Abstract
Objectives
The influential factors for anti-severe acute respiratory syndrome coronavirus 2 spike protein antibody (S-ab) levels were assessed after the administration of BNT162b2 mRNA coronavirus disease-2019 (COVID-19) vaccine at short and medium terms.
Methods
A total of 470 healthcare workers (118 males, mean age 41.0±11.9 years) underwent serum S-ab level measurement at 3 and 8 months after two inoculations of BNT162b2 vaccine given 3 weeks apart, who had no history of COVID-19 were enrolled in this study. The changes and differences after vaccination due to gender and adverse reactions of S-ab were analyzed.
Results
Systemic adverse reactions incidence (48%) was significantly higher after the second dose than after the first dose (8%). S-ab levels decreased as the age increased (from the 20s to 60s) in both measurements. S-ab level 8 months after the second inoculation [median 476.3 (interquartile range (IQR) 322.4-750.6) U/mL] was significantly lower than that after 3 months [977.5 (637.2-1,409.0) U/mL; p< 0.001]. The median decrease rate of S-ab levels in 5 months was 50.3% (IQR 40.3-62.6) and those differences were not observed among all generations. Gender-associated differences in S-ab levels were not observed; however, a significant relationship between higher S-ab levels and the systemic adverse reactions was observed at both measurements.
Conclusions
The systemic adverse reaction is an independent factor for higher S-ab levels at short and medium terms after BNT162b2 vaccination as demonstrated in our data.
Keywords: COVID-19, BNT162b2 mRNA COVID-19 vaccine, anti-SARS-CoV-2 spike protein antibody
Introduction
The coronavirus disease-2019 (COVID-19) pandemic has been severely damaging the global health and economic structures caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection continuing for about 2 years. Under these circumstances, great expectations are placed on mass vaccination that might control the spread of the virus. In Japan, the two-dose administration of BNT162b2 mRNA COVID-19 vaccine was given to healthcare professionals in February 2021, followed by two doses of Moderna mRNA-1273 COVID-19 vaccine that was started in May 2021. About 78% of the total population had completed two inoculations by the end of January 2022 (1). SARS-CoV-2 consists of four structural proteins: spike, envelope, membrane, and nucleocapsid encoded by a single, positive-standard RNA genome (2). Viral invasion is achieved through membrane fusion of the virus and host cells as the spike protein mediates receptor-binding on the host cells (3). BNT162b2 vaccine was mRNA-based encoding the receptor-binding domain of the spike protein with 95% efficacy in preventing symptomatic COVID-19 among individuals without previous infection (4). The association between reactogenicity and anti-SARS-CoV-2 spike protein antibody (S-ab) levels after the vaccination has been discussed in several studies as much research progresses on the factors that can influence S-ab levels after inoculation of BNT162b2 vaccine (5). It has actually been reported that the second inoculation obtains higher S-ab levels than the first inoculation of BNT162b2 vaccine, but the frequency of adverse reactions also increases (4). This implies that reactogenicity might be related to S-ab levels after vaccination. However, no uniform view has been obtained while epidemiological data regarding the relevance between reactogenicity and immunogenicity after administration of BNT162b2 vaccine remains limited. We report a single-center study here that measured S-ab levels in the short and medium terms after two inoculations of the BNT162b2 vaccine in all kinds of healthcare workers at our hospital. We then analyzed the change of S-ab levels, and the factors that influence them.
