441-1446, 1971. [DOI] [PubMed] [Google Scholar]
- 18.Hicks KA, Mahaffey KW, Mehran R, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 137: 961-972, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 173: 489-495, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natella PA, Le Corvoisier P, Paillaud E, et al. Long-term mortality in older patients discharged after acute decompensated heart failure: a prospective cohort study. BMC Geriatr 17: 34, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamauchi T, Sakata Y, Miura M, et al. Prognostic impact of atrial fibrillation and new risk score of its onset in patients at high risk of heart failure - a report from the CHART-2 study. Circ J 81: 185-194, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 378: 417-427, 2018. [DOI] [PubMed] [Google Scholar]
- 23.Zepeda I, Li DL, Quispe R, Taub CC. Clinical characteristics of young patients with heart failure with reduced ejection fraction in a racially diverse cohort. Crit Pathw Cardiol 18: 80-85, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 15: 602, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Ovbiagele B, Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci 351: 380-386, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiasa KI, Kaku H, Inoue H, et al. Age-related differences in the clinical characteristics and treatment of elderly patients with atrial fibrillation in Japan - insight from the ANAFIE (All Nippon AF In Elderly) Registry. Circ J 84: 388-396, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 381: 752-762, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J 166: 887-894, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa D, Aladio M, Girado CA, Pérez de la Hoz R, Sara Berensztein C. Frailty is independently associated with 1-year mortality after hospitalization for acute heart failure. Int J Cardiol Heart Vasc 21: 103-106, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail 18: 869-875, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Kanenawa K, Isotani A, Yamaji K, et al. The impact of frailty according to Clinical Frailty Scale on clinical outcome in patients with heart failure. ESC Heart Fail 8: 1552-1561, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54: 991-1001, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Chien CV, Lee CS. Frequency of and significance of physical frailty in patients with heart failure. Am J Cardiol 119: 1243-1249, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146-M156, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Kato M, Mori Y, Watanabe D, et al. Relationship between average daily rehabilitation time and decline in instrumental activity of daily living among older patients with heart failure: a preliminary analysis of a multicenter cohort study, SURUGA-CARE. PLoS One 16: e0254128, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, et al. Body mass index is an independent predictor of long-term outcomes in patients hospitalized with heart failure in Japan. Circ J 74: 2605-2611, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Zhou BF, Stamler J, Dennis B, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 17: 623-630, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 14: 542-559, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 33: 929-936, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katano S, Yano T, Kouzu H, et al. Energy intake during hospital stay predicts all-cause mortality after discharge independently of nutritional status in elderly heart failure patients. Clin Res Cardiol 110: 1202-1220, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akita K, Kohno T, Kohsaka S, et al. Current use of guideline-based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event-free survival. Int J Cardiol 235: 162-168, 2017. [DOI] [PubMed] [Google Scholar]
- 42.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 333: 1190-1195, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis 42: 1093-1101, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Modin D, Jørgensen ME, Gislason G, et al. Influenza vaccine in heart failure. Circulation 139: 575-586, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A comparison of prescribed anticoagulants among patients with AF