Materials and Methods
The study included all hospital workers who were two-dose vaccinated with BNT162b2 vaccine for 3 weeks, from February to March 2021, measured S-ab levels two times at 3 and 8 months after vaccination on request, were judged to have no SARS-CoV-2 infection before the second examination, and agreed to the analysis. History of SARS-CoV-2 infection was judged based on their medical history including daily evaluation of body temperature and related symptoms and results of SARS-CoV-2 antigen test of nasopharyngeal swabs using LumipulseⓇ (FUJIREBIO Co., Tokyo, Japan), chemiluminescent enzyme immunoassay system, when fever is ≥37.5°C. Current infection was diagnosed based on the polymerase chain reaction test of nasopharyngeal swabs using Ampdirect 2019-nCoV kit (SHIMADZU Co., Kyoto, Japan) if participants declared a close contact with patients with SARS-CoV-2 or had suggestive symptoms, such as fever, sore throat, headache, cough, nasal congestion, ageusia, and anosmia. Blood samples were collected twice during regular medical examinations at 3 months and 8 months after inoculation, and serum antibody levels were measured immediately after sampling with SARS-CoV-2 IgG assay using ElecsysⓇ Anti-SARS-CoV-2 S RUO (Roche Diagnostics Co., Rotkreuz, Switzerland). This system targets the receptor-binding domain of the SARS-CoV-2 spike protein and allows the quantitative evaluation of antibodies, predominantly IgG. Vaccine adverse reactions were recorded separately for local and systemic events based on the structured self-reported questionnaire. Notable local adverse reactions were defined as pain that requires treatment, redness, swelling, or induration with a diameter of ≥2 cm, itching and heat at the administration site, and blisters. Notable systemic adverse reactions were defined as fever of ≥38°C, rashes, moderate to severe headache, fatigue, runny nose requiring treatment, anaphylaxis, severe or widespread pruritus, vomiting of more than twice a day, and diarrhea of ≥6 times a day. The study was approved by the local institutional ethics review board, and all participants signed an informed consent.
Statistical analysis
Categorical variables in participant characteristics are presented as absolute numbers (n), relative frequencies (%), and mean and standard deviation. Non-parametric continuous data are expressed as the median with interquartile range (IQR). The frequency of vaccine adverse reactions between age groups was compared using by the chi-square test, and between-group comparisons of S-ab levels were performed using Wilcoxon Mann-Whitney U test. Significant predictors of S-ab levels after vaccination were identified using the multivariate log-normal analysis. A p value significance threshold of p<0.05 was used. All statistical analyses were performed using the Statistical Package for the Social Sciences (IBM, SPSS version 25; Armonk, USA).
Results
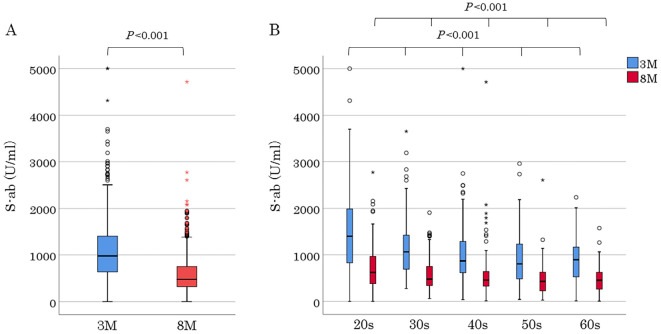
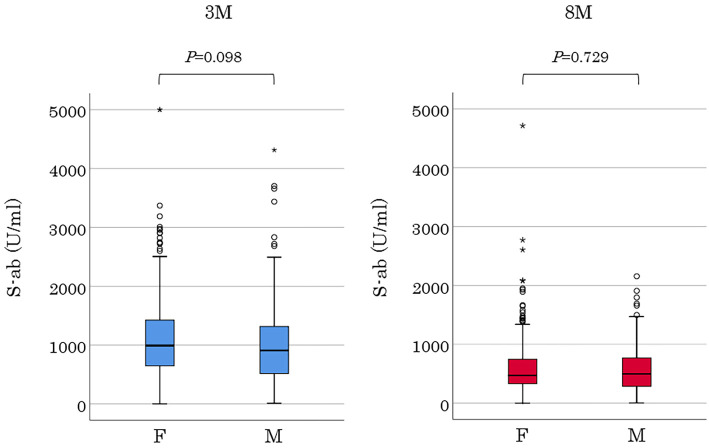
Of the 505 hospital employees who received two inoculations of the BNT162b2 mRNA COVID-19 vaccine, 17 and 15 moved or left before the first and second serum S-ab measurements, respectively. Two participants who did not receive the second dose due to allergic reactions during the first one and a participant who was infected with SARS-CoV-2 before the second S-ab measurement were excluded from the analysis. Finally, 470 employees aged 22-69 (mean, 41.0±11.9) years comprising of 118 males were included for analysis. All participants consisted of 51 doctors, 207 nurses, 10 pharmacists, 146 para-medical staffs, and 56 clerks and nursing school staffs. Baseline characteristics are presented in Table 1. The numbers of participants were almost the same from the 20s to 50s, except for the 60s which had fewer participants, and all generations had a female predominance distribution with a female ratio of 75%. Hypertension was the most common comorbidity in 20 participants (4%), followed by dyslipidemia in 8 participants (1%). Furthermore, there were participants with rheumatoid arthritis, systemic lupus erythematosus, Graves' disease, and primary aldosteronism; 3, 1, 1, and 1, respectively. The occurrence of vaccine adverse reactions is shown in Table 2. Both notable local and systemic adverse reactions significantly occurred more frequently after the second dose than after the first one. Although no participant had a fever of ≥38°C after the first vaccination, 22% had a fever after the second vaccination (20s, 33%; 30s, 24%; 40s, 19%; 50s, 15%; and 60s, 7%). Moreover, the younger generation had more systemic adverse reactions including fever. Serum S-ab level 8 months after the second inoculation [median 476.3 (IQR 322.4-750.6) U/mL] was significantly lower than that after 3 months (977.5 [637.2-1,409.0] U/mL; p<0.001) (Fig. 1A). S-ab levels decreased as the age increased from the 20s to 60s both at 3 months in the analysis by age [20s, 1,401 (IQR 826.5-1,988); 30s, 1,061 (IQR 689.3-1,427.0); 40s, 867.0 (IQR 614.5-1,280.5); 50s, 803.8 (IQR 482.8-1,227.0); and 60s 891.1 (IQR 528.4-1,099.3) U/mL; p<0.001] and 8 months [20s, 621.4 (IQR 379.0-965.4); 30s, 476.3 (339.1-747.6); 40s, 456.0 (328.2-634.6); 50s, 426.4 (226.7-624.6); and 60s, 452.5 (265.3-616.6) U/mL; p<0.001] after vaccination (Fig. 1B). The median decrease rate in S-ab levels from 3 to 8 months after vaccination (the ratio of S-ab level after 8 months to that after 3 months) was 50.3 (IQR 40.3-62.6)% on all participants, and no significant difference was observed among generations [20s, 47.6 (IQR 38.8-57.5); 30s, 49.7 (IQR 38.5-61.4); 40s, 50.1 (IQR 40.7-66.0); 50s, 51.9 (IQR 43.5-63.1); and 60s, 46.7 (IQR 34.6-67.5)%]. No significant difference in the antibody level was observed between men and women at two measurements (Fig. 2). The antibody levels 8 months after vaccination in 13 participants [median 812.0 (IQR 495.0-986.3) U/mL] were higher than after 3 months [median 529.2 (IQR 439.4-873.1) U/mL], although it was insignificant. The distribution of S-ab level peaked at the range of 600-799 U/mL and spread widely 3 months after vaccination; however, the majority of the population had a low antibody level of 200-399 U/mL 8 months after it. Regarding the analysis about the relationship between vaccine adverse reactions and S-ab level, the antibody levels of participants with systemic adverse reactions after the first dose were significantly higher than those without it at both measurements. Moreover, similar results were obtained regarding the systemic adverse reactions after the second dose (Fig. 3). The age of 20s was significantly associated with S-ab level 3 months after vaccination and the presence of systemic adverse reactions after the second dose was significantly associated with high S-ab level at two measurements in multivariate log-normal analysis (Table 3). However, no significant association was observed between the local adverse reactions and S-ab levels in any analysis.
Table 1.
Baseline Characteristics of Participants Data are Presented as N (%), Except for Displaying Age as Mean [Standard Deviation (SD)].
| Participants characterisicts (n=470) | values | |
|---|---|---|
| Age y.o., mean (SD) | 41.0 (11.9) | |
| Male, n (%) | 118 (25) | |
| Generation | ||
| 20-29 y.o., n (%) | 108 (23) | |
| male, n (%) | 20 (19) | |
| 30-39 y.o., n (%) | 105 (22) | |
| male, n (%) | 35 (33) | |
| 40-49 y.o., n (%) | 128 (27) | |
| male, n (%) | 28 (22) | |
| 50-59 y.o., n (%) | 105 (22) | |
| male, n (%) | 27 (26) | |
| 60 y.o -., n (%) | 24 (5) | |
| male, n (%) | 8 (33) | |
| Hypertension, n (%) * | 20 (4) | |
| Dyslipidemia, n (%) * | 8 (1) | |
| Collagen disease, n (%) * | 5 (1) | |
| Bronchial asthma, n (%) * | 3 (1) | |
| Diabetes mellitus, n (%) * | 2 (1) | |
| Endocrine disease, n (%) * | 2 (1) | |
| Depression, n (%) * | 3 (1) | |
| Previous history of malignancy disease, n (%) † | 3 (1) | |
| Immunosuppressant therapy, n (%) | 1 (1) |
* Prescribed as requiring year-round medication. † Included a participant after a lung cancer surgery and two participants after breast cancer surgery.
Table 2.
the Occurrence of Vaccine Adverse Reactions after the First and Second Doses of Vaccination.
| Age | n | 1st dose | 2nd dose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Focal reaction | Systemic reaction | Focal reaction | Systemic reaction | |||||||||||
| 20-29 y.o., n (%) | 108 | 52 (48) | 11 (10) | 63 (58) | 66 (61) | † | ||||||||
| 30-39 y.o, n (%) | 106 | 42 (40) | 14 (13) | 51 (48) | 53 (50) | † | ||||||||
| 40-49 y.o., n (%) | 130 | 55 (42) | 7 (5) | 65 (50) | 61 (47) | † | ||||||||
| 50-59 y.o., n (%) | 103 | 33 (32) | 6 (6) | 56 (54) | § | 40 (39) | † | |||||||
| 60 y.o. -, n (%) | 27 | 11 (41) | 1 (4) | 13 (48) | 6 (22) | ‡ | ||||||||
| total, n (%) | 470 | 193 (41) | 39 (8) | 248 (53) | 226 (48) | |||||||||
† p<0.001; ‡ p=0.048; § p=0.001 vs 1st dose
Figure 1.
S-ab levels 3 and 8 months after the second vaccination dose in all participants and participants in each generation. The top and bottom of a box represent IQR; a horizontal line, the median; and whiskers, the minimum and maximum values. Circles and asterisks represent outliers and extreme outliers, respectively. A: S-ab levels at two measurements in all participants. Undetectable S-ab levels of <0.4 (at two measurements of 1 participant) and ≥5,000 U/mL (at the first measurements of 2 participants) were imputed with 0.4 U/mL and 5,000 U/mL, respectively. B: Generational S-ab levels at two measurements. 3M: 3 months, 8M: 8 months
Figure 2.
S-ab levels by gender at 3 and 8 months after the second dose of vaccination. F: female, M: male
Figure 3.
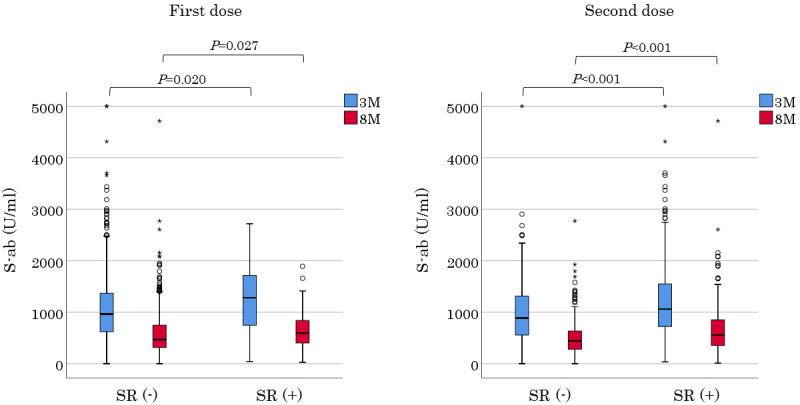
S-ab levels of participants from each group after 3 and 8 months, divided into those who had a systemic adverse reaction at the time of vaccination and those who did not. SR: systemic adverse reaction
Table 3.
Multivariate Log-normal Analysis of Factors Contributing to S-ab Levels 3 and 8 Months after Vaccination.
| Variables | 3M | 8M | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | t value | p value | Value | t value | p value | |||||||
| Age (20s) | 454.00 | 2.95 | 0.003 | 179.76 | 1.76 | 0.079 | ||||||
| Gender, female | 30.01 | 0.41 | 0.686 | -17.02 | -0.35 | 0.724 | ||||||
| Systemic adverse reaction after the 1st dose | -129.24 | -1.13 | 0.259 | -54.30 | -0.73 | 0.466 | ||||||
| Systemic adverse reaction after the 2nd dose | -0.21 | -3.11 | 0.002 | -149.65 | -3.37 | 0.001 | ||||||
Discussion
S-ab levels significantly decreased by approximately 50% from 3 to 8 months after vaccination in all generations in this survey with two serum S-ab level measurements in the short and medium term after two administrations of BNT162b2 mRNA COVID-19 vaccine for the hospital staff. This change of S-ab level was consistent with the results in previous reports. A previous study in Japan also reported a 68.9% reduction of S-ab levels from 3 to 6 months after the two-dose BNT162b2 vaccine (6). Although no evidence has been reported regarding the association of decreased SARS-CoV-2 antibodies with increased infection risk, this finding showing a rapid decline in antibody levels to near depletion in 8 months following inoculation is very meaningful supportive data when considering the validity of the third booster inoculation. The most impressive finding in our study is the significant relationship between systemic adverse reactions and serum S-ab level after vaccination. A significant association between reactogenicity and immune response after vaccination has been reported in some studies (7). Antigenic priming of the immune system during the first vaccination can increase reactogenicity following the booster inoculation (8). This might be the mechanism that results in the significant association between systemic reactogenicity and intense immune response after the second vaccination as in this study. The overall low incidence frequency and the low severity of adverse reactions in the older cohort with immunosenescence may be consistent with this result. Remarkably, the number of participants in their 60s with moderate or greater systemic adverse reactions after the second vaccination was one-third of those in their 20s in this study. However, the epidemiological study results of after COVID-19 vaccination are inconsistent based on the association between systemic adverse reactions and anti-SARS-CoV-2 antibody levels. A study showed a fever of ≥38°C after two doses of the BNT162b2 vaccine was related with higher S-ab levels, but no association between systemic adverse reaction grades and S-ab levels (5). In another study, there was no difference in S-ab levels according to local and systemic adverse reaction grades after the BNT162b2 or AZD1222 two-dose vaccine (9). The small sample size on these studies could be a reason for the dissociation from our study results. Further analysis buildup from large scale studies are needed. Nevertheless, it is noted that immunoreactivity to the vaccine might be predicted based on the presence of the systemic adverse reactions. Another important finding in this study is the relationship between gender and S-ab levels. Our study showed that antibody levels tended to be higher in females than in males both at 3 and 8 months after vaccination; however, no significant difference was confirmed. A study investigating antibody levels showed that the serum levels of IgG antibody against SARS-CoV-2 was lower by 16% in males than females up to 5 weeks after vaccination in healthcare workers (10). In addition, a study reporting a high aggravation rate of SARS-CoV-2 infection in males suggested a poor immune response to SARS-CoV-2 (11). The main reason why no gender difference was observed in S-ab levels in this study is probably due to the limited number of participants and the unique cohort characteristics with the female-dominated uneven gender distribution. The analysis in the general population is needed to clarify the gender difference of immune response to SARS-CoV-2 vaccine. The relationship between age and S-ab levels is a controversial issue in our study. Several studies have suggested an association between age and post-inoculation antibody levels during the early phase after vaccination (6,12). The younger generation also tended to have higher S-ab levels both at 3 and 8 months after vaccination in our study, and the decreased rates of S-ab levels in 5 months were similar for all generations. However, the elderly ratio of over 60 years of age is low, and we have no data for those over 70 years of age. Therefore, we could not prove immunogenicitic inferiority of the elderly to the vaccine in this study. Nonetheless, it is clear from the results of randomized trial that vaccine efficacy of 86% to 100% can be obtained in all generations for SARS-CoV-2 infection prevention (13). Interestingly, 13 of 400 participants had higher S-ab levels at 8 months than at 3 months after vaccination. All had no evidence of infection or close contact with an infected individual during the observation period. This is significantly different from the general antibody level movement, which peaks just after the second dose and then gradually declines thereafter, as revealed in previous studies (14). Some lifestyle habits such as drinking, weight gain, smoking and immunosuppressants have also been reported to affect antibody levels (12). Further examination is needed to elucidate the pathology of unique S-ab level movement in these participants. It should be recognized that some individuals could continue to maintain the same antibody level after vaccination. This study has several limitations. First, as mentioned above, this study has a marked bias in the female-dominated cohort, and is for healthcare professionals. It does not reflect worldwide data. Furthermore, healthcare workers could have more opportunities for high exposure to COVID-19 rather than the general population. It is quite difficult to differentiate previous asymptomatic-infected persons based on their medical history and physical findings alone. Second, no data was available on participants' preferences such as drinking or smoking, so that their effects on antibody levels could not be included in the analysis. Third, we could not speculate the mechanism of the relationship between systemic adverse reactions and S-ab level after vaccination as we did not have immunological data such as cytokines. In conclusion, this report highlights that the presence of systemic adverse reactions after the second dose was significantly associated with S-ab levels after the administration of the BNT162b2 mRNA COVID-19 vaccine in all generations. We hope that more research will be undertaken to prove this because evidence is inconsistent in the previous studies.
This study was approved by the independent ethics committee of National Hospital Organization Shinshu Ueda National Hospital and was based on the principles of the Declaration of Helsinki. Informed consent was obtained from all participants after explaining the study protocol.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We appreciate all participants who agreed to this study and all staff involved in the vaccination schedule at the National Hospital Organization Shinshu Ueda National Hospital as well as Mr. Toshitaka Maejima, Mr. Ryuichi Matsumoto, and Mr. Hiroya Mizusawa for cooperating and conducting the survey.
References
- 1. Prime minister of Japan and His cabinet. Information related to COVID-19 [Internet]. Available from: https://japan.kantei.go.jp/ongoingtopics/vaccine.html
- 2.Xia X. Domains and functions of spike protein in SARS-CoV-2 in the context of vaccine design. Viruses 13: 109, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y, Chan Y, Xin-Feng X, Wei X, Shu-Wen L. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin 41: 1141-1149, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas ST, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 385: 1761-1773, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto S, Fukunaga A, Tanaka A, et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine 18: 1924-1927, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mochizuki T, Hori T, Yano K, Ikari K, Okazaki K. Factors associated with change in SARS-CoV-2 antibody titers from three to six months after the administration of the BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Japan: a prospective study. Intern Med 61: 1139-1143, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyebanji OA, Wilson B, Keresztesy D, et al. Does a lack of vaccine side effects correlate with reduced BNT162b2 mRNA vaccine response among healthcare workers and nursing home residents? Aging Clin Exp Res 33: 3151-3160, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florian K, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 384: 1372-1374, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang YH, Song KH, Choi Y, et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med 36: 1486-1491, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaniv L, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med 9: 999-1009, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giacomo G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323: 1574-1581, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kageyama T, Ikeda K, Tanaka S, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect 27: 1861.e1-1861.e5, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 385: 1761-1773, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya JG, Amy EA, Valérie B, et al. Differences in IgG antibody responses following BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines. Microbiol Spectr 9: e0116221, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]